Novel fortified rice formulations enhance dietary iron bioavailability in deficient populations in sub-Saharan Africa.
Abstract
Iron deficiency and anemia are prominent contributors to the preventable disease burden worldwide. A substantial proportion of people with inadequate dietary iron rely on rice as a staple food, but fortification efforts are limited by low iron bioavailability. Furthermore, using high iron fortification dosages may not always be prudent in tropical regions. To identify alternative fortification formulations with enhanced absorption, we screened different iron compounds for their suitability as rice fortificants, measured in vitro gastric solubility, and assessed dietary iron bioavailability using stable isotopic labels in rural Ghanaian children. Isotopic incorporation in red blood cells indicates that in the two age groups of children investigated (4 to 6 and 7 to 10 years), formulations provided 36 and 51% of the median daily requirement in absorbed iron, respectively. We describe approaches to enhancing iron bioavailability from fortified rice, which can substantially contribute to the prevention of iron deficiency in rice-eating populations.
INTRODUCTION
Anemia is estimated to affect almost 2 billion people globally (1), and despite its multifactorial etiology, including other nutritional deficiencies such as vitamin A, folic acid, and vitamin B12 deficiencies, iron deficiency (ID) is considered its most common cause (1). The prevalence of ID in tropical countries is likely due to a combination of low dietary diversity (2) resulting in monotonous micronutrient-deprived diets, the high prevalence of infections, which can inhibit iron absorption via the hepcidin axis (3), and the high prevalence of parasites increasing iron losses (4). People living in areas with high ID anemia (IDA) prevalence may also be at increased risk for zinc deficiency (5).
Rice is a staple food for almost half of the world’s population, and its consumption is rapidly expanding in Africa (6). West African countries have estimated rice per capita intakes ranging from 77 to 281 g/day in adults (7). There is a high prevalence of iron and zinc deficiencies in many rice-consuming countries (8, 9), but the implementation of rice fortification has been limited. Rice is mainly consumed as intact polished grains, making its fortification technically demanding. In addition, because of the white color of polished rice, only organoleptically inert, white-colored, poorly water-soluble iron compounds, such as iron phosphates, are currently recommended fortificants (10). Ferric pyrophosphate (FePP) is the most widely used iron fortificant in rice and has shown low relative bioavailability (RBV) (≈50%) in humans compared to water-soluble ferrous sulfate (FeSO4) (11). Because of its low bioavailability, the recommended iron fortification level using FePP in rice is twice that of wheat flour fortified with FeSO4 (12). The higher fortification level could increase costs, could affect product appearance, and may not be prudent, as it has been reported to shift the colonic microbiome composition unfavorably in infants (13). Furthermore, fortification and supplementation levels of Fe (≈2 mg/kg body weight), while being efficacious in treating anemia, have been associated with increased hospitalizations in infants and preschool children, in particular, due to diarrhea, pneumonia (14, 15), and malaria. Thus, formulations with higher iron bioavailability yet lower fortification levels and acceptable sensory properties are needed. Furthermore, the premix approach used for rice fortification, where 0.5 to 1% of kernels are fortified and blended with natural unfortified rice (blending ratio, 1:100 to 1:200), causes high local nutrient concentrations per fortified kernel, which induce peculiar nutrient-nutrient as well as nutrient-solubilizing agent interactions in fortified rice (16, 17). We have recently shown a considerable enhancement in iron bioavailability upon addition of a citric acid and trisodium citrate combination (CA + TSC) before extrusion of fortified kernels (17). Furthermore, while the majority of studies suggests no effect of the zinc fortification compound on human iron bioavailability at typical concentrations found in foods, we reported reduced iron bioavailability from fortified rice cofortified with zinc oxide (ZnO) compared to fortification with zinc sulfate (ZnSO4) (16, 17). Sodium pyrophosphate (NaPP) has been suggested to enhance iron bioavailability from FePP in bouillon cubes (18), and its suitability for rice fortification has not been investigated. Furthermore, the addition of EDTA was reported to increase in vitro solubility of FePP from fortified rice (19).
To investigate the potential of optimized iron fortification formulations in rice, we conducted a series of simulated in vitro digestion experiments and characterized the color of the extruded rice kernels in comparison to unfortified rice, a proxy for their acceptability (20). We then tested five promising formulations in a dietary multiple meal study in Ghanaian children living in a rural area affected by infectious diseases and malaria. All participants consumed the five-meal series in randomized order and acted as their own controls throughout the study. Iron bioavailability of each formulation was assessed by labeling fortified rice with stable iron isotope tracers and measuring their incorporation in red blood cells (RBCs) 14 to 32 days after meal series administration. We hypothesized that (i) iron from rice formulations containing iron-solubilizing compounds would be absorbed similarly to readily water-soluble FeSO4 and would provide a substantial proportion of the absorbed iron requirement while maintaining an acceptable color profile and (ii) iron-solubilizing compounds would overcome the inhibitory effect of ZnO on FePP absorption.
RESULTS
Visual scores
Using the summary color score ΔE, rices with scores below 7 were considered hardly distinguishable from unfortified basmati rice (Table 1), while those with scores between 13 to 27 were discolored compared to unfortified basmati rice (Table 2). Compared to unfortified basmati rice, ΔE color scores were lowest when rice was fortified with FePP, ZnSO4, NaPP, and EDTA in 1:0.7:1:1 molar ratios.
Table 1. Composition of extruded rice formulations fortified with iron and zinc including relative iron solubility and visual scores.
Solubility values without a common script differ significantly (P < 0.05) using univariate general linear model with multiple comparisons Bonferroni correction. All rices were fortified with Fe (4 mg/g) except otherwise stated.
| Fe compound | Zn compound | Solubilizing agent |
Molar ratios (Fe/Zn/solubilizing agent) |
Relative solubility* | ΔE to basmati rice† |
| FeSO4 | – | – | – | 81.6a ± 3.22 | 14.6 |
| FePP | – | – | – | 7.68b,c ± 0.90 | 5.09 |
| ZnO | – | 1:0.7 | 13.6b,c ± 0.35 | 6.46 | |
| ZnSO4 | – | 1:0.7 | 4.35c ± 0.43 | 6.83 | |
| NaPP | 1:0.7:1 | 15.1b,d ± 0.84 | 5.59 | ||
| NaPP | 1:0.7:1.5 | 37.0e ± 0.67 | 6.61 | ||
| NaPP | 1:0.7:3.0 | 63.9f ± 6.15 | 6.74 | ||
| NaPP and EDTA | 1:0.7:1:1 | 112g,h ± 6.17 | 4.60 | ||
| NaPP, EDTA, CA + TSC | 1:0.7:1:1:0.1:1.7 | 102.7g ± 1.23 | 6.27 | ||
| EDTA, CA | 1:0.7:1:0.1 | 99.2g ± 3.28 | 5.36 | ||
| EDTA | 1:0.7:1 | 108g ± 0.5 | 7.37 | ||
| EDTA | 1:0.7:0.3 | 37.3d ± 0.35 | 5.94 | ||
| EDTA, CA + TSC | 1:0.7:1:0.1:1.7 | 118.9h ± 3.64 | 6.24 | ||
| – | CA + TSC‡ | 1:0.1:1.7 | 50.9i ± 3.9 | 7.55 | |
| CA + TSC + 20%‡ | 1:0.12:2.0 | 59.9i ± 6.9 | 7.39 | ||
| CA + TSC + 40%‡ | 1:0.15:2.5 | 60.3i ± 5.06 | 7.91 | ||
| FePP | ZnSO4 | NaFeEDTA | 1:0.7:0.05 | 24.0d,l ± 2.48 | 8.34 |
| ZnSO4 | NaFeEDTA | 1:0.7:0.17 | 43.9e,i ± 1.66 | 8.55 | |
| FeOP | – | – | – | 17.3b,l ± 0.93 | 7.31 |
| ZnSO4 | – | 1:0.7 | 10.4b,c ± 0.52 | 8.19 | |
| CA + TSC | 1:0.7:0.1:1.7 | 39.4e ± 1.03 | 8.74 |
*Solubility relative to unfortified basmati rice fortified with FeSO4 after cooking.
