SUMMARY
High-throughput electron microscopy has started to reveal synaptic connectivity maps of single circuits and whole brain regions, for example, in the Drosophila olfactory system. However, efficacy, timing, and frequency tuning of synaptic vesicle release are also highly diversified across brain synapses. These features critically depend on the nano-meter-scale coupling distance between voltage-gated Ca2+ channels (VGCCs) and the synaptic vesicle release machinery. Combining light super resolution microscopy with in vivo electrophysiology, we show here that two orthogonal scaffold proteins (ELKS family Bruchpilot, BRP, and Syd-1) cluster-specific (M)Unc13 release factor isoforms either close (BRP/Unc13A) or further away (Syd-1/Unc13B) from VGCCs across synapses of the Drosophila olfactory system, resulting in different synapse-characteristic forms of short-term plasticity. Moreover, BRP/Unc13A versus Syd-1/Unc13B ratios were different between synapse types. Thus, variation in tightly versus loosely coupled scaffold protein/(M)Unc13 modules can tune synapse-type-specific release features, and “nanoscopic molecular fingerprints” might identify synapses with specific temporal features.
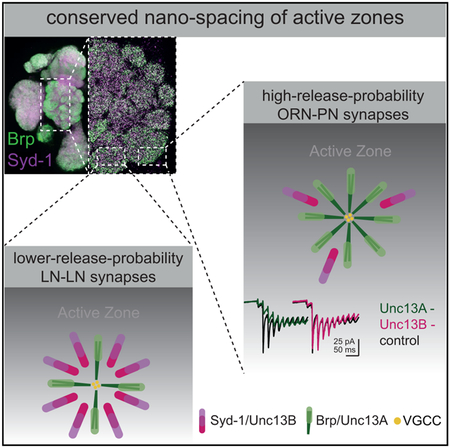
Graphical Abstract
In Brief
Fulterer et al. demonstrates that the scaffold proteins Bruchpilot and Syd-1 cluster (M)Unc13 release factor isoforms either close (BRP/Unc13A) or further away (Syd-1/Unc13B) from voltage-gated Ca2+ channels in the Drosophila olfactory system. These scaffold/release factor “modules” varied significantly between different synapse types, thereby tuning release features toward depression or facilitation.
INTRODUCTION
Synapses are highly specialized structures responsible for the flow of information between neurons. Synapses can exhibit high or low release probabilities. Different release probabilities often distinguish synapses of distinct neuronal populations and account for the diversity of synapse response time (fast and slow synapses), signal strength, and the adaption to signal trains (short-term plasticity) (Gjorgjieva et al., 2016; Jackman and Regehr, 2017). Biophysical and electrophysiological analyses have suggested that both release probability and short-term plasticity depend greatly on the nanometer-scale distance (coupling distance) between synaptic vesicles (SVs) with their Ca2+ sensor Synaptotagmin, and the voltage-operated Ca2+ channels (VGCCs). VGCCs mediate Ca2+ influx in response to membrane depolarizations caused by action potentials (Egger-mann et al., 2011; Stanley, 2016; Vyleta and Jonas, 2014; Wadel et al., 2007; Wang et al., 2016). Essential release factors of the (M)Unc13 protein family thereby seem to define SV release sites and position SVs relative to the VGCCs (Böhme et al., 2016; Sakamoto et al., 2018).
Effective coupling distances vary across mammalian brain synapses, resulting in major functional differences (Eggermann et al., 2011). The molecular mechanisms and proteins controlling these coupling distances remained rather enigmatic for a long time. Notably, only a few conserved families of large scaffold proteins organize the presynaptic active zone (AZ), giving AZs their typical ultrastructural shape and conferring stability to release sites (Petzoldt et al., 2016; Südhof, 2012). The AZ scaffold proteins in both rodents and Drosophila cluster Ca2+ channels and release factors, particularly (M)Unc13 which is essential for SV release (Böhme et al., 2016; Wang et al., 2016). Consequently, these molecules are crucial for patterning evoked SV release.
The functional diversity of specific synapses ultimately must be encoded within their molecular makeup, and molecular diversity exists even within traditional neurotransmitter type classifications (O’Rourke et al., 2012). However, whether molecular “design principles” exist that tune functional diversity over a spectrum of synapse types, remain relevant, but unresolved questions.
We show here that the ratio between two AZ scaffold proteins (ELKS family Bruchpilot [BRP] and Syd-1) varies widely across synapse types of the Drosophila brain and thereby tunes their effective coupling distance. BRP was responsible for clustering the (M)Unc13 release factor isoform Unc13A close to the VGCCs (≈70 nm), while Syd-1 clustered Unc13B further away (> 100 nm). High BRP/Unc13A levels promoted a high release probability at the first relay synapse of the olfactory system and, consequently, supported a fast but depressing release component. By contrast, neighboring interneuron synapses and the second relay projection neuron synapse expressed low Unc13A levels and depended more strongly on Unc13B. Varying tightly versus loosely coupled scaffold protein/(M) Unc13 complexes at presynaptic AZs might, thus, be a principle for tuning synaptic release features. AZ scaffold proteins therefore operate close to the top of an epistatic molecular hierarchy diversifying the release features of synapse types via a differential positioning of Unc13 isoforms.
RESULTS
AZ Scaffold Composition Diversity across Drosophila Brain Synapses
We started by asking whether variation in presynaptic AZ scaffold architecture might contribute to synapse diversity, and stained adult Drosophila brains for two major organizing scaffold proteins: BRP, a key component of the T-bar organization within the AZ center (Kittel et al., 2006), and Syd-1, a conserved AZ protein (Wentzel et al., 2013) localized at the edge of the AZ nanoarchitecture (Owald et al., 2010).
While both scaffold proteins were expressed in all synaptic areas of the brain (not shown), the relative intensity of Syd-1 and BRP varied between CNS regions and within them (Figure 1A). For example, the central brain ellipsoid (Figure 1B) and fan-shaped bodies (Figure 1B’), higher integrative centers for control of locomotion, showed differential staining patterns between the two scaffold proteins. These patterns were observed robustly across experiments and were present both in immunostainings using different antibodies (e.g., various antibodies against BRP), and in the fluorescence signals of GFP knock-in lines (not shown). Thus, these patterns are not artifacts caused by fixation or penetration differences, but instead, reflect real differences in the molecular composition between AZs belonging to distinct synapse types.
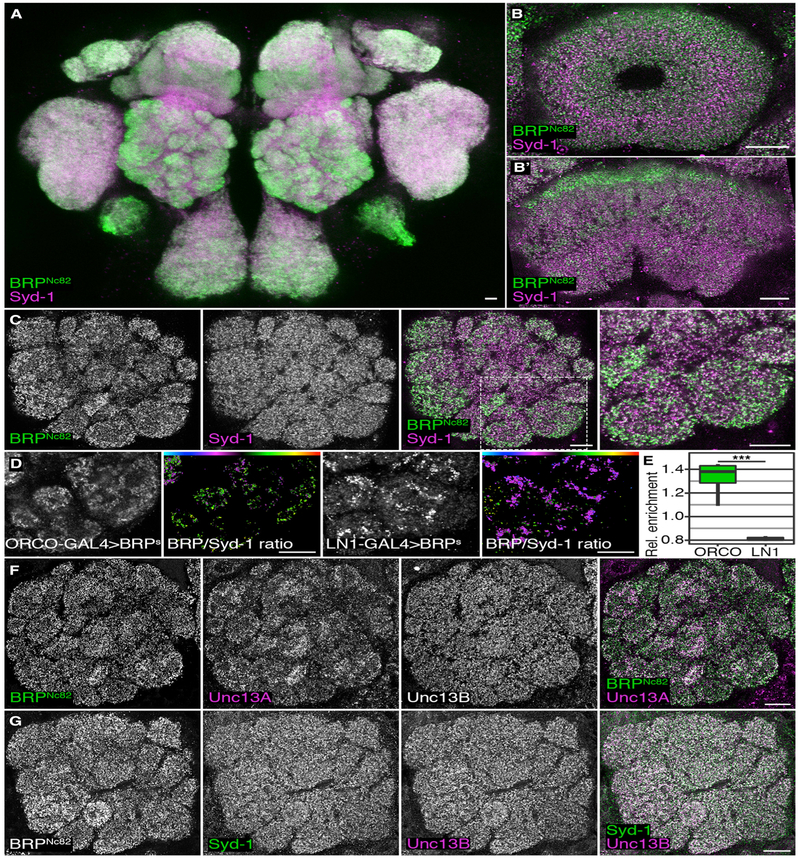
Figure 1. Distribution of Scaffold Proteins Syd-1 and Brp and Release Factor Unc13 Isoforms A and B in Different Neuron Populations of Adult Drosophila melanogaster Brains.
(A–C) Confocal images of adult Drosophila w1118 brains stained against Syd-1 (magenta) and BRPNc82 (green). All scale bars, 10 μm. Central brain maximum intensity projection of a confocal stack (A). Ellipsoid body (B). Fan-shaped body (B’). (C) Antennal lobe (AL), the blow-up shows AL glomeruli VA2 and VA3 (C).
(D) False color-coded median BRP/Syd-1 ratio in the AL glomeruli VA2 and VA3. The AZs of ORNs were highlighted using BRP-short-GFP (BRPS) expressed by ORCO-GAL4 and AZs of inhibitory LNs using LN1-GAL4. Color gradients visualize the ratio between Syd-1 and BRPNc82 (high BRP/Syd-1: green-red; low BRP/Syd-1: magenta-cyan).
