Abstract
Purpose
To estimate the potential near-term population impact of alternative second opinion breast biopsy pathology interpretation strategies.
Methods
Decision analysis examining 12-month outcomes of breast biopsy for nine breast pathology interpretation strategies in the U.S. health system. Diagnoses of 115 practicing pathologists in the Breast Pathology Study were compared to reference-standard-consensus diagnoses with and without second opinions. Interpretation strategies were defined by whether a second opinion was sought universally or selectively (e.g., 2nd opinion if invasive). Main outcomes were the expected proportion of concordant breast biopsy diagnoses, the proportion involving over-or under-interpretation, and cost of care in U.S. dollars within one-year of biopsy.
Results
Without a second opinion, 92.2% of biopsies received a concordant diagnosis. Concordance rates increased under all second opinion strategies, and the rate was highest (95.1%) and under-treatment lowest (2.6%) when all biopsies had second opinions. However, overtreatment was lowest when second opinions were sought selectively for initial diagnoses of invasive cancer, DCIS, or atypia (1.8 vs. 4.7% with no 2nd opinions). This strategy also had the lowest projected 12-month care costs ($5.907 billion vs. $6.049 billion with no 2nd opinions).
Conclusions
Second opinion strategies could lower overall care costs while reducing both over-and under-treatment. The most accurate cost-saving strategy required second opinions for initial diagnoses of invasive cancer, DCIS, or atypia.
Keywords: Pathology, Cost, Decision analysis, Second opinion, Overdiagnosis, Breast cancer diagnosis
Introduction
Increased awareness of diagnostic variation among pathologists has raised concerns that healthcare decisions based on inaccurate pathologic diagnosis could be compromised [1]. Recently, the Breast Pathology Study (B-Path) reported the extent of variation in pathologists’ diagnostic interpretation of breast biopsy tissue [2], and explored implications for individual women [3]. With overall concordance as low as 48% for atypia [2], there is clearly potential for improvement.
Appropriate clinical care is dependent on accurate pathologic diagnosis; diagnostic variation—a surrogate measure of accuracy—can lead to over-or under-interpretation. Absolute diagnostic accuracy is difficult to determine because true biologic standards remain elusive in clinical practice. Misclassification (over-or under-interpretation) directs patients to clinical care pathways that may result in over-or under-treatment and the attendant consequences. For pathologic interpretations of breast tissue, women diagnosed with more severe disease than they actually have may undergo unnecessary procedures and treatments, while women diagnosed with less severe disease may miss potentially life-saving interventions. Therefore, misclassification and misdiagnosis reduce the clinical effectiveness of breast cancer screening and increase potential harms to women.
Improving breast biopsy diagnostic interpretation accuracy is an important approach to enhancing quality of care and patient outcomes. Previously, we estimated the diagnostic accuracy of breast biopsy interpretations by practicing pathologists relative to expert consensus-reference diagnosis for invasive carcinoma, ductal carcinoma in situ (DCIS), atypia (atypical ductal hyperplasia [ADH]; ADH in a papilloma), and benign without atypia (proliferative and non-proliferative changes) [2]. These estimates were established using breast biopsy test sets designed to improve statistical confidence for diagnoses with low prevalence. We subsequently reported the clinical relevance of these estimates for individual women, showing that an estimated 4.6% of women receive more severe diagnoses and 3.2% receive less severe diagnoses compared with the reference-consensus diagnosis [3]. We also evaluated potential improvements in diagnostic accuracy using defined strategies for obtaining second opinions [4]. This paper builds on previous B-Path work by comparing second opinion strategies for pathologic breast tissue interpretation to determine the extent of over-and under-treatment and associated health care costs in the year following biopsy from a U.S. screening population perspective.
Methods
Decision analysis was used to estimate the effect of different second opinion strategies that could improve breast pathology diagnostic interpretation on over-and under-treatment and cost for the U.S. screening population. We estimated the expected probability that breast biopsy interpretations would agree with reference-standard diagnoses obtained in the B-Path Study under nine different strategies. We used interpretive performance of 115 pathologists participating in B-Path as a representation of diagnostic accuracy across the U.S [2, 3]. The probability that breast biopsy specimens within each reference-standard diagnosis would lead to over-or under-treatment was estimated based on standardized care pathways. Results were applied to the number of women undergoing breast biopsies each year in the U.S. to estimate the total numbers of women affected and the direct medical costs associated with each strategy in the year following a specific pathology diagnosis.
