Abstract
The temporomandibular joint (TMJ) disc, a fibrocartilaginous structure between the mandible and temporal bone, is implicated in temporomandibular disorders (TMDs). TMDs symptomatically affect approximately 25% of the population, of which 70% have internal derangement of the disc. Treatments lack efficiency, motivating novel therapies, including tissue-engineering toward TMJ disc regeneration. Recent developments in scaffold-based or scaffold-free approaches, cell sources, and biochemical and mechanical stimulation result in constructs exhibiting native tissue mechanics. Safety and efficacy of tissue-engineered implants show promising results in orthotopic animal studies. However, many hurdles need to be overcome in tissue-engineering approaches, and clinical and regulatory pathways. Future studies present an opportunity for clinicians and researchers to work together toward safe and effective clinical trials.
Keywords: tissue-engineering, temporomandibular joint disc
Motivation for Tissue-Engineering of the Temporomandibular Joint Disc
The temporomandibular joint (TMJ) is a ginglymoarthrodial joint (see Glossary), central to speaking and chewing functions [1]. The TMJ contains a disc between a condyle and the glenoid fossa-articular eminence region [2] (Figure 1). The TMJ disc is biconcave and fibrocartilaginous in nature [2]. As the TMJ articulates, the TMJ disc may distribute the stresses that develop within the joint [3] (Figure 1). Trauma [4] and age-related degeneration [5] can cause abnormal loading in the TMJ, leading to temporomandibular disorders (TMDs). TMDs are characterized by orofacial pain and/or limitation in jaw movement [6–8], and symptoms are present in approximately 25% of the population [9]. Perplexingly, TMDs affect females up to 8.0-fold more than males [9–12]. In addition, TMDs affect mostly younger patients between 20-50 years of age [12–14]. As the second most common musculoskeletal condition resulting in pain and disability, TMDs cost an estimated $4 billion per annum in the United States (https://www.nidcr.nih.gov/research/data-statistics/facial-pain).
Figure 1: TMJ disc anatomy.
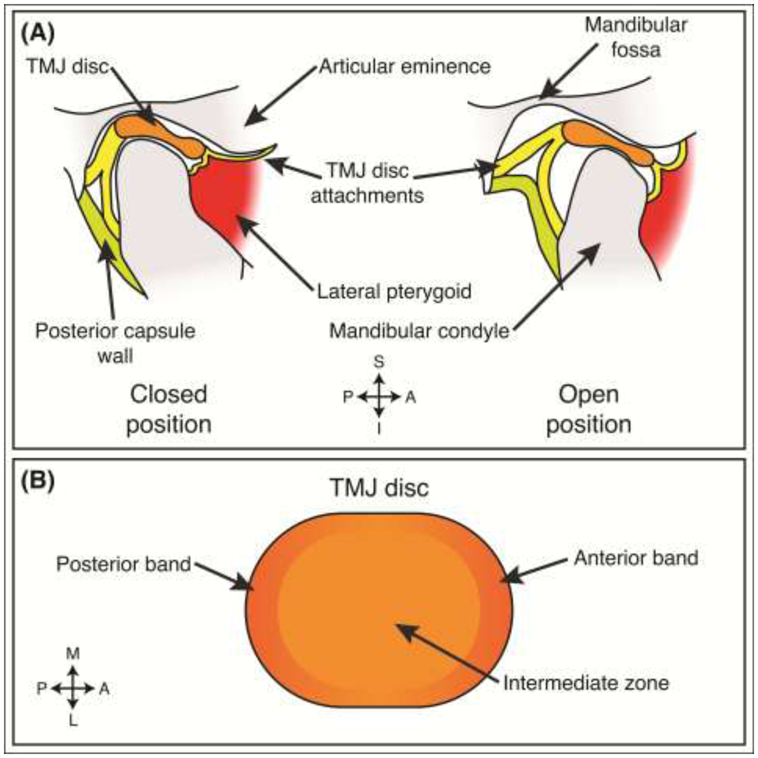
(A) Depending on the open or closed position of the joint, the TMJ disc is situated between the mandibular condyle and the articular eminence-mandibular fossa region. In this sagittal view, the disc is held in place by disc attachments, present at all angles (e.g., lateral, medial, posterior, anterior), surrounding the disc. The joint is separated into two joint capsules delineated by the TMJ disc. (B) The disc is regionally composed of two bands in the anterior and posterior portions of the disc. The middle portion of the disc is referred to as the intermediate zone. S – superior, I – inferior, A – anterior, P – posterior, M – medial, L – lateral.
A specific subset of TMDs involve discal pathologies such as internal derangement (ID), disc thinning, and disc perforation. ID affects about 70% of TMD patients [15]. Severe cases of ID present disc thinning and eventual disc perforation (Figure 2) in approximately 5-15% of ID patients [5,16,17]. However, ID and disc perforation can occur independently; the independent cases of disc perforation can be due to age-related wear [5]. These discal pathologies are the most prevalent manifestation of TMDs [15]. Osteoarthritis (OA) is also commonly seen in conjunction with ID [16,18], but the relationship between ID and OA is not understood; it is not known whether one precedes the other or if both share common causative events [18]. However, it is thought that TMJ disc pathologies such as ID or disc perforation are the first steps in a series of degenerative changes (i.e., OA) seen throughout the adjacent articulating, soft tissue surfaces [19].
Figure 2: Internal derangement of the TMJ disc.
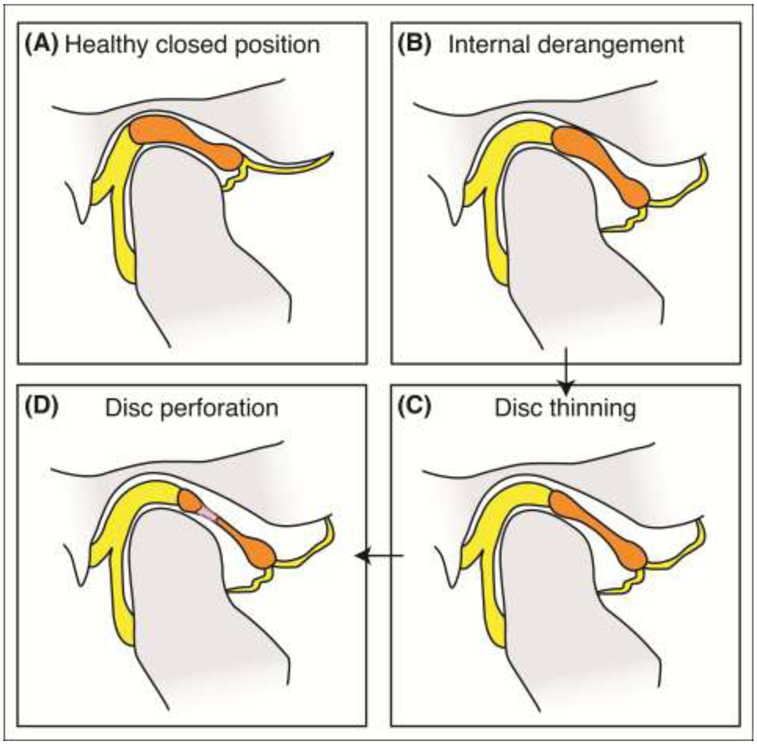
(A) A healthy closed jaw position is shown. (B) The most common type of internal derangement is shown, where the disc is displaced anteriorly. Progression of the joint in this configuration often causes (C) disc thinning and (D) eventual disc perforation.
