Abstract
Background:
mAb114 is a single monoclonal antibody targeting the receptor binding domain of Ebola virus glycoprotein that prevents mortality in rhesus macaques treated after lethal challenge with Zaire ebolavirus. We present expedited data from a phase 1 study to evaluate mAb114 safety, tolerability, pharmacokinetics, and immunogenicity.
Methods:
VRC 608 is a phase 1, dose-escalation study performed at the National Institutes of Health (NIH) Clinical Center. Healthy adults ages 18-60 were sequentially enrolled into dose groups of 5, 25, and 50 mg/kg and infused intravenously (IV) with mAb114 over 30 minutes and followed for 24 weeks. Safety and tolerability were assessed through soliciting infusion site and systemic symptoms by self-reporting, direct clinician assessment, and clinical laboratory data. All participants have completed the 28 day adverse event reporting period and are currently either in long-term follow up or have completed study visits. The primary study outcome was safety and tolerability, with pharmacokinetic and anti-drug antibody evaluation as secondary objectives.
Findings:
Nineteen participants were enrolled between May 16, 2018, and September 27, 2018. One participant was not infused because intravenous access was not adequate. Eighteen participants received a single infusion of 5 mg/kg (n=3), 25 mg/kg (n=5), or 50 mg/kg (n=10) of mAb114. All infusions were well tolerated at infusion rates between 209-375 mL per hour over 30-37 minutes with zero infusion reactions or rate adjustments. No participants had infusion site symptoms. Systemic symptoms were all mild and present only in 22% of participants across all dosing groups. There were no unsolicited adverse events (AEs) related to mAb114 and one serious adverse event (SAE) unrelated to mAb114. mAb114 has linear pharmacokinetics and a half-life of approximately 24 days with no evidence of anti-drug antibody development.
Interpretation:
mAb114 was well-tolerated, demonstrated linear pharmacokinetics, and was easily and rapidly infused making it an attractive and deployable option for treatment in outbreak settings.
Funding:
The VRC 608 clinical trial was supported by the intramural research program of the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), NIH. mAb114 production was funded by the Defense Advanced Research Projects Agency.
Keywords: Ebola Virus, Emerging Infectious Diseases, Filovirus, Monoclonal Antibody, Phase 1 Clinical Trial, Passive Immunization
Research in Context
Evidence Before This Study:
Zaire ebolavirus (Ebola) is a filovirus recognized since 1976 for its ability to cause Ebola virus disease (EVD) with mortality rates exceeding 50% in most outbreaks. The 2014 outbreak in West Africa highlighted its virulence and potential for global spread and hastened the development of preventative and therapeutic options. The Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD, USA, has experience in developing vaccines and monoclonal antibodies against infectious diseases. On October 11, 2018, we searched PubMed with the terms “Ebola” AND (“monoclonal antibody” OR “monoclonal antibodies”), restricting the article type to clinical trials and species to human. We also searched “Ebola” AND (“monoclonal antibody” OR “monoclonal antibodies”) AND “phase 1.” In the above searches, we excluded articles with convalescent plasma, non-human primates, and vaccine-related trials. Two papers described clinical trial studies with Ebola monoclonal antibody cocktails, both targeting the Ebola virus glycoprotein. Both cocktails utilize the combination of three monoclonal antibodies to confer the protection observed in nonhuman primate models. In the PREVAIL II study, Ebola infected patients in West Africa were randomized to receive either IV infusions of a cocktail of three humanized murine monoclonal antibodies which target two distinct viral glycoprotein epitopes (ZMapp®) plus standard of care, or standard of care alone. Patients in the ZMapp treatment group received a three dose regimen every third day, with infusion times of at least 5-8 hours. Patients receiving ZMapp had improved survival compared to standard of care but did not meet the statistical threshold for efficacy. In the second study, REGN3470-3471-3479, a coformulated cocktail of three human monoclonal antibodies derived from humanized mice targeting non-overlapping glycoprotein epitopes was tested in a phase 1 randomized trial of healthy adults as a single IV infusion administered over four hours. Headache or myalgia were the most frequent treatment-related adverse events reported and either mild or moderate in severity. One infusion reaction was observed and the mean half-lives of the antibodies ranged from 21.7 – 27.3 days, with no evidence of development of anti-REGN3470-3471-3479 antibodies. There were no studies describing a single mAb delivered and effective as a monotherapy.
Added Value of This Study:
Our study assessed the safety, tolerability, pharmacokinetics, and immunogenicity of mAb114, a single monoclonal antibody targeting the Ebola virus glycoprotein, in healthy adults. mAb114 is a fully human monoclonal antibody that binds and blocks receptor binding to the receptor binding domain (RBD) of the Ebola glycoprotein. mAb114 was found to be safe and well tolerated after IV infusion, with a mean serum half-life of 24 days, and low pharmacokinetic variability among study participants. mAb114 has several advantages in the treatment of an Ebola outbreak when compared to antibody cocktails. First, mAb114 uniformly protects macaques when given as a single 50 mg/kg infusion. Second, mAb114 affords this protection as a human monoclonal antibody isolated from a human survivor, whereas ZMapp antibodies were isolated from immunized mice and REGN3450-3471-3479 were isolated from humanized mice. Third, mAb114 targets a highly conserved epitope in the receptor binding domain (RBD) region and may have a lower risk of causing Ebola escape mutants compared to other monoclonal antibodies. Finally, in this phase 1 study, mAb114 was delivered rapidly (over approximately 30 minutes) without infusion reactions, lending itself to ease of use within Ebola treatment units (ETUs) compared to modalities that require multiple infusions and longer infusion times.
Implications of All the Available Evidence:
mAb114 was found to be safe and well tolerated with a robust pharmacokinetic profile in this phase 1 study. Non-human primate (NHP) data supports its therapeutic efficacy. Additionally, the Democratic Republic of Congo (DRC) Ministry of Health and Ethics Committee approved its use in an expanded access protocol of Ebola infected patients. Since August 10, 2018, 54 Ebola patients have received mAb114 to date. This data supports the further research and development of mAb114 as a treatment for Ebolavirus disease.