Breast pathology interpretation strategies
Strategies were defined based on the initiating trigger for a second opinion (Table 1), including: (1) the initial pathologist’s diagnostic interpretation (e.g., obtain a second opinion if the diagnosis is invasive cancer or DCIS); or (2) the pathologist’s desire for a second opinion for each case; (3) and/or each pathologist’s interpretation of the case relative to their laboratory’s current second opinion policy requirements (e.g., policy requires a second opinion for all invasive carcinoma diagnoses). The second opinion strategies did not require referral to centralized or expert pathologists, and are based on our prior evaluation of second opinion strategies [4]. While the percent of cases that will undergo secondary review differs under each strategy, we evaluate the impact that such strategies have on the 1-year outcomes for interpretation of all breast biopsy specimens—not only those requiring 2nd opinions.
Table 1.
The percent of cases requiring second (2nd) and third (3rd) reviews, misclassification rates (MCR), false positive (FP), and false negative (FN) proportions by pathology interpretation strategy and expert consensus diagnosis*
| Pathology interpretation strategy | Proliferative/non-prolif. |
Reference standard expert consensus diagnosis |
||||||
|---|---|---|---|---|---|---|---|---|
| Atypia |
DCIS |
Invasive |
||||||
| Reviews 2nd, 3rd | MCR (FN, FP) | Reviews 2nd, 3rd | MCR (FN, FP) | Reviews 2nd, 3rd | MCR (FN, FP) | Reviews 2nd, 3rd | MCR (FN, FP) | |
| No second opinion | 0.0, 0.0 | 5.8 (0,5.8) | 0.0, 0.0 | 52.2 (34.7,17.4) | 0.0, 0.0 | 15.9 (13.3,2.6) | 0.0, 0.0 | 3.9 (3.9,0) |
| Second opinion based on initial diagnosis | ||||||||
| 2nd if invasive | 1.0, 0.8 | 5.4 (0,5.4) | 0.4, 0.3 | 51.9 (34.4,17.5) | 2.6, 2.4 | 13.6 (13.2,0.4) | 96.1, 1.9 | 4.7 (4.7,0) |
| 2nd if invasive or DCIS | 3.2, 2.9 | 4.7 (0,4.7) | 17.4, 13.1 | 43.7 (35.9,7.9) | 86.7, 12.2 | 15.8 (15.2,0.6) | 99.6, 3.3 | 3.9 (3.9,0) |
| 2nd if invasive, DCIS or atypia | 12.9, 10.4 | 2.1 (0,2.1) | 65.3, 36.5 | 51.9 (41.8,10) | 93.7, 17.8 | 12.1 (11.4,0.6) | 99.6, 3.3 | 3.9 (3.9,0) |
| Second opinion based on pathologist desire or policy | ||||||||
| 2nd if desired | 26.7,10.1 | 3.3 (0,3.3) | 55.9, 31.3 | 47.7 (33.7,14) | 30.5, 11.2 | 11.4 (9.9,1.5) | 15.5, 1.5 | 3.6 (3.6,0) |
| 2nd if considered borderline | 19, 7.5 | 4.0 (0, 4.0) | 45.3, 25.6 | 48.9 (34.6,14.2) | 21.4, 9.3 | 11.5 (9.9,1.5) | 3.5,0.8 | 3.8 (3.8,0) |
| 2nd if required by policy | 33.8, 7.6 | 4.7 (0,4.7) | 40.6, 23 | 47.1 (33.9,13.2) | 55.3, 10.0 | 13.7 (12.1,1.6) | 59.9, 2.1 | 4.0 (4.0,0) |
| 2nd if desired or required | 54, 14.9 | 2.9 (0,2.9) | 80.4, 45.3 | 44.3 (32.8,11.4) | 75.5, 18.0 | 10.3 (9.5,0.9) | 69.8, 3 | 3.7 (3.7,0) |
| Second opinion for all | 100, 19.7 | 2.9 (0,2.9) | 100, 55.9 | 40.9 (29.9,11.1) | 100, 21.6 | 9.9 (9.3,0.6) | 100, 3.7 | 3.5 (3.5,0) |
Second opinion strategies require resolution by the third reviewer if the first two disagree
Decision-analytic model and assumptions
The model evaluated each strategy based on a common underlying distribution of reference-standard diagnoses in four diagnostic categories: (1) invasive carcinoma; (2) DCIS; (3) atypia, which comprised ADH; and (4) benign pathology with or without proliferative changes (Fig. 1). Age-specific diagnosis distributions were those observed among women undergoing needle core or excisional breast biopsy following a screening mammogram using data from the Breast Cancer Surveillance Consortium (BCSC) over the period 2004 through 2008 (See Table 3 in Appendix).