Management of disc-related TMDs varies with disease severity [20]. Non- and minimally-invasive strategies include physical therapy [21], occlusal splints or adjustments [22], pharmacologic agents [23], sodium hyaluronate and corticosteroid injections [24], arthrocentesis [25], and arthroscopy [16]. However, these treatments are only palliative. Only 5% of TMDs are candidates for surgical intervention [26]; surgeries for TMDs include discectomy with or without disc replacement [27] and partial or full joint reconstruction with autologous [28] or alloplastic materials [29]. Discectomy has shown promise for symptom reduction but has shown degenerative remodeling of the joint as a result [30,31]. Costochondral rib grafts are used to reconstruct the mandibular condyle [28], but no autologous grafts exist for the complete joint [14]. Alloplastic total joint prostheses have been indicated for severe ankylosis, failure of autologous grafts, failure of Proplast-Teflon implants, or severe OA [32]. Most TMD patients range between 20-50 years of age [12–14], but the typical lifetime of alloplastic total joint prostheses is 10-15 years [33], making revisions likely within a patient’s lifetime [14]. The use of alloplastic total joint prostheses is reserved as an option of last resort for a small subset of patients, creating a gap in terms of treatment options between non-invasive or minimally invasive strategies and end-stage surgical techniques.
The treatments described above do not provide mid-stage intervention for patients. To fill this gap, novel treatment strategies to improve patient outcomes must be developed. Tissue-engineering aims to regenerate the pathological tissues in TMD with biological neotissues to restore long-term function. Here, we focus on TMJ disc pathologies due to their overarching prevalence in TMDs [15]. In particular, we discuss recent tissue-engineering efforts (Table 1) and remaining hurdles for TMJ disc tissue-engineering.
Table 1: Recent tissue-engineering studies of the TMJ disc published since 2013.
Summary of the scaffold-based or scaffold-free approaches, cell sources, species, biochemical stimuli, mechanical stimuli, and implantation sites of the constructs are provided.
| Author, Year |
Reference | Scaffold- based or Scaffold- free Approach |
Cell Sources |
Species of Cell Sources |
Biochemical Stimuli |
Mechanical Stimuli |
Animal Model Tested (Implantation Site) |
|---|---|---|---|---|---|---|---|
| Vapniar sky, et al. (2018) | [39] | Self-assembling process | CCs expanded to passage 3 | Yucatan Minipig | TGF-β1, C-ABC, LOXL2 | Passive axial compression | Yucatan Minipig (Orthotopic) |
| Matsuka, et al. (2018) | [57] | Decellularized TMJ discs | Wharton’s jelly-derived MSCs | Human | None | None | None |
| Bousnaki, et al. (2018) | [55] | Chitosan and alginate scaffolds | Dental pulp stem cells or human nucleus pulposus cells | Human | Unidentified* | None | None |
| Wang, et al. (2018) | [51] | Coculture cell sheet seeded on PLGA electrospun scaffolds | TMJ disc cells and synovium-derived MSCs | Rabbit | TGF-β3 | None | None |
| Ronald & Mills (2016) | [34] | Titanium dioxide nanofilms | TMJ disc cells | Cow | None | None | None |
| Tarafder, et al. (2016) | [36] | Polycaprolactone scaffolding with PLGA microspheres | Bone marrow-derived and synovium derived MSCs | Human/Rabbit | CTGF, TGF-β3 | None | Rabbit (Orthotopic) |
| Legemate, et al. (2016) | [35] | PCL scaffolding with PLGA microspheres | Bone marrow-derived MSCs | Human | CTGF, TGF-β3 | None | None |
| Juran, et al. (2015) | [56] | Decellularized TMJ discs with laser micropatterning | Wharton’s jelly-derived MSCs | Pig | Epidermal growth factor, platelet-derived growth factor BB | None | None |
| Wu, et al. (2014) | [40] | Fibrin gel and chitosan scaffold | Synovium derived-MSCs | Rat | TGF-β3 | None | Nude Mice (Subcutaneous) |
| MacBarb, et al. (2013) | [38] | Self-assembling process | ACs and MCs | Cow | TGF-β1, C-ABC | Passive axial compression | None |
| Ahtiainen, et al. (2013) | [41] | Poly(lactic acid) scaffold | Subcutaneous adipose-derived MSCs | Rabbit | TGF-β1 | None | Rabbit (Orthotopic) |
It is unclear what biochemical stimuli are in the chondrogenic medium used in the study by Bousnaki, et al. because it is a proprietary formulation.
Recent Tissue-Engineering Efforts
Tissue-engineering employs scaffolds, cells, and various signals such as biochemical and mechanical stimuli (Figure 3). As discussed in this section, advances in materials engineering have resulted in a variety of scaffolds [34–36], while scaffold-free approaches, such as the selfassembling process [37–39], have also emerged in TMJ disc tissue-engineering. In terms of cell sources, primary chondrocytes, mesenchymal stem cells (MSCs), and cell expansion technologies are also reviewed below (Table 1). Signals such as biochemical and mechanical stimuli for mechanical improvement of the TMJ disc (Table 1) are also discussed. This section also examines small animal models that have been used for examining the performance of these implants [36,39–43].
Figure 3: Tissue-engineering paradigm of TMJ disc constructs.
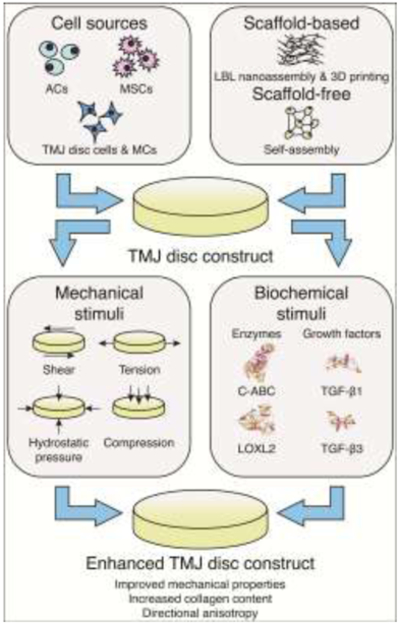
Combination of an appropriate cell source and scaffold-based or scaffold-free approaches can be used for fabrication of a TMJ disc construct (upper panels). Via the application of various biochemical and mechanical stimuli, an enhanced, biomimetic construct can be tissue-engineered (lower panels). ACs – hyaline articular chondrocytes, MSCs – mesenchymal stem cells, MCs – knee meniscus cells, LBL – layer-by-layer, 3D – three-dimensional, C-ABC – chondroitinase ABC, LOXL2 – lysyl oxidase-like 2, TGF-β – transforming growth factor beta.
Novel Scaffold-based and Scaffold-free Approaches
The primary purpose of scaffolds is to provide a template for cells to form tissues. Scaffolds can be functionalized with biomolecules to direct cell behavior and manufactured with mechanical properties similar to the tissues they are intended to replace. Ideally, scaffold degradation rates would match the rate of tissue formation. Scaffolds recently used in tissue-engineering the TMJ disc include natural materials and synthetic materials (Table 1). Two particularly interesting developments include novel scaffold fabrication methods and the emergence of scaffold-free approaches.
