Abstract
The clinical syndrome referred to as viral hemorrhagic fever (VHF) can be caused several different families of RNA viruses, including select members of the arenaviruses, bunyaviruses, filoviruses and flaviviruses. VHF is characterized by malaise, fever, vascular permeability, decreased plasma volume, coagulation abnormalities and varying degrees of hemorrhage. Study of the filovirus Ebola virus has demonstrated a critical role for suppression of innate antiviral defenses in viral pathogenesis. Additionally, antigen presenting cells are targets of productive infection and immune dysregulation. Among these cell populations, monocytes and macrophages are proposed to produice damaging inflammatory cytokines, while infected dendritic cells fail to undergo proper maturation, potentially impairing adaptive immunity. Uncontrolled virus replication and accompanying inflammatory responses are thought to promote vascular leakage and coagulopathy. However, the specific molecular pathways that underlie these features of VHF remain poorly understood. The arenavirus Lassa virus and the flavivirus yellow fever virus exhibit similar molecular pathogenesis suggesting common underlying mechanisms. Because non-human primate models that closely mimic VHF are available for Ebola, Lassa and yellow fever viruses, we propose that comparative molecular studies using these models will yield new insights into the molecular underpinnings of VHF and suggest new therapeutic approaches.
Viral hemorrhagic fever.
The clinical syndrome classically referred to as viral hemorrhagic fever (VHF) is characterized by malaise, fever, vascular permeability, decrease plasma volume, coagulation abnormalities and varying degrees of hemorrhage [1–3]. VHF can be caused in human by select members of several RNA virus families including arenaviruses (e.g. Lassa, Junin, and Lujo virus), bunyaviruses (Crimean Congo hemorrhagic fever and hemorrhagic fever with renal syndrome virus), filoviruses (Ebola and Marburg virus), and flaviviruses (yellow fever virus and dengue virus). Among those viruses that cause VHF, the frequency of hemorrhage manifestations can vary, but generally represents the most severe form of disease caused by these pathogens. Because disease which progresses to VHF has high fatality rates, the viruses associated with this syndrome are of particular concern from a public health perspective; this is in part because of the threat posed by these agents as bioweapons but also because outbreaks arising by natural means can have devastating impacts on populations [4]. This is evidenced by the 2013-2016 West Africa Ebola epidemic which killed more than 11,000 people, the annual burden of 100,000 to 300,000 Lassa virus cases in Africa and the 2016 yellow fever virus outbreak in Angola which caused more than 3,700 infections and 364 deaths [5-8]. This review will summarize our current understanding of the pathogenesis of VHF by focusing Ebola virus, as this is probably the most intensively studied VHF and which has the most developed non-human primate model of VHF. We will also, however, draw comparisons to two other VHF viruses for which there are well-developed animal models that closely mimic human VHF, Lassa virus and yellow fever virus (YFV).
Ebola virus.
Among viruses classified as VHF agents, Ebola virus is the most extensively studied. Ebola virus is a member of the filovirus family. The Filoviridae are classified into three genera: Ebolavirus, Marburgvirus and Cuevavirus [9]. The Ebolavirus and Marburgvirus genera include human pathogens. Ebolavirus includes five species: Zaire ebolavirus (Ebola virus), Sudan ebolavirus, Bundibugyo ebolavirus, Tai Forrest ebolavirus and Reston ebolavirus. The Marburgvirus genus contains a single species, Marburg marburgvirus (Marburg virus). The filoviruses are filamentous, enveloped, negative-sense RNA virus with a genome of approximately 19,000 nucleotides [10]. Ebola virus encodes eight primary transation products from seven genes. The proteins are known as nucleoprotein, viral protein of 35 kDa (VP35), VP40, soluble glycoprotein (sGP), glycoprotein (GP), VP30, VP24 and large protein (L)-which is the enzymatic component of the viral RNA dependent RNA polymerase [10].
Filoviruses are zoonotic pathogens. For Marburg virus, Rousettus aegyptiacus bats almost certainly serve as reservoir hosts, as these animals have been found to harbor live Marburg virus in nature [11], Circumstantial evidence including serological studies and detection of filoviral nucleic acids in tissue samples, implicate bats as reservoir hosts for Ebola virus and other filovirus family members [12], Expectations are that a reservoir host should not typically suffer severe disease or high lethality from infection. Consistent with this, bats experimentally-infected with Marburg virus or Ebola virus do not suffer overt signs of disease, despite replication in vivo, and infections seem to be cleared, presumably due to bat immune responses [13], This scenario differs dramatically from the situation in humans and non-human primates. In humans, Ebola virus is notorious for causing severe disease with high fatality rates [10], In some Ebola virus outbreaks, case fatality rates have been reported to be as high as 90 percent; and a Marburg virus outbreak in Angola in 2005 was reported to have an 88 percent case fatality rate [10], Whereas prior filovirus outbreaks had resulted in as many as a few hundred human cases, a much larger Ebola virus event occurred in West Africa. An outbreak which is thought to have begun in late 2013, expanded into an epidemic that extended into 2016, becoming by far the largest outbreak on record. This involved more than 28,000 cases and more than 11,000 deaths [8].
Ebola virus disease.
Long known as Ebola hemorrhagic fever, the clinical syndrome caused by Ebola virus is now called Ebola virus disease (EVD). This reflects the fact that, as in other VHFs, Ebola virus does not uniformly cause overt signs of hemorrhage. Typical cases of EVD present as follows [14], The incubation period is generally 3–13 days with abrupt onset of nonspecific symptoms such as fever, chills, fatigue, headache, myalgia, nausea, vomiting, and diarrhea. Hypotension is common and can progress to shock and death over the course of the illness. Rash is common but not always present. Leukopenia and thrombocytopenia occur. Evidence of liver damage including serum alanine and aspartate aminotransferase (ALT, AST) elevation is seen. Coagulation defects occur as evidenced by prolonged prothrombin time (PT), partial thromboplastin time (PTT), and bleeding, with patients often meeting the formal criteria for disseminated intravascular coagulation (DIC). Further, elevated D-dimers are detected in plasma indicative of fibrin degradation corresponding to blood clot formation. Pathology specimens from autopsy show hepatocellular necrosis, loss of lymphocytes from spleen and lymph nodes and tubular necrosis in the kidneys.
Lymphopenia is a characteristic feature of severe Ebola virus infection and lymphoid depletion and necrosis are common in the spleen, thymus, and lymph nodes in fatal human infection and in experimentally infected NHPs [15-19], Despite the loss of lymphocytes during Ebola virus infection, the lymphocytes themselves are not infected, due in part to an inability of Ebola virus to successfully enter these cell types [20], In macaque models, the lymphocyte loss is greatest among T-lymphocytes and natural killer (NK) cells [21], The mechanism(s) that underlie lymphocyte apoptosis is not clear but may be triggered via several different agonists or pathways, including the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and Fas death receptor pathways [19,22,23], impairment of DC function [19,22,24,25], abnormal production of soluble mediators such as nitric oxide that have proapoptotic properties [19,22,26,27], or by direct interactions between lymphocytes and EBOV proteins [28], EBOV infection of humans and NHPs triggers the expression of a number of inflammatory mediators including the IFNs, IL-6, IL-8, IL-10, IL-12, IFN-inducible protein (IP)-10, monocyte chemoattractant protein-1 (MCP-1), normal T-cell expressed and secreted (RANTES), TNF-α, and reactive oxygen and nitrogen species [27,29,30,22,31,26,32,23], Infection of various primary human cells in vitro also shows that EBOV infection can trigger the production of many of these same inflammatory mediators [22,33,34], The virus-induced expression of these mediators may result in an immunologic imbalance that contributes to failure of adaptive immune responses to clear infection in fatal cases, but the details of such a model remain to be worked out.
