Abstract
Inborn errors of metabolism (IEMs) are particularly frequent as diseases of the nervous system. In the pediatric neurologic presentations of IEMs neurodevelopment is constantly disturbed and in fact, as far as biochemistry is involved, any kind of monogenic disease can become an IEM. Clinical features are very diverse and may present as a neurodevelopmental disorder (antenatal or late-onset), as well as an intermittent, a fixed chronic, or a progressive and late-onset neurodegenerative disorder. This also occurs within the same disorder in which a continuum spectrum of severity is frequently observed. In general, the small molecule defects have screening metabolic markers and many are treatable. By contrast only a few complex molecules defects have metabolic markers and most of them are not treatable so far. Recent molecular techniques have considerably contributed in the description of many new diseases and unexpected phenotypes. This paper provides a comprehensive list of IEMs that affect neurodevelopment and may also present with neurodegeneration.
Keywords: antenatal brain malformation, inborn error of metabolism, neurodegenerative disorder, neurodevelopment disorder, neurological manifestation of IEMs, outcome of IEMs
Abstract
Las enfermedades hereditarias del metabolismo (EHM) afectan con gran frecuencia al sistema nervioso. En sus formas neuropediátricas el neurodesarrollo se encuentra siempre afectado. En realidad, cualquier enfermedad monogénica cuya fisiopatología implique una alteración bioquímica puede ser considerada como una EHM. Las presentaciones clínicas son muy diversas en forma de trastorno del desarrollo antenatal o tardío, o bien de una enfermedad neurodegenerativa a brotes intermitentes, de carácter crónico o progresivo de debut tardío. En una misma enfermedad pueden darse diferentes espectros de gravedad. En general, las EHM que afectan a las moléculas pequeñas tienen marcadores metabólicos diagnósticos y muchas de ellas son tratables. Por contra, las EHM de las moléculas complejas tienen raramente marcadores metabólicos conocidos y la mayoría no tienen un tratamiento a día de hoy. Las técnicas de secuenciación masiva han permitido la descripción de numerosas nuevas enfermedades y fenotipos inesperados. Este artículo ofrece una lista completa de EHM que afectan el neurodesarrollo y pueden presentarse también como enfermedades neurodegenerativas.
Abstract
Les maladies héréditaires du métabolisme (MHM) affectent très fréquemment le système nerveux. Dans leurs formes neuropédiatriques, le neurodéveloppement est toujours perturbé et dès l'instant qu'elle implique un mécanisme biochimique, toute maladie monogénique peut devenir une MHM. Les présentations cliniques sont très diverses et peuvent s'exprimer sous la forme d'un trouble du neurodéveloppement (anténatal ou à début tardif) ou d'une maladie neurodégénérative intermittente, chronique stable ou progressive à début tardif. Ceci peut aussi s'observer au sein d'une même maladie, ou un continuum de sévérité est fréquemment constaté. En général, les MHM affectant les petites molécules biochimiques ont des marqueurs métaboliques de dépistage et beaucoup sont traitables. Au contraire, les MHM affectant les molécules biochimiques complexes ont rarement des marqueurs métaboliques et la plupart d'entre elles ne sont pas traitables jusqu'à présent. Les techniques moléculaires récentes ont permis la description de nombreuses nouvelles maladies et de phénotypes inattendus. Cet article donne une liste complète des MHM affectant le neurodéveloppement et pouvant aussi se présenter comme des maladies neurodégénératives.
Introduction
Inborn errors of metabolism (IEMs) have a strong predilection for the nervous system. From the estimated 300 “new” disorders described in the 5 years between the 5th (2011) and the recent 6th (2016) edition of the classic clinical textbook Inborn Metabolic Diseases: Diagnosis and Treatment,1 85% display predominantly neurological manifestations. Neurodevelopment is constantly interrupted in the pediatric neurologic presentations of IEMs. The precise neurobiological stage of development that is affected and the associated clinical manifestations are both terms related to the main groups of pathophysiological categories found in these diseases, as well as to the specific mechanisms of brain damage linked to every particular IEM. Additionally, neurodevelopment and neurodegeneration can behave as opposites throughout disease evolution, but they may also run in parallel.2,3
Neurodevelopment and metabolism: some basic concepts
Neurodevelopment begins in the early prenatal stage with a complex process that starts with the proliferation of distinct cell types, followed by differentiation into various fates, migration to their proper locations, and integration into functional circuitries.4 These steps continue to evolve in the postnatal years due to functions such as plasticity, myelination, and the maturation of brain cell connectivity. Therefore, neurodevelopment encompasses an important period of time in the life of a human being that goes from early fetal life until the beginning of adulthood. If everything goes well, the highly specialized human brain will be capable of developing a series of intriguing abilities, such as complex language, cognition, emotion, and a repertoire of precise and coordinated movements.5 Neurodevelopment disorders can arise as cognitive, neuropsychiatric, or motor problems6 and in general traits include, intellectual disability, learning difficulties, attention deficit hyperactivity disorder, autism, and cerebral palsy.7 The abnormal functioning of molecules involved in IEM can disrupt neurodevelopment at different stages producing a wide repertoire of clinical manifestations that oscillate from severe brain malformations to mild neurological signs such as learning difficulties (Tables I and II).