(E) GFP-positive median ratios were normalized by median ratios of the surrounding AL to illustrate the relative difference between GFP-positive AZs and the surrounding AL. The AZs positive for ORCO-GAL4 > BRPS were enriched for BRP in comparison to LN1-derived AZs; Mann-Whitney U (MWU) test result: p = 0.000155, n = 8 each. The graph shows medians, interquartile ranges, and min/max values.
(F) AL stained against BRPNc82 (green), Unc13A (magenta), and Unc13B.
(G) AL stained against BRPNc82, Syd-1 (green), and Unc13B (magenta).
See also Figures S1, S2, and S6.
AZ Scaffold Composition Predicts Unc13 Isoform Diversity in the Olfactory System
The Drosophila olfactory system is an important model for studying synaptic principles of sensory information processing, routing, and encoding (Wilson, 2013). Olfactory receptor neurons (ORNs) in the antennal lobe (AL) convey olfactory information from the antenna onto projection neurons (PNs), which carry the information processed within the AL circuit to higher brain centers, called the lateral horn (LH) and mushroom body (MB). Information processing is tuned by local interneurons (LNs) (Chou et al., 2010; Nagel et al., 2015). BRP levels in the AL were high at the cortex of individual AL glomeruli (Figure 1C, see magnified image to the right), a position typical for ORN terminals (Mosca and Luo, 2014; Rybak et al., 2016). Syd-1, however, was distributed rather homogeneously over individual glomeruli and the whole AL. We expressed the AZ scaffold marker BRP-short-GFP (Schmid et al., 2008) within the principal AL neuron populations to test directly to which neuron type these BRP-rich presynaptic AZs belong. Note that BRP-short integrates into AZ scaffolds, but does not contain the C-terminal BRP epitope recognized by the antibody used for our BRP immuno-staining (Kremer et al., 2010). When using an ORN-specific driver line (ORCO-GAL4), the BRP expression pattern followed the distribution of ORN-derived AZs (Figure 1D), and BRP/Syd-1 ratios were high in ORN-derived AZs (Figures 1D, second image, and 1E). By contrast, when using the interneuron-specific LN1-GAL4 line, GFP was expressed primarily toward the center of individual glomeruli, and BRP/Syd-1 ratios were low at inter-neuron-derived AZs (Figures 1D, fourth image, and 1E). BRP is, thus, highly expressed at ORN-derived AZs, but less highly at interneuron-derived AZs.
We have shown previously that expression of BRP-short does not influence AZ number, AZ density, or the terminal area in the MB calyx of adult Drosophila (Kremer et al., 2010). Nevertheless we wanted to rule out that the observed differences in BRP/Syd-1 levels across AL AZ populations might have been influenced by expression of brp-short constructs. We therefore compared BRP/Syd-1 ratios in neuronal populations expressing either BRP-short-GFP or membrane-associated GFP (mCD8-GFP). Neither average BRP and Syd-1 intensities nor the BRP/Syd-1 ratio changed significantly between BRP-short-GFP- and mCD8-GFP-expressing animals (Figure S1A). The differences in BRP/Syd-1 ratios observed using BRP-short-GFP could be replicated when using mCD8-GFP (Figure S1B).
BRP and Syd-1 operate as “molecular spacers” at peripheral neuromuscular junction (NMJ) synapses of Drosophila larvae, and they define Ca2+ channel coupling distances via differential positioning of either the Unc13 isoform Unc13A (via BRP, more closely coupled) or Unc13B (via Syd-1, more loosely coupled) (Böhme et al., 2016). Thus, we tested whether AZs were differentially associated with the release factor Unc13 isoforms A and B within the AL. Unc13A matched BRP expression closely throughout the AL (Figure 1F). As BRP is highly expressed at ORN-derived AZs (Figures 1D and 1E), we conclude that Unc13A is also enriched at ORN-derived AZs (see also Figures 5A and 5B) and confirmed this observation using Spearman correlation-based colocalization analysis (Figure S2A). Unc13B and Syd-1 colocalized as well but were distributed more homogeneously over AL AZs (Figure 1G). Notably, the ORN-to-PN synapse is characterized by a high release probability (Kazama and Wilson, 2008), while LN synapses onto both ORNs and other LNs are rather slow and facilitating (Liu and Wilson, 2013; Nagel et al., 2015; Nagel and Wilson, 2016). Thus, the distribution of Unc13 isoforms and their associated central (BRP/Unc13A) and peripheral (Syd-1/Unc13B) scaffolding proteins correlates with the known properties of AL synapses.
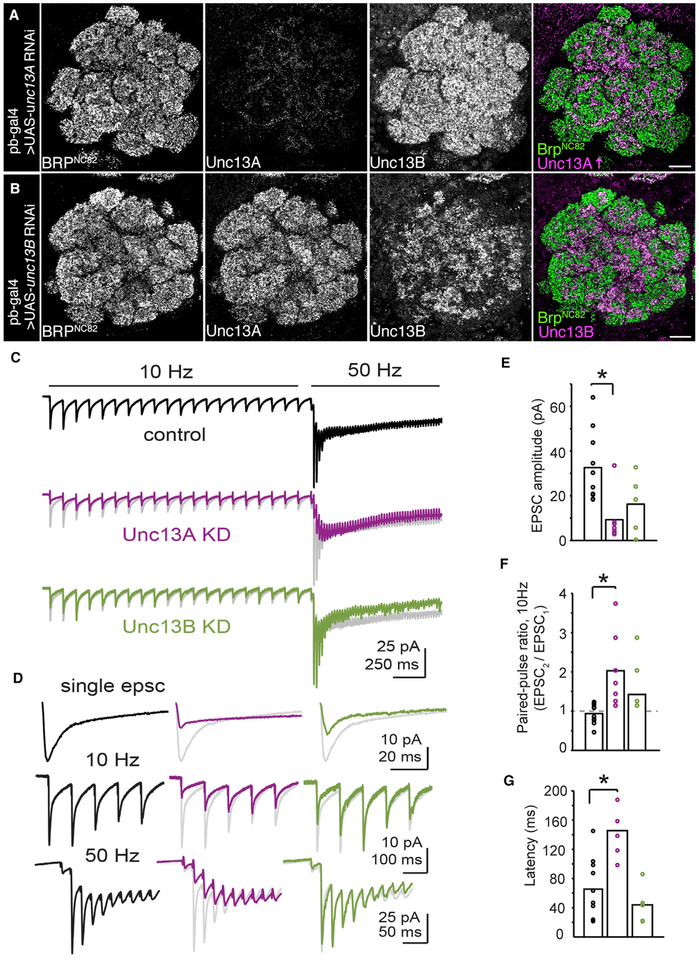
Figure 5. Unc13A and Unc13B Isoforms Play Distinct Roles in Synaptic Transmission at ORN-to-PN Synapses.
(A and B) Confocal sections of adult ALs. All scale bars, 10 mm. Staining against BRPNc82, Unc13A, and Unc13B in pb-GAL4 > UAS-unc13A RNAi flies. The Unc13A brightness was increased in the merged image for visibility (A). Anti-BRPNc82, Unc13A, and Unc13B staining in pb-GAL4 > UAS-unc13B RNAi flies (B).
(C) Group-averaged evoked synaptic currents in control (n = 10 cells), unc13A KD (n = 7 cells), and unc13B KD (n = 5 cells) flies during 10 Hz and 50 Hz stimulation of the antennal nerve. The average response from control recordings is shown in gray behind the average unc13A KD and unc13B KD traces for direct comparison.
(D) Group-averaged data from (C) showing the first EPSC (top), the onset of the 10 Hz response (middle), and the onset of 50 Hz response (bottom), reproduced on zoomed-in timescales. In (C) and (D), stimulus artifacts were minimized for clarity by linearly extrapolating between the pre- and post-stimulation artifact periods, typically 2–3 ms. At the transition from 10 Hz to 50 Hz stimulation, there is facilitation of the EPSC amplitude in control and unc13B KD experiments. This property is lost following unc13A KD and varies across cells.
(E) Quantification of the first EPSC amplitude across genotypes. EPSC amplitudes were significantly different between groups (F = 6.28; p < 0.01, one-way ANOVA with Tukey post hoc comparison). The first EPSC was significantly smaller in unc13A KD flies than in controls (p < 0.01, unpaired Student’s t test) and not significantly reduced after unc13B KD.
(F) Quantification of the paired-pulse ratio during 10 Hz stimulation across genotypes. The paired-pulse ratio varied between groups (F = 6.21; p < 0.01, one-way ANOVA with Tukey post hoc comparison). The paired-pulse ratio was significantly elevated in unc13A KD flies (p < 0.01, unpaired Student’s t test), but not in unc13B KD.
(G) The latency to peak evoked current from the onset of 50 Hz stimulation. The time to peak current was different between groups (F = 15.07; p < 0.001, one-way ANOVA with Tukey post hoc comparison), and was increased relative to controls in unc13A KD flies (p < 0.001). Bar charts represent mean values.
See also Figures S4 and S5.