Thrombocytopenia, consumption of clotting factors, and increased levels of fibrin degradation products indicate impairment of the coagulation system during EBOV infection. Clinical laboratory data suggest that coagulation is impaired by Ebola virus infection in humans [31,35-37] and NHPs [38,39], Notably, D-Dimer levels in blood specimens were substantially increased in patients with fatal and nonfatal EBOV infections but were four times higher in patients with fatal cases than in patients who survived [35], Infection triggers expression of tissue factor (TF) which activates coagulation cascades, and an intervention in NHPs designed to inhibit TF triggered blood coagulation, recombinant nematode anticoagulant protein c2 (rNAPc2), was able to protect some animals from an otherwise lethal infection. This treatment also modified the NHP cytokine response and viral titers, suggesting links between inflammation, coagulation and antiviral responses [39].
Most current models suggest that excessive pro-inflammatory cytokine production and absence of an adaptive immune response are major factors in Ebola virus pathogenesis. However, the most important in vivo sources of the cytokine storm, the relevant signaling pathways that trigger these responses, the mechanisms by which these signals are activated and the specific contribution of cytokine production to dysregulation of antiviral immunity, disseminated intravascular coagulation and circulatory shock all remain to be defined.
EBOV evasion of host antiviral responses.
Ebola virus and other filoviruses have a broad tissue tropism in primates, infecting many different cell types in the liver, spleen, adrenal glands and other organs [40,15,17,41], As noted above, Ebola virus and other filoviruses readily infect antigen-presenting cells (macrophages, dendritic cells (DCs)) and these cell types have been shown, in temporal studies in nonhuman primates (NHPs), to be early targets of EBOV replication [41,19,24,33,25,34,22]. Evidence suggests that monocyte and macrophage infection results in proinflammatory cytokine production which may contribute to manifestations of disease [22,34,42]. In contrast to macrophages, DCs are not activated by infection, and impair activation of T cells [25,24,43]. The loss of DC antigen-presenting function may why patients with fatal Ebola hemorrhagic fever (HF) show persistent high viremia in the absence of detectable virus-specific antibodies [44], although survivors do mount a potent antiviral adaptive immune response [45].
Ebola virus encodes multiple mechanisms to block interferon (IFN) responses. Most relevant to innate antiviral defense are the type I IFNs (IFNβ and multiple IFNαs). These serve as a major arm of the innate antiviral immune response [46]. IFN expression is triggered by several different pattern recognition receptors, including the RIG-I-like receptors (RLRs) RIG-I, MDA5 and LGP2, and several toll-like receptors (TLRs) such as TLR3, TLR4, TLR7 and TLR9. Upon their expression, IFNs are released from the producing cells and can signal in autocrine or paracrine fashion by binding the IFN apha receptor (IFNAR) and activating a JAK-STAT signaling pathway and induces expression of numerous IFN-stimulated genes (ISGs), triggering an antiviral state that renders cells refractory to viral infection [47].
The VP35 protein of Ebola virus is a multifunctional dsRNA binding protein that participates in interactions with the viral RNA polymerase and nucleoprotein that are critical for viral genome replication and production of viral mRNAs [48-50]. In addition, however, VP35 potently suppresses IFN production that would otherwise be triggered by RNA virus infection [51]. This function appears to be critical for virulence [52,53], For RNA viruses that replicate in the cytoplasm, the RIG-I-like receptors (RLRs) RIG-I and MDA5 are major sensors of infection, as they detect RNA products of viral replication that possess features that mark them as “foreign” [54]. Among such features, double-strandedness and the presence of 3’-triphophates are sensed by RIG-I. Activation of RLRs results in signaling that leads to activation of the serine/threonine kinases TBK-1 and/or IKKε, the phosphorylation of transcription factors interferon regulatory factor (IRF)-3 or −7 and IFN gene expression. VP35 has been described to antagonize RLR signaling at several points. It interacts with TBK-1 and IKKε, displacing their interaction with IRF-3 or IRF-7, contributing to inhibition of their activation [55]. VP35 has also been reported to inhibit IRF-7 mediated transcription by a mechanism involving VP35 interaction with IRF-7, the SUMO conjugating enzyme Ubc9 and the SUMO E3 ligase PIAS1 [56]. Despite these mechanisms, potent suppression of RLR signaling and IFN production requires VP35’s dsRNA binding activity [48,57,52]. Point mutations that disrupt this function while leaving VP35 viral RNA synthesis functions intact severely impair suppression of IFN responses [52,58]. This may reflect the capacity of VP35 to bind to RLR activating dsRNAs, as wild-type but not dsRNA binding mutant VP35 can directly interfere with activation of RIG-I by dsRNAs in vitro [59]. However, VP35 residues required for dsRNA binding are also required for its interaction with cellular protein PACT, which facilitates RIG-I activation [59]. By binding PACT, VP35 prevents PACT interaction with and activation of RIG-I. Disruption of dsRNA binding greatly disrupts VP35 suppression of IFN production in transfection studies. Further, when mutant VP35s are built into recombinant Ebola viruses, robust IFN responses are activated by infection. These data suggest that the dsRNA-dependent functions of VP35 are the major suppressors of IFN production.
VP35 also likely interferes with development of adaptive immune responses. As noted above, Ebola virus impairs DC maturation and function. VP35-mediated suppression of RLR signaling makes a major contribution to DC inhibition. As indicated earlier, DCs are early targets of Ebola virus [19]. DCs function to present antigen and promote adaptive immunity [60]. Infection of DCs with Ebola virus disrupts normal DC maturation processes that are required for efficient DC maturation [24,25,43]. However, Ebola viruses built to encode dsRNA binding mutants of VP35 trigger a robust DC maturation as well as robust IFN responses [43]. Expression of VP35 alone in DCs reproduces the inhibition of maturation seen with wild-type Ebola virus, while dsRNA binding mutants lose this suppressive capacity, indicating that VP35 inhibition of RLR signaling is the major mechanism by which Ebola virus inhibits DCs [61–63].