†Color differences relative to unfortified basmati rice. Rices with discoloration above ΔE values of ≥13 were distinguishable from unfortified basmati rice. In previous studies, rices with ΔE in the range of 5.1 to 6.7 compared to basmati rice were undistinguishable from unfortified basmati rice in triangle tests (20).
‡Fe (5 mg/g).
Table 2. Novel iron compounds screened for suitability for rice fortification using a premix approach.
Rice was fortified with Fe (5 mg/g).
| Iron compounds used for fortification | ΔE to basmati rice* |
| Fe glycinate taste free | 38.8 |
| Fe (III) tartrate 1 hydrate | 36.1 |
| Fe (II) bisglycinate | 27.1 |
| Fe (II) succinate | 11.4 |
| Fe (II) ammonium phosphate | 6.73 |
| Soluble FePP | 17.4 |
| Soluble FePP (III) + citrate | 10.8 |
| FeSO4 | 14.6 |
| FeSO4 + CA + TSC | 20.1 |
*Color differences relative to unfortified basmati rice. ΔE values of ≥13 were distinguishable from unfortified basmati rice. In previous studies, rices with ΔE in the range of 5.1 to 6.7 compared to basmati rice were undistinguishable from unfortified basmati rice in triangle tests (20).
In vitro iron solubility
Fortified rice with FeSO4 showed 81% iron solubility compared to unfortified rice with FeSO4 added as a solution after cooking. In contrast, rice fortified with FePP without any solubilizing agents had a relative solubility of 7.68 to 13.6%, depending upon the addition of the zinc compound (Table 1). Iron solubility was higher when rice was fortified with ZnO compared to ZnSO4. Adding solubilizing agents such as CA in combination with TSC increased solubility (50.9 to 60.3%). This was also the case for EDTA at varying concentrations (37 to 108%; Table 1). CA and TSC addition enhanced iron solubility of both FePP and ferric orthophosphate (FeOP).
Combining solubilizing agents generally did not result in additive effects on in vitro iron solubility (Table 1). This was not the case for NaPP and EDTA, as well as for EDTA, NaPP, and CA + TSC, which both had similar solubility to EDTA alone. Nonetheless, combining CA + TSC and EDTA resulted in the highest iron solubility compared to EDTA alone and EDTA, NaPP, and CA + TSC, but did not differ from NaPP and EDTA. Notably, formulations containing EDTA at 1:1 molar ratio to iron, with CA + TSC and/or NaPP, had iron solubility comparable to or higher than for the readily soluble FeSO4. The enhancement of iron solubility when adding a solubilizing agent was dose dependent for EDTA and NaPP but did not reach significance for CA + TSC (molar ratio Fe/Zn/solubilizing agent, 1:0.7:1.7 versus 1:0.15:2.5; P = 0.052; Table 1). This was also the case for formulations containing FeOP for which we found a fourfold increase (39.4 versus 10.1% solubility; Table 1), suggesting that FeOP could be similarly enhanced by CA + TSC addition, as we reported a four- to fivefold increase upon addition of CA + TSC.
At similar molar ratios, the addition of NaPP to extruded rice fortified with FePP seemed to be a less potent solubilizing agent than CA + TSC: To reach a similar increase in solubility, a higher concentration of NaPP had to be used, whereas those formulations still showed generally acceptable visual properties. The most potent solubilizing agent in vitro was found to be EDTA, which substantially increased iron solubility.
Choice of rice formulations for the human absorption study
We conducted a human absorption study to assess iron bioavailability of the most promising formulations with enhanced in vitro solubility and smallest possible color difference to unfortified rice. CA and TSC are made of low-cost, food-grade chemical compounds, and contrary to EDTA, no tolerable upper intake levels have been defined. Specifically, we investigated the effect of the presence of CA + TSC and the zinc compound (ZnO or ZnSO4), aiming to assess whether the presence of CA + TSC would overcome potential inhibitory effects of zinc on FePP bioavailability. In addition, we tested one formulation containing EDTA, as our solubility data strongly suggested enhanced in vitro iron solubility. Thus, the following formulations, which were also compared to a FeSO4 reference, were tested: FePP in combination with ZnO or ZnSO4 with (54FeZnOCT or 57FeZnSO4CT) or without addition of CA + TSC (54FeZnO or 57FeZnSO4) and FePP in combination with ZnO, CA, and EDTA (58FeZnOCE).
Composition of test meals
The study test meals contained 68 ± 1.8 or 6 ± 0.32 mg of phytic acid (PA) and 0.7 ± 0.02 or 0.7 ± 0.06 mg of ascorbic acid (AA) per serving containing bean or tomato sauce, respectively. The polyphenol (PP) contents in each serving of sauce were 11.1 ± 0.66 mg and 12.3 ± 2.2 mg as gallic acid equivalents in bean and tomato sauce, respectively. This resulted in a dietary phytate/Fe ratio of ~1:1. The dietary AA and PP contents were on average 0.7 mg and 11.7 mg per serving, respectively.
In vitro iron solubility from rice selected for the human absorption study
In vitro iron solubility from 54FeZnO (3.6%) significantly differed from 54FeZnOCT (19.8%), 57FeZnSO4CT (27%), and 58FeZnOCE (24%) (for all, P < 0.01), but not from 57FeZnSO4 (4.7%), which differed from all other conditions (for all, P < 0.01). Iron solubility did not significantly differ between 54FeZnOCT, 54FeZnSO4CT, and 58FeZnOCE (Table 3).
Table 3. Composition of extruded rice, administered study meals, human iron absorption, and in vitro iron solubility.