The PN boutons synapse onto MB Kenyon cell (KC) dendrites form so-called PN microglomeruli in the MB calyx (Kremer et al., 2010), the second relay of the olfactory system. These synapses operate slowly and need to adapt to the very high action potential (AP) frequencies typical for PNs (above 100 Hz) (Gruntman and Turner, 2013). The AZs derived from either KCs or LNs are positioned in between the microglomeruli (Christiansen et al., 2011). The BRP staining showed a rather homogeneous distribution over the calyx (Figure 2A). By contrast, Syd-1 labeling was very strong within microglomeruli (Figure 2A, yellow circles and arrows), where PN boutons synapse onto KC dendrites. We quantified BRP/Syd-1 ratios expressing BRP-short with PN- and KC-specific (Figure 2B) driver lines. The AZs of PNs were strongly enriched for Syd-1 (Figure 2C). Next, we compared Unc13A and Unc13B staining to BRP and Syd-1 (Figures 2D and 2E). As in the AL, the Unc13A distribution matched the BRP distribution (Figure 2D). Similarly, Unc13B strictly followed the Syd-1 pattern (Figure 2E), as confirmed by colocalization analysis (Figure S2B). Unc13B, thus, accumulated at the AZs of PN boutons and was only weakly expressed at the AZs outside microglomeruli (see the magnified image in Figure 2E).
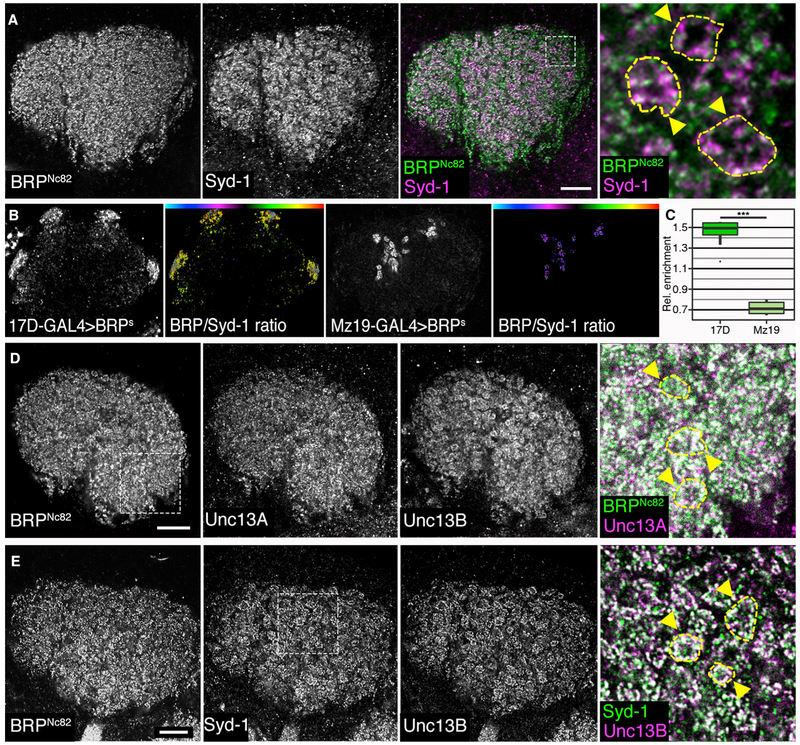
Figure 2. Distribution of Scaffold Proteins Syd-1 and BRP and the Unc13 Isoforms A and B in the Mushroom Body Calyx of Adult Drosophila.
Confocal images of adult Drosophila w1118 mushroom body calyces. All scale bars, 10 μm.
(A) Staining against BRPNc82 (green) and Syd-1 (magenta); the blow-up highlights microglomerular structures labeled by yellow dashed lines and arrowheads.
(B) False color-coded median BRP/Syd-1 ratio in calyces. The AZs of KCs were highlighted using BRP-short-GFP (BRPS) expressed by 17D-GAL4 and AZs of PNs using Mz19-GAL4. Color gradients visualize the ratio between Syd-1 and BRPNc82.
(C) GFP-positive median ratios were normalized by median ratios of the surrounding calyx. The AZs positive for 17D-GAL4 > BRPS were enriched for BRP in comparison to Mz19-derived AZs; MWU test result: p = 0.00000983, n = 8 for 17D and n = 13 for Mz19.
(D) Calyx stained against BRPNc82 (green), Unc13A (magenta), and Unc13B, blow-up highlights microglomeruli outlined by yellow dashed lines and arrowheads.
(E) Calyx stained against BRPNc82, Syd-1 (green), and Unc13B (magenta), blow-up shows microglomeruli indicated by yellow dashed lines and arrowheads. Graphs as explained for Figure 1.
See also Figure S2.
Thus, these two relays of the olfactory system feature an antagonistic expression pattern of Unc13 isoforms: Unc13A enriched in ORN-derived AZs while Unc13B enriched in PN-derived AZs within the calyx. At the same time, the scaffold protein BRP is enriched at ORN- and Syd-1 at PN-derived AZs. Each of these distribution patterns is consistent with the known dynamics of particular synapses, with ORN-to-PN synapses supporting fast transmission, and PN-to-KC synapses exhibiting slow transmission.
BRP Specifically Recruits Unc13A while Syd-1 Specifically Recruits Unc13B to AZs in the Drosophila Brain
We next analyzed whether BRP and Syd-1 recruit specific Unc13 isoforms to AZs. Knockdown (KD) of brp using RNAi constructs with a pan-neuronal driver line (elav-GAL4) drastically reduced Unc13A levels within the AL (Figures 3A and 3C), while Unc13B levels were not reduced (Figure 3C, left). Knockout of syd-1 (Figure S3) reduced Unc13B levels specifically (Figures 3B and 3C, middle). Thus, BRP and Syd-1 not only colocalize with Unc13A and B, respectively, but are necessary for their localization to specific AZs in a largely uncoupled, orthogonal manner. Notably, this relation was unidirectional: the scaffold proteins controlled Unc13 isoform AZ recruitment, but not vice versa. Elimination of either Unc13A or Unc13B had no major consequences on the levels or distribution of either scaffold protein (Figure 3C, right).
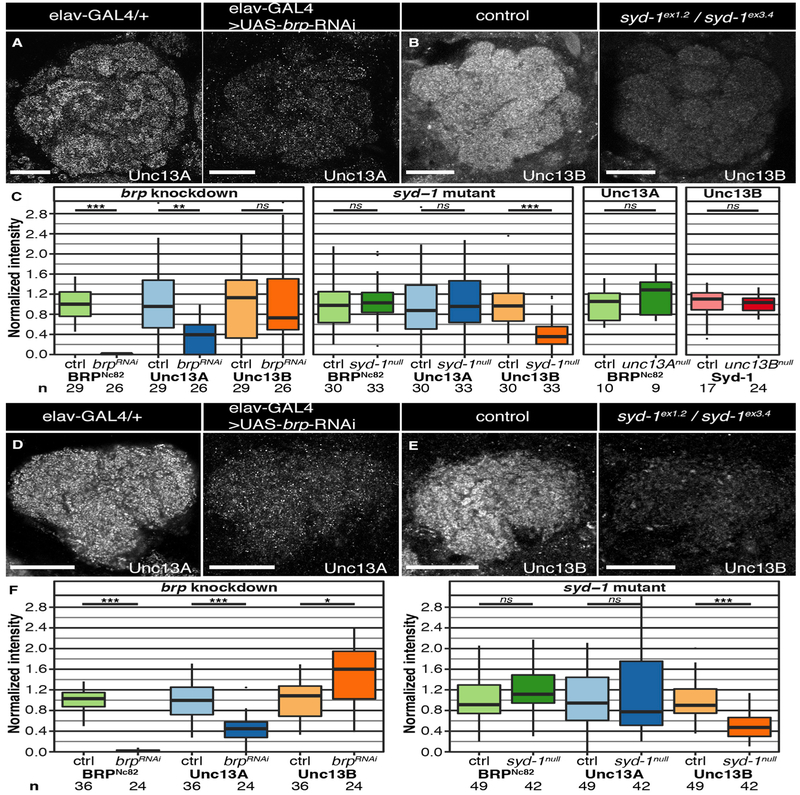
Figure 3. Reduction of Scaffold Proteins BRP and Syd-1 Results in Isoform-Specific Downregulation of Unc13s in the Adult Drosophila Brain.
(A and B) Confocal images of ALs stained against BRPNc82, Unc13A, and Unc13B. All scale bars, 20 μm. Flies with a pan-neuronal brp knockdown (KD; elav-GAL4 > UAS-brp-RNAi) in comparison to a control group (elav-GAL4/+); staining against Unc13A (A). syd-1null mutant flies (sydex1.2/sydex3.4) in comparison to w1118 controls; staining against Unc13B (B).
(C) Average staining intensities of BRP, Unc13A, and Unc13B in the KD or deletion situation normalized to controls show a significant downregulation of average intensity levels of Unc13A (p = 0.0016 for the brp KD). Syd-1 deletion results in downregulation of Unc13B (p = 0.000008). Average intensities of BRP and Syd-1 in unc13A and unc13B deletion mutants, respectively, normalized to controls (unc13B deletion: pacdel100B; unc13P84200; unc13A deletion: ems7.5/unc13P84200). The intensity levels of scaffold proteins were not altered in the mutants.
(D and E) Confocal images of calyces stained against BRPNc82, Unc13A, and Unc13B. All scale bars, 20 μm. Flies with a pan-neuronal brp KD in comparison to controls; staining against Unc13A (D). Syd-1null mutants in comparison to controls; staining against Unc13B (E).