New fabrication methods allow for surface modifications of scaffolding materials. Layer-by-layer nanoassembly is one such fabrication method [34,44]. Titanium dioxide nanofilms are used to modify surfaces of scaffolds for tissue-engineering of bone [44] as well as cartilage [34]. These nanofilms are created by layer-by-layer nanoassembly, based on the principle of electrostatic charge, to coat various surfaces allowing for increased cell attachment, control of cell phenotype, and control of differentiation. In a study using titanium dioxide surface modification with seeded TMJ disc cells, cell proliferation and extracellular matrix (ECM) deposition increased with increasing thickness of nanofilms [34]. The matrix was reminiscent of a fibrous ECM, in contrast to a cartilaginous ECM. Type I collagen and decorin, approximately 0.34mg/mL and 0.31mg/mL, were present in higher amounts than type II collagen and aggrecan, approximately 0.14mg/mL and 0.28mg/mL, after 14 days of culture on 20 layers of titanium dioxide nanofilms [34]. Additional work needs to be performed to couple layer-by-layer nanoassembly with typical scaffold materials such as polycaprolactone (PCL) or polylactic acid (PLA).
Three-dimensional (3D) printing is a fabrication technique that achieves microprecise placement of scaffolding materials and functional biomolecules. 3D printing can create regional variation in scaffolds reminiscent of the native TMJ disc. For example, a dual-nozzle setup in a PCL-poly(lactic-co-glycolic acid) (PLGA) microsphere system allowed spatiotemporal delivery of transforming growth factor beta 3 (TGF-β3) and connective tissue growth factor (CTGF) [35,36]. The 100mg dosages of growth factor-embedded microspheres resulted in increased intermediate zone type II collagen and aggrecan deposition by approximately 2-fold compared to the 50mg dosage when analyzing immunofluorescence images of constructs seeded with bone marrow-derived MSCs [35]. However, growth factor-embedded microsphere application decreased compressive modulus in both dosages by at least 2-fold when compared to empty microspheres in both areas analyzed [35]. Similar trends were apparent in instantaneous and relaxation moduli indicating that mechanical properties did not necessarily trend with growth factor application and ECM content [35]. Compared to traditional scaffold-based approaches, 3D printing offers the ability to create regional variation which can resemble native ECM content.
Scaffold-free approaches, such as the self-assembling process [37–39], have been developed to bypass issues related [45] to scaffold degradation products, e.g., acidity due to PLA degradation [46], fabrication byproducts, e.g., crosslinkers and plasticizers [46], and stressshielding of cells [47]. The self-assembling process recapitulates developmental aspects of cartilage formation to generate functional neotissues with characteristics resembling those of native tissues [45,48]. Specifically, it is the most prominent of these techniques for TMJ disc tissue-engineering because it has generated mechanically robust tissue [37]. Stimulation of self-assembled TMJ disc constructs by bioactive agents and mechanical compression resulted in values of approximately 3.5%, 2.75 MPa, and 2.25 MPa for collagen per wet weight, tensile Young’s modulus, and ultimate tensile strength (UTS), respectively. Additional analysis of constructs created from cocultures of hyaline articular chondrocytes (ACs) and knee meniscus cells (MCs) found collagen fibril alignment reminiscent of native TMJ discs, exhibiting direction-dependent strains in finite element analysis. This was promising because it showed anisotropic tissue on par with the alignment of native tissue [38], which further substantiates scaffold-free tissue-engineering as an alternative to scaffold-based approaches.
While scaffold-free approaches do not necessarily have the flexibility of scaffold-based approaches, e.g., scaffold functionalization with biomolecules, these limitations can be overcome with exogenous stimulation, which can have various effects on scaffold-free constructs such as increased mechanical properties [49,50]. In addition, variation of the cell source can also have a large influence on the eventual properties of the resulting constructs.
Cell Sources
Selection of a cell source is one of the most important considerations for TMJ disc tissue-engineering (Table 1). Options for primary cells range from native TMJ disc cells [34,51] to other cells from hyaline articular cartilage and the knee meniscus [38]. In addition, recent advances in cell expansion technologies [52–54] have allowed exploration of costal cartilage-derived cells [39]. MSCs are also heavily used [35,36,40,41,51,55–57].
Potential primary cell sources for TMJ disc tissue-engineering include TMJ disc cells, ACs, MCs, and costal chondrocytes (CCs). TMJ disc cells have been used in multiple studies [34,51], but the dearth of available, healthy tissue raises concerns for this source [58]. Thus, ACs and MCs have been considered [38]. Using AC-MC coculture with the self-assembling process resulted in a functional, anisotropic TMJ disc as discussed above [38]. With recent advances in cell expansion technologies that preserve chondrogenic phenotype [52–54], CCs might allow for either an autologous or allogeneic approach to replacing cartilages, as demonstrated previously in articular cartilage [59,60] and the TMJ disc [39]. Allogeneic CCs can be harvested from cadaveric tissue, while autologous tissue harvest procedures are conducted routinely for rhinoplasty and autologous TMJ reconstruction. Thus, existing surgical procedures may be sufficient for tissue regeneration purposes. The use of CCs can also remove or reduce donor site morbidity and virtually eliminate the potential of harvesting cells from OA tissue. When used in a hyaline articular cartilage model, CC constructs have attained a functionality index (FI, described in Box 1) of 55% compared to the medial condyle cartilage properties [60]. These techniques and results offer promise of an alternative source of chondrocytes that can create mechanically stable constructs for other parts of the body such as the TMJ disc.
Box 1: The functionality index compares constructs properties to native tissue values.
Values for biochemical content, such as overall collagen (Col) and glycosaminoglycan (GAG) content, accompany values for various mechanical properties such as ultimate tensile strength (UTS), Young’s modulus (ET), compressive relaxation modulus (Er), and compressive instantaneous modulus (Ei). Ranging from 0% to 100%, a value of 100% represents perfect recapitulation of native values. Subscripts serve to designate native (N) or tissue-engineered (TE) values.
An array of MSCs from both adult and fetal tissues have been used, as previously reviewed [61]. MSCs from various tissues (Table 1) offer an autologous or allogeneic approach and can be isolated in large quantities, making these sources clinically relevant for construct formation. Perhaps the most interesting MSCs are those derived from the synovium because they were shown to synthesize cartilage oligomeric matrix protein, link protein, and glycosaminoglycans (GAGs), similar to ACs [62]. For example, synovium-derived MSCs on fibrin-chitosan scaffolds increased type I collagen expression approximately 2-fold in vitro and ECM deposition in vivo as evidenced by histological analysis when compared to pure chitosan scaffolds [40]. Progress using MSCs has resulted in morphological and biochemical biomimicry evaluated via histology, gene expression, and other biochemical assays [36,40,41,51], but future research should next focus on assaying functional properties of MSC-derived constructs via mechanical testing.
The choice of cell source remains a challenge within the field of TMJ disc tissue-engineering. Lack of standardization of mechanical testing modalities makes it difficult to compare sources head-to-head and to determine if one cell source is more suitable than another. Perhaps the most important characteristic to consider when choosing a cell source is mechanical stability of the resulting tissue-engineered construct due to the dynamic joint environment.