Each rice meal serving contained 49.5 g of unfortified rice and 0.5 g of fortified rice with 50 g of either bean or tomato sauce. Values are means ± SDs or geometric means (95% CI). Labeled means in a column without a common letter differ. P < 0.05, Bonferroni-corrected linear mixed model. Designated micronutrient composition of fortified kernels can be found in the Supplementary Materials. 54FeZnO, extruded rice containing 54FePP, ZnO, and a micronutrient mix (MN); 57FeZnSO4, extruded rice containing 57FePP, ZnSO4, and MN; 54FeZnOCT, extruded rice containing 54FePP, ZnO, MN, CA, and TSC; 57FeZnSO4CT, extruded rice containing 57FePP, ZnSO4, MN, CA, and TSC; 58FeZnOCE, extruded rice containing 58FePP, ZnO, MN, CA, and EDTA; Reference, extruded rice containing MN and 58FeSO4 solution added before consumption. N/A, not applicable.
| Iron (mg/g)* | Zinc (mg/g)* |
Fe/Zn/CA/TSC/ EDTA (mol/mol)† |
Fe/Zn/PA/AA (mol/mol)‡ |
Fractional iron absorption (%)§ |
RBV (%)§,|| |
Relative iron solubility (%)¶ |
|
| 54FeZnO | 3.5 ± 0. 50 | 4.2 ± 0.08 | 1:1.0:0:0:0 | 1:1.0:0.8:0.0 | 2.3 (1.9–2.8)a | 36a | 3.6 ± 0.5a |
| 57FeZnSO4 | 3.2 ± 0.50 | 4.1 ± 0.19 | 1:1.1:0:0:0 | 1:1.1:1.0:0.0 | 3.5 (2.7–4.5)b | 54b | 4.7 ± 0.5a |
| 54FeZnOCT | 3.2 ± 0. 50 | 4.2 ± 0.1 | 1:1.1:0.3:6.0:0 | 1:1.1:0.9:0.0 | 4.5 (3.6–5.5)b | 70.4b | 19.8 ± 2.7b |
| 57FeZnSO4CT | 3.4 ± 0. 50 | 4.1 ± 0.19 | 1:1.1:0.3:5.5:0 | 1:1.1:0.9:0.0 | 6.3 (5.3–7.4)c | 98.3c | 27.1 ± 4.0b |
| 58FeZnOCE | 3.3 ± 0. 50 | 4.1 ± 0. 19 | 1:1.1:0.3:0:0.6 | 1:1.1:0.9:0.0 | 6.4 (5.1–8.1)c | 101c | 24.1 ± 3.1b |
| Reference | 3.6 ± 0. 10 | 1.3 ± 0.01 | N/A | 1:0.3:0.8:0.0 | 6.4 (5.2–7.8)c | N/A | N/A |
*Calculated iron and zinc contents in composite meals were based on the means from the analysis of single components [49.5 g of regular basmati rice, 0.5 g of extruded rice, and average mineral contents from the two different sauces (50 g of sauce); n = 3], and SDs for iron and zinc contents of composite meals were adapted by calculating the square root of the squared and summed SDs from each single component.
†Molar ratio in fortified rice.
‡Molar ratio per serving of rice—average mineral, PA, and AA contents from the two different sauces were calculated.
§n = 26.
||RBV calculation based on geometric mean fractional iron absorption (%) relative to fractional iron absorption from the reference meal for each subject.
¶Iron solubility was assessed in fortified rice kernels. For details on the calculation, please refer to the text, n = 3.
Participants’ characteristics in the human absorption study
Sixty-seven participants were screened and 31 subjects with iron-deficient erythropoiesis (IDE) and/or anemia were included in the study (Fig. 1). Five participants discontinued participation after the first meal administration; thus, 26 participants completed the study and were included in the analysis. At baseline, 58% of the participants were classified to have anemia, 39% to have IDA, and 15% to have ID without anemia (Table 4). At baseline, 11 participants had asymptomatic malaria parasitemia, 3 participants had elevated C-reactive protein (CRP) concentrations, and 6 participants had elevated α1-acid glycoprotein (AGP) concentrations. Two participants remained positive for malaria parasitemia throughout the whole study period, and one additional participant was positive for malaria parasitemia at endpoint only.
Fig. 1. Study flowchart for human absorption study.
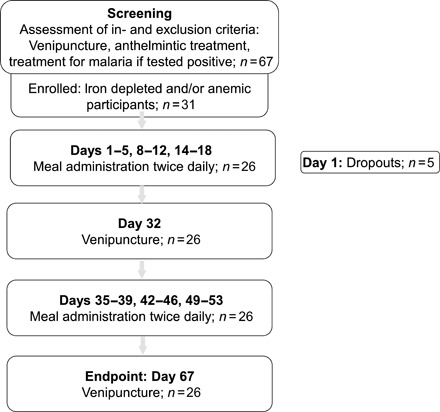
At inclusion, subjects who had anemia [defined as hemoglobin (Hb) <11.5 g/dl] and/or IDE [defined as zinc protoporphyrin/heme (ZnPP/H) >43 μmol/mol] were selected (42–44).
Table 4. Anthropometric, iron, and inflammatory variables of the Ghanaian children (n = 26), assessed before first meal administration.
Values are means ± SD if not otherwise stated. Vitamin A deficiency defined as RBP < 0.7 μM.
| Sex (female), % | 42 |
| Age, years | 7.6 ± 1.3 |
| Weight, kg | 22 ± 3.6 |
| Height, cm | 115 ± 9.3 |
| Weight for age, Z score | −1.3 ± 0.81 |
| Height for age, Z score | −1.2 ± 0.96 |
| Body mass index for age, Z score | −0.7 ± 0.65 |
| Zinc protoporphyrin/heme ratio*, μmol/mol |
52.0 (44.6–59.5) |
| Hemoglobin, g/liter | 112 ± 9 |
| Plasma ferritin*, μg/liter | 43.6 (32.5–58.4) |
| Body iron stores, mg/kg body weight |
4.2 ± 0.70 |
| Soluble transferrin receptor*, mg/liter |
8.8 (7.75–10.05) |
| Hepcidin*, nM | 12.0 (7.4–19.1) |
| Iron-deficient, non-anemic†, prevalence, n (%) |
4 (15) |
| Anemia‡,§ prevalence, n (%) | 15 (58) |
| Non-IDA | 5 (19) |
| IDA | 10 (39) |
| Retinol binding protein, μM | 0.79 ± 0.19 |
| Vitamin A deficiency prevalence, n (%) |
8 (24) |
| C-reactive protein >5 mg/liter, prevalence, n (%) |
3 (12) |
| α1-Acid glycoprotein >1 g/liter, prevalence, n (%) |
6 (23) |
| Positive malaria by microscopy, prevalence, n (%) |
11 (42) |
*Values are geometric means (95% CI).
†ID defined as sTfR >8.3 mg/liter and/or plasma ferritin <30 μg/liter.
‡Anemia was defined as Hb <115 g/liter.
§IDA defined as sTfR >8.3 mg/liter and/or plasma ferritin <30 μg/liter in combination with Hb <115 g/liter.
Human iron bioavailability enhanced upon addition of CA + TSC or EDTA
Overall, the type of meal affected X fractional iron absorption (FAFe) (P < 0.001). Specifically, the type of zinc compound and the presence of CA + TSC significantly affected FAFe (for all, P ≤ 0.001), but there was no interaction between CA + TSC and the zinc compound.
Geometric mean [95% confidence interval (CI)] FAFe from 54FeZnO (2.3%; 1.9 to 2.8) significantly differed from 57FeZnSO4 (3.5%; 2.7 to 4.5), 54FeZnOCT (4.5%; 3.6 to 5.5), 57FeZnSO4CT (6.3%; 5.3 to 7.4), 58FeZnOCE (6.4%; 5.1 to 8.1), and the FeSO4 reference (6.4%; 5.2 to 7.8) (for all, P ≤ 0.033; Table 3 and Fig. 2). 57FeZnSO4 significantly differed from all other conditions (P ≤ 0.001) except 54FeZnOCT. There was no significant difference between 54FeZnSO4CT, 58FeZnOCE, and the reference meal. However, they all differed significantly from 54FeZnOCT (for all, P ≤ 0.037). A similar pattern was seen for the bioavailability relative to FeSO4 (RBV): Mean RBV from 54FeZnO (36%) significantly differed from 57FeZnSO4 (54%), 54FeZnOCT (70%), 57FeZnSO4CT (98%), 58FeZnOCE (101%), and the reference (for all, P ≤ 0.004). 57FeZnSO4 differed from all other conditions (P ≤ 0.015), except for 54FeZnOCT. There was no significant difference between 54FeZnSO4CT, 58FeZnOCE, and the reference; however, 58FeZnOCE significantly differed from 54FeZnOCT (P ≤ 0.002).