Fig. 1.
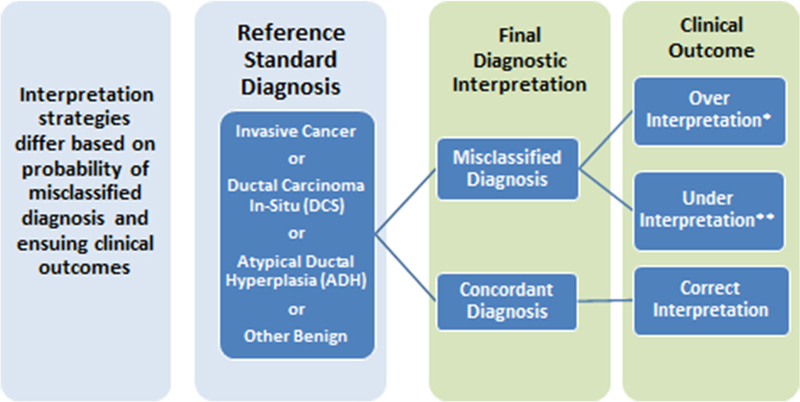
Schematic representation of interpretation strategies depicting a common distribution of reference-standard diagnoses for each interpretation strategy. Final diagnostic interpretation and ensuing clinical outcomes are affected by the interpretation strategy’s misclassification rate relative to the reference-standard diagnosis as well as conditional probabilities of over and under-interpretation when a diagnostic misclassification occurs. *The probability of over-interpretation is zero for women with reference-standard diagnosis of invasive cancer. **The probability of under-interpretation is zero for women with reference-standard diagnosis of other benign findings
For each strategy, the probability of obtaining a final pathology interpretation that was misclassified relative to the reference-standard diagnosis was estimated from B-Path [2] (Table 1). Reference-standard diagnoses in B-Path were based on a consensus of three experienced breast pathologists who independently interpreted a case, followed by a consensus meeting. B-Path misclassification estimates were applied to the prevalence of categorical in the U.S. population to estimate the expected probability that each woman’s diagnosis was concordant with the reference-standard diagnosis for each strategy.
A diagnosis hierarchy was used to establish over-and under-treatment, where the most extensive treatment was for invasive cancer, followed by DCIS, then atypia and then benign/non-proliferative findings without atypia. Over-treatment was defined as treatment for the diagnosis received relative to the reference-standard diagnosis. Under-treatment was considered to occur when the woman’s diagnosis was lower in the treatment hierarchy than the reference-standard diagnosis (e.g., atypia diagnosis when DCIS was the reference-standard diagnosis).
We assumed that each woman’s care pathway would be based on her final pathological diagnosis according to current clinical guidelines [5]. Women receiving an initial diagnosis of invasive cancer were assumed to receive invasive cancer care regardless of their true underlying disease state. Likewise, women with an initial DCIS diagnosis were assumed to receive care and associated costs for DCIS care regardless of true diagnosis. Women with an initial diagnosis of atypia were assumed to undergo an open surgical biopsy. On the basis of the open surgical biopsy, we assumed that the true underlying disease state would be recognized and that disease-concordant care would be provided. For women with atypia as the true underlying diagnosis, we assumed no further costs within the year under baseline assumptions. However, under alternative assumptions of more aggressive care, women with atypia were assumed to undergo breast MRI and treatment with hormonal therapy. Women with other benign diagnoses were assumed to undergo only a mammography within the subsequent year.