Improvement of Mechanical Properties of TMJ Disc Fibrocartilage
The TMJ disc functions in a dynamic environment of compression, tension, and shear [63,64]. Finite element analysis shows stresses in the TMJ disc during mouth opening to be greater than 7 MPa in compression, 4 MPa in tension, and 1 MPa in shear [65]. For comparison, the hip experiences approximately 7-10 MPa in compression and up to 18 MPa during stressful activities such as standing up [66,67]. Characterization of the native tissue should aim to define the gold-standard, design criteria for tissue-engineered TMJ disc constructs; the expectation is that replicating the native tissue’s mechanical properties would allow for restoration of mechanical function. Thus, to engineer constructs with physiological levels of mechanical stresses in mind, various biochemical and mechanical stimuli, and also changes in scaffold processing (Figure 3) have been developed. For scaffold-free approaches, self-assembled constructs have approached native values in mechanical properties due to synergistic effects of biochemical and mechanical stimulation [38,39]
A majority of recent scaffold-based studies use only biochemical stimuli to improve construct mechanical properties (Table 1). Constructs stimulated with biochemical stimuli have been previously found to exhibit native tissue structure-function relationships. For example, insulin-like growth factor I and TGF-β applied to constructs created from TMJ disc cells increased collagen synthesis by greater than 400% at 3 weeks of culture, leading to higher aggregate moduli of 5 kPa [68]. However, constructs sometimes do not follow native tissue structure-function relationships [35] (e.g., increased matrix deposition leading to increased mechanical properties). To overcome such deficiencies, mechanical stimulation may be considered. However, mechanical stimulation has not been employed in scaffold-based TMJ disc approaches, though it has been used in other fibrocartilages such as the knee meniscus. For example, hydrostatic pressure combined with TGF-β1 led to 4-fold higher collagen deposition and 3-fold higher GAG deposition, as compared to the unpressurized growth factor controls in MC-seeded PLA scaffolds [69]. Studies showing recapitulation of native tissue structure-function relationships should serve as models for future studies toward identifying additional stimuli. Biochemical stimuli must continue to be investigated, but, additionally, mechanical stimuli can be used to increase mechanical properties of engineered discs to withstand the dynamic in vivo environment.
Scaffold-free approaches have combined biochemical stimuli and mechanical stimuli to generate stiffer, stronger, anisotropic constructs, followed by examination of the resulting constructs in large animal models. Using a scaffold-free approach with AC-MC coculture, TGF-β1, chondroitinase ABC (C-ABC), and lysyl oxidase-like 2 (LOXL2) have been identified in the past as efficacious for fibrocartilage tissue-engineering, enhancing tensile Young’s modulus and UTS by 245% and 186%, respectively [70]. In a self-assembled TMJ disc model using AC-MC coculture stimulated with only TGF-β1 and C-ABC, tensile Young’s modulus, UTS, and collagen per wet weight increased by 2-fold or greater in the intermediate zone of the disc, as compared to controls [38]. Passive axial compression and these biochemical stimuli were combined and noted to exhibit synergism, showing 5.8-fold, 14.7-fold, and 13.8-fold increases in collagen per wet weight, tensile Young’s modulus, and UTS, respectively, compared to unstimulated controls [38]. Moving to in vivo studies, TMJ discs engineered using all three stimuli (TGF-β1, C-ABC, and LOXL2) coupled with passive axial compression, yielded an FI (Box 1) of 42% of native properties with a passaged, allogeneic CC source [39]. By combining these three biochemical stimuli with mechanical stimulation, increased functional properties were achieved as compared to either alone. Thus, further synergistic effects of other biochemical and mechanical stimuli should be explored.
As reviewed elsewhere [49], strategies for other tissues, such as hyaline articular cartilage, can help inform further mechanical improvement of constructs. Similar designs and models can be used to engineer the fibrocartilage of the TMJ disc. For example, in a recent study on tension and its effects for articular cartilage engineering, continuous stimulation combined with a bioactive regimen increased the tensile properties by 5.8-fold over unstimulated controls in AC-derived, self-assembled constructs [71]. By improving mechanical stability using biochemical and mechanical stimuli, constructs continue to approach native tissue values. Attaining mechanical biomimicry is a crucial characteristic for constructs to perform satisfactorily when implanted into the orthotopic environment.
Current Animal Models
Prior to human clinical trials, tissue-engineered implants are examined in relevant animal models to demonstrate initial safety and efficacy. Similar to TMJ disc tissue-engineering, development of animal models is based on design criteria. For the TMJ, similar anatomies, chewing patterns, and diets compared to humans, and ease of surgical access are included in the design criteria. In addition, relative size of TMJ structures and animal cost may also determine which model to use. Animal models exist for various purposes such as observing the adverse reactions to an implant subcutaneously to examining surgically induced pathologies in orthotopic studies. Small animals such as mice and rats are economical, serve as pain models [72,73], and simulate OA and associated degenerative changes in the joint [74,75]. However, their small TMJ disc size limits studies to simple subcutaneous implantation as opposed to orthotopic studies in larger animals such as rabbits [43]. Moving toward orthotopic studies, rabbits allow for additional biochemical and histological analysis, and reliable mechanical testing [42], but present substantial differences from human size and loading conditions [43]. This motivates the use of large animal models that more closely resemble human anatomies and conditions [42].
Many preliminary studies involve subcutaneous implantation to examine possible adverse reactions and establish proof-of-concept. These studies, as reviewed [43], are commonly performed in mice or rats due to their low cost, without much consideration of anatomical or dietary similarities. For example, a fibrin-chitosan scaffold with synovium-derived rat MSCs was embedded into explanted TMJ discs with perforation defects and implanted into nude mice subcutaneously in a xenogeneic approach [40]. Histological analysis showed increased type I and II collagen deposition in the fibrin-chitosan scaffold, compared to the pure chitosan scaffold [40]. Although this study represents a disc perforation model, additional biochemical and mechanical analyses must be performed in larger animals to show reparative ability in the fully loaded orthotopic environment.
Recent studies employed the rabbit for orthotopic evaluation of tissue-engineered TMJ discs [36,41]. For example, 3D printed PCL-PLGA microsphere scaffolds seeded with allogeneic, synovium-derived MSCs were implanted into the disc and noted histologically to degrade by 6 weeks [36]. Cells retained their chondrocyte-like phenotype in vivo [36]. Scoring of the condylar surfaces with an OA score resulted in values of approximately 3.9 and 2.4 for the scaffolds without and with growth factors, respectively, where a lower score represents a better outcome [36]. While these studies [36,41] demonstrate feasibility for implantation of tissue-engineered TMJ discs via histological analysis, mechanical testing is of paramount importance to show the integrity of tissue-engineered constructs.
Strides in animal studies are promising to the research community as they point to a feasible translation pathway for tissue-engineered constructs. The use of ectopic small animal and larger orthotopic models (e.g., the mouse and rabbit models) is a crucial first step in proof-of-concept work for the field. However, it will ultimately be regenerative studies in orthotopic animal models in species such as the minipig that will be most impactful for translation of tissue-engineered TMJ discs toward human clinical studies.
The Path to Translation
Translational hurdles that remain (see Outstanding Questions) include tuning of construct mechanical properties toward biomimicry (Figure 3) as well as scale-up of area and thickness of implants (Figure 4, Key Figure). A recent minipig study, showing safe and efficacious implantation of TMJ constructs [39], establishes this orthotopic large animal model as a cogent element in the translational pathway (Figure 4). Clinical and regulatory hurdles are also significant for translation of TMJ disc constructs (Figure 4).
Outstanding Questions.
How do researchers achieve tuning of tissue-engineered constructs to the mechanical environment of the TMJ disc?
Can researchers scale-up constructs, in area and thickness, to be relevant to human discal pathologies and size?
For what cases will tissue-engineered products be indicated (or contraindicated)?
Can novel surgical procedures be developed for accessing the TMJ, and fixing and implanting tissue-engineered TMJ disc constructs orthotopically?
What is the local and systemic responses to tissue-engineered TMJ discs in vivo?
How would tissue-engineered constructs for the TMJ disc be regulated by the FDA?
Figure 4: Toward the path to translation.