VP35 RLR inhibition is also critical for Ebola virus virulence. This has been demonstrated in both mouse and guinea pig models, where infection with a dsRNA binding mutant of Ebola virus results in substantial attenuation relative to wild-type VP35 [52,53]. While the molecular basis of attenuation of VP35 mutants in vivo have not yet been reported, decreased viral loads correlate with reduced disease. Because the IFN response can substantially suppress growth of VP35 mutant Ebola viruses in cell culture and in vivo; the VP35 defect in RLR signaling likely results in potent innate immune responses that control infection in vivo, thereby explaining attenuation. In this regard, it is notable that the related Marburg virus does encode a VP35 protein that impairs IFN responses [64]. However, the Marburg VP35 protein is somewhat less suppressive of IFN responses than is the Ebola virus VP35 [65]. This may reflect in part differences in how the Marburg virus VP35 protein antagonizes IFN responses. Further, Marburg virus induces more IFN response than does Ebola virus in cell culture studies. Nonetheless, some outbreaks of Marburg virus, particular one on Angola in 2005, resuting in high reported case fatality rates, comparable to what has been reported for Ebola virus [66]. This suggests that absolute suppression of IFN responses is not necessary for a virus to cause VHF. It may be that Marburg virus has evolved other mechanisms that allow it to replicate efficiently when IFN is produced.
Ebola virus also suppresses the capacity of cells to respond to exogenous IFNs. Addition of IFN to cells results in activation of a Jak-STAT pathway in which STAT1 and STAT2 become phosphorylated by Jak family tyrosine kinases. This allows the interaction of STAT1 with the NPI-1 subfamily of karyopherin alpha (KPNA) (also known as importin alpha) proteins, allowing the nuclear accumulation of protein complexes containing STAT1 and activation of IFN stimulated genes (ISGs) [67]. The Ebola virus VP24 blocks the nuclear accumulation of tyrosine phosphorylated STAT1 by binding to the NPI-1 subfamily of KPNAs, blocking their binding to the non-classical nuclear localization signal on STAT1 and preventing expression of ISG expression [68-71]. This blocks the antiviral effects of IFNs. Interestingly, Marburg virus also differs from Ebola virus in how it blocks IFN signaling. Instead of its VP24 protein serving as a suppressor of IFN responses, the Marburg virus matrix protein VP40 blocks the activity of the kinase Jak1. This prevents the tyrosine phosphorylation events that typically occur after IFN addition to cells. This prevents ISG induction and blocks antiviral responses [72,73]. The extent to which these different inhibitory strategies influence the outcome of infection remains to be determined. However, it seems likely that these functions protect infected cells from the antiviral effects of IFNs produced in vivo and may contribute to the relative poor efficacy of IFNs administered to treat these infections.
In addition to blocking signaling pathways related to RLR and IFN responses, Ebola virus encodes proteins that can counteract specific IFN-induced antiviral proteins and that may interfere with adaptive immunity by other means. Notable examples, include the inhibition of the IFN-induced antiviral kinase PKR, which is inhibited by VP35; the ability of the Ebola virus glycoprotein (GP) to counteract BST-2, also known as tetherin, which can prevent release of budding virus particles from infected cells; and the capacity of GP to potentially mask the presence of class I major histocompatibility complex on the cell surface [74-76]. The relative contribution of these functions to pathogenesis also requires further investigation.
Induction of inflammation.
As noted above, a major feature of VHF is the induction of a systemic inflammatory response, sometimes referred to as “cytokine storm.” That this occurs despite the capacity of Ebola virus to potently suppress innate immune responses in infected cells is striking and incompletely understood. In DCs for example, VP35 appears to suppress not only IFN production but also inflammatory cytokine production [61,62]. In contrast, numerous studies have infected peripheral blood mononuclear cells (PBMC), monocytes or macrophages and detected robust cytokine responses, suggesting that these cells may be important sources of damaging soluble mediators in vivo [34,22,42,33,77,78]. The pathways involved in the inflammatory responses and the viral products that trigger them remain to be defined. It is also unclear whether infected cells are the primary sources of cytokines or whether cytokines may be triggered largely via a bystander effect. One possibility is that GP triggers these responses, as GP activates signaling via Toll like receptor 4 [79]. Further GP is present on the surface of viral particles, on the surface of infected cells and is released in various forms as soluble protein; providing several potential mechanism to trigger systemic inflammation. Recent work correlates induction of human primary macrophage inflammatory cytokine production by the virulent Ebola virus but not by the more attenuated Reston virus with the capacity of Ebola versus Reston virus GPs to activate TLR4 signaling [42]. However, the contribution of GP-mediated inflammation to in vivo disease remains to be validated and other mechanisms may also be in play. Further defining proinflammatory pathways and defining which cytokines are most relevant to disease versus promoting effective antiviral responses could provide new avenues to therapeutic intervention.
Similarities of other VHFs to Ebola virus.
Lassa virus.
Despite belonging to a different virus family, Lassa virus can cause VHF with many similarities to Ebola virus disease. Lassa virus belongs to the arenavirus family [80]. It is a pleomorphic, enveloped RNA virus with a bi-segmented, ambisense genome. Like Ebola virus, Lassa virus is a zoonotic pathogen. The reservoir for Lassa virus is the commensal rodent known as the multi-mammate rat (Mastomys natalensis). Once infected, this rodent displays no overt signs of disease and is thought to shed the virus throughout its lifespan. Peridomestic infestations by M. natalensis can lead to outbreaks of Lassa virus disease, known as Lassa Fever, where over 300,000 cases occur annually with an estimated 3000 deaths [6]. Hospitalized patients presenting with Lassa fever have a case fatality rate of about 70-80%, but only about 1-2% of Lassa virus infections result in death. Transmission is through to occur through mucosal exposure to rodent excreta or by nosocomial means. After a 1-3 week incubation, Lassa fever presents initially as a non-specific febrile illness but can then progress to pharyngitis, vomiting, diarrhea, conjunctival injection, mucosal bleeding, pleural effusion and pericardial effusion which can lead to hypovolemia, shock and death. A transient thrombocytopenia is commonly observed and in rare cases petechial rash can also be present. Elevated liver enzyme levels (ALT, AST) is also common. A marked neural component of disease is also common and can present as sensorineural hearing deficit, tremors, encephalitis, and marked seizures.
Like Ebola virus, Lassa virus exhibits broad tissue tropism infecting liver, spleen, adrenal glands and other organs and infected antigen-presenting cells, with DCs once again being important in vivo targets of infection [81-83]. Similar to Ebola virus, Lassa virus also inhibits IFN responses and DC maturation. Lassa virus infection does not activate DCs or macrophages and Lassa virus-infected DCs fail to stimulate strong T cell responses and only induce weak memory responses [25,82,84]. The Lassa virus nucleoprotein (NP) has the capacity to block IFN induction which involves, at least in part, NP 3’-5’ exonuclease activity [85-87]. Lassa virus NP is also involved in the inhibition of antigen-presenting cell (DC and macrophage)-mediated NK cell responses [88]. It has also been shown that the LASV Z protein can inhibit RIG-I and Melanoma Differentiation-Associated protein 5 (MDA5) [89,90].
Yellow fever virus.