Fig. 2. Boxplots for the absolute iron absorption (mg/day) assessed measuring absorption from six different rice meals in iron-deficient and/or anemic Ghanaian children.
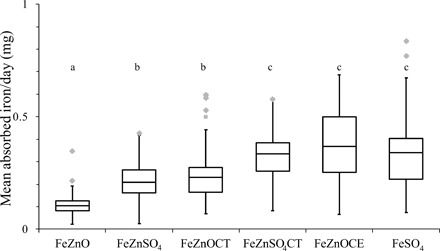
Horizontal bars show the median iron absorption for each day (100 g of rice); each box represents the first to third quartile, and whiskers represent the lowest and highest data points regardless of outliers. Gray dots indicate outliers 1.5 interquartile ranges above the third quartile. Different letters indicate significant differences. P < 0.05, Bonferroni-corrected linear mixed model; n = 26. 54FeZnO, rice extruded with 54FePP, ZnO, and a vitamin premix; 57FeZnSO4, rice extruded with 57FePP, ZnSO4, and a vitamin premix; 54FeZnOCT, rice extruded with 54FePP, ZnO, a vitamin premix, CA, and TSC; 57FeZnSO4CT, rice extruded with 54FePP, ZnO, a vitamin premix, CA, and TSC; 58FeZnOCE, rice extruded with 54FePP, ZnO, a vitamin premix, CA, TSC, and EDTA; Reference, rice extruded with a vitamin premix, 58FeSO4 added before consumption.
DISCUSSION
We screened several formulations of iron and zinc cofortified rice and identified rices containing EDTA alone or in combination with CA + TSC as most promising to test in a target population for a fortification program. We then conducted a study simulating school-based iron fortification with conventional fortification levels (4 mg of Fe/100 g of rice) and kernel premix ratios (1:100).
In Ghanaian children consuming a rice-based diet, iron absorption from fortified rice containing CA + TSC or CA + EDTA was substantially enhanced (Fig. 2). These differences in bioavailability are nutritionally relevant: Iron from the formulation currently widely used to fortify rice (54FeZnO) was absorbed at only 30 to 40% of that from 57FeZnSO4CT and 58FeZnOCE (Table 3). On the basis of our FAFe data, our study population of African school-age children consuming 100 g of rice daily fortified with formulations containing solubilizing agents CA/TSC and EDTA (FeZnSO4CT and FeZnOCE) absorbed ≈0.26 mg of iron per day, providing ≈36 and 51% of the median iron requirements for children aged 7 to 10 and 4 to 6 years, respectively (21). In contrast, a rice fortified without solubilizing agents (FeZnO) would provide between 13 and 18% of the median absorbed iron requirement in the respective age groups. In the future, these results may contribute in the revision of rice fortification guidelines toward lower, yet equally effective, iron fortification levels, which are closer to levels recommended for wheat flour fortification with FeSO4. These lower fortification levels may also be more prudent and safe, in view of the reported side effects and gastrointestinal inflammation caused by high iron doses in fortification and supplementation (13).
This is the first study to directly quantify and compare aggregate iron bioavailability from iron-fortified rice by labeling multiple meals that are consumed over several weeks by the target population and that are likely representative of the local diet. We provided 10 meals per formulation and a total of 60 labeled fortified meals per subject. This approach has several advantages over traditional stable iron isotope studies (22, 23). It measures the cumulative iron absorption from different meal characteristics of the local diet: We included traditional sauces with the rice meals to mimic the local balance of iron absorption inhibitors (such as PA and PP) and enhancers (such as AA). The multiple meal approach allows testing a rice kernel premix level of 1:100, which is commonly used in rice fortification programs. Moreover, using this design over several weeks in African children with poor iron status and common infections integrates not only dietary factors but also the countervailing effects of anemia driving higher iron absorption, while infection/inflammation reduces absorption and utilization through elevated plasma hepcidin. As we treated participants against malaria, these results provide an estimate of iron bioavailability from the fortified food as part of the local diet in the context of malaria control. While we treated all children against helminths, it is unlikely that this treatment affected iron bioavailability (24) but would instead be expected to affect long-term iron losses. This study design may be used as a preparatory study design for both efficacy and larger, more complex effectiveness trials, as it requires fewer subjects, incurs in lower costs, and avoids the dependence on indirect iron biomarkers that are often confounded by inflammation.
Our findings show that CA + TSC addition to FePP-fortified extruded rice improves iron solubility and consequently absorption regardless of the zinc fortificant used in the rice kernels. We recently reported the enhancing effect of CA + TSC on iron absorption from FePP in extruded rice in healthy Swiss women and attributed this effect to an in situ generation of soluble FePP-citrate complexes during extrusion (17). We also reported an inhibitory effect of ZnO on iron absorption from FePP-fortified rice in Swiss women with poor iron status, whereas ZnSO4 showed no such effect (16). However, in both studies, the PA content of the meals was low (12, 13). In the current study, the diet contained moderate amounts of PA as 50% of the meals in the diet also contained legumes, with an overall dietary PA/Fe ratio in the administered test meals of 1:1, a ratio likely to be considerably inhibitory (25). Findings from the current study show that addition of CA + TSC to the extruded rice does not fully overcome the inhibitory effect of ZnO relative to ZnSO4: About 30% less iron was absorbed from FeZnOCT compared to FeZnSO4CT (Table 3). A recent review on the effect of zinc on iron bioavailability from foods containing both minerals concluded that there is no significant effect at zinc/iron molar ratios below 2:1, whereas higher molar ratios decrease iron absorption (26). In the current study, the molar ratio of zinc/iron per fortified kernel was 1.3:1, highlighting the peculiar effects that occur in rice fortified with a grain premix approach, where high local concentrations may support stronger Fe-Zn interactions. One potential drawback of using ZnSO4 instead of ZnO in extruded rice containing vitamin A may be increased vitamin A degradation during storage (27); however, rice may not be the preferred vehicle for vitamin A fortification (28).
EDTA is an effective iron chelator that binds to iron at gastric pH, protecting it from interacting with inhibitory dietary ligands, but releases iron for absorption at near-neutral pH in the duodenum (29). Studies on the effects of EDTA on dietary iron absorption have mainly used NaFeEDTA or Na2EDTA and consistently show increased iron absorption in meals rich in iron absorption inhibitors, such as PA (30). However, NaEDTA addition to FePP-fortified wheat-based infant cereal had no enhancing effect on human iron bioavailability (31). In addition, EDTA can not completely overcome the inhibitory effects of PP on iron absorption (32). EDTA/iron molar ratios of 1:2 to 1:1 have been suggested for iron fortification (29), and in the current study, a ~1:2 ratio was used. In view of the peculiar interaction effects expected to take place in the extruded fortified rice kernels, we decided to test this extra arm in spite of an earlier study testing EDTA addition on FePP-fortified food, which found no significant enhancing effect on iron absorption in humans (31). The amount of EDTA in rice meals administered to participating children was well below the acceptable daily intake (ADI) and would remain below the ADI even if the children had met their entire caloric requirements with fortified rice (33) or if younger children (1 to 6 years) had been included. Nevertheless, CA + TSC may have advantages over EDTA as a chelator in extruded rice because it has no ADI, has a lower price and production costs, and is generally recognized as safe.