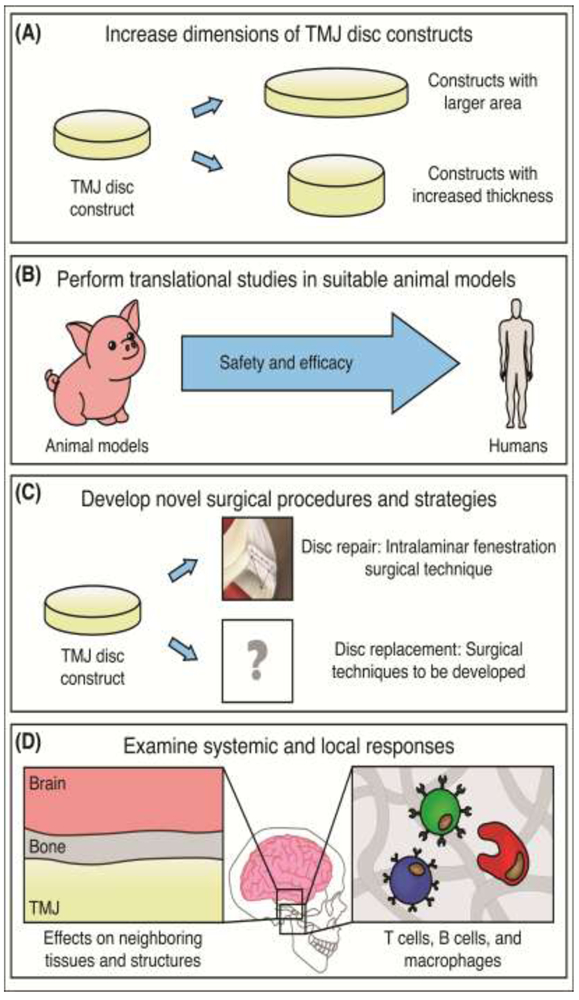
(A) Constructs should be tailored for human discal pathologies and size, potentially increasing in both area and thickness. (B) Prior to translation through regulatory bodies such as the FDA, animal studies must be performed in proper large animals, such as the minipig. (C) Novel surgical procedures for disc repair and disc replacement need to be developed as well. (D) Additional studies also need to be performed to examine local and systemic responses to tissue-engineered TMJ discs in the orthotopic environment. Upon overcoming these hurdles, the TMJ disc tissue-engineering field will be closer to human clinical trials.
Application of Proper Tissue-Engineering Parameters for Tuning of TMJ Disc Constructs to the TMJ Mechanical Environment
Constructs must be tuned to the mechanical environment of the TMJ disc because they will be subject to compressive, tensile, and shear forces [63,64]. Theoretically, the required mechanical properties will depend on surgical technique, model, and animal. For example, it was shown that an FI (Box 1) of 42% was shown to be sufficient when implanted via the intralaminar fenestration surgical technique (Figure 5) in a focal thinning model in the Yucatan minipig [39]. When moving toward perforation or larger defects, this implant might be insufficient. On the opposite end, some constructs might be too stiff or strong compared to native values, as observed in some scaffold-based approaches [35], causing stress concentrations and possible degeneration on the articulating surfaces. Also, a mismatch in the rates of scaffold degradation versus tissue formation can lead to failure. Therefore, it is important to consider tuning mechanical properties by application of proper stimulation regimens, whether using a scaffold-based or scaffold-free tissue-engineering approach (Figure 3).
Figure 5: The intralaminar fenestration surgical technique.
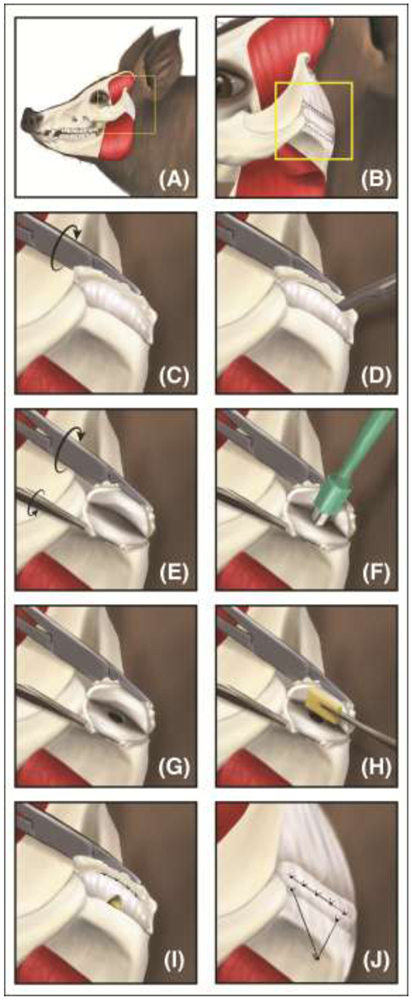
(A-B) Through a preauricular incision, the TMJ was exposed. (C-E) Surgeons fileted the disc open with a scalpel, and (F-G) created a one-sided thinning defect via a biopsy punch. (H) A tissue-engineered disc was placed between the two laminae and (I) sutured back together. Sutures attached to the side of the disc instead of on the articulating surfaces allowed for continued loading of the TMJ disc while healing; this placement avoided possible stress concentrations and resulting degeneration. (J) The lateral attachment is recreated by use of an anchoring system. From Vapniarsky, et al., 2018 [39]. Reprinted with permission from AAAS.
Tailoring of Tissue-Engineered TMJ Discs to Human Discal Pathologies and Size
As the translational direction points to additional large animal orthotopic studies before human clinical trials commence, defect models must increase in size. As such, constructs must also scale-up (Figure 4). In the recent minipig study [39], a one-sided 3mm defect, mimicking disc thinning, was used. Future studies need to scale-up to a larger defect area to mimic increased disc thinning, in addition to two-sided defects to mimic disc perforation. To scale-up constructs to larger thicknesses, one might consider using larger scaffolds. But as scaffolds and constructs trend upward in thickness, it should be kept in mind that diffusion limitations increase. Decreased diffusion can result in shell-like neotissues with necrotic centers, that display inadequate mechanics. However, scaffold-free approaches might prove advantageous for creation of larger constructs to mimic disc thinning. Self-assembled articular cartilage constructs made of passaged ACs up to 25 mm dia. have been made by combining cytochalasin D, TGF-β1, C-ABC, and LOXL2, under a compressive load and in mechanical confinement [76]. This approach may allow for examining TMJ disc healing in larger defects that mimic clinically observed disc thinning. As such, a significant portion of future TMJ disc studies should investigate the scale-up of defects and constructs for relevance to human TMJ anatomy.
Novel and Cogent Translational Studies
Orthotopic large animal models need to be performed to examine the safety and efficacy of tissue-engineered constructs prior to translation. Toward selection, possible species for performing regenerative studies include sheep [77], goats [78], dogs [79], farm pigs [80], and minipigs [81]. The farm pig and minipig are two suitable models that have been recently used for regenerative studies due to their similarities to humans in chewing patterns, diet, and anatomy [3,81–85].