Defects in the proliferation of progenitors in the ventricular zone8 lead to microcephaly and are predominantly those of centrosomes and centrioles, involving structural proteins and microtubules, which in turn affect proliferation and cell migration. However, most of these defects are not considered as metabolic disorders. IEM that disturb proliferation are related to amino acid, fatty acid synthesis, and complex lipid metabolism defects (Table I).
TABLE I. Small molecule defects and vitamin-related disorders: neurodevelopmental phenotypes, brain MRI, and clinical outcome.
| GROUP OF DISEASES OR MOLECULES | ANTENATAL BRAIN ABNORMALITIES Structural defects, abnormal head growth | POSTNATAL BRAIN ABNORMALITIES at anytime of the postnatal development | MAIN NEUROLOGICAL SYMPTOMS Mimicking what kind of neurodevelopmental disorder? | OUTCOME Early death? Stability - Improvement? Neurodegeneration? | REF |
| Small molecules:ACCUMULATION | In general no antenatal manifestations | In general normal or subtle abnormalities (except decompensations) | Free interval. Most are efficiently treatable and can be screened at birth | In general, with treatment, constant improvement | 1, 29 |
| AMINOACID CATABOLISM. Diagnosis on AA chromatography. Postnatal neurodevelopmental disruption. Abnormal synaptic signaling, excitotocity (and other specific mechanisms), connectivity alterations, mostly circuitries involved in executive functions. Under treatment, in general mimicking ADHD and learning disabilities | |||||
| Phenylalanine PKU | Not described Maternal PKU: congenital microcephaly, ventriculomegaly, hypoplastic cerebral white matter, and delay of myelination | White matter T2 reversible hyperintensities | - Untreated: profound ID. Neuropsychiatric and behavioral AUTISM-like manifestations - Even under strict diet may still present ADHD, NPSY (anxiety, depression) Learning disabilities | Stability. NDEV disorder Outcome in long-term treated patients at older ages not reported. Diet discontinuation may produce NDEG in adults/elderly patients | 1 |
| Homocysteine Homocystinurias | Not described in CBS Remethylation defects may present as congenital hydro-cephalus | - Reversible WM lesions in CBS - Hemorrhagic/ischemic stroke | - Untreated: ID, NPSY, stroke - Even on strict treatment: ADHD, NPSY (obsessions, depression), Learning disabilities | Stability. NDEV disorder although unknown evolution in elderly patients | 1 |
| Leucine MSUD | Not described | WM T2 reversible hyperintensities | - Untreated: ID, spasticity - Even under strict treatment: ADHD, NPSY, Learning disabilities | Stability. NDEV disorder although unknown evolution in elderly patients | 1 |
| Ammonia, Glutamate, Glutamine (in UCD) | Not described | WM T2 reversible hyperintensities | - Even under strict treatment: ADHD, NPSY, Learning disabilities - Cerebral palsy-like in arginase deficiency and HHH | Stability. NDEV disorder although unknown evolution in elderly patients | 1 |
| ORGANIC ACIDURIAS (OA): Diagnosis on OA/Acylcarnitines (GCMS) Postnatal neurodevelopmental disruption. Neurobiological mechanisms similar to AA accumulation but associated with energy depletion. Classic OA under treatment mimic ADHD and learning disabilities whereas “cerebral” OA mimic cerebral palsy (CP) and some of them produce NDEG. | |||||
| Propionic, Methylmalonic, Isovaleric acidurias | Not described | WM T2 and basal ganglia hyperintensities that may be reversible | - Untreated: ID, Cerebral palsy-like (spasticity, movement disorders)- Even under strict treatment: ADHD, NPSY, Learning disabilities, Optic atrophy | Stability. NDEV disorder although unknown evolution in elderly patients | 1 |
| Cerebral organic acidurias (COA):Glutaric type 1 | Immature gyration pattern Bitemporal (biopercular) hypoplasia | Macrocephaly Subdural hematoma Basal ganglia lesions WM hyperintensities | - Acute encephalopathy (first 2 years of life), BG lesions. Dyskinetic CP - Mild forms: motor delay versus normality - Tremor, orofacial dyskinesia in adolescence in previously asymptomatic patients | - After acute BG injury, very similar to dyskinetic CP of other causes (stability but episodes of exacerbation that may lead to status dystonicus) - Unknown in older patients | 1 |
| Other COA: L2 OH glutaric; D2 OH glutaric; DL OH glutaric aciduria* Canavan disease (N-AAA) | L2OH glutaric: cerebellar hypoplasia N-AAA: Soft, gelatinous WM Loss of myelinated arcuated fibres | Characteristic white matter and cerebellar involvement, cortical atrophy | L2OH: Progressive cognitive deterioration in infancy with ataxia, seizures, pyramidal signs. Mild forms may mimic ID D2OH: Severe neonatal encephalopathy (seizures, early death) N-AAA: macrocephaly, early regression, progressive spasticity, optic atrophy, death | All these diseases evolve towards NDEG, with different degrees of severity and natural history | 1 |