Scale-up
Hot extrusion is the method of choice for large-scale rice fortification (34) and can be implemented cost-effectively when using a premix approach to fortify rice. The color scores of the tested fortified rices suggest acceptable levels of discoloration, indistinguishable from unfortified rice by consumers, as discoloration was within the range of previously reported acceptable formulations (20). Our results show a two- to threefold increase in iron bioavailability when using solubilizing agents CA+TSC and EDTA when compared to current rice fortification formulations. Current recommended iron fortification levels for the general population range between 70 and 120 parts per million (ppm) depending on the rice intake (higher than 300 g/day and higher than 75 g/day, respectively) (12). Our current and previous data in adults (17), indicating increased bioavailability when using CA, TSC, and/or EDTA suggest an iron fortification level of 40 ppm in the general population (for daily rice intakes between 150 g and higher than 300 g in adults) and of 70 ppm (for daily rice intakes between 75 g and 149 g) (35). These intake levels can be effective in providing a substantial contribution to absorbed iron requirements in rice-consuming populations using food-grade and low-cost ingredients. In general, no cost increase of using CA TSC and/or EDTA would be expected, which are low-cost bulk ingredients in the food industry, as the cost increase would be compensated by the lower amount of iron required in premixes. In general, ingredient costs only contribute to a minor part (3 USD per metric ton) to the cost of rice fortification, which was previously estimated to account for approximately 15 to 19 USD per metric ton of rice, when produced at scale (29). Other approaches such as the use of quick-cooking rice formulations, which also resulted in organoleptically acceptable rice providing nutritionally relevant iron bioavailability (36), did not use a premix approach and are likely to be more expensive and less suitable for wide implementation in low-resource settings.
Our study has several strengths: (i) the highly precise and accurate measures of iron bioavailability; (ii) the integration of fortified rice into the regularly consumed diet over a high number of administered servings; (iii) the used 1:100 premix/unfortified rice blending ratio, which is common in large-scale rice fortification programs; (iv) we studied school-age children with poor iron status; and (v) our study design using multiple stable isotope labels allowed within-subject comparisons of all five rice formulations compared to a reference meal. Our study also had limitations: (i) We did not assess zinc absorption from the fortified rice, and the different formulations may affect zinc absorption; however, it has been previously shown that zinc absorption is generally adequate from similar formulations (37) and effective in increasing zinc status (38). (ii) We tested a simulated diet composed of rice and two sauces, which may not fully reflect the long-term dietary composition in this population. However, we do not expect different dietary components to affect the relative differences between the different formulations as reported. Similarly, by including a moderately inhibitory test meal, we have attempted to replicate the effect of inhibitory dietary components. (iii) The antimalarial treatment that children received likely lowered their initially high hepcidin levels and influenced their absorption. Thus, our results estimating the percentage of absorbed iron contribution of the rice formulations with solubilizing agents are applicable to settings where malaria control measures are in place. (iv) While our study shows a high contribution to iron requirements of fortified rice, our study was not designed to assess impact on the potential decrease in ID prevalence following a program of rice fortification. Further effectiveness or careful monitoring and evaluation of ongoing fortification programs would be required to assess this.
We recommend that future studies determine whether (i) the strong enhancing effect of EDTA is also present in composite meals of lower PA content, (ii) fortification with ZnSO4 instead of ZnO combined with EDTA would lead to an increased iron absorption, and (iii) zinc absorption would be influenced differently by EDTA depending on the zinc source (ZnO or ZnSO4). Furthermore, other potential fortification formulations, notably containing the solubilizing agent NaPP, or other iron phosphate compounds such as FeOP should be investigated in humans with the aim of further broadening the technical portfolio for rice fortification.
In conclusion, our findings show that consuming 100 g of rice of these optimized formulations can provide ~36 to 51% of the absorbed iron requirement in school-age children. In west Africa, per capita daily rice consumption in adults has been reported to be as high as 260 g in several countries (7), suggesting these formulations to have a large potential in covering a substantial proportion of the absorbed iron requirement in these populations and other rice-consuming communities.
MATERIALS AND METHODS
Experimental design
To assess the potential of iron-fortified rice for optimized iron bioavailability, we identified formulations of fortified rice with enhanced in vitro iron solubility and tested the most promising formulations in a deficient population in rural Ghana.
Production of fortified rice batches
For the human iron absorption study, six different batches of rice grains were prepared from hot extrusion (~90°C) using stable iron isotopes (54FePP, 57FePP, or 58FePP), as described earlier (17). Batches that were only used for assessment of in vitro iron solubility were produced similarly using nonlabeled iron. The composition of the different batches can be found in table S1. Labeled 54FePP, 57FePP, and 58FePP were prepared in separate batches by Dr. Paul Lohmann GmbH from elemental isotopically enriched iron (Chemgas, Boulogne, France) using a scaled-down process as used for the synthesis of the commercial FePP compound. The batches were then mixed with commercial FePP (Dr. Paul Lohmann GmbH), resulting in an isotopic enrichment of 47.2% 54FePP, 19.8% 57FePP, and 8.6% 58FePP, respectively.
In vitro iron solubility assessment
In vitro solubility was assessed in triplicate using amylase and pepsin enzymes, as previously described (17), modified from Miller et al. (39), and relative iron solubility was calculated with FeSO4 as a reference sample (17).
Measurement of visual scores
Visual scores were assessed as the summary difference in color compared to basmati rice. The measurement was performed with a Minolta Chroma meter (CR-210, Minolta Camera Co., Osaka, Japan). Color was measured in the CIELAB coordinate system, and color difference between two samples (1 and 2) was expressed as the summary parameter: ΔE = [(L1 – L2)2 + (a1 – a2)2 + (b1 – b2)2]1/2, where L indicates lightness, a indicates the red/green coordinate, and b indicates the yellow/blue coordinate. Fortified rices with values of ΔE = 5.1 and ΔE = 6.6 compared to basmati rice could not be identified as different in triangle tests in previous studies (20).
Human iron absorption assessment
In the human absorption study, we used a randomized, crossover, single-blind study design. The order of the test meal and reference meal administration was randomized. The primary outcome was FAFe, which was determined as incorporation of isotopic iron labels into erythrocytes 14 to 32 days after the administration of iron-stable isotope-labeled fortified test meals to fasting Ghanaian children (n = 26).
Each participant consumed six different kinds of isotopically labeled test meals, and one kind of meal was provided in a series of 10 servings over five consecutive days (two meals per day) followed by the administration of a different kind of meal on days 8, 14, 36, 43, 50 following the same regimen (Fig. 1), resulting in a total number of 60 servings per participant. The six different meals were based on rice extruded with (i) 57FePP and ZnSO4 (57FeZnSO4); (ii) 54FePP and ZnO (54FeZnO); (iii) 57FePP, ZnSO4, CA, and TSC (57FeZnSO4CT); (iv) 54FePP, ZnO, CA, and TSC (54FeZnOCT); (v) 58FePP, ZnO, CA, and EDTA (58FeZnOCE); and (vi) no iron or zinc, but fortified with 58FeSO4 added as solution before consumption (reference). Each extruded rice batch also contained a vitamin premix (vitamin A, folic acid, and vitamins B1, B3, B6, and B12; table S2B), and the meals were accompanied with a bean or a tomato sauce. Each meal contained 2 mg of iron as labeled FePP or FeSO4 and 3 mg of zinc as ZnO or ZnSO4. As the fortification level was consistent with a moderate dietary iron content, we did not expect changes of iron status during the duration of the feeding study. Furthermore, isotopic signals in RBC are constant after approximately 10 days of incorporation (consistent with RBC precursor maturation in the bone marrow) and are then invariant for the duration of RBC life span (120 days on average).