In a recent study demonstrating safety and efficacy of a self-assembled, allogeneic, tissue-engineered implant for disc repair, a novel TMJ disc thinning model was created in the Yucatan minipig [39]. Because the implants were created from a CC source, implantation into the TMJ disc represented non-homologous use. Implants approaching native tissue values were stimulated by a regimen of biochemical and mechanical stimulation. To affix implants securely, the intralaminar fenestration surgical technique was developed (Figure 5) [39]. Although this was an allogeneic, non-homologous use which has potential to elicit an immune response, implant safety was shown by minimal to no immune response to the constructs, as assayed by histological staining for CD3, CD20, and CD68 for T cells, B cells, and macrophages. However, it was specified that additional work needs to further elucidate the immunological response [39], such as macrophage activation due to tissue-engineered implants [86–88] (Figure 4). In terms of efficacy, results showed that the tensile Young’s modulus, integration at the repair-to-native tissue interface, and percent of defect closure were 3.4-fold, 3.2-fold, and 4.4-fold higher, respectively, compared to empty defect controls [39]. OA scores of the condylar surface treated with implants were 3.0-fold less than the empty defect controls [39], yielding a better clinical outcome overall. Together, these results demonstrate the feasibility of allogeneic TMJ disc tissue-engineered constructs in the orthotopic environment and pave the way for additional orthotopic large animal studies and future human clinical trials (Figure 4).
Overcoming Additional Clinical and Regulatory Hurdles
In stark contrast to diarthrodial joints such as the knee, there is limited knowledge surrounding the TMJ, especially when it comes to developing new processes and products for repair or replacement of the TMJ disc. Compared to the TMJ, a greater variety of products, treatments, and studies exist for the knee. To illustrate these differences, one can consider indications and contraindications in the TMJ versus the knee. For example, in the knee, there are clear guidelines as to what constitutes small, large, partial thickness, and full thickness defects with concomitant treatment algorithms [89]. In contrast, it is not clear when a tissue-engineered treatment would be indicated in the TMJ. Currently, in the knee, tissue-engineered products are contraindicated for the OA milieu [90]. This has not been confirmed for the TMJ, though the expectation is that the constructs under OA conditions might succumb to the same fate as the native tissue [91]. Development of treatment guidelines and additional studies specific to the TMJ should continue, toward bringing TMJ-related knowledge to levels of other diarthrodial joints.
One must also consider fixation and associated surgical approaches. The intralaminar fenestration surgical technique (Figure 5) was successful in treating early-stage disc thinning, but in the minipig [39]. However, in 5% of TMD cases requiring surgery [26], it is not yet obvious how one may be able to attach a partial or whole, tissue-engineered disc (Figure 4). Surgeons and researchers must continue to collaborate to develop surgical approaches for implantation of tissue-engineered implants, as they are of utmost importance to the success of the tissue-engineered treatment.
With regard to clearing the regulatory hurdle, the TMJ’s proximity to the brain (Figure 4) may necessitate more stringent safety requirements than products for other joints such as the knee. These requirements may include analysis of the synovial fluid in the TMJ, but also the neighboring cerebrospinal fluid. Notoriously, mechanical failure and resulting degradation of the Proplast-Teflon disc implants resulted in exposure of the brain cavity [92–94]. Additionally, current large animal work has yet to investigate fully immunological implications related to TMJ disc implants (Figure 4) or how immunomodulation may be used in a proinflammatory environment [95]. In terms of regulation, the FDA has not previously guided a tissue-engineered TMJ disc product [96], thus raising the question of establishing TMJ-specific safety and efficacy guidance documents. Future research in the field needs to establish the safety of tissue-engineered TMJ discs by elucidating the immune response. Additionally, researchers need to communicate with regulatory bodies, such as the FDA, to obtain guidance on how tissue-engineered TMJ disc products need to be demonstrated as safe and efficacious.
Concluding Remarks
While recent advances propel TMJ disc tissue-engineering forward, many hurdles still exist. To summarize, the pressing challenges include improvement of mechanical properties of constructs, scale-up of implant dimensions, determination of indications for tissue-engineered discs, development of surgical techniques, analysis of the immunological response, and regulation by the FDA (see Outstanding Questions). Tissue-engineering and basic science investigations for TMDs will continue to drive the field. The field should focus toward addressing questions in the clinical and regulatory spaces. Specifically, studies should pay attention to developing novel surgical techniques and associated fixation methods toward human clinical trials. For each new tissue-engineering approach, regulatory requirements need to be satisfied by demonstration of TMJ-specific safety and efficacy in large animal models. As regulatory bodies turn their attention toward clinical trials, these data will be the primary preclinical assessment of implants. Considering the momentum toward significant preclinical studies, it is an exciting time to be in the field of TMJ disc tissue-engineering. After the early success shown in the orthotopic study performed in the Yucatan minipig [39] and the identification of clinical and regulatory hurdles discussed here, there is new impetus to develop tissue-engineering solutions to begin addressing the various intractable TMJ trauma and degenerative ailments. The possibility of translating tissue-engineered TMJ discs is increasingly being realized.
Supplementary Material
Highlights.
Current treatments for TMJ disorders lack long-term efficacy and are palliative, motivating tissue-engineering for repair or replacement of the injured or ailing tissues in the TMJ, such as the disc.
Scaffold-based or scaffold-free approaches, cell sources, biochemical stimuli, and mechanical stimuli are all elements of the tissue-engineering process that need to be considered to tailor TMJ disc construct properties.
Large animals can serve as models of human TMD; orthotopic implantation in large animal models is a necessary translational step.
The first successful orthotopic study of the TMJ disc in a large animal model has primed the field for translation of tissue-engineered constructs; however, there are still numerous hurdles prior to human clinical trials.
Acknowledgement
The authors would like to acknowledge support from the following funding sources: National Institutes of Health R01 DE015038.
Glossary
- Ginglymoarthrodial joint
a joint functioning in both rotation and translation
- Internal derangement (ID)
misalignment or displacement of the TMJ disc from a normal anatomic position
- Mastication
the mechanical grinding of food into smaller pieces by teeth
- Osteoarthritis (OA)
a slowly progressing joint disease characterized by degenerative changes in the cartilage and subchondral bone; presents through wear of the cartilage or underlying bone and presence of osteophytes; commonly affects large diarthrodial joints such as the knee, but also joints such as the TMJ
- OA score
a semi-quantitative measure of the severity of osteoarthritis based on histomorphological analysis of cartilage, underlying bone, and degenerative marks such as osteophytes; a higher number indicates increased degeneration; standardized by various groups including the Osteoarthritis Research Society International (OARSI) or the International Cartilage Regeneration and Joint Preservation Society (ICRS)
- Ruminants
an even-toed, hoofed mammal (e.g., bovine, ovine) that chews regurgitated food from its first stomach
- Young’s modulus