Background
Ebolaviruses are negative-strand RNA viruses with five species,1,2 three of which are known to induce hemorrhagic fever in humans: Bundibugyo ebolavirus (BDBV), Sudan ebolavirus (SUDV), and Zaire ebolavirus (EBOV).3 Outbreaks likely begin by zoonotic transmission to humans after exposure to fruit bats or other infected animals with later spread through the community by direct contact with blood, secretions, organs, or other bodily fluids of infected patients or corpses.4-7 Ebolavirus disease (EVD) occurs after a 2-21 day incubation period and causes severe systemic illness characterized by fever, fatigue, myalgia, headache, pharyngitis, vomiting, diarrhea, rash, kidney and liver dysfunction, and bleeding diathesis, with case fatality rates averaging above 50%.3,8 The 2014 West Africa EBOV outbreak was the largest to date with a total of 28,616 confirmed, suspected and probable cases and 11,310 deaths.3,9 More recently, on August 1, 2018, the Ministry of Health of the Democratic Republic of the Congo (DRC) declared the ongoing EVD outbreak in the North Kivu Province just days after a previous outbreak in the Equateur Province was declared over. An increased frequency of EVD in humans emphasizes the urgent need for effective therapies in the face of sporadic and unpredictable outbreaks.10
Passive immunization during an Ebola outbreak was shown to be a feasible treatment approach as early as 1995 in Kikwit, DRC.11 During the 2014 West African EBOV outbreak mixed results were reported regarding the efficacy of convalescent whole blood or plasma for post-infection treatment to improve survival outcomes.12,13 Passive immunization of nonhuman primates (NHP) using EBOV-convalescent whole blood,14 purified and concentrated IgG,15 as well as species-matched convalescent IgG16 and vaccine-generated polyclonal IgG17 have additionally generated mixed results and limited success in challenge models. However, passive immunization of macaques with species-matched convalescent IgG16 or monoclonal antibodies (mAbs) are associated with virus clearance and resolution of EVD. ZMapp and REGN3470-3471-3479 are anti-EBOV mAb cocktails of three mAbs isolated from immunized wildtype or HumAb18 mice, respectively, each of which have been reported to successfully treat EBOV infection in NHPs.19,20 REGN3470-3471-3479 has been well tolerated in a phase 1 clinical trial in healthy adults,21 and ZMapp was administered to patients in a randomized control trial during the 2014 West Africa EBOV outbreak. Despite an estimated beneficial effect of ZMapp administration based on an observed difference in mortality from 37% in patients receiving standard of care to 22% in the ZMapp-treated group, the trial failed to meet the prespecified statistical threshold for efficacy.22
There are several challenges for an antibody, or combination of antibodies, to overcome if Ebola infection is to be blocked.23 The ebolavirus is large and pleomorphic, and may require high antibody concentrations to fully occupy stable viral glycoprotein (GP) trimer that coats the virion surface at high density. The GP receptor-binding domain (RBD) is recessed beneath highly glycosylated structural features, likely protecting the critical RBD from antibody access until after the virus is internalized by the target cell via micropinocytosis, where a low pH environment and enzymatic digestion of large portions of GP by cathepsins disfavor the retention of any antibodies previously bound to GP. Indeed, mAbs targeting the digested structures or remaining epitopes at the base of GP after cleavage show poor ability to individually block infection.24 Virus-host cell fusion and replication occur only after exposure of the RBD on cathepsin-cleaved GP and engagement of the host cell receptor protein, Niemann-Pick C1 (NPC1) in late endosomes. 25-28
The virus-receptor binding event represents an attractive functional target for mAbs with potent blocking activity. Thus, an ideal antibody to block EBOV would bind to the native virion GP trimer prior to micropinocytosis, remain bound at low pH following cathepsin cleavage and inhibit virus replication by blocking GP-NPC1 engagement. To address these requirements and the global need for an effective therapy against EVD, the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), developed the fully human monoclonal antibody mAb114. mAb114 was identified from a survivor of the 1995 EBOV outbreak in Kikwit, DRC, approximately 11 years after infection.29 It was selected from a panel of memory B cells based on its binding to the EBOV (GP) and potency using in vitro functional assays.24,29 mAb114 binds to a highly conserved region of amino acids in the RBD of Zaire ebolavirus variants24,30 and prevents the interaction of GP with the NPC1 receptor, thus blocking virus entry into the host cell cytoplasm. mAb114 has several advantageous properties which allow it to remain functional during numerous steps of the viral life cycle, including the ability to remain bound to RBD in both physiologic and low pH intracellular environments, and after glycoprotein cathepsin cleavage events.24 Because mAb114 binds to the RBD, the risk of escape mutants may be reduced as mutations in the RBD would risk a subsequent decrease in viral fitness.
These features of mAb114 are believed to contribute to its ability to protect rhesus macaques after infection with a uniformly lethal dose of EBOV.24 In this infectious challenge model, mAb114 reversed viremia, clinical symptoms, laboratory abnormalities, and prevented death.29 mAb114 was first shown to provide this protection when given as a 50 mg/kg intravenous (IV) infusion daily for 3 days beginning as late as five days after exposure.29 Subsequent studies in the same lethal NHP challenge model showed that a single infusion of 50 mg/kg or 30 mg/kg up to 5 days post challenge provided full protection from death (Nancy J. Sullivan – personal communication/unpublished data presented at the 47th Annual Meeting of the German Society for Immunology, Erlangen, Germany, September 14, 2017). These NHP studies provided preclinical evidence that one administration of mAb114 could be an effective means to treat EVD.
This phase 1 study evaluated the safety, tolerability and pharmacokinetics (PK), of mAb114, and also screened for potential induction of anti-drug antibodies. The major goal of the study was to determine whether the 50 mg/kg dose capable of fully protecting NHPs in a lethal challenge model could be rapidly administered to healthy adults and display a PK profile predicted to provide protection.