The expected care costs were modeled under each alternative interpretation strategy using 2013 U.S. dollars. Medicare national fee schedules were used to estimate the cost associated with each procedure and included physician and technical reimbursement amounts. To ascertain the direct medical care costs of each care pathway, costs of breast imaging, additional pathological interpretations, core biopsies, excisional biopsies, and treatment costs for DCIS or invasive cancer in the year following such diagnoses were included in the model based on estimates from published studies [6, 7] (see Table 3 in Appendix).
Population projections were made by applying the estimated probabilities of concordant diagnoses, over-interpretation and under-interpretation, and expected costs to the number of women undergoing breast biopsies in the U.S. each year. We estimated the population of women undergoing diagnostic breast biopsy based on 2013 population counts from U.S. Census data [8], the 2013 National Health Interview Survey (NHIS) [9], and breast biopsy rates [10]. Based on these data sources, we estimated that between 738,000 and 843,000 diagnostic breast biopsies are done in the U.S. each year, and we assumed a base case number of 790,500 women undergoing breast biopsies each year.
Estimated savings
To estimate the potential savings associated with alternative strategies, we compared the total care costs for each strategy with care costs when second opinions were obtained according to policy requirements as reported by B-Path participants. The proportion of participating pathologists reporting that they would ask for a second opinion based on their laboratory’s policy was 33% for benign without atypia, 40% for atypia (ADH), 58% for DCIS, and 60% for invasive cancer diagnoses [11]. We report the incremental savings that could be achieved over and above current policy requirements for obtaining a second opinion for each strategy that resulted in cost savings. Lower and upper bounds for the estimated savings were based on the 95% confidence interval for the misclassification rates in B-Path.
Sensitivity analyses
We undertook sensitivity analyses to ascertain the effect of different assumptions and model parameters on the estimated savings associated with second opinion strategies. In one analysis, we modified care pathways to include more intensive care involving use of breast MRI and hormonal therapy with tamoxifen following an atypia diagnosis. In other analyses, we considered the effects that doubling the cost of obtaining second opinions or increasing the number of women affected each year to 1.6 million, a commonly cited figure for annual U.S. breast biopsies [12].
Results
Based on assumptions in the model, in the absence of a second opinion for breast pathology interpretations, 92.2% of breast biopsies would receive a reference-standard-concordant diagnosis resulting in a projected 729,078 concordant diagnoses in the U.S. each year (Table 2). Concordant diagnoses can be considered clinical estimates of diagnostic accuracy (i.e., correct diagnoses). Misclassified diagnoses, estimated at 61,422 each year, can be considered clinical estimates of the total number of breast biopsies with an incorrect diagnosis. The number of misclassified diagnoses decreased under all second opinion strategies evaluated in the model. Misclassification was lowest when either all biopsies were subjected to a second opinion (N = 39,051 with misclassified diagnoses), or when second opinions were obtained only when the initial pathologists’ diagnosis was invasive cancer, DCIS, or atypia (N = 39,683). When pathologists desired a second opinion or policy requirements called for one, misclassifications were similar in magnitude (N = 40,316).
Table 2.