A randomization schedule was designed allocating a random number to a list of possible meal administration sequences. This took into account that meal sequences administered with the same stable isotopes had to be administered at least 21 days apart. Eligible children were individually randomly assigned a number corresponding to one of the generated meal administration sequences.
Inclusion criteria for the human absorption study assessed at baseline were as follows: (i) ages 6 to 8 years and (ii) presence of IDE [defined as erythrocyte zinc protoporphyrin/heme (ZnPP/H) >43 μmol/mol] with or without anemia [defined as hemoglobin (Hb) <11.5 g/dl]. Exclusion criteria were as follows: (i) severe underweight or wasting (Z score weight-for-age and weight-for-height < −3), (ii) chronic or acute illness, (iii) regular intake (>2 days) of iron-containing mineral and vitamin supplements within 2 months before onset of the study, and (iv) blood donation or comparable blood loss in the 4 months preceding the study.
On study days, the first serving was administered in the morning between 0700 and 0800 after an overnight fast and the second serving was administered ~3.5 hours after completion of the first serving. All meal administrations were supervised. After meal consumption, the empty bowls were rinsed with a total of 20 ml of water and the participants received 250 ml of water; all water had to be consumed by the participants to ensure complete intake of isotopes. Participants were not allowed to eat or drink between the test meals. Approximately 1.5 hours after the second serving, the participants received a standardized snack (wheat flour cookies, Mass Industries Ltd., Tema, Ghana) and had to refrain from eating and drinking for another 1.5 hours. Thereafter, they were allowed to eat and drink ad libitum. If the next day was a study day, ad libitum eating and drinking were allowed until 2000 and 0000, respectively.
During screening (baseline measurements), body weight and height of the participants were measured and blood samples were collected to assess iron status [Hb, ZnPP/H, plasma ferritin (PF), plasma CRP, soluble transferrin receptor (sTfR), body iron stores (BIS), hepcidin, AGP, and retinol binding protein (RBP)] and malaria parasitemia. Before each venipuncture, the body temperature of the children was measured with a digital thermometer (OMRON Healthcare Europe B.V., Nigeria) to help identify children who may have symptomatic malaria. All participants received anthelmintic treatment (albendazole, 400 mg), and participants positive for malaria antigens received antimalarial treatment (arthesunate-amodiaquine, 100 mg/270 mg). Measurements for body weight and height, body temperature, as well as blood collections were repeated on days 33 and 68.
The study test meals were designed on the basis of commonly consumed rice meals in the study area and consisted of two meals per day (50 g of raw rice each) served alternately with either a tomato sauce or a cowpea-based sauce. The composite test meals were prepared at the school kitchen of SOS children’s village in Tamale, Ghana, and contained 49.5 g of raw unfortified rice and about 500 mg of raw extruded rice (433 ± 1.4, 529 ± 0.5, 508 ± 0.6, 481 ± 0.5, 509 ± 0.6, and 498 ± 2.0 mg of extruded rice for meals 54FeZnO, 57FeZnSO4, 54FeZnOCT, 57FeZnSO4CT, 58FeZnOCE, and reference, respectively). Raw unfortified rice and extruded rice were mixed and cooked in a small industrial oven for 37 min at ~150°C in separate glass bowls for each participant. After cooking the rice, 499 ± 4.0 μl of a FeSO4 solution containing 2 mg of Fe labeled with 0.2 mg 58Fe was added to each reference meal. The solution was produced from enriched elemental iron (99.86% 58Fe enrichment), as previously described (23).
Tomato and bean sauces were prepared in three and two batches, respectively (table S3), and kept frozen until 1 day before a meal administration when they were thawed. On each administration day, the sauces were reheated and 50 g of sauce was added to each cooked rice meal. Each sauce was given once per day (with either the first or second serving). The meals were transported in cooling boxes to Dungu Primary School, where each meal was administered to its corresponding participant.
Iron contents in the unfortified rice, extruded rice, and vegetable sauce were analyzed by atomic absorption spectrophotometry (Agilent Technologies GTA 120 or AA240FS) after mineralization by microwave digestion (TurboWave, MLS GmbH) using nitric acid. PA and PP contents were determined as previously described (40) with the only difference that fat was extracted from the sauces with petroleum ether before PA determination. The AA content in the vegetable sauce was analyzed by high-performance liquid chromatography (Acquity H-Class UPLC System, Waters AG) after stabilization and extraction in metaphosphoric acid and reduction by dithiothreitol.
Ethics
Informed consent was obtained from the participant’s caregivers by signature or finger print. The ethical committees of the Kintampo Health Research Centre Institutional Ethics Committee, Ghana, and ETH Zürich, Switzerland, reviewed and approved the study protocol (2016-3 and EK 2015-N-73, respectively) and its amendment. The trial was registered at clinicaltrials.gov as NCT02176759.
Test meal composition
The iron and zinc concentrations and the Zn/Fe molar ratios in the different test meals and the reference meal are summarized in Table 2. The targeted fortification levels per fortified rice batch were 400 mg/100 g of iron and 600 mg/100 g of zinc, respectively. The mean ± SD iron concentrations in the isotopically labeled extruded rice grains from meals 54FeZnO, 57FeZnSO4, 54FeZnOCT, 57FeZnSO4CT, and 57FeZnOCT were 395 ± 9.1, 331 ± 5.2, 331 ± 17.8, 368 ± 12.3, and 339 ± 3.3 mg/100 g, respectively. The zinc concentrations in the same grains were 595 ± 1.8, 558 ± 36.5, 591 ± 14.9, 598 ± 6.9, and 570 ± 2.4 mg/100 g from meals 54FeZnO, 57FeZnSO4, 54FeZnOCT, 57FeZnSO4CT, and 58FeZnSOCE, respectively. The extruded rice grains in the reference meal contained 6 ± 3 mg/100 g of iron and 8 ± 0.6 mg/100 g of zinc. The native iron content was 1.5 ± 0.06 mg of iron in each composite test meal, which also contained 1.3 ± 0.25 mg of zinc and 0.1 ± 0.02 g of PA. AA and PP contents were determined only in vegetable sauce (freeze-dried), as their contents in rice were assumed to be negligible.
Laboratory measurements
Hb (HemoCue AB, Ängelholm, Sweden) and ZnPP/H (Hematofluorometer 206d, AVIV Biomedical Inc., USA) were measured on blood collection days. Hepcidin [DRG Hepcidin 25 (bioactive) HS, DRG Instruments, Germany] was measured in plasma samples. PF, sTfR, BIS, CRP, AGP, and RBP were analyzed (VitMin Lab, Freiburg, Germany) by sandwich enzyme-linked immunosorbent assay (ELISA), as described elsewhere (41). Detection of malarial parasites in blood smears was done using the Giemsa staining method, and results were supported by rapid malaria diagnostic test kits (SD Bioline). The analysis of blood iron isotopic composition was performed as previously described (23).