Methods
Study Design and Participants
VRC 608 is a first-in-human phase 1, open-label, dose-escalation clinical trial of the human monoclonal antibody mAb114 (VRC-EBOMAB092-00-AB). Eligible participants were healthy adults aged 18-60 years as defined by inclusion and exclusion criteria related to clinical laboratory tests, medical history, and physical examination with no history of prior receipt of an investigational Ebola virus vaccine. All subjects were recruited through institutional review board (IRB) approved print and electronic informational advertisements and were sequentially enrolled into groups according to a protocol specified dose-escalation plan. Participants were specifically screened to be negative for HIV-1, hepatitis B and C, and were excluded if they weighed greater than 100 kg due to limited quantities of study product. Full inclusion and exclusion criteria are enumerated in the protocol supplied in the appendix. The trial was conducted at the NIH Clinical Center by the VRC Clinical Trials Program (CTP). The protocol was reviewed and approved by the NIAID IRB. The US Department of Health and Human Services human experimental guidelines for conducting clinical research were followed. All participants gave written informed consent before enrollment.
mAb114 is a human IgG1 antibody targeting the RBD domain of EBOV GP.29 mAb114 was developed by the VRC and manufactured at Cook Pharmica LLC d.b.a. Catalent Indiana, LLC (Bloomington, IN) operating under contract by MedImmune LLC (Gaithersburg, MD) according to current Good Manufacturing Practice regulations. mAb114 is supplied as a lyophilized product in a glass vial at 400 mg per vial. Long-term storage temperature is 2-8°C, stability testing has also shown that mAb114 remains stable at 40°C for up to 6 months. mAb114 is reconstituted with sterile water and added to 100mL normal saline for IV infusion over 30 minutes.
Preclinical toxicology studies supported mAb114 use in humans. There was no in vitro reactivity to phospholipid cardiolipin using an anti-cardiolipin ELISA. Additionally, mAb114 demonstrated no in vitro autoreactivity using an anti-nuclear antigen HEp-2 system. A tissue cross-reactivity study demonstrated no cross reactivity with a panel of normal human tissues. In vivo toxicology studies for safety and pharmacology in rhesus monkeys receiving up to 500 mg/kg mAb114 weekly for 4 weeks showed no mAb114-related effects on safety pharmacology parameters.
Study Procedures
Three open-label groups received mAb114 (group 1: 5 mg/kg, group 2: 25 mg/kg, and group 3: 50 mg/kg) IV in a dose-escalation study design. Participants were enrolled in any open group by the study coordinator or study nurse. mAb114 infusions were administered via peripheral IV over approximately 30 minutes. The participants were monitored by a study clinician during infusion and for 4 hours post infusion prior to being discharged. Participants recorded prespecified systemic reactogenicity parameters assessing fever, malaise, myalgia, headache, chills, nausea, and arthralgia for 3 days after infusion. Clinicians assessed the infusion site for pain and/or tenderness, pruritis, swelling, erythema, and bruising after administration and then on days 1, 2, and 7. Baseline safety laboratory tests including white blood cell count and differential, hemoglobin, hematocrit, platelets, electrolytes, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and creatinine were obtained prior to product administration and then after infusion on protocol specified time points. All adverse events (AEs) occurring through 28 days after mAb114 administration, serious adverse events (SAEs) and any new chronic medical conditions occurring throughout the trial were recorded and assessed by study clinicians. AEs were coded with the Medical Dictionary for Regulatory Activities (MedDRA) and severity was graded using the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.1, March 2017. Participants are followed for 24 weeks after mAb114 administration, and follow up remains ongoing.
Quantification of mAb114 serum concentrations was performed with a Beckman Biomek based automation platform. The anti-idiotype monoclonal antibody was added to Immulon-4HXB microtiter plates overnight prior to blocking. Threefold dilutions, from 1:100 to 1:218,700, were analyzed in duplicate. Biotin-labeled anti-human IgG1 was added, and Streptavidin conjugated with horseradish peroxidase and TMB (3, 5’, 5, 5’-tetra-methylbenzidine) substrate was used to develop the reaction. Sulfuric acid was then added to halt color development. Plates were read within 30 minutes at 450nm with a Molecular Devices Paradigm plate reader. Sample concentrations were quantified using linear regression of a mAb114 standard curve covering a range of 5-100 ng/mL.
Evaluation for the presence of anti-drug antibodies was based upon the Meso Scale Discovery (MSD) electrochemiluminescence (ECL) homogenous bridging assay, as previously described.31 Trial participant sera was evaluated for all available samples at the pre-mAb114 administration and 4 and 8 weeks post administration.
Outcomes
The primary endpoints were safety and tolerability of mAb114. The secondary endpoints were PKs and assessment of anti-drug antibody to mAb114. All subjects have completed the adverse event reporting period of the study, and all specified primary and secondary outcomes listed in the protocol are reported.
Statistical Analyses
Group samples sizes in this small trial with descriptive statistics were selected for their ability to reasonably identify SAEs across a range of hypothetical true event rates. Within a group of n=3 (5 mg/kg), there is an 80% chance to observe at least one event if the true rate is no less than 0.42 and a 90% chance of observing no event if the true rate is no bigger than 0.035. Within a group of n=10 (50 mg/kg) there is at least an 80% chance of observing at least one event if the true rate is no less than 0.15 and at least a 90% chance of observing no event if the true rate is no bigger than 0.1.