(F) Average staining intensities of BRP, Unc13A, and Unc13B in the KD or deletion situation normalized to controls show a significant downregulation of average intensity levels of Unc13A (p = 0.000018) and a significant upregulation of Unc13B (p = 0.0049) in the brp KD animals. Deletion of syd-1 results in downregulation of Unc13B (p = 0.000002). Association tests were conducted using linear mixed models with imaging batch and animal as nested random effects. Bonferroni-corrected significance thresholds α = 0.0167 for the brp KD and the syd-1 deletion and α = 0.025 for the unc13A/B deletions. Graphs as explained for Figure 1.
See also Figure S3.
We also conducted the same analysis in the MB calyx. As expected, Unc13A clustering was strongly affected by brp KD, but not by the elimination of Syd-1 (Figures 3D–3F), while the Unc13B localization was specifically impeded in syd-1 mutants (Figures 3E and 3F, right). Unc13B staining levels were higher in the PN boutons of brp KD animals than in the controls (Figure 3F, left). Thus, BRP clusters Unc13A and Syd-1 clusters Unc13B across several synapse types of the Drosophila olfactory system. This mechanism likely extends over the whole brain (not shown).
Super Resolution at ORN-to-PN Synapses: Unc13A Is Closer to Ca2+ Channels Than Unc13B
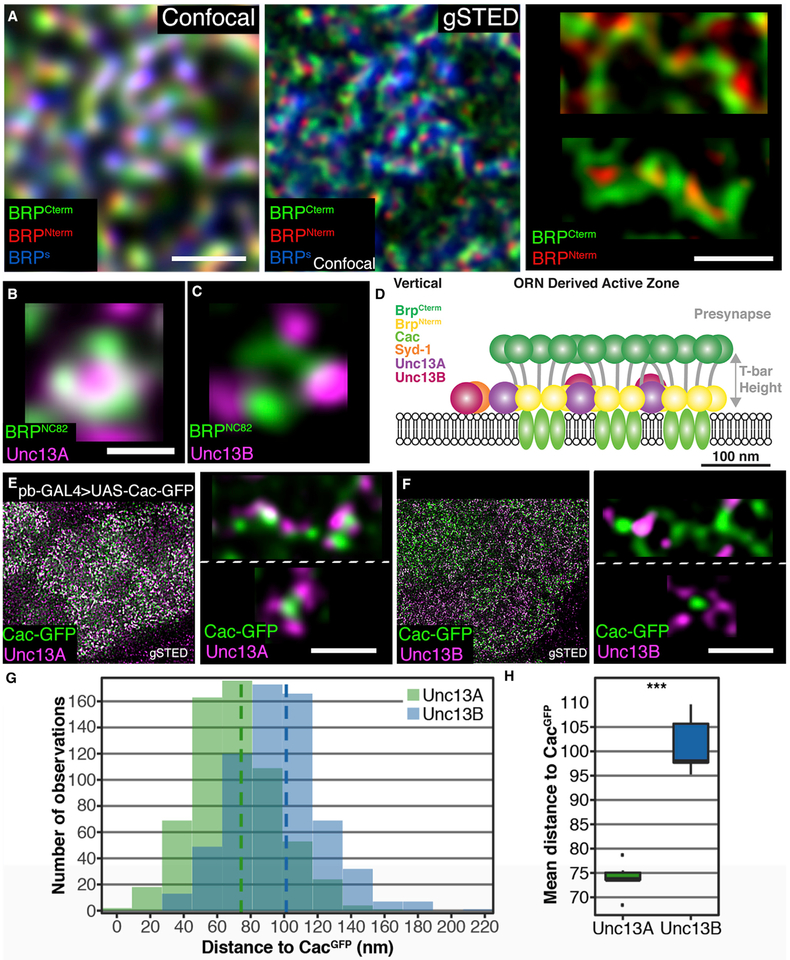
At NMJ synapses, Unc13A clusters more closely to Ca2+ channels than Unc13B, conferring a high release probability to individual APs (Böhme et al., 2016). To examine whether the same clustering principle applies to central cholinergic synapses, we analyzed the nanoscopic pattern of the AZ scaffold at the ORN output synapses with stimulated emission depletion (STED) microscopy. Using this technique (Kittel et al., 2016), we examined whether—similar to NMJ synapses—Unc13A localizes more closely to AZ centers than Unc13B at central ORN-derived AZs.
Due to the rather superficial position of the AL synapses (Spühler et al., 2016), STED imaging with a high resolution of 50 nm (instead of 250 nm achievable in confocal scans) was possible in the AL (Figure 4A). BRP-short-GFP was expressed in ORNs and imaged in parallel to the STED channels to identify ORN-derived AZs (Figure 4A, blue label). The ORN-derived synapses form elongated AZs, at which multiple T bars are fused and arranged opposite to multiple postsynaptic profiles (“dyad synapses”) (Mosca and Luo, 2014; Rybak et al., 2016). Elon-gated BRP staining patterns were thus expected, as BRP is the major building block of T bars. The BRP C terminus, labeled by the Nc82 monoclonal antibody, maps to the distal part (“roof”) of T bars at NMJ synapses (Fouquet et al., 2009). Planar views of T bars show a ring-like distribution of BRP Nc82 epitopes (Fouquet et al., 2009; Mosca and Luo, 2014). By contrast, the BRP N terminus labels the pedestal (“foot”) structure of the T bar above Ca2+ channels at the center of the AZ. Accordingly, multiple T bars nearby should thus be visible as an extended rod-like BRP C-term label with periodic foci of BRP N-term signal. This was indeed observed: the BRP short-labeled ORN-derived AZs featured extended BRP signals with multiple BRP N-term foci (Figures 4A and 4D). Depending on the angle of imaging, BRP C-term signals (Nc82) appeared as either elongated rod-like structures or concatenated ring structures (Figure 4A, upper and lower image, respectively; see Figure 4D for a schematic representation). BRP formed simple rings in between the clusters of the elongated ORN-derived AZs, where interneuron synapses are located (not shown). We investigated the nanospacing of Unc13 isoforms at ORN output AZs, concentrating on planar-imaged AZs appearing as rings (Figures 4B and 4C, planar images). Unc13A colocalized tightly with the BRP C-term signals, while Unc13B was located at greater distances.
Figure 4. Nanoscopic Organization of AZs at ORN-to-PN Synapses.
(A) Sections of adult ALs at confocal and STED resolution. All scale bars, 500 nm. ORCO-GAL4 > UAS-brp-short-GFP brains with staining against GFP (confocal resolution), BRPC-Term (BRPNc82), and BRPN-Term.
(B and C) Magnified planar AZs. Scale bars, 200 nm. Staining against BRPNc82 and Unc13A (B). Staining against BRPNc82 and Unc13B (C).
(D) Cartoon depicting the assumed AZ composition in a side view.
(E) VA2 glomeruli in pb-GAL4 > UAS-Cac-GFP flies showing GFP with an Unc13A staining. Blow-up top: magnified elongated multimeric AZ, Cac-GFP, and Unc13A. Bottom: magnified planar AZ, Cac-GFP, and Unc13A.</p/>(F) VA2 glomeruli in pb-GAL4 > UAS-Cac-GFP flies showing GFP with an Unc13B staining. Blow-up top: magnified elongated multimeric AZ, Cac-GFP, and Unc13B. Bottom: magnified planar AZ, Cac-GFP, and Unc13B.
(G) Histogram of distance bins of either Unc13A to Cac-GFP (green) or Unc13B to Cac-GFP (blue).</p/>(H) Unc13B is further away from Cac-GFP than Unc13A is, p = 9.99 × 10−15, n = 619 measurements for Unc13A and n = 647 for Unc13B across six animals each. Association tests were conducted using linear mixed models with scan, hemisphere, and animal as nested random effects. Graphs as explained for Figure 1.
The BRP C-term signal surrounds Ca2+ channels at the center of AZs at NMJ synapses, while Syd-1 localizes more toward the AZ edge (Owald et al., 2010). Quantification of STED data at these peripheral synapses revealed an average distance of 120 nm between Unc13B and GFP-labeled Ca2+ channels (the voltage-gated α1 subunit Cacophony expressed using the GAL4 system, Cac-GFP), while Unc13A was positioned only 70 nm away (Böhme et al., 2016). To test whether similar differences in coupling distances might exist at ORN-derived AZs, we expressed Cac-GFP within ORNs and co-stained brains with either Unc13A (Figure 4E) or Unc13B (Figure 4F) to quantify the coupling distances (Figures 4G and 4H). A higher overlap between Cac-GFP and Unc13A than with Unc13B was already discernable at low magnification (compare overlap given by the white signal in Figures 4E and 4F, left images). Cac-GFP formed discrete dots at STED resolution, corresponding to the center of single BRP rings at a position approximately at the AZ center. Unc13A localized significantly closer to Cac-GFP dots than Unc13B (compare the planar image in Figures 4E with 4F), as confirmed by quantification (Figure 4H). Distances were very similar to the ones at NMJ synapses (Böhme et al., 2016). Thus, the sub-AZ nanospacing is similarly organized at the cholinergic ORN output synapses, as it is at glutamatergic NMJs: a cluster of Ca2+ channels at the center of the AZ localizes beneath an elongated stretch of BRP proteins, which is surrounded by discrete Unc13A and Unc13B clusters at distinct distances. We next asked whether the two Unc13 isoforms support different release components.