| SMALL CARBOHYDRATE MOLECULE ACCUMULATION: diagnosis on plasma and urine biomarkers | |||||
| Galactosemias (Galactose, Galactose-1-P Galactitol) | Not reported | White matter T2 reversible hyperintensities | Even on strict diet delayed speech development, verbal dyspraxia, difficulties in spatial orientation and visual per-ception, ID, anxiety, depression. Mimics ID and learning difficulties | Stability. NDEV disorder although unknown evolution in elderly patients | 1 |
| METAL ACCUMULATION: Diagnosis on specific metals, protein carriers, brain MRI features, molecular analysis. Postnatal developmental disruption, mainly motor signs mimicking CP but later on progressive neurodegeneration | |||||
| Copper ATP7B transporter | Not described | “Eye of the panda” sign (lenfiform nuclei, thalamus, midbrain and pons). Atrophy of cerebral and cerebellar cortex | Wilson disease: bulbar dysfunction, tremor, rigidity, behavioral, and mood disturbances in 1st decade | NDEG without treatment | 30 |
| Iron: NBIA (PKAN, CoASY, PLA2G6, C19oef12, FAH2, WDR45, ATP13A2, FTL, DCAF17, SCP2, GTPBP2) | Not described (every defect has different pathophysiological mechanisms; brain iron accumulation is probably a paraphenomenon) | T2 pallidum hypointensity, leading to the “eye of the tiger” sign. WM alterations may be also present. Cortical and cerebellum atrophy | More than 10 genetic recognized conditions so far included in the NBIA syndromes. Some of them mimic NDEV disorders:- INAD due to PLA2G6 and early forms of PKAN mimic CP- BPAN mimic Retts | NDEG is the natural evolution. No effective treatments so far | 30 |
| Manganese SLC30A10 and SLC30A14 transporters | Not described | Hyperintense T1, hypointense T2 signals on basal ganglia | Early dystonia-parkinsonism mimicking dystonic CP; hepatic dysfunction is associated | NDEG is the natural evolution. But may improve on chelation treatment | 30 |
| Small molecules: DEFICIENCIES | Frequent antenatal manifestations, in particular congenital microcephaly | Wide variety of findings, from normal to severe alterations | Most have congenital or very early manifestations, mimicking CP or ID with NPSY signs. Late-onset presentations may occur | Many of them have a treatment with a variable response depending on the particular defect | 1, 29, 32 |
| AMINO ACID DEFECTS: Diagnosis on AAC (B, U, CSF), molecular analysis. Prenatal disruption of crucial neurodevelopmental programs. While BCAA deficiencies produce ID, ASD and the clinical spectrum of the synaptopathies, the other AA defects may cause severe antenatal malformations | |||||
| Large neutral AA transporter (SLC7A5) and BCDHK Low BCAA | Congenital microcephaly may be present with no other alterations in the brain MRI | Hypomyelination | Mimicking NDEV disorders, in particular ID with NPSY signs, mostly autism spectrum disorder (ASD) | NDEV disorder: Stability towards improvement with treatment (BCAA supplementation) | 33, 35 |
| Glutamine synthetase Low P glutamine | Complete agyria, hypomyelination, periventricular cysts, Cerebral atrophy | The same abnormalities found at early stages | Severe encephalopathy. Severe epilepsy | Early death. Severe disease involving crucial biological processes and probably incompatible with life | 37 |
| Serine defects 3-PGDH(**) PSAT1, PSP Serine (SL-C1A4) transporter Low P serine | Lissencephaly, microcephaly in Neu Laxova syndrome (congenital form) HM. Hypoplasic cerebellum CC Absent vs hypoplasia | Mild forms may display microcephaly and hypomyelination | - Severe encephalopathy: microcephaly, spastic tetraparesis, cataracts, epilepsy. Mimicking congenital infections - Mild forms: ID and epilepsy | NDEV disorder: stability towards improvement with L-serine supplementation | 36 |
| Asparagine synthetase Low to Nl P asparagine | Simplified gyri Decreased cerebral volume, congenital microcephaly | The same abnormalities found at early stages | Profound developmental delay, early-onset intractable seizures, axial hypotonia with severe appendicular spasticity | Early death. Severe disease involving crucial biological processes and probably incompatible with life | 38 |