PK analysis of the mAb114 concentration data was performed using both compartmental and non-compartmental approaches. Maximum concentration (Cmax) and time of maximum concentration (Tmax) were taken directly from the observed concentration-time data. Coefficient of variation (CV: (standard deviation/mean)*100%) was used to compare variability in concentrations within dosing groups. Average concentration (Cave) over the first 4 weeks of therapy was calculated from the area under the concentration vs. time curve over the interval, Cave = AUC0-28D / 28, with AUC0-28D determined using the linear trapezoidal method. mAb114 PK data were also fitted to a standard two-compartment PK model using the computer program NONMEM v7.3 (ICON, Dublin) to calculate typical population PK parameters and their variances. Individual participants’ estimated clearance (CL), volume of distribution at steady-state (Vdss) and beta half-life (t1/2β) were generated using the post-hoc empiric Bayesian subroutine from the population PK model. The population PK model was assessed for appropriateness graphically, by goodness of fit statistics and bootstrap analysis with Wings for NONMEM v7.2 (using 1000 replicates). Given the limited number of participants studied, no exploratory covariate analysis was performed to assess clinical factors as fixed effects that might be associated with mAb114 PK.
Role of the funding source
The VRC 608 clinical trial was supported by the intramural research program of the VRC, NIAID, NIH. mAb114 manufacturing was funded by the United State Department of Defense, Defense Advanced Research Projects Agency, and the VRC conducted the study. The VRC 608 Study Team and authors were responsible for the study design, data collection, data analysis, data interpretation, and writing of the report. The principal investigator, MRG, and associate investigators had full access to all the data in the study and JEL had final responsibility for the decision to submit for publication.
Results
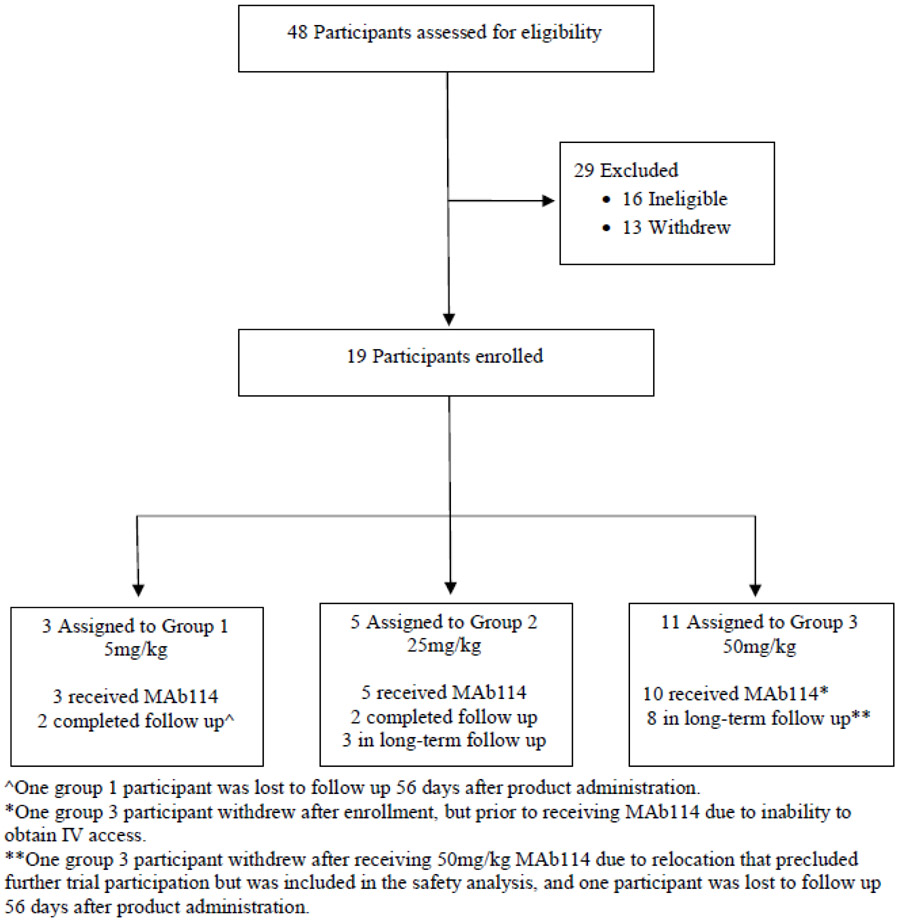
A total of 48 participants were screened and 19 were enrolled from May 16, 2018 to September 27, 2018 (Figure 1). The study population demographics (Table 1) consisted of 12 (63%) women and 7 (37%) men with a mean age of 38.4 years (range 22-56 years) and a mean weight of 73.9 kg (range 47.9 – 97.9 kg). Eighteen participants received one dose of mAb114 administered IV: 5 mg/kg (n=3), 25 mg/kg (n=5), and 50 mg/kg (n=10). All 18 participants completed the safety assessment of local and systemic reactogenicity. One participant enrolled but was terminated from the study before receiving mAb114 due to inadequate IV access. One participant in the 50 mg/kg group received mAb114 and subsequently withdrew from the trial after 28 days due to employment relocation. One participant in the 5 mg/kg group and one participant in the 50 mg/kg group were lost to follow up 56 days after product administration. All other participants remain in long-term follow up (post the 28 day AE reporting period) or have completed study visits (Figure 1).
Figure 1.
CONSORT Diagram for the VRC 608 Trial
Table 1:
Baseline demographics of participants
| 5 mg/kg IV (n=3) |
25 mg/kg IV (n=5) |
50 mg/kg IV (n=11) |
Overall (n=19) |
|
|---|---|---|---|---|
| Gender | ||||
| Female | 1 (33%) | 4 (80%) | 7 (64%) | 12 (63%) |
| Male | 2 (67%) | 1 (20%) | 4 (36%) | 7 (37%) |
| Age (years) | ||||
| Mean (SD) | 39.7 (7.4) | 36.4 (9.8) | 39.0 (13.9) | 38.4 (11.7) |
| Range | 34-48 | 25-50 | 22-56 | 22-56 |
| Race | ||||
| Asian | 0 | 0 | 1 (9%) | 1 (5%) |
| Black or African American | 0 | 0 | 1 (9%) | 1 (5%) |
| Multiracial | 1 (33%) | 0 | 2 (18%) | 3 (16%) |
| White | 2 (67%) | 5 (100%) | 7 (64%) | 14 (74%) |
| Ethnic origin | ||||
| Non-Hispanic or Latino | 3 (100%) | 4 (80%) | 10 (91%) | 17 (90%) |
| Hispanic or Latino | 0 | 1 (20%) | 1 (9%) | 2 (11%) |
| Weight (kg) | ||||
| Mean (SD) | 88 (8.8) | 66.3 (12.6) | 73.5 (12.4) | 73.9 (13.4) |
| Range | 81-97.9 | 47.9-83 | 52.7-92.4 | 47.9-97.9 |
| Education | ||||
| College/University | 2 (67%) | 3 (60%) | 8 (73%) | 13 (68%) |
| Advanced degre | 1 (33%) | 2 (40%) | 3 (27%) | 6 (32%) |
Data are number (%) unless otherwise specified.