YFV belongs to the flavivirus family. It is an enveloped, positive-sense RNA virus with an ~11 KB genome. It is transmitted by mosquitoes of the Haemagogus and Aedes genera. It can be maintained through two life cycles: an urban cycle in which it is transmitted between humans via Aedes aegypti and in a jungle cycle where transmission occurs between non-human primates (NHP) via Hemagogus mosquitos in South America and Aedes africanus in Africa; humans can be infected by mosquitoes that previously fed on an infected monkey [91]. YFV is endemic in central Africa and South America and results in approximately 200,000 cases and 30,000 deaths annually despite the existence of an effective vaccine [92]. A re-emergence of YFV in December 2015 in Angola resulted in 3,748 suspected cases and 364 deaths. Although a vaccination campaign was implemented, depleted vaccine supplies hindered this effort [7]. In January 2017, a total of 110 suspected cases of yellow fever, including 30 deaths, were reported in Brazil [93]. Approximately 15% of infected YFV patients become severely ill, entering the “intoxication stage” [94]. Of patients that develop severe visceral disease, case fatality rates range from 20%-50% [95]. The VHF caused by YFV shares many features with Ebola virus disease [94,96]. Its incubation period is usually 3–6 days but like Ebola virus, it has an abrupt onset with non-specific symptoms such as fever, chills, and headache. Other symptoms include myalgia, nausea, vomiting, dizziness and hemorrhage. As during Ebola virus disease, leukopenia and thrombocytopenia occur, evidence for coagulation defects is present and AST and ALT levels increase. Distinguishing YFV from other VHFs, patients become severely jaundiced.
Mechanisms of YFV pathogenesis.
Pathogenesis is incompletely understood. Like other flaviviruses, YFV inhibits type I IFN responses. NS4B, whose function is conserved among flaviviruses, can block STAT1 activation and interferon stimulated gene (ISG) expression in Vero cells after addition of IFNβ [97]. Further, NS5 can interact with STAT2 to inhibit IFN responses [98]. Lastly, the sequence of the YFV E protein influences the extent to which the virus triggers innate antiviral responses [99]. The importance of innate immune evasion to the pathogenesis of YFV is suggested by the reduced mortality in rhesus macaques treated with IFNα inducers such as polyI:C [100]. Similarly, administration of IFNγ reduced viremia and hepatitis severity in squirrel monkeys and prolonged survival time in rhesus macaques [101]. Similar to hemorrhagic fever caused by EBOV, YFV infection results in profound lymphopenia and depletion of lymphocytes in germinal centers of spleen, LN tonsils and Peyer’s patches [102,103]. Similar to Ebola hemorrhagic fever, cytokine dysregulation during may mediate lymphopenia, endothelial damage, disseminated intravascular coagulation and circulatory shock observed in the terminal stage of YFV. Levels of pro-inflammatory modulators were significantly higher in patients with fatal yellow fever compared to patients who survived [104]. Similarly, levels of IL-6, IFNγ, MCP-1 and IL-15 were elevated in rhesus macaques infected withYFV [103]. Thrombocytopenia, prolonged clotting and prothrombin times have been observed in human patients and nonhuman primates due to diminished liver production of fibrinogen and clotting factors [94,103]. However, as with other VHFs, the source of the cytokine storm and its connection to disseminated intravascular coagulation and circulatory shock remains to be defined.
A working model of VHF pathogenesis.
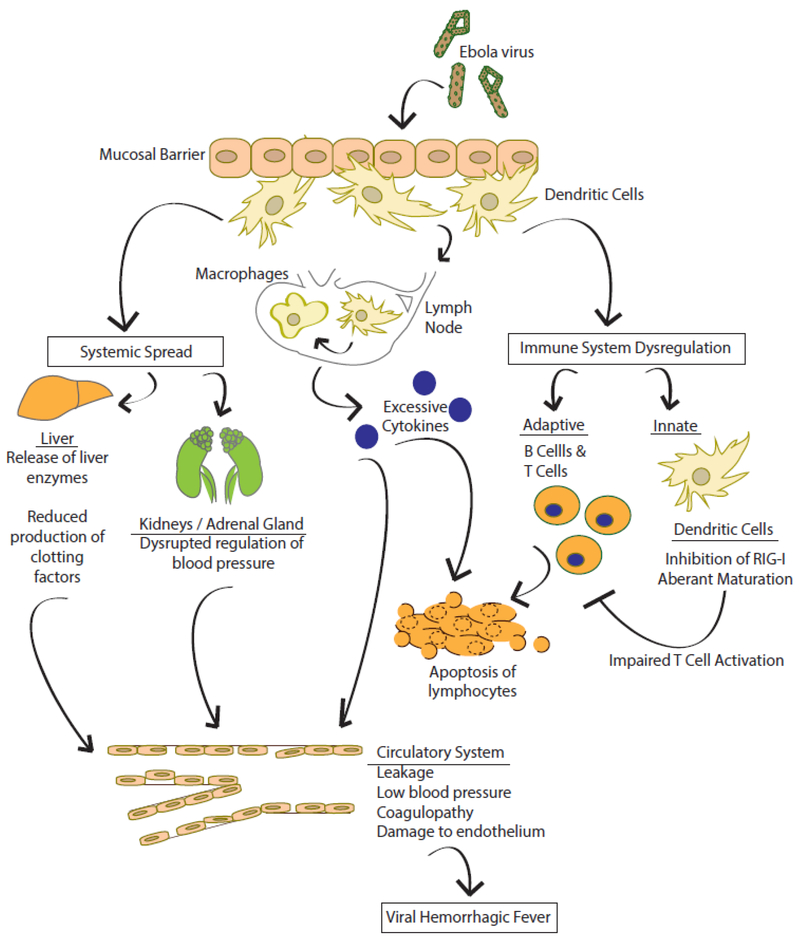
Based on the available data, the following model of VHF is suggested (Fig. 1). Infection with VHF viruses may occur by exposure of mucous membranes or breaks in the skin to infectious virus such as may occur for Ebola virus; through exposure to excreta from infected rodents, as in Lassa virus; or through the bite of an infected insect, such as occurs for YFV. After introduction, macrophages and DCs are early targets of infection. Macrophages and DCs support productive replication and also traffic to local lymph nodes and to other tissues and organs, promoting systemic dissemination. Infection of and damage to different organs promotes the indicated pathologic processes; for example liver damage may depress production of clotting factors, which may result in hemorrhage. Infection of macrophages also results in unbridled production of cytokines, commonly referred to as cytokine storm. This not only promotes vascular leakage and hypotension but can also activate coagulation pathways that may ultimately lead to disseminated intravascular coagulation. Also, these cytokines likely contribute to apoptosis of lymphocytes. Infection of DCs leads to a dysregulated phenotype where IFN responses are suppressed and maturation of DCs is impaired. This likely inhibits activation of T cells, further preventing control of the infection.
Figure 1. Model of viral hemorrhagic fever.
This model is largely based on Ebola virus, but is consistent with what is also known about Lassa and yellow fever virus pathogenesis. Ebola virus infects at a mucosal surface and infects macrophages and dendritic cells which migrate to lymph nodes. The infection suppresses innate and adaptive immune responses, allowing the virus to disseminate systemically. This systemic spread leads to damage in a variety of tissues, excessive cytokine responses, vascular leakage and disseminated intravascular coagulation. See text for details.
The need for a comparative systems biology approach to define common underlying features of VHF.