| NEUROTRANSMITTER DEFECTS: Monoamines and amino acids that behave as neurotransmitters MONOAMINES: Diagnosis mostly on CSF pterins and NT metabolites. Some BH4 deficiencies and DNAJC12 have high plasma phe levels. Low brain dopamine disrupt neurodevelopmental programs probably at early prenatal stages, symptoms may appear at diverse ages and mimic CP AMINO ACIDS that behave as neurotransmitters: Diagnosis based on P, U, CSF analysis. Molecular analysis. Probably prenatal disruption of neurodevelopmental programs although brain malformations are not common. They present with the clinical spectrum of the synaptopathies and some particular defects as severe global encephalopathies. | |||||
| Monoamine synthesis defects: (TH, AADC, DNA-JC12, BH4 deficiencies: GTPCH, SR, DHPR, PTPS) | No antenatal malformations described. In severe forms congenital microcephaly can be present | In general normal brain MRI although mild nonspecific findings have been found in some cases. BG calcifications can appear in DHPR | - Very severe forms may cause congenital microcephaly and may be incompatible with life - Early encephalopathies with hypokinetic-rigid and dystonia mimicking CP - Parkinsonism, - L-Dopa responsive dystonia | NDEV disorder: Stability towards improvement with treatment (L-Dopa, 50HT and related molecules) | 44 |
| Monoamine transport defects: DAT, VMAT2 | Not described | In general normal brain MRI | - DAT may present as a severe dyskinetic CP that evolves towards parkinsonism - VMAT2 presents as dystoniaparkinsonism | DAT may have a NDEG progression. VMAT2 has a similar outcome and treatment than biogenic amine defects | 44 |
| GABA defects SSADH (High P/CSF GABA) GABAT (Low CSF GABA) | Spongiform leukodystrophy in GABAT | Pallidum hyperintensity, WM lesions, cerebellar atrophy in SSADH | - SSADH may present as ID and NPSY signs (ASD and others), epilepsy and ataxia - GABAT Early mortality and profound developmental impairment | SSADH: Stability although progression of epilepsy has been reported in adults Outcome in patients at older ages not reported | 44 |
| Glycine NKH: GCS and lipoate disorders High P/CSF Glycine | Cerebellar hypoplasia Cysts CC absent vs hypoplasia Diffusion restriction pattern in the brain stem (these diseases are also related to AA accumulation defects) | WM lesions in particular in lipoate related hyperglycinemias | - Severe neonatal encephalopathy, epilepsy, hypotonia, spasticity, profound mental retardation - Mild forms: Moderate ID, NPSY signs (ADHD, behavioral problems), seizures, chorea episodes | Early death in the severe forms Stability in mild forms although no long-term outcomes have been reported | 1 |
| GABA, glutamate and glycine receptors and transporters | Congenital polymicrogyria has been described in glutamatergic NMDA receptors. In G I uta mate transporter (SLC25A22): abnormal gyration. CC hypoplasia and congenital microcephaly | No specific patterns have been reported | Diverse signs in the spectrum of the synaptopathies: epilepsy, ID, NPSY problems Glycine receptors and transporters: hyperekplexia Glutamate transporter: severe congenital encephalopathy, epilepsy, severe psychomotor delay | Severe mutations in the glutamate receptors lead to early death Stability versus improvement with clonazepam in some forms of hyperekplexia | 1, 44 |
| AGC1 (SLC25A12) Low CSF NAA, high lactate | Global hypomyelination (this disease is also an energy defect) | BG hyperintensity, delayed myelination, reduced NAA in brain MRS, cerebellar atrophy | Arrested psychomotor development, hypotonia, infantile onset epilepsy | Severe encephalopathy. Long-term outcome not reported | 1, 44 |
| METAL DEFICIENCIES: Diagnosis on specific metals and protein disruption since they present with severe early encephalopath | |||||
| Copper (low serum copper and ceruleoplasmin ATP7A) | Brain vascular tortuosities | Cephalhematomas, vascular tortuosity | Menkes disease is a severe early encephalopathy. Seizures, hypotonia, loss of developmental milestones Mild forms (occipital horn syndrome) may present with mild to moderate ID | Menkes disease is a NDEG disorder | 30 |
| AP1S1 (Low serum cerulopasmin and copper) | Not reported (MEDNIK syndrome is also a trafficking disorder) | Mild T2 hyperintensity of caudate and putamen, brain atrophy | Multisystem disease. Dysmorphic features, Ichthyosis, ID, deafness, peripheral neuropathy, hepatopathy | Liver copper overload treatable by zinc acetate therapy This disease may be progressive, but there are no outcome reports | 43 |
| Manganese (Low blood Mn and Zn) SLC39A8 | Cerebellar atrophy | Severe atrophy of cerebellar vermis and hemispheres | Severe encephalopathy, hypotonia, seizures, strabismus, profound ID | Stability, mild NDEV achievements Progressive cerebellar signs reported in one family (NDEG?) | 41, 42 |