a material property defining the stiffness of a material when deformed by uniaxial tension or compression; measured as the ratio of stress (force per unit area) to strain (change in length divided by original length)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aryaei A et al. (2016) Recent Tissue Engineering Advances for the Treatment of Temporomandibular Joint Disorders. Curr. Osteoporos. Rep 14, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alomar X et al. (2007) Anatomy of the Temporomandibular Joint. Semin. Ultrasound, CT, MRI 28, 170–183 [DOI] [PubMed] [Google Scholar]
- 3.Sindelar BJ and Herring SW (2005) Soft tissue mechanics of the temporomandibular joint. Cells, Tissues, Organs 180, 36–43 [DOI] [PubMed] [Google Scholar]
- 4.Arnett GW et al. (1996) Progressive mandibular retrusion-idiopathic condylar resorption. Part II. Am. J. Orthod. Dentofac. Orthopeics 110, 117–127 [DOI] [PubMed] [Google Scholar]
- 5.Katzberg RW and Westesson P-L (1993) Diagnosis of the temporomandibular joint, (1st edn) W.B. Saunders. [Google Scholar]
- 6.Dubner R et al. (2016) The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res 95, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slade GD et al. (2016) Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res 95, 1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scrivani SJ et al. (2008) Temporomandibular Disorders. N. Engl. J. Med 359, 2693–2705 [DOI] [PubMed] [Google Scholar]
- 9.Solberg WK et al. (1979) Prevalence of mandibular dysfunction in young adults. J. Am. Dent. Assoc 98, 25–34 [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves DA de G et al. (2010) Symptoms of temporomandibular disorders in the population: an epidemiological study. J. Orofac. Pain 24, 270–278 [PubMed] [Google Scholar]
- 11.Martins-Júnior RL et al. (2010) Temporomandibular disorders: A report of 124 patients. J. Contemp. Dent. Pract 11, 71–78 [PubMed] [Google Scholar]
- 12.Wilkes CH (1989) Internal derangements of the temporomandibular joint. Pathological variations. Arch. Otolaryngol. - Head Neck Surg 115, 469–477 [DOI] [PubMed] [Google Scholar]
- 13.Warren MP and Fried JL (2001) Temporomandibular disorders and hormones in women. Cells, Tissues, Organs 169, 187–192 [DOI] [PubMed] [Google Scholar]
- 14.Murphy MK et al. (2013) Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implants 28, 393–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar WB and McCarty WLJ (1979) The TMJ dilemma. J. Alabama Dent. Assoc 63, 19–26 [PubMed] [Google Scholar]
- 16.Muñoz-Guerra MF et al. (2013) Temporomandibular joint disc perforation: Long-term results after operative arthroscopy. J. Oral Maxillofac. Surg 71, 667–676 [DOI] [PubMed] [Google Scholar]
- 17.Kuribayashi A et al. (2008) MRI findings of temporomandibular joints with disk perforation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 106, 419–425 [DOI] [PubMed] [Google Scholar]
- 18.American Society of Temporomandibular Joint Surgeons (2003) Guidelines for diagnosis and management of disorders involving the temporomandibular joint and related musculoskeletal structures. CRANIO 21, 68–76 [PubMed] [Google Scholar]
- 19.Ballesteros LE and León-S FE (1999) [Anatomical and pathological study of the temporomandibular joint disk in Colombian individuals]. Rev. Med. Chil 127, 1469–1474 [PubMed] [Google Scholar]
- 20.Romero-Reyes M and Uyanik JM (2014) Orofacial pain management: Current perspectives. J. Pain Res 7, 99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armijo-Olivo S et al. (2016) Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther 96, 9–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pficer JK et al. (2017) Occlusal stabilization splint for patients with temporomandibular disorders: Meta-analysis of short and long term effects. PLoS One. DOI: 10.1371/journal.pone.0171296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurita Varoli F et al. (2015) Analgesia evaluation of 2 NSAID drugs as adjuvant in management of chronic temporomandibular disorders. Sci. World J DOI: 10.1155/2015/359152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mountziaris PM et al. (2009) Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods 47, 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Riu G et al. (2013) Arthrocentesis and temporomandibular joint disorders: Clinical and radiological results of a prospective study. Int. J. Dent DOI: 10.1155/2013/790648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolwick MFF and Dimitroulis G (1994) Is there a role for temporomandibular joint surgery? Br. J. Oral Maxillofac. Surg 32, 307–313 [DOI] [PubMed] [Google Scholar]
- 27.Eriksson L and Westesson PL (2001) Discectomy as an effective treatment for painful temporomandibular joint internal derangement: A 5-year clinical and radiographic follow-up. J. Oral Maxillofac. Surg 59, 750–758 [DOI] [PubMed] [Google Scholar]
- 28.Sharma H et al. (2015) Costochondral Graft as Interpositional material for TMJ Ankylosis in Children: A Clinical Study. J. Maxillofac. Oral Surg 14, 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolford LM et al. (2003) Comparison of 2 temporomandibular joint total joint prosthesis systems. J. Oral Maxillofac. Surg 61, 685–690 [DOI] [PubMed] [Google Scholar]
- 30.Hinton RJ (1992) Alterations in Rat Condylar Cartilage Following Discectomy. J. Dent. Res 71, 1292–1297 [DOI] [PubMed] [Google Scholar]
- 31.Agerberg G and Lundberg M (1971) Changes in the temporomandibular joint after surgical treatment. A radiologic follow-up study. Oral Surgery, Oral Med. Oral Pathol 32, 865–875 [DOI] [PubMed] [Google Scholar]
- 32.Mercuri LG (2000) The use of alloplastic prostheses for temporomandibular joint reconstruction. J. Oral Maxillofac. Surg 58, 70–75 [DOI] [PubMed] [Google Scholar]
- 33.Ingawalé S and Goswami T (2009) Temporomandibular joint: Disorders, treatments, and biomechanics. Ann. Biomed. Eng 37, 976–996 [DOI] [PubMed] [Google Scholar]
- 34.Ronald S and Mills D (2016) Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films. J. Funct. Biomater DOI: 10.3390/jfb7020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legemate K et al. (2016) Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. J. Dent. Res 95, 800–807 [DOI] [PubMed] [Google Scholar]
- 36.Tarafder S et al. (2016) Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. DOI: 10.1088/1758-5090/8/2/025003 [DOI] [PubMed] [Google Scholar]
- 37.Hu JC and Athanasiou KA (2006) A Self-Assembling Process in Articular Cartilage Tissue Engineering. Tissue Eng. 12, 969–979 [DOI] [PubMed] [Google Scholar]
- 38.MacBarb RF et al. (2013) Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials 34, 9980–9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vapniarsky N et al. (2018) Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med DOI: 10.1126/scitranslmed.aaq1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y et al. (2014) The pilot study of fibrin with temporomandibular joint derived synovial stem cells in repairing TMJ disc perforation. Biomed Res. Int DOI: 10.1155/2014/454021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahtiainen K et al. (2013) Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. J. R. Soc. Interface. DOI: 10.1098/rsif.2013.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almarza AJ et al. (2018) Preclinical Animal Models for Temporomandibular Joint Tissue Engineering. Tissue Eng. Part B Rev 24, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helgeland E et al. (2018) Scaffold-based TMJ tissue regeneration in experimental animal models: a systematic review. Tissue Eng. Part B Rev 24, 300–316 [DOI] [PubMed] [Google Scholar]
- 44.Kommireddy DS et al. (2006) Stem cell attachment to layer-by-layer assembled TiO2 nanoparticle thin films. Biomaterials 27, 4296–4303 [DOI] [PubMed] [Google Scholar]
- 45.Athanasiou KA et al. (2013) Self-Organization and the Self-Assembling Process in Tissue Engineering. Annu. Rev. Biomed. Eng 15, 115–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X and Ma PX (2004) Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng 32, 477–486 [DOI] [PubMed] [Google Scholar]
- 47.Bryant SJ et al. (2004) Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J. Orthop. Res 22, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 48.Lee JK et al. (2017) The self-assembling process and applications in tissue engineering. Cold Spring Harb. Perspect. Med DOI: 10.1101/cshperspect.a025668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salinas EY et al. (2018) A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part B Rev 24, 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon H et al. (2016) Articular cartilage tissue engineering: The role of signaling molecules. Cell. Mol. Life Sci 73, 1173–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CH et al. (2018) Layering Poly (lactic-co-glycolic acid)-based electrospun membranes and co-culture cell sheets for engineering temporomandibular joint disc. J. Biol. Regul. Homeost. Agents 32, 55–61 [PubMed] [Google Scholar]