Expected annual number of women with concordant and misclassified diagnoses and expected costs of care in the year following biopsy by strategy
| Pathology interpretation strategy | Number of women per year |
Care costs in year following diagnosis ($ in billions) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Concordant diagnoses | Lower bound | Upper bound | Misclassified diagnoses | Lower bound | Upper bound | Costs | Lower bound | Upper bound | |
| No second opinion | 729,078 | 715,403 | 743,149 | 61,422 | 47,351 | 75,098 | $6.049 | $6.018 | $6.084 |
| Second opinion based on initial diagnosis | |||||||||
| 2nd if invasive | 731,292 | 710,185 | 749,552 | 59,208 | 40,948 | 80,315 | $5.976 | $5.894 | $6.023 |
| 2nd if invasive or DCIS | 737,141 | 717,695 | 753,505 | 53,359 | 36,995 | 72,805 | $5.913 | $5.817 | $5.971 |
| 2nd if invasive, DCIS or atypia | 750,817 | 735,165 | 763,307 | 39,683 | 27,193 | 55,335 | $5.907 | $5.804 | $5.973 |
| Second opinion based on pathologist’s desire or current policy | |||||||||
| 2nd if desired | 746,390 | 726,944 | 759,038 | 44,110 | 31,462 | 61,422 | $5.992 | $5.930 | $6.023 |
| 2nd if considered borderline | 741,963 | 723,070 | 760,935 | 48,537 | 32,806 | 67,430 | $5.980 | $5.902 | $6.019 |
| 2nd if required by policy | 737,457 | 729,078 | 760,935 | 53,043 | 36,126 | 72,884 | $6.036 | $5.968 | $6067 |
| 2nd if desired or required | 750,185 | 717,616 | 754,374 | 40,316 | 26,640 | 56,758 | $6.002 | $5.929 | $6.045 |
| Second opinion for all | 751,449 | 735,165 | 764,809 | 39,051 | 25,691 | 55,335 | $6.060 | $5.992 | $6.098 |
Lower and upper bounds are based on 95% confidence interval for misclassification rate
The improvement in diagnostic accuracy with the addition of second opinion strategies was accompanied by reduced rates of over-treatment relative to under-treatment (Fig. 2). As depicted in Fig. 2, the number of women experiencing over-treatment was lowest when second opinions were sought selectively for cases initially diagnosed with invasive cancer, DCIS, or atypia.
Fig. 2.
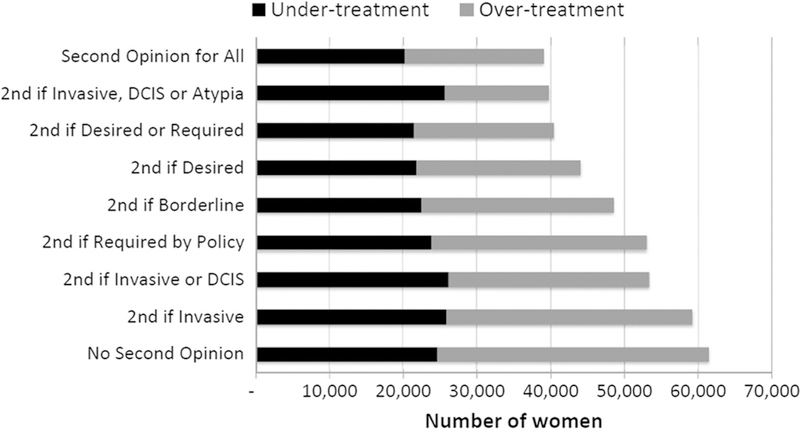
Estimated number of cases misclassified by diagnostic interpretation strategy with number of over-and under-treated shown for each pathologic interpretation strategy
Under base case assumptions, the no second opinion strategy resulted in breast-related direct medical costs of $6.049 billion in the year following the breast biopsy diagnosis (Table 2). In comparison, costs were lowest at $5.907 billion when second opinions were sought for cases initially interpreted as invasive, DCIS, or atypia—an estimated savings of $142 million per year with improved outcomes for potentially over 20,000 women.
Many pathology laboratories in the US have policies requiring that a second opinion be obtained on specific cases, usually new diagnoses of invasive cancer. Accuracy improved for over 17,000 women (misclassified diagnoses dropped to 44,110) when these policies were analyzed in the model. However, when the cost savings of improved treatment were considered, the care costs were similar to results of strategies that considered not obtaining second opinions at all ($6.036 billion/year vs. $6.049 billion/year with no second opinions, respectively).
Despite adding costs for second interpretations in the model, all second opinion strategies projected overall savings except for the strategy of obtaining a second opinion for all breast biopsies each year (Table 2). Estimated cost savings were greatest for the strategy that triggered a second opinion when the initial pathologist reported that invasive cancer, DCIS, or atypia was present with an expected annual savings of $142 million per year relative to no second opinion or of $129 million per year relative to current policy requirements. As analytic assumptions were varied in sensitivity analyses (Fig. 3), projected cost savings for this strategy remained high, increasing to an expected annual savings of over $250,000,000 per year if the actual total number of breast biopsies is 1.6 million per year as has been estimated by some [12]. When the cost of obtaining a second opinion was doubled (from base case of $140 to $280), the strategy of obtaining a second opinion if desired or required by current policies no longer saved money under lower bound misclassification estimates.