| BRAIN ENERGY MOLECULE TRANSPORTER DEFECTS: Diagnosis on B and CSF glucose/lactate/KB. RBC glucose and lactate uptake. Due to the energy characteristics of the prenatal brain and the newborn, these diseases should produce post-natal neurodevel-opmental disruptions. They mimic CP and ID | |||||
| GLUT 1 deficiency (low CSF glucose) | Not reported | Normal brain MRI. Severe forms may have microcephaly | - Early infantile severe epilepsy - Motor dysfunction that may be diverse and combined: cerebellar ataxia/dyspraxia, pyramidal signs, dyskinesias, mimicking CP - ID may be associated | Improvement with ketogenic diet and other anaplerotic treatments (such as triheptanoin) | 62 |
| Monocarboxylicacid transporter (lactate, pyruvate, ketone bodies) SLC16A1 | Not reported | Recurrent attacks of ketoacidosis. Moderate ID in homozygous forms | Stability of ID although no long-term outcomes reported | 63 | |
| FATTY ACID TRANSPORTER and SYNTHESIS DEFECTS: Diagnosis on FFA profile, lipidomics, molecular investigations | |||||
| DHA transporter: MFSD2A High plasma LPC (Low brain DHA?) | Cortical malformation, gross hydrocephaly, hugely dilated lateral ventricles cerebellar/brain stem hypoplasia/atrophy | The same antenatal manifestations, microcephaly | Severe encephalopathy: developmental delay, hypotonia, hyperreflexia, spastic quadriparesis, seizures | Lethal microcephaly syndrome | 34 |
| FA elongation: ELOVL4 (NL serum FA) | Not reported | Cerebellar atrophy | Severe encephalopathy: Ichthyosis, seizures, ID, spasticity resembling Sjogren Larsson (mimicking CP) Spinocerebellar ataxia. Retinopathy | NDEG disease Neonatal death reported in a mouse model | 40, 49 |
| Fatty aldehyde dehydrogenase (FALDH) | Not reported | Not reported | Sjogren Larsson syndrome: Congenital pruritic ichthyosis with bilateral spastic paraparesis, dysarthria, mild ID Mimic CP | Not enough information about outcome | 49 |
| ELOVL5 Low serum C20-4, C22-6 | Not reported | Cerebellar atrophy | Spinocerebellar ataxia | NDEG disease | 49 |
| VITAMIN/COFACTOR DEFECTS: Diagnosis on AAC, OAC, folates, B12, molecular investigations1 | |||||
| Biotin Biotinidase | Not reported | WM and BG abnormalities have been described | Biotin responsive MCD deficiency Seizures, developmental delay. Hearing loss. Crisis. Optic atrophy | Early treatment with biotin is very efficient. Stability towards improvement. | 1 |
| B12 Cobalamin C | Congenital hydrocephalus Hypomyelination and ventriculomegaly in HCFC1 | Similar to antenatal | Progressive neurological deterioration crisis, abnormal movements, seizures, microcephaly | HCFC1 may cause early death Other causes: Stability towards improvement | 1 |
| Folic acid (FOLR) MTHFR | Not reported | Hypomyelination, cerebellar atrophy, microcephaly, enlarged ventricles | Progressive encephalopathy, seizures, microcephaly, psychiatric disorders | If treated, stability towards improvement | 1 |
| B6 (pyridoxine) Antiquitin PNPO | May have hypoxicencephalopathy like features. Thin CC | Subtle, nonspecific findings | Neonatal pyridoxine-dependent epilepsy Some cases with encephalopathy PLP dependent epilepsy | Stability towards improvement PNPO has been related to early death | 1 |
| Mitochondrial TPP transporter (SLC25A19) Thiamine transporter (SLC19A3) | SLC25A19: Extreme microcephaly lissencephaly, dysgenesis of the corpus callosum, spinal dysraphic state | SLC19A3: Basal ganglia necrosis | SLC25A19: Amish lethal microcephaly: extreme microcephaly SLC19A3: Recurrent BG involvement, thiamine-responsive | SLC25A19: Early death. One case of long-term survival with profound ID and microcephaly has been reported SLC19A3: may be fully reversible with thiamine (improvement) | 1 |
| TPP kinase | Not reported | Increased signal intensities in the corticospinal tract at the medulla oblongata, and increased intensities in the dorsal pons. BG necrosis | Psychomotor retardation, severe encephalopathy, dystonia, ataxia | Stability after symptom onset although not enough information about outcome has been reported | 1 |