mAb114 administrations were safe and well tolerated with no infusion reactions (Table 2 and 3). All infusion rates were between 209 and 375 mL/hr, rate alterations were not required, and infusions were completed in 30-37 minutes. There were no local infusion site solicited symptoms in the 7-day reporting period after mAb114 administration, and no fevers associated with infusion. Four of 18 participants (22%) reported mild solicited systemic reactogenicity in the three days after mAb114 administration, all which resolved between 1 and 4 days: malaise (n=3, 17%), myalgia (n=2, 11%), headache (n=4, 22%), chills (n=2, 11%), nausea (n=2, 11%), and joint pain (n=2, 11%). There were no moderate or severe solicited systemic symptoms.
Table 2:
mAb114 infusion related parameters
| Participant ID | Total Dose Infused (mg) |
Administration Duration (min) |
Total Volume Administered (mL) |
Average Administration Rate (mL/hr) |
Number of Infusion Reaction Symptoms |
|---|---|---|---|---|---|
| Group 1: 5 mg/kg IV | |||||
| A1 | 405 | 31 | 108.10 | 209.2 | 0 |
| A2 | 426 | 30 | 111.50 | 223.0 | 0 |
| A3 | 490 | 31 | 109.79 | 212.5 | 0 |
| Group 2: 25 mg/kg IV | |||||
| A4 | 2080 | 31 | 141.50 | 273.9 | 0 |
| A5 | 1610 | 37 | 132.20 | 214.4 | 0 |
| A6 | 1200 | 32 | 123.95 | 232.4 | 0 |
| A7 | 1755 | 30 | 135.20 | 270.4 | 0 |
| A8 | 1650 | 31 | 133.00 | 257.4 | 0 |
| Group 3: 50 mg/kg IV | |||||
| A9 | 4380 | 30 | 187.50 | 375.0 | 0 |
| A10 | 3910 | 30 | 178.20 | 356.4 | 0 |
| A11 | 3950 | 31 | 179.00 | 346.5 | 0 |
| A12 | 3400 | 31 | 168.00 | 325.2 | 0 |
| A13 | 3300 | 30 | 166.00 | 332.0 | 0 |
| A14 | 4620 | 30 | 177.40 | 354.8 | 0 |
| A15 | 4090 | 30 | 181.80 | 363.6 | 0 |
| A16 | 3460 | 30 | 169.20 | 338.4 | 0 |
| A17 | 2640 | 30 | 152.80 | 305.6 | 0 |
| A18 | 3020 | 30 | 160.40 | 320.8 | 0 |
Table 3:
Maximum local and systemic reactogenicity
| 5 mg/kg IV (n=3) |
25 mg/kg IV (n=5) |
50 mg/kg IV (n=10) |
Overall (n=18) |
|
|---|---|---|---|---|
| Local Symptoms | ||||
| Pain or tenderness | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Bruising | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Swelling | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Redness | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Pruritus | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Any local symptom | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Systemic symptoms | ||||
| Malaise | ||||
| None | 3 (100%) | 4 (80%) | 8 (80%) | 15 (83%) |
| Mild | 0 | 1 (20%) | 2 (20%) | 3 (17%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Myalgia | ||||
| None | 3 (100%) | 4 (80%) | 9 (90%) | 16 (89%) |
| Mild | 0 | 1 (20%) | 1 (10%) | 2 (11%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Headache | ||||
| None | 3 (100%) | 3 (60%) | 8 (80%) | 14 (78%) |
| Mild | 0 | 2 (40%) | 2 (20%) | 4 (22%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Chills | ||||
| None | 3 (100%) | 4 (80%) | 9 (90%) | 16 (89%) |
| Mild | 0 | 1 (20%) | 1 (10%) | 2 (11%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Nausea | ||||
| None | 3 (100%) | 4 (80%) | 9 (90%) | 16 (89%) |
| Mild | 0 | 1 (20%) | 1 (10%) | 2 (11%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Temperature | ||||
| None | 3 (100%) | 5 (100%) | 10 (100%) | 18 (100%) |
| Mild | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Joint Pain | ||||
| None | 3 (100%) | 4 (80%) | 9 (90%) | 16 (89%) |
| Mild | 0 | 1 (20%) | 1 (10%) | 2 (11%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Any systemic symptom | ||||
| None | 3 (100%) | 3 (60%) | 8 (80%) | 14 (78%) |
| Mild | 0 | 2 (40%) | 2 (20%) | 4 (22%) |
| Moderate | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
Data are number (%). Each vaccine recipient is counted once at worst severity for any local and systemic parameter. There was no local reactogenicity and no moderate or severe systemic reactogenicity.
There were no unsolicited AEs related to mAb114. Eight subjects reported 10 unrelated unsolicited AEs. There was one unrelated SAE for an unscheduled hospitalization due to vomiting and syncope 84 days after mAb114 infusion. There were no deaths and no suspected unexpected serious adverse reactions (SUSARs).