Unc13A Steers Fast, Phasic Release and Plasticity at ORN-to-PN Synapses
To test the functional role of each Unc13 isoform at a central synapse, we generated transgenic RNAi lines that specifically reduced Unc13A and Unc13B levels. When expressed only in ORNs (pan-ORN driver pb-GAL4), unc13A RNAi eliminated nearly all the Unc13A label within the AL (Figure 5A). This indicates that most of the Unc13A in the AL is localized at ORN output AZs, in line with our finding that Unc13A is strongly enriched at ORN output synapses (Figures 1F and S2A). The Unc13B label was not altered by ORN-specific KD of Unc13A (Figure 5A). ORN-driven RNAi against unc13B generated holes in the Unc13B AL staining (Figure 5B), at the positions typical for ORN synapses (at the cortex of glomeruli). However, substantial label remained, consistent with our finding that Unc13B is rather homogeneously distributed across AL synapse populations. Thus, neurons other than ORNs, particularly inter-neurons, contribute to the Unc13B label in the AL.
To test the role of each isoform in ORN-PN synaptic transmission, we expressed either the unc13A or unc13B RNAi in ORNs and recorded synaptic currents from postsynaptic PN neurons while electrically stimulating the antennal nerve, which contains ORN axonal fibers. The antennal nerve was stimulated at 10 Hz and then 50 Hz to simulate spontaneous and odor-evoked firing rates, respectively (Kazama and Wilson, 2008; Nagel et al., 2015). Postsynaptic PNs were identified by their characteristic location and size. The PN input resistances, which have been shown to correlate with EPSC size (Kazama and Wilson, 2008), were similar across control and KD flies (600–1,200 MΩ, Figure S4A). The stimulation current (10–100 μA) was gradually increased until a reliable EPSC was evoked. Recordings in which the stimulation current was >100 μA were excluded.
Synaptic transmission at the ORN-PN synapse is typically fast and depresses with repeated stimulation (Figures 5C, 5D, and 5F) (Kazama and Wilson, 2009; Nagel et al., 2015). However, RNAi-mediated KD of unc13A reduced the amplitude of single EPSCs (Figures 5C–5E) and produced synaptic facilitation in response to a 10 Hz train of stimuli, both of which suggest a reduction in release probability (Figures 5C, 5D, and 5F). Of note, significantly larger currents were required to elicit EPSCs in these flies (Figure S4B). Increasing the stimulation frequency from 10 to 50 Hz in control flies evokes fast EPSCs with renewed depression (Figures 5C, 5D, and 5F). These fast EPSCs were absent or reduced in unc13A KD flies, resulting in a significantly longer latency to peak current (Figure 5G). A very similar pheno-type was observed in flies overexpressing an N-terminal fragment of Unc13A (Figure S5), which lacks the catalytic MUN domain, in ORNs. We have previously shown that this fragment displays a dominant-negative phenotype at NMJ synapses (Reddy-Alla et al., 2017). These data provide independent evidence that Unc13A promotes a fast, transient component of SV release at the ORN-to-PN synapse.
In clear contrast to the loss of unc13A, KD of unc13B spared fast ORN-PN transmission (Figures 5A–5E). Here, paired-pulse dynamics and the latency to peak response during 50-Hz stimulation were not significantly different from control recordings. Moreover, a fast component was observed in all recordings during 50-Hz stimulation. These data are consistent with a model in which Unc13B is dispensable for fast synaptic transmission.
Taken together, Unc13A is of specific importance for fast release at this synapse. The data strongly imply that the BRP-mediated positioning of Unc13A close to Ca2+ channels promotes the high release probability typical for this synapse.
Loss of this component interferes with the fast phasic, quickly depressing release component, shifting the short-term plasticity of the SV release toward a facilitating mode (Figure 5F).
Unc13B Dominates Transmission at Slow Interneuron Synapses
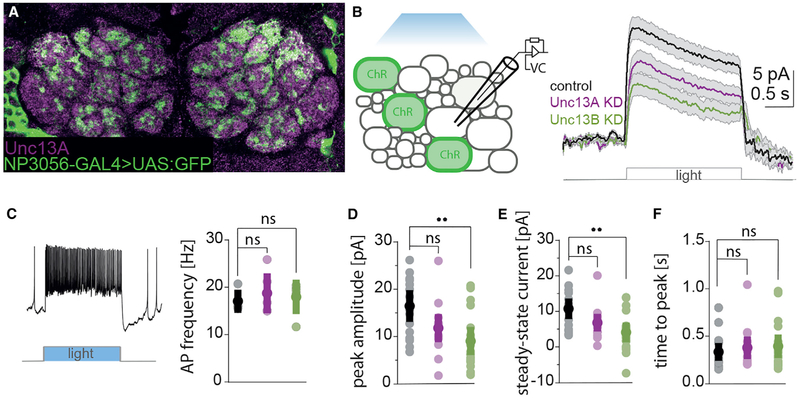
LNs also contribute a major fraction of AZs in the AL. We showed (Figure 1D) that these synapses, in contrast to the ORN-to-PN synapses, display a low BRP/Syd-1 ratio and a low Unc13A level and that they express Unc13B at levels comparable to ORN-derived synapses. While ORN-to-PN synapses are fast and depressing, LN-LN synapses are rather slow and facilitating (Nagel and Wilson, 2016). To examine the role of Unc13 isoforms at LN-LN synapses, we recorded LN-derived synaptic currents after knocking down the Unc13A or B isoforms in these neurons (Figures 6A–6F). We co-expressed Channelrhodopsin (ChR2)-GFP and unc13 isoform-specific RNAi in a subset of LNs using the NP3056-GAL4 line (Chou et al., 2010) (Figure 6A). Previous work established that light-stimulation of Channelrhodopsin results in the activation of inhibitory synaptic currents in non-ChR2-expressing LNs (Nagel and Wilson, 2016). These currents are absent in genetic controls lacking the LN driver (Nagel and Wilson, 2016); similar currents activated chemogenetically can be blocked by GABA antagonists (Liu and Wilson, 2013). To ensure that the firing pattern induced by ChR2 activation in pre-synaptic cells is not affected by the unc13 knockdown itself or by accompanying developmental changes, we first recorded from GFP-positive ChR2-expressing cells. The firing pattern was the same in control and in unc13a/unc13b RNAi expressing lines (Figures 6C).
Figure 6. An Unc13B Knockdown in Unc13B-Dominated LN:LN Synapses Drastically Reduces Release Amplitude.
(A) Staining against Unc13A and anti-GFP in the fly AL. The GFP-positive LNs barely show an Unc13A signal.
(B) Scheme of the experimental procedure. Left: recordings were made from LNs postsynaptic to Channelrhodopsin 2 (ChR2+, GFP+) expressing cells. Voltage command (VC, right) measured average postsynaptic currents in response to optogenetical activation of the presynapse (ChR2+) in control (n = 15), unc13A KD (n = 13), and unc13B KD (n = 18) flies. Traces are mean ± SEM.
(C) Whole-cell current-clamp recordings of presynaptic (ChR2+, GFP+) LNs. Left: example of a spike train in presynaptic LNs evoked by a light pulse. Right: light-evoked firing rate of Channelrhodopsin-expressing cells over a 2-s stimulation period. The action potential frequency in presynaptic LNs (ChR2+) is not affected by the expression of unc13A/unc13B-RNAi constructs. (Kruskal-Wallis test, ns = not significant). From left to right: controls (black), unc13A KD (magenta), unc13B (green).
(D) Average peak amplitudes of postsynaptic currents are significantly different between control and unc13B KD flies (Kruskal-Wallis followed by Dunn’s multiple comparison test, Bonferroni-corrected, controls (black): n = 16, unc13A KD (magenta): n = 14, outliers were removed with the fourth spread method [Devore, 2011], p = 0.069, unc13B KD (green): n = 18, p = 0.001).
(E) Average steady-state current (SSC) measured at 1.5 s after light onset, averaged over a period of 0.5 s. SSC was significantly smaller in unc13B KD flies compared to control animals (Kruskal-Wallis followed by Dunn’s multiple comparison test, Bonferroni-corrected, controls (black): n = 16, unc13A KD (magenta): n = 15, p = 0.10, unc13B KD (green): n = 18, p = 0.001).
(F) Time until outward currents reached the maximum after onset of the light stimulus. The time to reach the peak amplitude remained the same in all of the recorded groups (Kruskal-Wallis followed by Dunn’s multiple comparison test, Bonferroni-corrected, controls (black): n = 16, unc13A KD (magenta): n = 14, outliers were removed with the fourth spread method, p = 0.83, unc13B KD (green): n = 18, p = 0.79). Values represent mean ± SD.
We then patched neighboring unlabeled LNs and recorded light-evoked inhibitory currents (Figure 6B). LNs were identified by their size, location, and characteristic electrophysiological properties. For example, LNs have larger cell bodies than PNs, have lower input resistances, and fire large spikes that surpass 0 mV. In contrast to ORN-to-PN synapses, where the KD of unc13A had the strongest effect and changed the timing of release, the KD of unc13A did not change the timing of release and reduced release in only a nonsignificant manner (Figures 6D–6F). The KD of unc13B, instead, reduced peak amplitudes and steady-state currents significantly compared to controls (Figures 6D and 6E). Notably, the time to peak remained unchanged in LNs (Figure 6F). This may reflect the method of stimulation: Single LNs form only weak synaptic connections onto neighboring LNs (Liu and Wilson, 2013) and here we used optogenetics to stimulate a large population of LNs. Alternatively, it might reflect other differences between ORN-to-PN and LN-LN synapses. The LN receptor sites, for example, could be distant to the presynaptic release sites, leading to uniformly slow responses to both Unc13A- and Unc13B-mediated release. In either case, our data indicate that Unc13B is of greater importance than Unc13A at this synapse type, consistent with their expression pattern. In contrast to the observations in ORNs, where unc13A KD produced the strongest effect on amplitude and slowed down release, the unc13B KD had a larger effect on response amplitude in the LNs.