- 52.Murphy MK et al. (2013) Enhancing Post-Expansion Chondrogenic Potential of Costochondral Cells in Self-Assembled Neocartilage. PLoS One. DOI: 10.1371/journal.pone.0056983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy MK et al. (2015) Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Transplant. 24, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy MK et al. (2015) TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 33, 762–773 [DOI] [PubMed] [Google Scholar]
- 55.Bousnaki M et al. (2018) Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J. Mater. Sci. Mater. Med DOI: 10.1007/s10856-018-6109-6 [DOI] [PubMed] [Google Scholar]
- 56.Juran CM et al. (2015) Engineered Microporosity: Enhancing the Early Regenerative Potential of Decellularized Temporomandibular Joint Discs. Tissue Eng. Part A 21, 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matuska AM et al. (2018) Approaches to improve integration and regeneration of an ex vivo derived temporomandibular joint disc scaffold with variable matrix composition. J. Mater. Sci. Mater. Med DOI: 10.1007/s10856-018-6164-z [DOI] [PubMed] [Google Scholar]
- 58.Johns DE et al. (2008) Clinically relevant cell sources for TMJ disc engineering. J. Dent. Res 87, 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huwe LW et al. (2017) Using Costal Chondrocytes to Engineer Articular Cartilage with Applications of Passive Axial Compression and Bioactive Stimuli. Tissue Eng. Part A 24, 516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huwe LW et al. (2018) Characterization of costal cartilage and its suitability as a cell source for articular cartilage tissue engineering. J. Tissue Eng. Regen. Med 12, 1163–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S et al. (2015) Stem Cells for Temporomandibular Joint Repair and Regeneration. Stem Cell Rev. Reports 11, 728–742 [DOI] [PubMed] [Google Scholar]
- 62.Recklies AD et al. (1998) Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 41, 997–1006 [DOI] [PubMed] [Google Scholar]
- 63.Tanaka E and Van Eijden T (2003) Biomechanical behavior of the temporomandibular joint disc. Crit. Rev. Oral Biol. Med 14, 138–150 [DOI] [PubMed] [Google Scholar]
- 64.Wu Y et al. (2015) Viscoelastic shear properties of porcine temporomandibular joint disc. Orthod. Craniofacial Res 18, 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q et al. (2014) Effect of jaw opening on the stress pattern in a normal human articular disc: Finite element analysis based on MRI images. Head Face Med. DOI: 10.1186/1746-160X-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Athanasiou KA et al. (2017) Articular Cartilage, (2nd edn) CRC Press. [Google Scholar]
- 67.Hodge WA et al. (1989) Contact pressures from an instrumented hip endoprosthesis. J. Bone Jt. Surg. Am Vol. 71, 1378–1386 [PubMed] [Google Scholar]
- 68.Detamore MS and Athanasiou KA (2005) Evaluation of three growth factors for TMJ disc tissue engineering. Ann. Biomed. Eng 33, 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunja NJ et al. (2009) Effects of TGF-β1 and hydrostatic pressure on meniscus cell-seeded scaffolds. Biomaterials 30, 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makris EA et al. (2014) Combined use of chondroitinase-ABC, TGF-β1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials 35, 6787–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JK et al. (2017) Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater 16, 864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roveroni RC et al. (2001) Development of a behavioral model of TMJ pain in rats: The TMJ formalin test. Pain 94, 185–191 [DOI] [PubMed] [Google Scholar]
- 73.Nicoll SB et al. (2010) A rat model of temporomandibular joint pain with histopathologic modifications. J. Orofac. Pain 24, 298–304 [PubMed] [Google Scholar]
- 74.Cledes G et al. (2006) Validation of a chemical osteoarthritis model in rabbit temporomandibular joint: a compliment to biomechanical models. Int. J. Oral Maxillofac. Surg 35, 1026–1033 [DOI] [PubMed] [Google Scholar]
- 75.Güler N et al. (2011) Sodium iodoacetate induced osteoarthrosis model in rabbit temporomandibular joint: CT and histological study (Part I). Int. J. Oral Maxillofac. Surg 40, 1289–1295 [DOI] [PubMed] [Google Scholar]
- 76.Huang BJ et al. (2018) Overcoming Challenges in Engineering Large, Scaffold-Free Neocartilage with Functional Properties. Tissue Eng. Part A 24, 1652–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyamoto H et al. (1999) A sheep model for temporomandibular joint ankylosis. J. Oral Maxillofac. Surg 57, 812–817 [DOI] [PubMed] [Google Scholar]
- 78.Li L et al. (2015) Establishment and histological evaluation of a goat traumatic temporomandibular joint model. J. Oral Maxillofac. Surg 73, 943–950 [DOI] [PubMed] [Google Scholar]
- 79.Miyamoto H et al. (2007) The effect of etodolac on experimental temporomandibular joint osteoarthritis in dogs. J. Cranio-Maxillo-Facial Surg 35, 358–363 [DOI] [PubMed] [Google Scholar]
- 80.Lowe J et al. (2018) Properties of the Temporomandibular Joint in Growing Pigs. J. Biomech. Eng DOI: 10.1115/1.4039624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vapniarsky N et al. (2017) The Yucatan Minipig Temporomandibular Joint Disc Structure-Function Relationships Support Its Suitability for Human Comparative Studies. Tissue Eng. Part C. Methods 23, 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herring SW and Liu ZJ (2001) Loading of the temporomandibular joint: anatomic and in vivo evidence from the bones. Cells, Tissues, Organs 169, 193–200 [DOI] [PubMed] [Google Scholar]
- 83.Herring SW et al. (2002) Temporomandibular joint in miniature pigs: Anatomy, cell replication, and relation to loading. Anat. Rec 266, 152–166 [DOI] [PubMed] [Google Scholar]
- 84.Kalpakci KN et al. (2011) An Interspecies Comparison of the Temporomandibular Joint Disc. J. Dent. Res 90, 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herring SW (1976) The dynamics of mastication in pigs. Arch. Oral Biol 21, 473–480 [DOI] [PubMed] [Google Scholar]
- 86.Mosser DM and Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaffney L et al. (2017) Macrophages’ role in tissue disease and regeneration In Macrophages: Origin, Functions and Biointervention (Kubiak JZ and Kloc M, eds), pp. 245–271, Springer; [DOI] [PubMed] [Google Scholar]
- 88.Ogle ME et al. (2016) Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med 241, 1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Falah M et al. (2010) Treatment of articular cartilage lesions of the knee. Int. Orthop 34, 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basad E et al. (2015) Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surgery, Sport. Traumatol. Arthrosc 23, 3729–3735 [DOI] [PubMed] [Google Scholar]
- 91.Salash JR et al. (2016) Potential Indications for Tissue Engineering in Temporomandibular Joint Surgery. J. Oral Maxillofac. Surg 74, 705–711 [DOI] [PubMed] [Google Scholar]
- 92.Smith RM et al. (1993) Erosion of a Teflon-Proplast implant into the middle cranial fossa..J. Oral Maxillofac. Surg 51, 1268–1271 [DOI] [PubMed] [Google Scholar]
- 93.Berarducci JP et al. (1990) Perforation into middle cranial fossa as a sequel to use of a proplast-teflon implant for temporomandibular joint reconstruction. J. Oral Maxillofac. Surg 48, 496–498 [DOI] [PubMed] [Google Scholar]
- 94.Chuong R and Piper MA (1992) Cerebrospinal fluid leak associated with proplast implant removal from the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol 74, 422–425 [DOI] [PubMed] [Google Scholar]
- 95.Diehl R et al. (2017) Immunosuppression for in vivo research: State-of-The-Art protocols and experimental approaches. Cell. Mol. Immunol 14, 146–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donahue RP et al. (2018) Considerations for translation of tissue engineered fibrocartilage from bench to bedside. J. Biomech. Eng DOI: 10.1115/1.4042201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