Fig. 3.
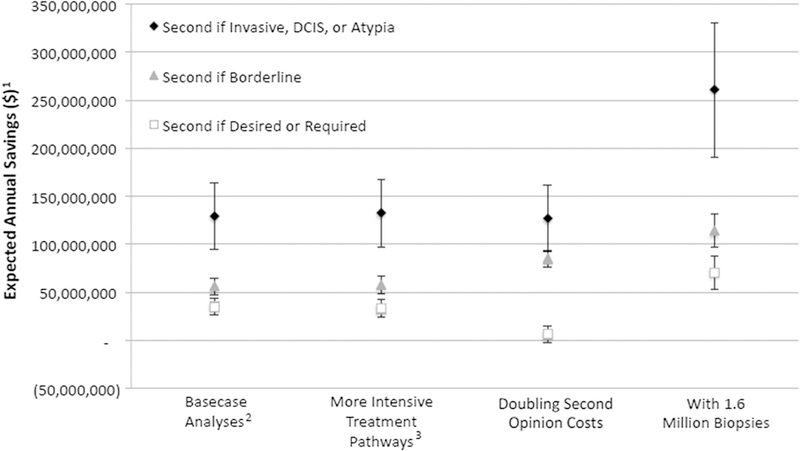
Expected annual savings for specified strategies relative to current policy requirements for second opinion as base case assumptions are varied. Bars reflect the lower and upper bound estimates (1) Title of Y axis (‘‘Relative to if a second opinion was required by policy’’; and on X Axis: (2) Basecase Analyses (‘‘Based on 790,500 women undergoing breast biopsies every year according to baseline care pathways (see Appendix)’’; and (3) More Intensive Treatment Pathways (‘‘Based on 790,500 women undergoing breast biopsies every year but with breast MRI and hormonal therapy used in the year following a breast diagnosis involving Atypia’’)
Discussion
Our decision analysis evaluated second opinion strategies for pathologic interpretation of breast tissue to determine the extent of over-and under-treatment and associated costs of health care in the year following biopsy from a U.S. screening population perspective. Our population projections indicate that the vast majority of women who undergo breast biopsy each year in the United States receive pathological interpretations that are concordant with consensus expert opinion diagnoses. While this is reassuring [3], our analyses also suggest that there is potential for improvement in pathologic diagnosis. Compared with current second opinion policies, apart from a strategy of second opinion for all biopsy interpretations, the highest number of additional concordant diagnoses was projected when second opinions were triggered by an initial diagnosis of invasive cancer, DCIS, or atypia. Under that strategy, an additional 13,360 women were projected to receive concordant diagnoses. Importantly, that strategy was also estimated to result in a lower expenditure of healthcare resources relative to current policies for second opinions. Overall, our analysis suggests that annual potential savings of approximately $129 million could be realized if second opinions were routinely sought following an initial pathology interpretation of invasive cancer, DCIS, or atypia.
When compared with current policy requirements for obtaining second opinions, we found that several strategies may reduce overall expenditures on breast-related care in the year following a biopsy. However, it is important to consider the balance of women projected to receive over-versus under-treatment under each strategy. The lowest costs were estimated for second opinions triggered by initial interpretations of invasive cancer, DCIS, or atypia, but this strategy had relatively more under-versus over-treated women. While this suggests a lower chance of over-diagnosis, our analysis did not directly address the issue of over-diagnosis, which is defined as the diagnosis of a dis-ease that will not come to medical attention or adversely affect an individual during his or her lifetime [13]. Given the 1-year time horizon for our analysis, we were not able to evaluate over-diagnosis, which is challenging to evaluate in both clinical and model-based studies [14, 15].
There is growing awareness that diagnoses of DCIS may not all pose a threat to a woman’s expected survival and that active surveillance may be a reasonable course of action [16]. Our study used established care guidelines and care pathways to estimate costs of care in the year following the biopsy and, therefore, did not consider the option of active surveillance which may be less costly. Estimates of costs associated with over-diagnosis in the year following a DCIS diagnosis may be lower in settings of active surveillance.