A Generic Design Rule for Scaffold Protein/Unc13 Isoform Modules
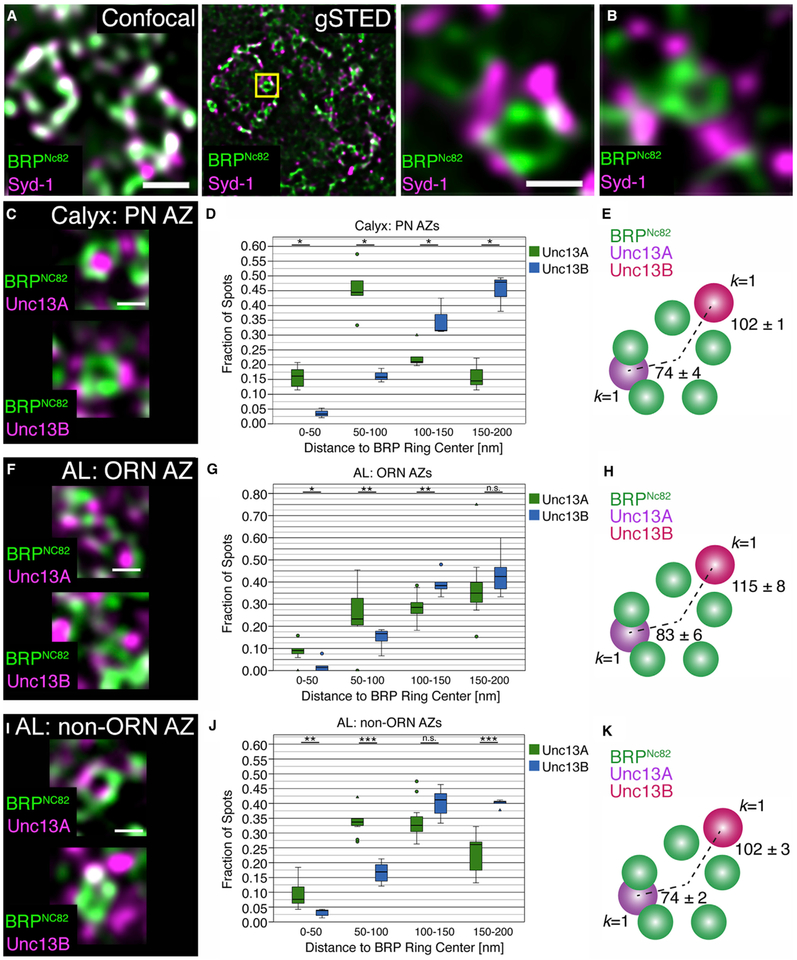
Our electrophysiological and STED data imply collectively that domain spacing on a nanometer scale might be a major contributor to synapse diversity, at least in the Drosophila brain. We finally tested this hypothesis by comparing the sub-AZ spacing of Unc13A and B across synapse types directly and quantitatively. To this end, we analyzed PN-derived AZs within the calyx. Given that Unc13B is the dominant species here, we wondered whether Unc13B also resides distant from the AZ center at PN-KC synapses. The calyx microcircuitry could easily be resolved with STED analysis. The PN-derived AZs within the calycal microglomeruli form single T bars similar to the ones at NMJ synapse AZs (Christiansen et al., 2011; Yasuyama et al., 2002). Accordingly, typical donut-type BRP signals were observed using STED and Syd-1 clustered to the edge of these (Figure 7A), similarly to the ORN-derived AZs in the AL (Figure 7B). We quantified the nanodomain spacing of Unc13A and Unc13B relative to the AZ center where the Ca2+ channels are located (i.e., the center of the BRP rings; Figures 7C–7K). Very similar distances were found for PN- (Figures 7C–7E), ORN- (Figures 7F–7H), and non-ORN AL-derived AZs (Figures 7I–7K): Unc13B localized at a 102–115 nm distance from the center and Unc13A more centrally, at a 74–83 nm distance. These values are similar to the distances found for NMJ AZs (Böhme et al., 2016).
Figure 7. Comparative STED Analysis for ORN-, LN-, and PN-Derived AZs.
(A) Magnified section of an adult wild-type calyx imaged by conventional confocal (left) and time-gated STED (gSTED) microscopy. Scale bar, 500 nm.
(B) Staining against BRPNc82 and Syd-1. The magnified insets show a planar AZ within the calyx microglomeruli and in the AL. Scale bar, 200 nm.
(C) Planar view of BRPNc82 and Unc13A (above) or Unc13B (below) at a PN AZ in the calyx (gSTED). Scale bar, 200 nm.
(D and E) Cluster distance (D) and k nearest-neighbor analysis (E). Boxplot shows the number of Unc13A and Unc13B puncta at defined distances to the center of the BRP ring, normalized to the total number of puncta for each isoform up to 200 nm from the BRP ring center (D; Unc13A: n = 5 brains, 190 AZs; Unc13B: n = 3 brains, 150 AZs). (E) Scheme representing the k nearest-neighbor analysis (k = 1). At the PN AZs, the first Unc13A spot was found closer to the BRP ring center than any Unc13B spot (p < 0.05, Mann-Whitney U (MWU) test).
(F) Planar view of BRPNc82 and Unc13A (upper) or Unc13B (lower) at ORN-derived AZs (gSTED). Note the concatameric structure of the BRP rings. Scale bar, 200 nm.
(G) Cluster distance analysis at ORN AZs in the AL as in (D; Unc13A: n = 11 brains, 128 AZs; Unc13B: n = 5 brains, 97 AZs).
(H) Scheme representing the k nearest-neighbor analysis (k = 1). At the ORN AZs, the first Unc13A spot was found closer to the BRP ring center than any Unc13B spot (p < 0.01, MWU test).
(I) Example of gSTED images of BRPNc82 and Unc13A (upper) or Unc13B (lower) in planar view at non-ORN AZs in the AL. Scale bar, 200 nm.
(J) Cluster distance analysis at non ORN AZs in the AL as in (D; Unc13A: n = 11 brains, 446 AZs; Unc13B: n = 5 brains, 298 AZs).
(K) Scheme representing the k nearest-neighbor analysis (k = 1). At the non-ORN synapses in the AL, the first Unc13A spot was found closer to the BRP ring center than any Unc13B spot (p < 0.001, MWU test). *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant, Mann-Whitney U (MWU) tests. Values indicate mean ± SEM. Graphs as explained for Figure 1.
Taken together, the BRP/Unc13A and Syd-1/Unc13B modules appear to be stereotyped nanospacing modules for either tighter or looser coupling. Their proportions per AZ differ systematically between synapse types in the Drosophila brain. Thus, our analysis identified a basic principle: the AZ scaffold protein composition steers functional diversity by controlling the effective coupling distance, probably to adapt synapse types to their computational tasks within circuits. Notably, we found other essential release factors, Unc18 and Syntaxin, to be equally distributed over the AZ population of the Drosophila brain (Figure S6), emphasizing the specific role of Unc13 family proteins in AZ diversity, at least in this system.
DISCUSSION
Synapses are highly specialized structures mediating the flow of information between neurons and are thought to be cellular substrates for learning and memory. One hallmark of synapses is their functional heterogeneity: synapses can exhibit either high or low transmission fidelity and this diversity results in synapse-specific differences in response kinetics and short-term plasticity. Functional synaptic diversity, particularly regarding synaptic short-term plasticity, is considered critical for routing and encoding sensory information within neuron networks in the brain (Chabrol et al., 2015; Jackman and Regehr, 2017). It plays a major role in the temporal coding of multisensory integration and extraction of specific sensory features. Accordingly, specific synaptic alterations can result in devastating neurological and psychiatric diseases.
Functional synaptic diversity is influenced by pre- and post-synaptic factors: the number, density and location of SVs, VGCCs, receptors, and fusion machinery proteins (O’Rourke et al., 2012). Specific interactions between these molecular players at the nanometer (“nanoscale”) level are believed to define synaptic efficacy and plasticity. Presynaptic factors, particularly the physical distance between presynaptic VGCCs and the sensor for SV fusion, determine the functional coupling distance that influences release probability and, hence, short-term plasticity (Eggermann et al., 2011). Indeed, this coupling varies widely across synapses. Tight coupling has been associated with high release probability and synaptic depression, but loose coupling with low release probabilities and greater paired-pulse facilitation (Eggermann et al., 2011; Vyleta and Jonas, 2014). However, despite the physiological evidence of this kind of presynaptic diversity for brain function, its cellular and molecular underpinnings are poorly understood. Consequently, deciphering the molecular basis of synaptic functional diversity has been named as a major challenge remaining in synapse research (Südhof, 2012). Here, we find evidence for a generic molecular principle that regulates coupling strength to generate functional diversity at Drosophila central synapses.