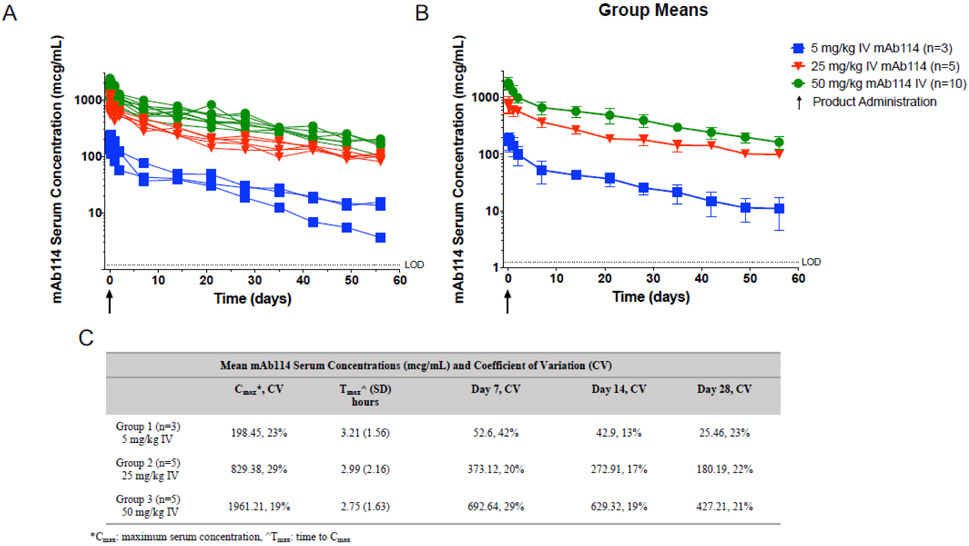
mAb114 PK, with the exception of half-life, were analyzed for all participants with at least 28 days of PK data, this included all subjects in groups 1 (n=3) and group 2 (n=5) and five subjects in group 3. Half-life calculations were performed on participants with at least 56 days of PK data (n=9). mAb114 PK profiles show predictability in vivo (Figure 2), with low variability between participants as seen by the coefficients of variation for mAb114 concentrations at Day 14 and Day 28 which were all below 25% (range 13-23%) across dose levels as shown in Figure 2C. The mean (± SD) maximum serum concentration (Cmax) for the intended treatment dose of 50 mg/kg was 1961 ± 340 mcg/mL, which occurred 2.8 ± 1.6 hours after mAb114 administration. Dose-dependent linearity was apparent when comparing these results to the 25 mg/kg mean Cmax of 829 ± 237 mcg/mL that was approximately one-half the 50 mg/kg group mean Cmax, and the 5 mg/kg mean Cmax of 26 ± 6.0 mcg/mL that was approximately one-tenth of the 50 mg/kg Cmax. The coefficients of variation of the Cmax ranged from 19%-29% across dosing groups (Figure 2C). The average concentration over the first 28 days following the 50 mg/kg mAb114 infusion was 664 ± 130 mcg/mL with a clearance rate of 1.5 ± 0.2 mL/day/kg. The mean day 28 serum concentration was 427 ± 88 mcg/mL for participants receiving 50 mg/kg, 180 ± 39 mcg/mL for participants receiving 25 mg/kg, and 25.5 ± 6 mcg/mL for participants receiving 5 mg/kg mAb114. Comparing to known protective levels in NHPs, the Cmax of the 50 mg/kg group was approximately 1.5-fold greater than that of a protective 50 mg/kg dose administered up to 5 days post lethal challenge (Nancy J. Sullivan – personal communication). The average half-life (±SEM) of the antibody for all dose levels was 24.2 ± 1.8 days (table 4).
Figure 2.
mAb114 serum concentrations (mcg/mL) of participants infused with 5 (blue), 25 (red), and 50 (green) mg/kg mAb114. Arrow indicates product administration, and horizontal line indicates the lower limit of detection (LOD) by ELISA assay. A) mAb114 serum concentration (mcg/mL) for individuals across all dosing groups, B) Group means (+/− SD) for mAb114 serum concentration (mcg/mL), C) Mean mAb114 serum concentrations (mcg/mL) and coefficient of variation (CV).
Table 4:
Pharmacokinetic parameters of mAb114
| mAb114 Treatment Group |
Clearance (CL) mL/day |
Area under the curve (AUC0-28D) mcg *day/mL |
Average Conc. (Cave)) day 0-28 mcg/mL |
Volume of Distribution (V dss) L |
Half-life (T1/2b) days* |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Group 1 (n=3) 5 mg/kg IV |
199 (45) | 1480(304) | 52.87 (10.87) | 5.08 (0.88) | 20.1 (6.9) |
| Group 2 (n=5) 25 mg/kg IV |
108 (21) | 8586 (900) | 306.65 (32.14) | 3.93 (0.50) | 26.7 (3.8) |
| Group 3 (n=5) 50 mg/kg IV |
115(15) | 18,588 (3627) | 663.87 (129.55) | 4.16 (0.74) | 23.6 (n=1) |
| Overall IV Half-life (T1/2) Average (SEM) (n=9)* | 24.2 (1.8) days | ||||
for all participants with 56 days of PK data
There were no anti-drug responses to mAb114 detected in any of the trial participants in the evaluation of serum collected 4 and 8 weeks post mAb114 administration.