To demonstrate the value of obtaining second opinions for outside pathology diagnoses referred to a tertiary care setting, one study evaluated all pathology cases in a single month and reported the frequency of diagnostic discrepancies that may have affected care [17]. Among 297 outside breast cases, there were 40 (13.47%) discrepancies, with 14 (4.73%) characterized as potentially significant. Of the 14 cases, 8 were documented to have changes in individual clinical management costs ranging from a savings of $90,642 to an added cost of $115,832. Another study from the same institution focused on breast pathology cases for which second opinions were sought and found significant discrepancies in 226/1970 cases (11.47%) [18]. While intraductal lesions were identified among the most problematic cases, this study also included pathology diagnoses not considered in B-Path (lobular carcinoma, metaplastic carcinomas, and phyllodes tumors), which were also noted as often being discrepant.
For second opinion strategies to succeed, it is critical that health systems support pathologists in obtaining second opinions. The B-Path study showed that nearly all participating pathologists (96%) believed second opinions improve diagnostic accuracy and that many seek second opinions even in the absence of institutional policies [11]. The manpower demands for obtaining second opinions were not considered in our study, but it is possible that second opinions could be obtained from within the same institution. With the growth of telepathology, it is increasingly possible that second opinions could be efficiently instituted through centralized services [18, 19]. Time lags in the diagnostic evaluation can be stressful for women, and digital imaging might help to speed up this process. Digital whole slide imaging was approved by the FDA in 2017 for primary diagnosis.
While our results suggest potential cost savings for second opinion strategies, these findings must be interpreted with caution due to several limitations inherent in our analysis. First and foremost, our population projections were based on a simulation study of second opinion strategies and involved a limited set of slides and only a single slide per case. We also did not consider the expertise of the pathologists from whom a second opinion was sought. However, changes in overall misclassification rates have been shown to differ little by expertise [4].
We conclude that relative to current second opinion policies, strategies that require breast biopsy specimens initially read as invasive cancer, DCIS, or atypia have the potential to improve diagnostic accuracy for more than 13,000 women each year, while also reducing both over-and under-treatment, and lowering overall care costs by an estimated $129 million per year. Implementation of second opinion strategies in clinical settings should be studied to evaluate efficient best practices for improving pathologic interpretation of breast biopsy specimens.
Acknowledgements
This work was supported by the National Cancer Institute (NCI) (R01 CA140560, R01 CA172343 and K05 CA104699) and by the NCI-funded Breast Cancer Surveillance Consortium (BCSC, HHSN261201100031C). The content is solely the responsibility of the authors and does not necessarily represent the views of the NCI or the National Institutes of Health. The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of BCSC sources see: http://www.breastscreening.cancer.gov/work/acknowledgement.html. The authors thank Linn Abraham for analyzing the BCSC pathology data and Mackenzie Bronson for her research assistance.
Appendix
See Table 3.
Table 3.
Model parameters, data sources and assumptions
| Pathology diagnosis distribution from the Breast Cancer Surveillance Consortium (BCSC)a | Invasive | DCIS | Atypia | Benign |
|---|---|---|---|---|
| Ages 40–49 | 11.0% | 4.9% | 3.2% | 80.9% |
| Ages 50–59 | 20.2% | 8.5% | 4.3% | 67.1% |
| Ages 60–69 | 29.6% | 8.8% | 3.1% | 58.4% |
| Ages 70–79 | 35.9% | 9.2% | 3.0% | 51.9% |
| Ages ≥80 | 47.0% | 11.3% | 3.1% | 38.6% |
| Cost components in 2013 U.S. Dollars | Costs | Source | Assumptions |
|---|---|---|---|
| Treatment for DCIS within year following diagnosis | $13,376.08 | Yabroff (2008) [7] | |
| Treatment for invasive carcinoma within year following diagnosis | $27,043.02 | Yabroff (2008) [7] | Prevalence-weighted care costs for regional and distant breast cancer |
| Hormone therapyd | $1469.65 | Average wholesale price for Tamoxifen 20 mg/day | |
| ‘‘Follow-up’’ digital mammogram | $277.62 | Medicare | Digital screening mammogram with associated professional and technical fees |
| Bilateral screening breast MRI | $1103.34 | Medicare | |
| Core breast biopsyb | $936.90 | Plevritis (2006) [6] | Ultrasound-guided core needle biopsy and stereotactic biopsy, weighted based on their respective probabilities |
| Open surgical biopsy | $1657.39 | ||
| Second opinion breast pathology interpretationc | $140.17 | Medicare | Tissue exam by pathologist with associated professional and technical fees |
Based on observed breast pathology outcomes among women 504,032 women undergoing 994,085 screening mammograms in 2004 through 2008
The cost of core breast biopsy is a combined value from the costs of ultrasound-guided core needle biopsy and stereotactic biopsy, weighted based on their respective probabilities
The cost of core biopsy’s additional reading is the summation of costs from the CPT codes for a tissue exam by pathologist and the associated professional and technical components
Based on average wholesale price for Tamoxifen given at a regimen of 20 mg/day
Footnotes
Compliance with ethical standards
Conflicts of interest The authors declare that they have no conflicts of interest.