Nanoscopic Scaffold Diversity Tunes Release Probability and Short-Term Plasticity
Short-term plasticity causes synapses to act as temporal filters, allowing them to transmit a certain range of signal frequencies preferentially. Excitatory and inhibitory neurons especially often show distinct forms of short-term plasticity, as do synapses from the same cell onto different types of interneurons (Beierlein et al., 2003; Stokes and Isaacson, 2010). However, the molecular basis of this synaptic diversity remained mysterious, making it difficult to interrogate the function of this diversity in a circuit context. Here, we show that in the Drosophila olfactory system, expression of unc13 isoforms and their corresponding scaffolding proteins correlates with temporal properties of synaptic transmission.
The first relay synapse of the Drosophila olfactory system displays a particularly high release probability (Kazama and Wilson, 2008). We found that this synapse is enriched for BRP/Unc13A complexes, and expression of unc13A is required for its high release probability. This was evident from the complete abrogation of fast, phasic post-synaptic currents when this specific Unc13 isoform was specifically reduced. Although this first synapse in the Drosophila olfactory system exhibits short-term depression due to its high release probability, it still transmits broadband signals, consistent with our finding that unc13B is expressed uniformly across the AL and that a slow component of transmission remains after unc13A KD. Interestingly, previous work using different pharmacological blockers of nicotinic acetylcholine (ACh) receptors could separate two components of evoked post-synaptic current—one with a fast, the other with a much lower rate of depression (Nagel et al., 2015). Thus, the fast, depressing, Unc13A-mediated release component might be matched by a different ACh receptor than the slow Unc13B component.
In contrast to ORN-to-PN synapses, AL synapses originating from LNs are slow and facilitating (Liu and Wilson, 2013; Nagel et al., 2015; Nagel and Wilson, 2016). Accordingly, we observed that these synapses are low in Unc13A and that, although unc13A KD tended to affect LN-LN synaptic signals as well, unc13B KD had a stronger effect on LN-LN synaptic transmission, indicating a more prominent role for Unc13B at these synapses. Interestingly, the slow time course of transmission was unchanged in either KD situation. This could be an artifact caused by the optogenetic stimulation protocol. However, this is unlikely, as the latency to the first light-induced spike was short in all presynaptic LN recordings (Figure 6C). Another possibility is that postsynaptic factors also contribute to the slowness of these synapses.
The second relay synapse of the olfactory system (PN-KC synapses) transmits high-frequency signals typical for PNs (>100 Hz), is slow and integrates convergent signals (Gruntman and Turner, 2013). Our finding that Unc13B (and accordingly also Syd-1) is particularly prominent at this synapse implies strongly that these synapses are, by their nanoscopic design, tuned toward a facilitating low depression mode, which might well be instrumental to their computational role. Future studies integrating behavioral and electrophysiological analyses at this synapse are warranted to explore this hypothesis.
Toward a “Nanoscopic Code” of Synapse Diversity for Circuit Modeling
An obvious question is how this cellular and molecular diversity is instructed; whether it takes place as a cell-autonomous function of the respective presynaptic neuron or whether postsynaptic partner neurons are involved in the developmental setup of molecular AZ diversity. Postsynaptic targeting specificity has been observed electrophysiologically in mouse neocortex at the AZs of pyramidal cells, connected to two classes of postsynaptic GABAergic neurons (Beierlein et al., 2003; Reyes et al., 1998). The Drosophila system with its unique possibilities for genetic intervention should be an ideal model for studying cellular interactions systematically in the context of establishing synaptic diversity. Cell adhesion proteins, for example, the newly discovered teneurins (Hong et al., 2012; Mosca and Luo, 2014) or LRP4 (Mosca et al., 2017), are attractive candidates for instructing and maintaining AZ diversity via scaffold protein clustering. Interestingly, postsynaptically expressed cell adhesion proteins have been shown to instruct short-term plasticity at hippocampal synapses (Sylwestrak and Ghosh, 2012). It will be interesting to see whether these regulations might involve differential spacing of (M)Unc13 isoforms.
Electron microscopy has been used recently to systematically reconstruct both the AL (Berck et al., 2016) and downstream integrative centers mediating olfactory learning and memory processes (Takemura et al., 2017). In these studies, the electron-dense AZ scaffold (“T bar”) was used as the crucial landmark to identify AZs and, consequently, synaptic connections between reconstructed neurons. Although this complete wiring diagram will clearly help bridging the gap between circuits and behavior, a satisfactory understanding and functional modeling of circuits will also depend on an in-depth knowledge of specific features of the synapse types present. We are confident that the “nanoscopic molecular fingerprints” of synapses, which we started to provide here using the abundances and ratios of specific presynaptic proteins, will be helpful in assessing specific synaptic features.
Scaffold Protein Composition Tuning Functional Diversity: a Generic Design Principle?
Will our results generalize to the mammalian brain? Our recent analysis demonstrated that Unc13 nanoclusters function topologically to generate stable SV release sites, with their defined spacing ensuring determined and reliable timing of SV release (Reddy-Alla et al., 2017). Moreover, a dramatic decrease of synaptic transmission upon disruption of the AZ scaffold has been described in several model systems (BRP/RBP in Drosophila and RIM/RBP or RIM/ELKS in mouse); this observed decrease is in line with a loss of release sites, which was paralleled by a severe reduction of Munc13/Unc13A in all cases (Acuna et al., 2016; Böhme et al., 2016; Wang et al., 2016).
Despite these analogies, future analyses will have to clarify to which degree the exact details of the molecular interactions between scaffold proteins and (M)Unc13 release factors have been conserved. For example, we do currently not know whether the mammalian Syd-1 homologs (Wentzel et al., 2013) are involved in clustering Munc13 isoforms as well. Moreover, given the different structural organization of the Unc13 family N termini, the details of the molecular interactions used in AZ localization and scaffold binding have likely changed over evolutionary time. While a generic role of AZ scaffolds in localizing and stabilizing release sites generated by Unc13 appears probable, modifications in the molecular setup might have led to evolutionary diversified discrete “solutions”. Notably, our findings illustrate how functional diversity might be executed varying the amounts of the same proteins (Nusser, 2018).
Previous work at C. elegans and Drosophila neuromuscular synapses have described a “linear assembly pathway” with Syd-1 targeting BRP/ELKS complexes into growing AZs (Dai et al., 2006; Owald et al., 2010). The fact that BRP/Syd-1 ratios are diversified between Drosophila CNS synapses now requires for modifications of this scheme. While a specific Neurexin-Neuroligin pair operating in conjunction with Syd-1 is crucial for NMJ synapse assembly, the evolutionary diversification of similar cell adhesion molecules might contribute to diversifying AZ assembly schemes.
Considerable biophysical evidence supports the diversification of coupling distances at mammalian synapses (Eggermann et al., 2011). Notably, seminal experiments, where Munc13 iso-forms were expressed in cultured hippocampal neurons in a munc13 null mutant background, have already shown that mammalian Munc13 isoforms differentially control release probability and short-term plasticity and, thus, contribute to the heterogeneity of synaptic information coding (Rosenmund et al., 2002). It should, therefore, be explored whether differential VGCC-coupling of these mammalian Munc13 isoforms also contributes to the functional diversity of synapses in the rodent brain. The well-documented interactions between the canonical AZ scaffold proteins and the extended, evolutionarily diversified N termini of Munc13 family proteins (Deng et al., 2011; Hu et al., 2013; Kawabe et al., 2017; Zhou et al., 2013) have a good chance to be critical for the definition of release sites in a generic manner, and could provide diversification by their differential coupling to Ca2+ channels. Notably, the mammalian BRP homolog ELKS was shown to be involved in the clustering of specific Munc13 isoforms (Kawabe et al., 2017).
Our results collectively elucidate a comparatively simple “molecular syntax” that explains synapse functional diversity in a systematic manner. Our nanoscopic fingerprints contain unique information crucial to integrate the electrophysiological, ultrastructural, and molecular analyses of synapses, and could, thus, become of real importance for our analysis of both the healthy and the diseased brain.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures.
Animal Rearing and Fly Strains
Fly strains were reared under standard laboratory conditions (Sigrist et al., 2003) at 25°C, 65%–70% humidity and constant 12/12 hr light/dark cycle in incubators. If not stated differently, 4–7 days female flies were used for the experiments.
Immunohistochemistry, Image Acquisition, and Analysis
Immunohistochemistry was performed according to our standard protocol (Andlauer et al., 2014). Conventional confocal and STED images were acquired with a TCS SP8 and TCS SP8 gSTED 3× microscope (Leica Microsystems, Wetzlar, Germany), respectively.
Electrophysiology
Whole-cell patch-clamp recordings from PNs and LNs were made as described in Nagel et al. (2015) and Nagel and Wilson (2016). LNs were identified based on morphology and electrophysiological properties.
Statistics
Antibody ratios were calculated using custom ImageJ plugins (http://ratios.andlauer.net). Normalized median ratios were compared using Mann-Whitney U tests in R v3.3.3.
Intensity (e.g., knockdowns and deletions) data as well as STED punctae distances were analyzed in R with linear mixed models using nested random effects; the associations were confirmed using non-parametric permutation tests.
For cluster distance analyses, statistical tests were conducted in SPSS (IBM, Armonk, USA). The nonparametric Mann-Whitney U test was used for analyses.
For colocalization analyses, Spearman rank correlation coefficients were compared using the paired Wilcoxon signed-rank test. For electrophysiological analyses, comparisons between groups were performed by Kruskal Wallis or one-way ANOVA tests, as indicated in the text.