Discussion
Ebolavirus outbreaks have occurred with increasing frequency since the discovery of the virus in 1976.3 No medical countermeasures have been licensed and populations in endemic regions remain vulnerable. The lethality of acute EVD has a devastating effect on the health of both individuals and communities. Immunotherapy with monoclonal antibodies has shown promise in experimental NHP models and has been used in past and current EVD outbreaks.19,20,22,29 ZMapp was deployed in the 2014 West Africa outbreak in a randomized control trial, and although the trial did not meet the prespecified criteria for superiority over supportive care alone, it was reported to confer therapeutic benefit with an overall day 28 absolute mortality that was 15% lower among those receiving ZMapp – corresponding to a 40% lower relative risk of death.22 Of note, all deaths which occurred in the treatment group occurred before all three doses of ZMapp were administered; seven out of eight before the second dose and one before the third dose, and it is likely patients were past the 5-day infection window for which the product showed protection in NHPs.19,22 In the recent outbreak in the North Kivu province of DRC, mAb114 was the first product to be used for treatment under an expanded access protocol, followed by Remdesivir, REGN3470-3471-3479 and ZMapp. This phase 1 trial in healthy adults evaluated mAb114 in a single-infusion regimen, and demonstrated favorable safety and PK in humans at doses show to be protective in NHPs. All mAb114 administrations were well tolerated at doses up to 50 mg/kg, with no evidence of infusion reactions. All 18 study participants completed the safety assessment of solicited local and systemic reactogenicity, of which there were no local infusion site symptoms and only mild systemic symptoms observed in four participants. There were no moderate or severe solicited systemic symptoms and no product-related unsolicited AEs throughout the reporting period. Furthermore, the PK profile of mAb114 shows dose-dependent linearity, low variability between participants within a given dose, and is consistent with the PK profile of NHPs receiving protective antibody doses.29
mAb114 has several features which facilitate its use for treatment in an outbreak setting. It is effective in NHPs as a single antibody and does not require an antibody cocktail for protection against lethal EBOV challenge.29 Coupled with being given as a single infusion at 50 mg/kg, it’s simplified dosing may ease issues associated with manufacturing treatment courses in response to large outbreaks as occurred during 2014-2016 in West Africa.32 It is currently the only monoclonal antibody therapeutic isolated from a human EVD survivor. The epitope targeted by mAb114 resides in a highly conserved region of amino acids in the RBD of Zaire ebolavirus. This may limit its vulnerability to mutational escape variant generation both within an outbreak and between different outbreaks. It is important to note that protection in NHPs from lethal EBOV challenge by ZMapp has been achieved through administration of a cocktail of three monoclonal antibodies that are not protective if given individually.19,33 Similarly, two of the three antibodies in REGN3470-3471-3479 are in the same class as ZMapp’s,20,34,35 and it is not known if the third class provides protection as monotherapy. Thus, in the case of ZMapp, and possibly REGN3470-3471-3479, mutational escape from any one antibody epitope may therefore result in breakthrough infection and not offer enhanced protection over a single monoclonal antibody therapy (e.g., mAb114). mAb114 has demonstrated protection in a single administration dose up to 5 days post EBOV infection in NHP (Nancy J. Sullivan – personal communication/unpublished data) and in this trial we demonstrate that comparable dosing can be achieved in an approximately 30 minute infusion. There were no mAb114 infusion reactions and no alterations of the infusion time were required. In contrast, ZMapp is a three-dose regimen administered at 50 mg/kg every third day.22 ZMapp infusion data are representative of Ebola-infected patients and may not be directly comparable to the that of healthy subjects presented here. We are not aware of any ZMapp data in healthy adults. REGN3470-3471-3479 has been tested at a single dose of up to 150mg/kg in healthy adults.36 Both cocktails are administered over approximately 4 hours or longer. Shorter administration times and lower doses can allow for more efficient treatment in the Ebola treatment units (ETUs) and for more doses to be made available during an outbreak setting. Furthermore, lyophilized mAb114 does not require storage in freezers, as it is stable at 2-8°C and the lyophilized product remains stable for six months at 40°C, which is another advantage for outbreak deployment.
This trial evaluated a mAb114 treatment regimen intended to be deployed in an outbreak setting. That regimen was chosen based on data from a series of dose-down lethal challenge studies of rhesus macaques treated with mAb114 after lethal EBOV infection. mAb114 was fully protective when administered as three 50mg/kg IV infusions 24, 48, and 72 hours after EBOV exposure, and was also equally effective when administered as three 50mg/kg infusions 120, 144, and 168 hours after exposure.29 Subsequent challenge studies employing the same model showed that a single 50mg/kg or 30mg/kg infusion 120 hours after exposure was effective in animals displaying high levels of plasma viremia. The main limitation to the presented study is the small number of subjects enrolled, which is typical of a phase 1 study. For accuracy of the analysis only subjects with 28 days of data were analyzed (n=13) for PK parameters, and for half-life calucations only subjects with 56 days of data were analyzed (n=9). Ongoing and planned future trials will add additional data from mAb114 recipients as well as data from populations living in potential outbreak settings.
At the request of the DRC Ministry of Health, and following expanded access protocol approval by the Ministry of Health and Ethics Committee, mAb114 was deployed for compassionate use as a single infusion dose of 50 mg/kg IV in the North Kivu outbreak beginning in August, 2018. mAb114 treatment courses have been provided to the DRC by NIAID. Since August 10, 2018, 54 EVD patients have received mAb114 to date. Outcome data are not yet analyzed, but any analysis of mortality rates amongst recipients of investigational treatments in expanded access protocols will require great care to discern efficacy signals (or lack thereof) amidst possible sources of bias. A multinational randomized-controlled trial (NCT03719586) is currently underway to assess multiple therapeutic agents including mAb114 in the current DRC EVD outbreak, with the design to roll over into subsequent outbreaks. These data should prove helpful to fully elucidate the safety and efficacy of Ebolavirus therapeutic agents and identify clinically useful products for future patient care worldwide.
Supplementary Material
Acknowledgements
We thank the vaccine trial volunteers for their contribution and commitment to vaccine research. We also acknowledge the contributions of our NIH Clinical Center and NIAID colleagues, including the NIAID Institutional Review Board, the EMMES Corporation, and colleagues at the NIAID VRC including Dr. Marybeth Daucher and the Vaccine Clinical Materials Program and VRC Pilot Plant. We especially thank colleagues at the Defense Advanced Research Projects Agency (DARPA) for collaborating to develop mAb114 (COL Matthew Hepburn, Amy Jenkins, Audrey Glynn and Dan Wattendorf). VRC appreciates the longstanding research collaboration with the DRC Institut National de Recherche Biomedicale which allowed this trial to occur.