| AA: amino acids; AAC: aminoacid chromatography; ADHD; attention deficient hyperactivity disorder; AGC1: aspartate glutamate mitochondrial carrier; AADC: aromatic amino acid decarboxylase; AD: autosomal dominant; AR: autosomal recessive; ASD: autism spectrum disorder; BCDHK: branched chain dehydrogenase kinase; BG: basal ganglia; BPAN: β propeller associated neurodegeneration; CC: corpus callosum; CoASY: coenzyme A synthetase; COA: cerebral organic acidurias; CP: cerebral palsy; CSF: cerebrospinal fluid; DAT: dopamine transporter; DHA: docosahexanoic acid; DHPR: dihydropterine reductase; D-P: dystonia parkinsonism; DNAJC12: chaperone associated with complex assembly, protein folding, and export; DRD: dopa responsive dystonia; ELOV: fatty acid elongase; FA: fatty acid; FOLR: folate receptor, mutations leading to brain folate depletion; GABA: γ-aminobutyric acid; GABAT: GABA transaminase; GCMS: Gas chromatography mass spectrometry; GCS: glycine cleavage system; GTPCH: guanosine triphosphate cyclohydroxilase; HCFO: gene responsible for X-linked cobalamin deficiency; HM: hypomyelination; HVA: homovanillic acid; ID: intellectual disability; INAD: infantile neuroaxonal dystrophy; KB: ketone bodies; LPC: lysophosphatidylcholine; MCD: multiple carboxylase; MHBD:2-methyl-3-Hydroxybutyryl-CoA dehydrogenase; MHPG: 3-Methoxy-4-hydroxyphenylglycol; MSUD: maple syrup urine disease; MTHFR: methylene tetrahydrofolate reductase; NAA:N-acetyl aspartate; NBIA: neurodegeneration with brain iron accumulation; NDEG: neurodegeneration; NDEV: neurodevelopmental; NPSY: neuropsychiatric; OAC: organic acid chromatography; SDE: syndrome; WM: white matter; NL: neu-laxova, severe form of serine synthesis deficiency. NKH: non ketotic hyperglycinemias; OA: organic acid; NR: not reported; OGC: oculogyric crisis; PHE: phenylalanine; PKAN: pantothenate kinase-associated neurodegeneration; P:plasma; PKU: phenylketonuria; PLP: pyridoxal 5'-phosphate; PNPO: pyridox(am)ine 5'-phosphate oxidase; PSAT1: phosphoserine aminotransferase; PSP: phosphoserine phosphatase; PTPS: PVC: paraventricular cysts; PVM: periventricular leucomalacia; RBC: red blood cell; SR: sepiapterine reductase; SSADH: succinyl adenosine dehydrogenase; TH: tyrosine hydroxylase; TPP: thiamine pyrophosphate; U: urine; UCD: urea cycle disorders; VMAT: brain vesicular monoamine transporter 2; WM: white matter; 3-PGDH: 3-Phosphoglycerate Dehydrogenase Deficiency; 5-HIAA: 5-hydroxyindolaceticacid; 5-OH-T5 hydroxytryptophane Important and frequent manifestations and outcome are in bold. (*) SLC25A1 citrate mito carrier. (**) This variation of clinical manifestations may be common in many other disorders. Severe defects affect early developmental stages and behave as brain malformations whereas mild forms may present as “synaptopathies.” |
Defects in neuronal migration, which occur from the 2nd to the 6th month of gestation,9 give rise to lissencephaly and other types of cortical gyration defects (pachygyria, polymicrogyria). IEM leading to deficiencies of both small molecules (amino acids, fatty acids) (Table I) and complex molecules (phosphatidyl inositides, cholesterol, peroxisomal metabolism, O-glycosylation, and trafficking defects) (Table II) are related to migration defects, as well as some energy defects (ie, fumarase deficiency). Flaws found in other neurobiological processes of brain development include:
Synapse formation and maturation 10 which occurs essentially in the last trimester as well as in the first 2 years of life. Synaptic dysfunction presents itself through a series of symptoms such as intellectual disability (ID), epilepsy, neuropsychiatric signs, and movement disorders in any combination.11
Myelination, that begins in the second half of gestation and goes on to adolescence.12 Defects in myelination are related to connectivity defects (ID, neuropsychiatric disorders, epilepsy) and motor problems (spasticity, peripheral neuropathy).
Cytoskeletal rearrangements are crucial for every aspect of neurodevelopment (regulation of cell division, migration, dendritic and axonal growth, transport of cargo along those fibers). Cytoskeletal dysfunction is related to migrational defects (malformations), axonal conduction (spasticity, peripheral neuropathy), and dendritic dysfunction (symptomatic spectrum of synaptopathies).13 Synapse formation and maturation, myelination and cytoskeletal functions are very likely to be affected by most IEM since metabolism is an important regulator of these functions. The role of metabolism in the biology of neurodevelopment has scarcely been explored. Some recent works point to relevant functions of glucose14 purines, pyrimidines,15 lipids, and autophagy16 in the molecular mechanisms which regulate brain growth and neuronal connectivity. From the “metabolic side,” very few IEMs have been explored from the neurodevelopmental perspective. However, neurodevelopment is constantly disturbed in the pediatric presentations of inherited metabolic diseases.