Supplementary Material
Highlights.
Active zone scaffold proteins systematically differ between synapse types in Drosophila
BRP localizes Unc13A 30–40 nm closer to voltage-gated Ca2+ channels than Syd-1 Unc13B
BRP/Unc13A dominates at fast, depressing, Syd-1/Unc13B at slow, facilitating synapses
ACKNOWLEDGMENTS
We thank Madeleine Brünner, Christine Quentin, and Anastasia Stawrakakis for excellent technical assistance. We also thank the Cellular Imaging Facility of the Leibniz Institute for Molecular Pharmacology and the Core Facility BioSupraMol of the Freie Universität Berlin for training, discussion, and support on the Leica TCS gSTED and the TCS SP8 systems. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (Exc 257, TP A3 and A06 SFB958, and TP09 SFB740) to S.J.S. and to A.M.W (Emmy Noether Program), and from NIH (R01MH109690), NSF (IOS1555933), and the NYU Whitehead Fellowship program to K.N. T.F.M.A. was supported by the German Federal Ministry of Education and Research (BMBF) through the Integrated Network IntegraMent, under the auspices of the e:Med Programme (01ZX1614J).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.03.126.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Acuna C, Liu X, and Südhof TC (2016). How to make an active zone: unexpected universal functional redundancy between RIMs and RIM-BPs. Neuron 91, 792–807. [DOI] [PubMed] [Google Scholar]
- Andlauer TF, Scholz-Kornehl S, Tian R, Kirchner M, Babikir HA, Depner H, Loll B, Quentin C, Gupta VK, Holt MG, et al. (2014). Drep-2 is a novel synaptic protein important for learning and memory. eLife 3, e03895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, and Connors BW (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol 90, 2987–3000. [DOI] [PubMed] [Google Scholar]
- Berck ME, Khandelwal A, Claus L, Hernandez-Nunez L, Si G, Tabone CJ, Li F, Truman JW, Fetter RD, Louis M, et al. (2016). The wiring diagram of a glomerular olfactory system. eLife 5, e14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme MA, Beis C, Reddy-Alla S, Reynolds E, Mampell MM, Grass-kamp AT, Lützkendorf J, Bergeron DD, Driller JH, Babikir H, et al. (2016). Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca(2+) channel-vesicle coupling. Nat. Neurosci 19, 1311–1320. [DOI] [PubMed] [Google Scholar]
- Chabrol FP, Arenz A, Wiechert MT, Margrie TW, and DiGregorio DA (2015). Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat. Neurosci 18, 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, and Luo L (2010). Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci 13, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen F, Zube C, Andlauer TF, Wichmann C, Fouquet W, Owald D, Mertel S, Leiss F, Tavosanis G, Luna AJ, et al. (2011). Presynapses in Kenyon cell dendrites in the mushroom body calyx of Drosophila. J. Neurosci 31, 9696–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, and Jin Y (2006). SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci 9, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, and Südhof TC (2011). RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron 69, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore JL (2011). Probability and Statistics for Engineering and the Sciences (Cengage Learning)
- Eggermann E, Bucurenciu I, Goswami SP, and Jonas P (2011). Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci 13, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, and Sigrist SJ (2009). Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol 186, 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorgjieva J, Drion G, and Marder E (2016). Computational implications of biophysical diversity and multiple timescales in neurons and synapses for circuit performance. Curr. Opin. Neurobiol 37, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntman E, and Turner GC (2013). Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat. Neurosci 16, 1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Mosca TJ, and Luo L (2012). Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Tong XJ, and Kaplan JM (2013). UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. eLife 2, e00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, and Regehr WG (2017). The mechanisms and functions of synaptic facilitation. Neuron 94, 447–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe H, Mitkovski M, Kaeser PS, Hirrlinger J, Opazo F, Nestvogel D, Kalla S, Fejtova A, Verrier SE, Bungers SR, et al. (2017). ELKS1 localizes the synaptic vesicle priming protein bMunc13–2 to a specific subset of active zones. J. Cell Biol 216, 1143–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama H, and Wilson RI (2008). Homeostatic matching and nonlinear amplification at identified central synapses. Neuron 58, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama H, and Wilson RI (2009). Origins of correlated activity in an olfactory circuit. Nat. Neurosci 12, 1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. (2006). Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051–1054. [DOI] [PubMed] [Google Scholar]
- Kremer MC, Christiansen F, Leiss F, Paehler M, Knapek S, Andlauer TF, Förstner F, Kloppenburg P, Sigrist SJ, and Tavosanis G (2010). Structural long-term changes at mushroom body input synapses. Curr. Biol 20, 1938–1944. [DOI] [PubMed] [Google Scholar]
- Liu WW, and Wilson RI (2013). Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc. Natl. Acad. Sci. USA 110, 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, and Luo L (2014). Synaptic organization of the Drosophila antennal lobe and its regulation by the Teneurins. eLife 3, e03726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Luginbuhl DJ, Wang IE, and Luo L (2017). Presynaptic LRP4 promotes synapse number and function of excitatory CNS neurons. eLife 6, e27347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KI, and Wilson RI (2016). Mechanisms underlying population response dynamics in inhibitory interneurons of the Drosophila antennal lobe. J. Neurosci 36, 4325–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KI, Hong EJ, and Wilson RI (2015). Synaptic and circuit mechanisms promoting broadband transmission of olfactory stimulus dynamics. Nat. Neurosci 18, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z (2018). Creating diverse synapses from the same molecules. Curr. Opin. Neurobiol 51, 8–15. [DOI] [PubMed] [Google Scholar]
- O’Rourke NA, Weiler NC, Micheva KD, and Smith SJ (2012). Deep molecular diversity of mammalian synapses: why it matters and how to measure it. Nat. Rev. Neurosci 13, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Körner J, et al. (2010). A Syd-1 homo-logue regulates pre- and postsynaptic maturation in Drosophila. J. Cell Biol 188, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzoldt AG, Lützkendorf J, and Sigrist SJ (2016). Mechanisms controlling assembly and plasticity of presynaptic active zone scaffolds. Curr. Opin. Neurobiol 39, 69–76. [DOI] [PubMed] [Google Scholar]
- Reddy-Alla S, Böhme MA, Reynolds E, Beis C, Grasskamp AT, Mampell MM, Maglione M, Jusyte M, Rey U, Babikir H, et al. (2017). Stable positioning of Unc13 restricts synaptic vesicle fusion to defined release sites to promote synchronous neurotransmission. Neuron 95, 1350–1364. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, and Sakmann B (1998). Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci 1, 279–285. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, and Rhee JS (2002). Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 33, 411–424. [DOI] [PubMed] [Google Scholar]
- Rybak J, Talarico G, Ruiz S, Arnold C, Cantera R, and Hansson BS (2016). Synaptic circuitry of identified neurons in the antennal lobe of Drosophila melanogaster. J. Comp. Neurol 524, 1920–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Ariyoshi T, Kimpara N, Sugao K, Taiko I, Takikawa K, Asanuma D, Namiki S, and Hirose K (2018). Synaptic weight set by Munc13–1 supramolecular assemblies. Nat. Neurosci 21, 41–49. [DOI] [PubMed] [Google Scholar]
- Schmid A, Hallermann S, Kittel RJ, Khorramshahi O, Frölich AM, Quentin C, Rasse TM, Mertel S, Heckmann M, and Sigrist SJ (2008). Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat. Neurosci 11, 659–666. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, and Schuster CM (2003). Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci 23, 6546–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spühler IA, Conley GM, Scheffold F, and Sprecher SG (2016). Super resolution imaging of genetically labeled synapses in Drosophila brain tissue. Front. Cell. Neurosci 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF (2016). The nanophysiology of fast transmitter release. Trends Neurosci 39, 183–197. [DOI] [PubMed] [Google Scholar]
- Stokes CC, and Isaacson JS (2010). From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron 67, 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2012). The presynaptic active zone. Neuron 75, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, and Ghosh A (2012). Elfn1 regulates target-specific release probability at CA1-interneuron synapses. Science 338, 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura SY, Aso Y, Hige T, Wong A, Lu Z, Xu CS, Rivlin PK, Hess H, Zhao T, Parag T, et al. (2017). A connectome of a learning and memory center in the adult Drosophila brain. eLife 6, e26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyleta NP, and Jonas P (2014). Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science 343, 665–670. [DOI] [PubMed] [Google Scholar]
- Wadel K, Neher E, and Sakaba T (2007). The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 53, 563–575. [DOI] [PubMed] [Google Scholar]
- Wang SSH, Held RG, Wong MY, Liu C, Karakhanyan A, and Kaeser PS (2016). Fusion competent synaptic vesicles persist upon active zone disruption and loss of vesicle docking. Neuron 91, 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel C, Sommer JE, Nair R, Stiefvater A, Sibarita JB, and Scheiffele P (2013). mSYD1A, a mammalian synapse-defective-1 protein, regulates synaptogenic signaling and vesicle docking. Neuron 78, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI (2013). Early olfactory processing in Drosophila: mechanisms and principles. Annu. Rev. Neurosci 36, 217–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, and Schürmann FW (2002). Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J. Comp. Neurol 445, 211–226. [DOI] [PubMed] [Google Scholar]
- Zhou K, Stawicki TM, Goncharov A, and Jin Y (2013). Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. eLife 2, e01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.