Definitions
ELISA assays could not be performed in the field; thus, for screening purposes, definitions were as follows: anemia, Hb <11.5 g/dl; IDE, ZnPP/H >43 μmol/mol (42–44). In the subsequent analyses and for reporting, definitions were as follows: ID, PF <30 μg/liter or sTfR >8.3 mg/liter, in combination with Hb <11.5 g/dl IDA, inflammation/infection CRP >5 mg/liter, and/or AGP >1 g/liter. Vitamin A deficiency was defined with an RBP concentration of <0.7 μM. The amount of absorbed isotopic label (=FAFe) in the blood was assessed by a shift of the isotopic iron ratio in RBCs to calculate total isotopic label using the participant’s blood volume (45) and assuming 80% incorporation of the absorbed iron into RBCs (46). The calculation was conducted using the principles of isotopic dilution and considering that isotopic labels are not monoisotopic (23). We corrected for previously incorporated 54Fe, 57Fe, or 58Fe by using the isotopic ratio value 12 to 16 days after a test meal administration as a new baseline value for the subsequent test meal administration (23).
Sample size calculation
A calculated sample size of 30 allowed the detection of an intrasubject difference in FAFe of 30% between the administered meals with a β of 0.8, α of 0.05, and an SD of 0.2 units in the log-transformed FAFe including and 25% attrition for potential dropouts.
Statistical analysis
Data were analyzed using SPSS (version 22.0, 2013; SPSS Inc., Chicago, IL) and Microsoft Excel (2013; Microsoft Corporation, Redmond, WA). FAFe from the different meals within the same participant was compared by linear mixed models followed by Bonferroni correction for multiple comparisons; RBV was compared with repeated-measures univariate analysis of variance (ANOVA) followed by Bonferroni correction for multiple comparisons. Univariate ANOVA was used to measure the effects of the zinc source, CA + TSC, and CA + EDTA addition. Results were presented as means ± SDs if normally distributed; otherwise, the results were presented as geometric means (95% CI). Calculated iron and zinc contents in composite meals were based on the means from the analysis of single components (regular basmati rice, extruded rice, and average mineral contents from the two different sauces; n = 3), and SDs for iron and zinc contents of composite meals were adapted by calculating the square root of the squared and summed SDs from each single component. In vitro solubility was analyzed with ANOVA, and multiple comparisons were conducted using Bonferroni correction. Non-normally distributed data were logarithmically converted for statistical analysis and reconverted for reporting. RBV from each meal was calculated based on geometric mean FAFe (%) relative to FAFe from the reference meal for each subject. Differences were considered as significant at P < 0.05.
Supplementary Material
Acknowledgments
We would like to thank the teachers of Dungu Primary School and the participating children as well as their parents. We are grateful to the kitchen staff at SOS children village (Tamale, Ghana) and the staff at the Public Health Laboratory at Tamale Teaching Hospital (Tamale, Ghana). Funding: This study was funded by the Laboratory of Human Nutrition, ETH Zürich (Zurich, Switzerland), Abbott Fund (Chicago, USA), DSM (Kaiseraugst, Switzerland), and USAID (Washington, DC, USA). This publication was made possible through support provided by the U.S. Agency for International Development under the terms of contract no. AID-641-Q-14-0001. Author contributions: L.S.H., D.M., M.B.Z., A.R.A., and C.I.C. designed the studies. L.S.H., D.M., A.R.A., H.Z.-G., and C.S. conducted the experiments. L.S.H. and C.Z. analyzed data. L.S.H., D.M., M.B.Z. and A.R.A. wrote the paper. L.S.H., D.M., and M.B.Z. had primary responsibility for the final content. All authors read and approved the final version of the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaau0790/DC1
Table S1A. Components (mg/g rice flour) used for extrusion.
Table S1B. Vitamin premix composition in % per gram premix.
Table S2. Target nutrient concentration in the fortified rice per gram of extruded rice kernels, corresponding to 100 g of finished product.
Table S3. Ingredients for two different sauces provided with cooked rice.
REFERENCES AND NOTES
- 1.Kassebaum N. J.; GBD 2013 Anemia Collaborators , The global burden of anemia. Hematol. Oncol. Clin. North Am. 30, 247–308 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Lebso M., Anato A., Loha E., Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: A community based cross-sectional study. PLOS ONE 12, e0188783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drakesmith H., Prentice A. M., Hepcidin and the iron-infection axis. Science 338, 768–772 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann M. B., Hurrell R. F., Nutritional iron deficiency. Lancet 370, 511–520 (2007). [DOI] [PubMed] [Google Scholar]
- 5.International Zinc Nutrition Consultative Group (IZiNCG); Brown K. H., Rivera J. A., Bhutta Z., Gibson R. S., King J. C., Lönnerdal B., Ruel M. T., Sandtröm B., Wasantwisut E., Hotz C., International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 25, S99–S203 (2004). [PubMed] [Google Scholar]
- 6.Seck P. A., Diagne A., Mohanty S., Wopereis M. C. S., Crops that feed the world 7: Rice. Food Secur. 4, 7–24 (2012). [Google Scholar]
- 7.Food Fortification Initiative, Global Alliance for Improved Nutrition, Feasibility and Potential Coverage of Fortified Rice in the Africa Food Supply Chain (FFI, Atlanta, GA, USA, 2016).
- 8.S. de Pee, Overview of evidence and recommendations for effective large-scale rice fortification, in Scaling up Rice Fortification in Asia, Sight and Life (Supplement, 2014), pp. 20–25.
- 9.Muthayya S., Hall J., Bagriansky J., Sugimoto J., Gundry D., Matthias D., Prigge S., Hindle P., Moench-Pfanner R., Maberly G., Rice fortification: An emerging opportunity to contribute to the elimination of vitamin and mineral deficiency worldwide. Food Nutr. Bull. 33, 296–307 (2012). [DOI] [PubMed] [Google Scholar]
- 10.L. Allen, B. De Benoist, O. Day, R. F. Hurrell, Guidelines for Food Fortification with Micronutrients. Food and Agriculture Organisation of the World Health Organisation (2006).