References
- 1.National Academies of Sciences, Engineering, and Medicine (2015) Improving diagnosis in health care The National Academies Press, Washington [Google Scholar]
- 2.Elmore JG, Longton GM, Carney PA et al. (2015) Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 313(11):1122–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmore JG, Nelson HD, Pepe MS et al. (2016) Variability in pathologists’ interpretations of individual breast biopsy slides: a population perspective. Ann Intern Med 164(10):649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmore JG, Tosteson AN, Pepe MS et al. (2016) Evaluation of 12 strategies for obtaining second opinions to improve interpretation of breast histopathology: simulation study. BMJ 353:i3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (2016) NCCN guidelines for treatment of cancer by site https://www.nccn.org/professionals/physician_gls/f_guidelines.asp - site
- 6.Plevritis SK, Kurian AW, Sigal BM et al. (2006) Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA 295(20):2374–2384 [DOI] [PubMed] [Google Scholar]
- 7.Yabroff KR, Lamont EB, Mariotto A et al. (2008) Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100(9):630–641 [DOI] [PubMed] [Google Scholar]
- 8.United States Census Bureau USC (2013) American FactFinder https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Accessed 16 March 2017
- 9.National Health Interview Survey (2013) http://www.cdc.gov/nchs/nhis/nhis_2013_data_release.htm
- 10.Soot L, Weerasinghe R, Wang L, Nelson HD (2014) Rates and indications for surgical breast biopsies in a community-based health system. Am J Surg 207(4):499–503 [DOI] [PubMed] [Google Scholar]
- 11.Geller BM, Nelson HD, Carney PA et al. (2014) Second opinion in breast pathology: policy, practice and perception. J Clin Pathol 67(11):955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman M (2009) Where’s the outrage? J Am Coll Surg 208(1):78–79 [DOI] [PubMed] [Google Scholar]
- 13.Welch HG, Black WC (2010) Overdiagnosis in cancer. J Natl Cancer Inst 102(9):605–613 [DOI] [PubMed] [Google Scholar]
- 14.Etzioni R, Gulati R (2016) Recognizing the limitations of cancer overdiagnosis studies: a first step towards overcoming them. J Natl Cancer Inst doi: 10.1093/jnci/djv345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati R, Feuer EJ, Etzioni R (2016) Conditions for valid empirical estimates of cancer overdiagnosis in randomized trials and population studies. Am J Epidemiol 184(2):140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esserman LJ, Thompson IM, Reid B et al. (2014) Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 15(6):e234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton LP, Feeley TW, Albright HW, Walters R, Hamilton SH (2014) Second-opinion pathologic review is a patient safety mechanism that helps reduce error and decrease waste. J Oncol Pract 10(4):275–280 [DOI] [PubMed] [Google Scholar]
- 18.Khazai L, Middleton LP, Goktepe N, Liu BT, Sahin AA (2015) Breast pathology second review identifies clinically significant discrepancies in over 10% of patients. J Surg Oncol 111(2): 192–197 [DOI] [PubMed] [Google Scholar]
- 19.Farahani N, Pantanowitz L (2015) Overview of telepathology. Surg Pathol Clin 8(2):223–231 [DOI] [PubMed] [Google Scholar]