TABLE II. Neurodevelopmental phenotypes, brain MRI and clinical outcome in complex molecule defects.
| GROUP OF DISEASES OR MOLECULES | ANTENATAL BRAIN ABNORMALITIES Structural defects, abnormal head growth | POSTNATAL BRAIN ABNORMALITIES at anytime of the post-natal development | MAIN NEUROLOGICAL SYMPTOMS Mimicking what kind of neurodevelopmental disorder? | OUTCOME Early death? Stability - Improvement? Neurodegeneration? | REF |
| I COMPLEX MOLECULE ACCUMULATION DEFECTS | In general no antenatal manifestations, although occasionally reported in very severe forms | Great variety of brain MRI abnormalities that evolve towards progressive cortical/subcortical atrophy | Progressive disorders with late-onset NDEG with or without obvious “storage” signs (like hepatosplenomegaly, coarse facies, cherry red spot, dysostosis multiplex, vacuolated lymphocytes) | These disorders are NDEG diseases. Some forms lead to early death. New therapies have been currently developed in order to modify these fatal outcomes | 1 |
| Glycogen (APBD, Lafora disease). NDEG starting in adolescence or adulthood / myoclonic epilepsy syndrome with polyglucosan deposition1 | |||||
| Sphingolipid catabolism: Sphingolipidoses (SPD) are a subgroup of lysosomal storage disorders in which sphingolipids accumulate in one or several organs. Most SPD present with post-natal progressive neurological regression. The clinical presentation and course of the most severe classic forms are often typical. Late-onset less typical forms (with movement disorder or psychiatric presentations) have been overlooked in the past. Diagnosis is mostly based on leukocytes, enzymes, and molecular analysis45 | |||||
| Gaucher type 2 (B cerebrosidase) | Brain atrophy | Acute neuronopathic perinatal form | Early death | 45 | |
| Gaucher type 3 | Brain atrophy | Myoclonic epilepsy, brainstem dysfunction starting at 5-8 years of age | In general, these disorders start with arrest of neurodevelopment in early forms and with learning disabilities in older children following by motor signs (ataxia, spasticity) and other neurological symptoms leading to a progressive NDEG Age at death ranges from childhood to adulthood depending on the age of first symptoms | 45 | |
| Niemann-Pick Disease type A (ASMD) | In general, no antenatal brain abnormalities are found in these diseases | Brain atrophy | At 5-10 months: hypotonia, loss of acquired motor skill, intellectual deterioration, spasticity, rigidity. Death below 3 years | 45 | |
| Niemann-Pick Disease type C (NPC1/2) | Cerebellar atrophy | - Early infantile: regression, spasticity - Late infantile: ataxia, MD, spasticity - Adult: ataxia, MD, psychiatric signs | 45 | ||
| Gangliosidosis GM1 (late onset) GM2 | Basal ganglia abnormalities, cerebellar atrophy | - Rapid regression, seizures, spasticity - Late infantile: ataxia, seizures - Adult: ataxia, MD, psychiatric signs | 45 | ||
| Krabbe disease | WM lesions, calcifications | Rapid global regression in early forms. Gait disturbances, optic atrophy, later forms | 45 | ||
| Metachromatic leukodystrophy | Characteristic leukodystrophy | Spastic tetraparesis, optic atrophy Late-onset: motor and/or psychiatric signs | 45 | ||
| Neuronal ceroid lipofuscinosis (NCL) Group of disorders with accumulation of autofluorescent ceroid lipopigments in neural tissue. Diagnosis is mostly based on molecular analysis. They are usually characterized by progressive psychomotor retardation, seizures, visual loss, and early death. Initial signs and outcome depend on the different subtypes: Congenital forms (Cathepsin D), Infantile NCL (6-24 months). Late infantile NCL (2-4 years). Juvenile NCL (4-10 years); Adult NCL (30 years)45 | |||||
| INCL, LINCL, JNCL, ANCL | Severe cortical/ subcortical atrophy may be present at prenatal stages in the congenital form | Cerebellar atrophy may be the initial sign followed by global, progressive brain atrophy | - Congenital: early death -INCL-CLN1: Microcephaly, optic atrophy, ataxia, myoclonus, regression -LINCL-CLN2: Jansky Bielschowsky. Seizures, ataxia, regression - JNCL: Batten disease, seizures, dysarthria, parkinsonism, dementia - ANCL: progressive myoclonic epilepsy: type A. Dementia with motor signs: type B | NDEG: Progressive motor and cognitive decline leading to death. | 45 |
| GAG and oligosaccharide catabolism. Mucopolysaccharidosis (MPS) and oligosaccharidosis are a subgroup of lysosomal storage disorders in which glycosaminoglycans (GAG) or glycoproteins accumulate in one or several organs. Most those disorders display post-natal progressive neurological regression but only a few present with predominant cognitive impairment. Diagnosis is based on GAG analysis and leukocyte enzyme analysis1 | |||||
| MPS type III (Sanfilippo disease) | In general, no antenatal brain abnormalities are found in these diseases | Progressive cortical/ subcortical atrophy. Enlarged perivascular (Virchow) spaces are also common | 2-6 years: Learning difficulties with behavioral problems followed by cognitive decline | NDEG: Progressive motor and cognitive decline leading to death | 1 |