Some have questioned the utility of the term VHF, because the syndrome it represents is caused by a diverse array of viruses with varying replication strategies, because hemorrhage is often not a major manifestation of disease and because for some viruses, full blown VHF is less common than are less severe outcomes [105]. Nonetheless, the most severe forms of illness caused by the so-called VHF viruses result in common clinical features which suggests common underlying mechanisms. Outbreaks caused by these viruses can be unpredictable, as in the case of Ebola virus and YFV infection and these infections often occur in remote locations, making laboratory study of human infections difficult. For this reason, treatments that target common mechanisms would be useful as a generic approach to VHF treatment. However, devising such therapeutic approaches requires a better understanding of VHF mechanisms of disease. For this, good animal models that closely replicate severe human disease are important. Among the VHF viruses, high quality macaque models exist for Ebola virus and other filoviruses, for Lassa virus and for YFV. These, coupled with modern molecular technologies and systems biology approaches, present opportunities for detailed comparisons of VHF that could clarify mechanisms of disease and suggest therapeutic approaches.
Among the most pressing questions that could be addressed, What pathway(s) direct excessive inflammation and how does this influence the outcome of disease? Massive pro-inflammatory cytokine production likely plays a major role in the pathogenesis of VHF [27,22]. In vitro studies suggest that infected monocytes and macrophages are a major source of inflammatory cytokines. However, the cell types most responsible for the inflammatory response that occurs in vivo and the most relevant signaling pathways that direct the inflammatory response remain undefined. Further, it is unclear if virus infection directs this response or whether a bystander effect makes a significant contribution to the inflammatory cytokine response.
How do inflammatory and immune responses contribute to vascular leakage and coagulopathy and what is the commonality between VHF from different virus families?
In vitro studies demonstrate that EBOV infection can elicit cytokines such as TNF that promote endothelial leakage [106,107,19,108]. The inflammatory response in monocytes and macrophages has been linked to production of tissue factor (TF) which can activate coagulation cascades and thereby contribute to DIC. Inhibition of TF activity can partially protect NHPs from lethal EBOV challenge [39]. These data therefore suggest a link between inflammation, vascular leakage and cytokine production, however the contribution of inflammatory cytokines to these other processes remains to be fully evaluated experimentally. Further, the contributions of liver damage or damage to the adrenal gland to coagulopathy or low blood pressure also need further examination [109]. To date, there is limited information comparing the coagulopathies between hemorrhagic fever virus infections. Dissection as to these commonalities may allow for more universal treatment approaches across all VHF infections.
What is the status of the adaptive immune response?
Viral impairment of DC maturation suggests that T cell responses to EBOV should be impaired in vivo. Consistent with this view, fatal Ebola infections have been associated with the lack of specific antibody responses and with the apoptotic loss of lymphocytes [18,110,23,21,111]. Similar findings are reported for Lassa fever and yellow fever. However, survivors develop specific T cell responses [45,112]. Therefore, the development of adaptive immune responses during VHF in vivo requires further assessment.
References
- 1.Fenton MB, Davison M, Kunz TH, McCracken GF, Racey PA, Tuttle MD (2006) Linking bats to emerging diseases. Science 311 (5764):1098–1099; author reply 1098-1099. doi:311/5764/1098c [pii] 10.1126/science.311.5764.1098c [DOI] [PubMed] [Google Scholar]
- 2.Paessler S, Walker DH (2013) Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol 8:411–440. doi: 10.1146/annurev-pathol-020712-164041 [DOI] [PubMed] [Google Scholar]
- 3.Schnittler HJ, Feldmann H (2003) Viral hemorrhagic fever--a vascular disease? Thromb Haemost 89 (6): 967–972. doi: 10.1267/THRO03060967 [DOI] [PubMed] [Google Scholar]
- 4.Channabasappa N, Johnson-Welch S, Mittal N De novo cholangiocarcinoma after liver transplantation in a pediatric patient. Pediatr Transplant 14 (8):E110–114. doi:PTR1220 [pii] 10.1111/j.1399-3046.2009.01220.x [DOI] [PubMed] [Google Scholar]
- 5.Birmingham K, Kenyon G (2001). Nature Medicine 7 (8):878–878. doi: 10.1038/90892 [DOI] [PubMed] [Google Scholar]
- 6.McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES (1987) A prospective study of the epidemiology and ecology of Lassa fever. Journal of Infectious Diseases 155 (3):437–444 [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos PF, Monath TP (2016) Yellow Fever Remains a Potential Threat to Public Health. Vector Borne Zoonotic Dis 16 (8):566–567. doi: 10.1089/vbz.2016.2031 [DOI] [PubMed] [Google Scholar]
- 8.Organization WH (2016) SITUATION REPORT-EBOLA VIRUS DISEASE 10 June 2016. [Google Scholar]
- 9.Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brauburger K, Rodney Brister J, Bukreyev AA, Cai Y, Chandran K, Davey RA, Dolnik O, Dye JM, Enterlein S, Gonzalez JP, Formenty P, Freiberg AN, Hensley LE, Hoenen T, Honko AN, Ignatyev GM, Jahrling PB, Johnson KM, Klenk HD, Kobinger G, Lackemeyer MG, Leroy EM, Lever MS, Muhlberger E, Netesov SV, Olinger GG, Palacios G, Patterson JL, Paweska JT, Pitt L, Radoshitzky SR, Ryabchikova EI, Saphire EO, Shestopalov AM, Smither SJ, Sullivan NJ, Swanepoel R, Takada A, Towner JS, van der Groen G, Volchkov VE, Volchkova VA, Wahl-Jensen V, Warren TK, Warfield KL, Weidmann M, Nichol ST (2013) Virus nomenclature below the species level: a standardized nomenclature for filovirus strains and variants rescued from cDNA. Arch Virol 159 (5):1229–1237. doi: 10.1007/s00705-013-1877-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann H, Sanchez A, Geisbert TW (2013) Filoviridae: Marburg and Ebola Viruses. Fields Virology, 6 edn. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 11.Towner JS, Pourrut X, Albarino CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM (2007) Marburg virus infection detected in a common African bat. PLoS One 2 (8):e764. doi: 10.1371/journal.pone.0000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leendertz SA, Gogarten JF, Dux A, Calvignac-Spencer S, Leendertz FH (2015) Assessing the Evidence Supporting Fruit Bats as the Primary Reservoirs for Ebola Viruses. Ecohealth 13 (1):18–25. doi: 10.1007/s10393-015-1053-0 10.1007/s10393–015-1053–0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones ME, Schuh AJ, Amman BR, Sealy TK, Zaki SR, Nichol ST, Towner JS (2015) Experimental Inoculation of Egyptian Rousette Bats (Rousettus aegyptiacus) with Viruses of the Ebolavirus and Marburgvirus Genera. Viruses 7 (7):3420–3442. doi:v7072779 [pii] 10.3390/v7072779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kortepeter MG, Bausch DG, Bray M (2011) Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis 204 Suppl 3:S810–816. doi: 10.1093/infdis/jir299 [DOI] [PubMed] [Google Scholar]