The VRC 608 Study Team includes Cynthia Starr Hendel, Sarah Plummer, Pamela Costner, Jamie Saunders, Floreliz Mendoza, Aba Eshun, Joseph Casazza, Abidemi Ola, William Whalen, Xiaolin Wang, Jennifer Cunningham, Olga Vasilenko, Catina Boyd, Olga Trofymenko, Maria Burgos Florez, Somia Hickman, Ro Shauna Rothwell, Iris Pittman, Lam Le, Brenda Larkin, Josephine Cox, Preeti Apte, Renunda Hicks, Cora Trelles Cartagena, Pernell Williams, LaShawn Requilman, Thuy Nguyen, Colin Tran, Sandra Vazquez, Michelle Conan-Cibotti, Judy Stein, and Tatiana Beresnev.
Footnotes
Registration: clinicaltrials.gov NCT03478891
Declaration of Interests
NJS, JEL, and BSG are listed as inventors on pending patent applications for mAb114 which has been non-exclusively licensed. All other authors have no conflicts of interest.
References
- 1.Kuhn JH, Bao Y, Bavari S, et al. Virus nomenclature below the species level: a standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch Virol 2013; 158(1): 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez A, Kiley MP, Holloway BP, Auperin DD. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus research 1993; 29(3): 215–40. [DOI] [PubMed] [Google Scholar]
- 3.Ebola Virus Disease Fact Sheet: World Health Oganization, Febrary 12th, 2018. [Google Scholar]
- 4.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med 2004; 10(12 Suppl): S110–21. [DOI] [PubMed] [Google Scholar]
- 5.Meslin FX. Global aspects of emerging and potential zoonoses: a WHO perspective. Emerg Infect DIs 1997; 3(2): 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okware SI, Omaswa FG, Zaramba S, et al. An outbreak of Ebola in Uganda. Trop Med Int Health 2002; 7(12): 1068–75. [DOI] [PubMed] [Google Scholar]
- 7.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196 Suppl 2: S142–7. [DOI] [PubMed] [Google Scholar]
- 8.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56(2): 271–93. [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Situation Report: Ebola virus disease. June 10th, 2016. http://apps.who.int/iris/bitstream/handle/10665/208883/ebolasitrep_10Jun2016_eng.pdf?sequence=1
- 10.World Health Organization. Ebola virus disease – Democratic Republic of the Congo, Disease outbreak news August 4th, 2018. http://www.who.int/csr/don/4-august-2018-ebola-drc/en/.
- 11.Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis 1999; 179 Suppl 1: S18–23. [DOI] [PubMed] [Google Scholar]
- 12.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med 2016; 374(1): 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahr F, Ansumana R, Massaquoi TA, et al. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect 2017; 74(3): 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahrling PB, Geisbert JB, Swearengen JR, Larsen T, Geisbert TW. Ebola hemorrhagic fever: evaluation of passive immunotherapy in nonhuman primates. J Infect Dis 2007; 196 Suppl 2: S400–3. [DOI] [PubMed] [Google Scholar]
- 15.Jahrling PB, Geisbert TW, Jaax NK, Hanes MA, Ksiazek TG, Peters CJ. Experimental infection of cynomolgus macaques with Ebola-Reston filoviruses from the 1989–1990 U.S. epizootic. Arch Virol Suppl 1996; 11: 115–34. [DOI] [PubMed] [Google Scholar]
- 16.Dye JM, Herbert AS, Kuehne Al, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A 2012; 109(13): 5034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan NJ, Hensley L, Asiedu C, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 2011; 17(9): 1128–31. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A 2014; 111(14): 5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514(7520): 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascal KE, Dudgeon D, Trefry JC, et al. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in non-human primates. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivapalasingam S, Kamal M, Slim R, et al. Safety, pharmacokinetics, and immunogenicity of a coformulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. The Lancet Infectious Diseases 2018. [DOI] [PubMed] [Google Scholar]
- 22.Group PIW, Multi-National PIIST, Davey RT Jr., et al. A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N Engl J Med 2016; 375(15): 1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ploquin A, Zhou Y, Sullivan NJ. Ebola Immunity: Gaining a Winning Position in Lightning Chess. J Immunol 2018; 201(3): 833–42. [DOI] [PubMed] [Google Scholar]
- 24.Misasi J, Gilman MS, Kanekiyo M, et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 2016; 351(6279): 1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cote M, Misasi J, Ren T, et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 2011; 477(7364): 344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misasi J, Chandran K, Yang JY, et al. Filoviruses require endosomal cysteine proteases for entry but exhibit distinct protease preferences. J Virol 2012; 86(6): 3284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carette JE, Raaben M, Wong AC, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011; 477(7364): 340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misasi J, Sullivan NJ. Camouflage and misdirection: the full-on assault of ebola virus disease. Cell 2014; 159(3): 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corti D, Misasi J, Mulangu S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016; 351(6279): 1339–42. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn JH, Radoshitzky SR, Guth AC, et al. Conserved receptor-binding domains of Lake Victoria marburgvirus and Zaire ebolavirus bind a common receptor. J Biol Chem 2006; 281(23): 15951–8. [DOI] [PubMed] [Google Scholar]
- 31.Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15(1): e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu X, Audet J, Lv M, et al. Two-mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci Transl Med 2016; 8(329): 329ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 2012; 4(138): 138ra81. [DOI] [PubMed] [Google Scholar]
- 34.Davidson E, Bryan C, Fong RH, et al. Mechanism of Binding to Ebola Virus Glycoprotein by the ZMapp, ZMAb, and MB-003 Cocktail Antibodies. J Virol 2015; 89(21): 10982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallesen J, Murin CD, de Val N, et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat Microbiol 2016; 1(9): 16128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sivapalasingam S, Kamal M, Slim R, et al. Safety, pharmacokinetics, and immunogenicity of a coformulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect Dis 2018; 18(8): 884–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.