- 11.Hurrell R. F., Preventing iron deficiency through food fortification. Nutr. Rev. 55, 210–222 (1997). [DOI] [PubMed] [Google Scholar]
- 12.de Pee S., Proposing nutrients and nutrient levels for rice fortification. Ann. N. Y. Acad. Sci. 1324, 55–66 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Jaeggi T., Kortman G. A. M., Moretti D., Chassard C., Holding P., Dostal A., Boekhorst J., Timmerman H. M., Swinkels D. W., Tjalsma H., Njenga J., Mwangi A., Kvalsvig J., Lacroix C., Zimmermann M. B., Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64, 731–742 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Zlotkin S., Newton S., Aimone A. M., Azindow I., Amenga-Etego S., Tchum K., Mahama E., Thorpe K. E., Owusu-Agyei S., Effect of iron fortification on malaria incidence in infants and young children in Ghana: A randomized trial. JAMA 310, 938–947 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Soofi S., Cousens S., Iqbal S. P., Akhund T., Khan J., Ahmed I., Zaidi A. K. M., Bhutta Z. A., Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 382, 29–40 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Hackl L., Zimmermann M. B., Zeder C., Parker M., Johns P. W., Hurrell R. F., Moretti D., Iron bioavailability from ferric pyrophosphate in extruded rice cofortified with zinc sulfate is greater than when cofortified with zinc oxide in a human stable isotope study. J. Nutr. 147, 377–383 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hackl L., Cercamondi C. I., Zeder C., Wild D., Adelmann H., Zimmermann M. B., Moretti D., Cofortification of ferric pyrophosphate and citric acid/trisodium citrate into extruded rice grains doubles iron bioavailability through in situ generation of soluble ferric pyrophosphate citrate complexes. Am. J. Clin. Nutr. 103, 1252–1259 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cercamondi C. I., Duchateau G. S. M. J. E., Harika R. K., van den Berg R., Murray P., Koppenol W. P., Zeder C., Zimmermann M. B., Moretti D., Sodium pyrophosphate enhances iron bioavailability from bouillon cubes fortified with ferric pyrophosphate. Br. J. Nutr. 116, 496–503 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Johns P. W., Patel G. C., Parker M. E., Lasekan J. B., Milani P., Nixon M. K., Tigner M., Schmitz D. J., Evaluation of the effect of Ultra Rice®EDTA supplementation on the soluble iron, visual acceptance and vitamin A stability of commercial milled rice blends. Int. J. Food Sci. Technol. 50, 1615–1624 (2015). [Google Scholar]
- 20.Moretti D., Lee T.-C., Zimmermann M. B., Nuessli J., Hurrell R. F., Development and evaluation of iron-fortified extruded rice grains. J. Food Sci. 70, S330–S336 (2005). [Google Scholar]
- 21.Institute of Medicine (US) Panel on Micronutrients, Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academies Press, 2001). [PubMed] [Google Scholar]
- 22.Cook J. D., Reddy M. B., Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am. J. Clin. Nutr. 73, 93–98 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Cercamondi C. I., Egli I. M., Mitchikpe E., Tossou F., Zeder C., Hounhouigan J. D., Hurrell R. F., Total iron absorption by young women from iron-biofortified pearl millet composite meals is double that from regular millet meals but less than that from post-harvest iron-fortified millet meals. J. Nutr. 143, 1376–1382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinz D., Hurrell R. F., Righetti A. A., Zeder C., Adiossan L. G., Tjalsma H., Utzinger J., Zimmermann M. B., N’Goran E. K., Wegmüller R., In ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am. J. Clin. Nutr. 101, 462–470 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Hurrell R., Egli I., Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 91, 1461S–1467S (2010). [DOI] [PubMed] [Google Scholar]
- 26.Olivares M., Pizarro F., Ruz M., López de Romaña D., Acute inhibition of iron bioavailability by zinc: Studies in humans. Biometals 25, 657–664 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Pinkaew S., Wegmuller R., Hurrell R., Vitamin A stability in triple fortified extruded, artificial rice grains containing iron, zinc and vitamin A. Int. J. Food Sci. Technol. 47, 2212–2220 (2012). [Google Scholar]
- 28.Kuong K., Laillou A., Chea C., Chamnan C., Berger J., Wieringa F. T., Stability of Vitamin A, iron and zinc in fortified rice during storage and its impact on future national standards and programs—Case study in Cambodia. Nutrients 8, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bothwell T. H., MacPhail A. P., The potential role of NaFeEDTA as an iron fortificant. Int. J. Vitam. Nutr. Res. 74, 421–434 (2004). [DOI] [PubMed] [Google Scholar]
- 30.MacPhail A. P., Patel R. C., Bothwell T. H., Lamparelli R. D., EDTA and the absorption of iron from food. Am. J. Clin. Nutr. 59, 644–648 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Hurrell R. F., Reddy M. B., Burri J., Cook J. D., An evaluation of EDTA compounds for iron fortification of cereal-based foods. Br. J. Nutr. 84, 903–910 (2000). [PubMed] [Google Scholar]
- 32.Cercamondi C. I., Egli I. M., Zeder C., Hurrell R. F., Sodium iron EDTA and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br. J. Nutr. 111, 481–489 (2014). [DOI] [PubMed] [Google Scholar]
- 33.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , Scientific Opinion on the use of ferric sodium EDTA as a source of iron added for nutritional purposes to foods for the general population (including food supplements) and to foods for particular nutritional uses. EFSA J. 8, 1414 (2010). [Google Scholar]
- 34.Steiger G., Müller-Fischer N., Cori H., Conde-Petit B., Fortification of rice: Technologies and nutrients. Ann. N. Y. Acad. Sci. 1324, 29–39 (2014). [DOI] [PubMed] [Google Scholar]
- 35.S. de Pee, D. Moretti, C. Fabrizio, Standards and Specifications for Fortified Rice, in Scaling up Rice Fortification in West Africa, Sight and Life (Supplement, 2018), pp. 63–67.
- 36.Chavasit V., Porasuphatana S., Suthutvoravut U., Zeder C., Hurrell R., Iron bioavailability in 8–24-month-old Thai children from a micronutrient-fortified quick-cooking rice containing ferric ammonium citrate or a mixture of ferrous sulphate and ferric sodium ethylenediaminetetraacetic acid. Matern. Child Nutr. 11 ( suppl. 4), 179–187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackl L., Speich C., Zeder C., Sánchez-Ferrer A., Adelmann H., de Pee S., Tay F., Zimmermann M. B., Moretti D., Cold extrusion but not coating affects iron bioavailability from fortified rice in young women and is associated with modifications in starch microstructure and mineral retention during cooking. J. Nutr. 147, 2319–2325 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Pinkaew S., Winichagoon P., Hurrell R. F., Wegmuller R., Extruded rice grains fortified with zinc, iron, and vitamin A increase zinc status of Thai school children when incorporated into a school lunch program. J. Nutr. 143, 362–368 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Miller D. D., Schricker B. R., Rasmussen R. R., Van Campen D., An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 34, 2248–2256 (1981). [DOI] [PubMed] [Google Scholar]
- 40.Cercamondi C. I., Icard-Vernière C., Egli I. M., Vernay M., Hama F., Brouwer I. D., Zeder C., Berger J., Hurrell R. F., Mouquet-Rivier C., A higher proportion of iron-rich leafy vegetables in a typical Burkinabe maize meal does not increase the amount of iron absorbed in young women. J. Nutr. 144, 1394–1400 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Erhardt J. G., Estes J. E., Pfeiffer C. M., Biesalski H. K., Craft N. E., Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 134, 3127–3132 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann M. B., Molinari L., Staubli-Asobayire F., Hess S. Y., Chaouki N., Adou P., Hurrell R. F., Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. Am. J. Clin. Nutr. 81, 615–623 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Hastka J., Lasserre J. J., Schwarzbeck A., Hehlmann R., Central role of zinc protoporphyrin in staging iron deficiency. Clin. Chem. 40, 768–773 (1994). [PubMed] [Google Scholar]
- 44.World Health Organization, Iron Deficiency Anemia: Assessment, Prevention, and Control (WHO, Geneva, Switzerland, 2001).
- 45.Linderkamp O., Versmold H. T., Riegel K. P., Betke K., Estimation and prediction of blood volume in infants and children. Eur. J. Pediatr. 125, 227–234 (1977). [DOI] [PubMed] [Google Scholar]
- 46.International Atomic Energy Agency, IAEA Human Health Series No. 21, Assessment of Iron Bioavailability in Humans Using Stable Isotope Techniques (IAEA, Vienna, Austria, 2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaau0790/DC1