- 15.Zaki SR, Goldsmith CS (1999) Pathologic features of filovirus infections in humans. Curr Top Microbiol Immunol 235:97–116 [DOI] [PubMed] [Google Scholar]
- 16.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB (2000) Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest 80 (2):171–186 [DOI] [PubMed] [Google Scholar]
- 17.Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR (2015) Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 235 (2):153–174. doi: 10.1002/path.4456 [DOI] [PubMed] [Google Scholar]
- 18.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ (1999) Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 5 (4):423–426. [DOI] [PubMed] [Google Scholar]
- 19.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ (2003) Pathogenesis of Ebola Hemorrhagic Fever in Cynomolgus Macaques: Evidence that Dendritic Cells Are Early and Sustained Targets of Infection. Am J Pathol 163 (6):2347–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dube D, Schornberg KL, Stantchev TS, Bonaparte MI, Delos SE, Bouton AH, Broder CC, White JM (2008) Cell adhesion promotes Ebola virus envelope glycoprotein-mediated binding and infection. J Virol 82 (14):7238–7242. doi:JVI.00425-08 [pii] 10.1128/JVI.00425-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW (2004) Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic Fever in cynomolgus macaques. Viral Immunol 17 (3):390–400 [DOI] [PubMed] [Google Scholar]
- 22.Hensley LE, Young HA, Jahrling PB, Geisbert TW (2002) Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett 80 (3):169–179. [DOI] [PubMed] [Google Scholar]
- 23.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM (2010) Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis 4 (10). doi: 10.1371/journal.pntd.0000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A (2003) Ebola and Marburg Viruses Replicate in Monocyte-Derived Dendritic Cells without Inducing the Production of Cytokines and Full Maturation. J Infect Dis 188 (11):1630–1638 [DOI] [PubMed] [Google Scholar]
- 25.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B (2003) Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol 170 (6):2797–2801 [DOI] [PubMed] [Google Scholar]
- 26.Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, Rollin PE (2004) Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol 78 (19):10370–10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, Lansoud-Soukate J, Mavoungou E (2002) Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol 128 (1):163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaddanapudi K, Palacios G, Towner JS, Chen I, Sariol CA, Nichol ST, Lipkin WI (2006) Implication of a retrovirus-like glycoprotein peptide in the immunopathogenesis of Ebola and Marburg viruses. FASEB J 20 (14):2519–2530 [DOI] [PubMed] [Google Scholar]
- 29.Ebihara H, Rockx B, Marzi A, Feldmann F, Haddock E, Brining D, LaCasse RA, Gardner D, Feldmann H (2011) Host response dynamics following lethal infection of rhesus macaques with Zaire ebolavirus. J Infect Dis 204 Suppl 3:S991–999. doi:jir336 [pii] 10.1093/infdis/jir336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson KL, Rollin PE (2007) Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis 196 Suppl 2:S357–363 [DOI] [PubMed] [Google Scholar]
- 31.McElroy AK, Harmon JR, Flietstra TD, Campbell S, Mehta AK, Kraft CS, Lyon MG, Varkey JB, Ribner BS, Kratochvil CJ, Iwen PC, Smith PW, Ahmed R, Nichol ST, Spiropoulou CF (2016) Kinetic Analysis of Biomarkers in a Cohort of US Patients With Ebola Virus Disease. Clin Infect Dis 63 (4):460–467. doi:ciw334 [pii] 10.1093/cid/ciw334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ (1999) Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis 179 Suppl 1:S188–191. [DOI] [PubMed] [Google Scholar]
- 33.Gupta M, Mahanty S, Ahmed R, Rollin PE (2001) Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology 284 (1):20–25. [DOI] [PubMed] [Google Scholar]
- 34.Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H (2001) Infection and activation of monocytes by Marburg and Ebola viruses. J Virol 75 (22):11025–11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollin PE, Bausch DG, Sanchez A (2007) Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis 196 Suppl 2:S364–371. doi:JID38435 [pii] 10.1086/520613 [DOI] [PubMed] [Google Scholar]
- 36.Isaacson MSP, Courteille G (1976) Clinical aspects of Ebola virus disease at the Ngaliema hospital, Kinshasa, Zaire, 1976 In: Ebola Virus Haemorrhagic Fever. Elsevier/North Holland Biomedical Press, New York, [Google Scholar]
- 37.Organization WH (1978) Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ 58:247–270 [PMC free article] [PubMed] [Google Scholar]
- 38.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE (2003) Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol 163 (6):2371–2382. doi: 10.1016/S0002-9440(10)63592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, Young HA, Fredeking TM, Rote WE, Vlasuk GP (2003) Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 362 (9400):1953–1958 [DOI] [PubMed] [Google Scholar]
- 40.Gibb TR, Bray M, Geisbert TW, Steele KE, Kell WM, Davis KJ, Jaax NK (2001) Pathogenesis of experimental Ebola Zaire virus infection in BALB/c mice. J Comp Pathol 125 (4):233–242 [DOI] [PubMed] [Google Scholar]
- 41.Hensley LE, Alves DA, Geisbert JB, Fritz EA, Reed C, Larsen T, Geisbert TW (2011) Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis 204 Suppl 3:S1021–1031. doi:jir339 [pii] 10.1093/infdis/jir339 [DOI] [PubMed] [Google Scholar]
- 42.Olejnik J, Forero A, Deflube LR, Hume AJ, Manhart WA, Nishida A, Marzi A, Katze MG, Ebihara H, Rasmussen AL, Muhlberger E (2017) Ebolaviruses associated with differential pathogenicity induce distinct host responses in human macrophages. J Virol doi:JVI.00179-17 [pii] 10.1128/JVI.00179-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubaki NM, Ilinykh P, Pietzsch C, Tigabu B, Freiberg AN, Koup RA, Bukreyev A (2013) The lack of maturation of Ebola virus-infected dendritic cells results from the cooperative effect of at least two viral domains. J Virol 87 (13):7471–7485. doi:JVI.03316-12 [pii] 10.1128/JVI.03316-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, Burt FJ, Leman PA, Khan AS, Rowe AK, Mukunu R, Sanchez A, Peters CJ (1999) Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 179 Suppl 1:S177–187. doi: 10.1086/514321 [DOI] [PubMed] [Google Scholar]
- 45.McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, Lyon GM, Ribner BS, Varkey J, Sidney J, Sette A, Campbell S, Stroher U, Damon I, Nichol ST, Spiropoulou CF, Ahmed R (2015) Human Ebola virus infection results in substantial immune activation. Proceedings of the National Academy of Sciences of the United States of America 112 (15):4719–4724. doi:1502619112 [pii] 10.1073/pnas.1502619112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider WM, Chevillotte MD, Rice CM (2014) Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32:513–545. doi: 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoggins JW (2014) Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol 6:40–46. doi:S1879-6257(14)00066-2 [pii] 10.1016/j.coviro.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardenas WB, Loo YM, Gale M, Jr., Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF (2006) Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol 80 (11):5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muhlberger E, Lotfering B, Klenk HD, Becker S (1998) Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J Virol 72 (11):8756–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlberger E, Weik M, Volchkov VE, Klenk HD, Becker S (1999) Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol 73 (3):2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basler CF, Wang X, Muhlberger E, Volchkov V, Paragas J, Klenk HD, Garcia-Sastre A, Palese P (2000) The Ebola virus VP35 protein functions as a type I IFN antagonist. Proceedings of the National Academy of Sciences of the United States of America 97 (22):12289–12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, Reid SP, Ramanan P, Cardenas WB, Amarasinghe GK, Volchkov VE, Basler CF (2010) Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol 84 (6):3004–3015. doi:JVI.02459-09 [pii] 10.1128/JVI.02459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartman AL, Bird BH, Towner JS, Antoniadou ZA, Zaki SR, Nichol ST (2008) Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of ebola virus. J Virol 82 (6):2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leung DW, Amarasinghe GK (2012) Structural insights into RNA recognition and activation of RIG-I-like receptors. Curr Opin Struct Biol 22 (3):297–303. doi:S0959-440X(12)00062-0 [pii] 10.1016/j.sbi.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prins KC, Cardenas WB, Basler CF (2009) Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol 83 (7):3069–3077. doi:JVI.01875-08 [pii] 10.1128/JVI.01875-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K (2009) Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog 5 (6):e1000493. doi: 10.1371/journal.ppat. 1000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, Basler CF, Amarasinghe GK (2010) Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol 17 (2):165–172. doi:nsmb.1765 [pii] 10.1038/nsmb.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartman AL, Ling L, Nichol ST, Hibberd ML (2008) Whole Genome Expression Profiling Reveals that Inhibition of Host Innate Immune Response Pathways by Ebola Virus can be Reversed by a Single Amino Acid Change in the VP35 Protein. J Virol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, Yen B, Liu G, Leung DW, Geisbert TW, Ebihara H, Amarasinghe GK, Basler CF (2013) Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe 14 (1):74–84. doi:S1931-3128(13)00226-6 [pii] 10.1016/j.chom.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106 (3):255–258 [DOI] [PubMed] [Google Scholar]
- 61.Yen B, Mulder LC, Martinez O, Basler CF (2014) Molecular basis for ebolavirus VP35 suppression of human dendritic cell maturation. J Virol 88 (21):12500–12510. doi:JVI.02163-14 [pii] 10.1128/JVI.02163-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yen BC, Basler CF (2016) Effects of Filovirus Interferon Antagonists on Responses of Human Monocyte-Derived Dendritic Cells to RNA Virus Infection. J Virol 90 (10):5108–5118. doi:JVI.00191-16 [pii] 10.1128/JVI.00191-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, Ganesh B, He B (2010) The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and lipopolysaccharide. J Gen Virol 91 (Pt 2):352–361. doi:vir.0.017343-0 [pii] 10.1099/vir.0.017343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albarino CG, Wiggleton Guerrero L, Spengler JR, Uebelhoer LS, Chakrabarti AK, Nichol ST, Towner JS (2015) Recombinant Marburg viruses containing mutations in the IID region of VP35 prevent inhibition of Host immune responses. Virology 476:85–91. doi:S0042-6822(14)00538-8 [pii] 10.1016/j.virol.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edwards MR, Liu G, Mire CE, Sureshchandra S, Luthra P, Yen B, Shabman RS, Leung DW, Messaoudi I, Geisbert TW, Amarasinghe GK, Basler CF (2016) Differential Regulation of Interferon Responses by Ebola and Marburg Virus VP35 Proteins. Cell Rep 14 (7):1632–1640. doi:S2211-1247(16)30027-4 [pii] 10.1016/j.celrep.2016.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Organization WH (2012) Marburg haemorrhagic fever. http://www.who.int/mediacentre/factsheets/fs marburg/en/. [Google Scholar]
- 67.McBride KM, Reich NC (2003) The ins and outs of STAT1 nuclear transport. Sci STKE 2003 (195):RE13. doi: 10.1126/stke.2003.195 2003/195/re13 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Mateo M, Carbonnelle C, Reynard O, Kolesnikova L, Nemirov K, Page A, Volchkova VA, Volchkov VE (2011) VP24 is a molecular determinant of Ebola virus virulence in guinea pigs. J Infect Dis 204 Suppl 3:S1011–1020. doi: 10.1093/infdis/jir338 [DOI] [PubMed] [Google Scholar]
- 69.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF (2006) Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol 80 (11):5156–5167. doi: 10.1128/JVI.02349-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF (2007) Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol 81 (24):13469–13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK (2014) Ebola Virus VP24 Targets a Unique NLS Binding Site on Karyopherin Alpha 5 to Selectively Compete with Nuclear Import of Phosphorylated STAT1. Cell Host Microbe 16 (2):187–200. doi:S1931-3128(14)00263-7 [pii] 10.1016/j.chom.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valmas C, Grosch MN, Schumann M, Olejnik J, Martinez O, Best SM, Krahling V, Basler CF, Muhlberger E (2010) Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS pathogens 6 (1):e1000721. doi: 10.1371/journal.ppat.1000721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valmas C, Basler CF (2011) Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol 85 (9):4309–4317. doi:JVI.02575-10 [pii] 10.1128/JVI.02575-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schumann M, Gantke T, Muhlberger E (2009) Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol 83 (17):8993–8997. doi:JVI.00523-09 [pii] 10.1128/JVI.00523-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P (2009) Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proceedings of the National Academy of Sciences of the United States of America 106 (8):2886–2891. doi:0811014106 [pii] 10.1073/pnas.0811014106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Francica JR, Varela-Rohena A, Medvec A, Plesa G, Riley JL, Bates P (2010) Steric shielding of surface epitopes and impaired immune recognition induced by the ebola virus glycoprotein. PLoS Pathog 6 (9):e1001098. doi: 10.1371/journal.ppat.1001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez O, Johnson JC, Honko A, Yen B, Shabman RS, Hensley LE, Olinger GG, Basler CF (2013) Ebola virus exploits a monocyte differentiation program to promote its entry. J Virol doi:JVI.02695-12 [pii] 10.1128/JVI.02695-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wahl-Jensen V, Kurz SK, Hazelton PR, Schnittler HJ, Stroher U, Burton DR, Feldmann H (2005) Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J Virol 79 (4):2413–2419. doi:79/4/2413 [pii] 10.1128/JVI.79.4.2413-2419.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okumura A, Pitha PM, Yoshimura A, Harty RN (2010) Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol 84 (1):27–33. doi:JVI.01462-09 [pii] 10.1128/JVI.01462-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buchmeier MJ, de la Torre JC, Peters CJ (2013) Arenaviridae. Fields Virology, 6 edn. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 81.Winn WC Jr, Walker DH(1975) The pathology of human Lassa fever. Bull World Health Organ 52 (4– 6):535–545 [PMC free article] [PubMed] [Google Scholar]
- 82.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V (2004) Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol 172 (5):2861–2869 [DOI] [PubMed] [Google Scholar]
- 83.Hensley LE, Smith MA, Geisbert JB, Fritz EA, Daddario-DiCaprio KM, Larsen T, Geisbert TW (2011) Pathogenesis of Lassa fever in cynomolgus macaques. Virol J 8:205. doi:1743-422X-8-205 [pii] 10.1186/1743-422X-8-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pannetier D, Reynard S, Russier M, Journeaux A, Tordo N, Deubel V, Baize S (2011) Human dendritic cells infected with the nonpathogenic Mopeia virus induce stronger T-cell responses than those infected with Lassa virus. J Virol 85 (16):8293–8306. doi:JVI.02120-10 [pii] 10.1128/JVI.02120-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qi X, Lan S, Wang W, Schelde LM, Dong H, Wallat GD, Ly H, Liang Y, Dong C (2010) Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature 468 (7325):779–783. doi:nature09605 [pii] 10.1038/nature09605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hastie KM, Kimberlin CR, Zandonatti MA, MacRae IJ, Saphire EO (2011) Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3’ to 5’ exonuclease activity essential for immune suppression. Proceedings of the National Academy of Sciences of the United States of America 108 (6):2396–2401. doi:1016404108 [pii] 10.1073/pnas.1016404108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynard S, Russier M, Fizet A, Carnec X, Baize S (2014) Exonuclease domain of the Lassa virus nucleoprotein is critical to avoid RIG-I signaling and to inhibit the innate immune response. J Virol 88 (23):13923–13927. doi:JVI.01923-14 [pii] 10.1128/JVI.01923-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russier M, Reynard S, Carnec X, Baize S (2014) The exonuclease domain of Lassa virus nucleoprotein is involved in antigen-presenting-cell-mediated NK cell responses. J Virol 88 (23):13811–13820. doi:JVI.01908-14 [pii] 10.1128/JVI.01908-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing J, Ly H, Liang Y (2015) The Z proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-I-like receptor-dependent interferon production. J Virol 89 (5):2944–2955. doi:JVI.03349-14 [pii] 10.1128/JVI.03349-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan L, Briese T, Lipkin WI (2010) Z proteins of New World arenaviruses bind RIG-I and interfere with type I interferon induction. J Virol 84 (4):1785–1791. doi:JVI.01362-09 [pii] 10.1128/JVI.01362-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monath TP, Vasconcelos PF (2015) Yellow fever. J Clin Virol 64:160–173. doi: 10.1016/j.jcv.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 92.Monath TP (2005) Yellow fever vaccine. Expert Rev Vaccines 4 (4):553–574. doi: 10.1586/14760584.4.4.553 [DOI] [PubMed] [Google Scholar]
- 93.Organization WH (2017) Yellow fever - Brazil. http://www.who.int/csr/don/13-ianuarv-2017-vellow-fever-brazil/en/. [Google Scholar]
- 94. Monath TP, Barrett AD (2003) Pathoenesis and pathophysiology of yellow fever. Adv Virus Res 60:343–395 [DOI] [PubMed] [Google Scholar]
- 95.Monath TP (2008) Treatment of yellow fever. Antiviral Res 78 (1):116–124. doi: 10.1016/j.antiviral.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 96.Monath TP (2001) Yellow fever: an update. Lancet Infect Dis 1 (1):11 −20. doi:S1473-3099(01)00016–0 [pii] 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- 97.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A (2005) Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol 79 (13):8004–8013. doi:79/13/8004 [pii] 10.1128/JVI.79.13.8004-8013.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laurent-Rolle M, Morrison J, Rajsbaum R, Macleod JM, Pisanelli G, Pham A, Ayllon J, Miorin L, Martinez-Romero C, tenOever BR, Garcia-Sastre A (2014) The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 16 (3):314–327. doi:S1931-3128(14)00294-7 [pii] 10.1016/j.chom.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez-Garcia MD, Meertens L, Chazal M, Hafirassou ML, Dejarnac O, Zamborlini A, Despres P, Sauvonnet N, Arenzana-Seisdedos F, Jouvenet N, Amara A (2016) Vaccine and Wild-Type Strains of Yellow Fever Virus Engage Distinct Entry Mechanisms and Differentially Stimulate Antiviral Immune Responses. MBio 7 (1):e01956–01915. doi:mBio.01956-15 [pii] 10.1128/mBio.01956-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stephen EL, Sammons ML, Pannier WL, Baron S, Spertzel RO, Levy HB (1977) Effect of a nuclease-resistant derivative of polyriboinosinic-polyribocytidylic acid complex on yellow fever in rhesus monkeys (Macaca mulatta). J Infect Dis 136 (1):122–126 [DOI] [PubMed] [Google Scholar]
- 101.Arroyo JI, Apperson SA, Cropp CB, Marafino BJ Jr, Monath TP, Tesh RB, Shope RE, Garcia-Blanco MA (1988) Effect of human gamma interferon on yellow fever virus infection. The American journal of tropical medicine and hygiene 38 (3):647–650 [PubMed] [Google Scholar]
- 102.Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB (1981) Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. The American journal of tropical medicine and hygiene 30 (2):431–443 [DOI] [PubMed] [Google Scholar]
- 103.Engelmann F, Josset L, Girke T, Park B, Barron A, Dewane J, Hammarlund E, Lewis A, Axthelm MK, Slifka MK, Messaoudi I (2014) Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model. PLoS Negl Trop Dis 8 (11):e3295. doi: 10.1371/journal.pntd.0003295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, Daffis S, Klewitz C, Bae HG, Niedrig M, Zeller H, Heinzel-Gutenbrunner M, Koivogui L, Kaufmann A (2004) Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis 190 (10):1821–1827. doi: 10.1086/425016 [DOI] [PubMed] [Google Scholar]
- 105.Kuhn JH, Clawson AN, Rodoshitzky SR, Wahl-Jensen V, Bavari S, Jahrling PB (2014) Viral Hemorrhagic Fevers: History and Definitions In: Singh SK, Ruzek D (eds) Viral Hemorrhagic Fevers. CRC Press, Boca Raton, FL, [Google Scholar]
- 106.Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ (1996) Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol 70 (4):2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geisbert TW, Hensley LE (2004) Ebola virus: new insights into disease aetiopathology and possible therapeutic interventions. Expert Rev Mol Med 6 (20):1–24 [DOI] [PubMed] [Google Scholar]
- 108.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE (2003) Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis 188 (11):1618–1629 [DOI] [PubMed] [Google Scholar]
- 109.Feldmann H (2010) Are we any closer to combating Ebola infections? Lancet 375 (9729):1850–1852. doi:S0140-6736(10)60597-1 [pii] 10.1016/S0140-6736(10)60597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baize S, Leroy EM, Mavoungou E, Fisher-Hoch SP (2000) Apoptosis in fatal Ebola infection. Does the virus toll the bell for immune system? Apoptosis 5 (1):5–7 [DOI] [PubMed] [Google Scholar]
- 111.Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW (2007) Lymphocyte death in a mouse model of Ebola virus infection. J Infect Dis 196 Suppl 2:S296–304. doi:JID38206 [pii] 10.1086/520602 [DOI] [PubMed] [Google Scholar]
- 112.Bradfute SB, Warfield KL, Bavari S (2008) Functional CD8+ T cell responses in lethal Ebola virus infection. J Immunol 180 (6):4058–4066. doi:180/6/4058 [pii] [DOI] [PubMed] [Google Scholar]