| B mannosidosis Fucosidosis Salla Disease | Variable neurodegenerative disorder seizures; learning difficulties, challenging behavior | In Sanfilippo disease, learning difficulties and NPSY signs mimicking NDEV disorders normally precede NDEG | 1 | ||
| Sialidosis (MLI) Mucolipidosis (ML IV) | Slowly progressive cherry red spot myoclonus developmental delay with progressive blindness | 1 | |||
| II COMPLEX MOLECULE SYNTHESIS DEFECTS | Antenatal abnormalities found in cholesterol defects and phosphatidylinositide defects | Diverse post-natal brain abnormalities. Brain and cerebellar atrophy common in NDEG defects | Great variety of manifestations. Most presenting complex motor problems, although ID and epilepsy are also present | Most of them NDEG. Only a few mimic NDEV diseases at early stages of the disease | |
| Phospholipid synthesis and recycling (PL). A few defects in de novo PL synthesis and many defects of phospholipases involved in the remodeling of lipid membranes display preponderant late-onset neurodevelopment/neurodegeneration presentations. Two defects present with congenital spondylometaphyseal dysplasia or Lenz-Majewski dwarfism39,46 | |||||
| Choline kinase | In general, antenatal brain abnormalities have not been described in these diseases | Thin CC (occasional) | Mimics NDEV disorders with ID and NPSY (ASD) but associates muscle dystrophy, early onset muscle wasting | All these diseases have a NDEG character. Initially they can mimic CP | 39, 46 |
| SERAC1 | Characteristic basal ganglia abnormalities | MEGHDEL syndrome (early dystonia spasticity) dyskinetic CP. Mild late forms | 39, 46 | ||
| ABHD12 | Cerebellar atrophy | PHARC syndrome. Demyelinating polyneuropathy, cataracts, hearing loss, and RP mimicking Refsum disease | 39, 46 | ||
| PLA2G6 | Cerebellar atrophy Pallidum hypointensities due to iron accumulation | 1) Infantile neuroaxonal dystrophy 2) Neurodegeneration with brain iron accumulation. Static encephalopathy 3) Early onset dystonia parkinsonism, cognitive decline, and psychiatric disorder (PARK 14) | 39, 46 | ||
| PNPLA6 | Cerebellar atrophy | Clinical spectrum of neurodegenerative disorders: HSP (SPG39), Boucher-Neuhauser, GordonHolmes, Oliver McFarlane and Laurence-Moon syndromes | 39, 46 | ||
| DDHD1 | ThinCC. WM abnormalities may be present | HSP (SPG28), cerebellar ataxia without ID | 39, 46 | ||
| DDHD2 | ThinCC. WM abnormalities may be present | Progressive complex HSP (SPG54),ID, developmental delay | 39, 46 | ||
| CYP2U1 | Basal ganglia calcifications | HSP with basal ganglia calcifications (SPG56) | 39, 46 | ||
| Phosphatidyl inositides (PI3K)/AKT. Phosphatidylinositol are PL synthesized from cytidyl diphosphate diacylglycerol. The PI3Kinases are a family of signaling enzymes that regulate a wide range of processes including cell growth and brain development47 | |||||
| AKT3, PIK3R2 and PIK3CA | Megalencephaly-capillary malformation and megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndromes | Congenital macrocephaly | Sporadic overgrowth disorders associated with markedly enlarged brain size and other recognizable features. Mimicking ID and overgrowth syndromes | Stability. Behave as NDEV disorders | 47 |
| Sphingolipids (SPL): At least 7 SPL synthesis defects display preponderant late-onset neurodevelopment/neurodegeneration presentations including HSP | |||||
| CERS I and II | In general, antenatal brain abnormalities have not been described in these diseases | Brain atrophy | Progressive myoclonic epilepsy and cognitive decline (childhood/ adulthood) | All these diseases have a NDEG character. Initially they can mimic CP; GM3 synthase may mimic Rett syndrome | 45 |
| FAHN | Cerebellar atrophy, WM lesions, pallidum hypointensity | Complex HSP (SPG35) starting in childhood | 45 | ||
| GM3 synthase | Initially normal but evolving towards diffuse brain atrophy | At 3 months: Amish epilepsy syndrome, profound developmental stagnation and regression. Salt and pepper syndrome Rett syndrome-like, mimicking NDEV disorders | 45 | ||
| GM2/GD2 synthase | Brain atrophy | Slowly progressive HSP with mild to moderate cognitive impairment (SPG26) | 45 | ||
| GBA2 | Cerebellar atrophy | Adolescence to adulthood: Autosomal recessive cerebellar ataxia and complex HSP (SPG46) | 45 | ||
| Serine palmitoyl transferase | Dominant hereditary sensory neuropathy (HSAN12). Starting in adulthood | 45 | |||
| Cholesterol and bile acids: Most cholesterol synthesis disorders may present with various multiple congenital and morphogenic anomalies including brain, and/or a marked delay in psychomotor development. These include Greenberg dysplasia, X-linked dominant chondrodysplasia punctata, lathosterolosis and desmosterolosis, Smith-Lemli-Opitz syndrome, CHILD syndrome, and CKS. Diagnosis is based on plasma oxysterol analysis by GC/MS48 | |||||
| MKD-MA | Cerebellar atrophy | Autoinflammatory disorder with ID, ataxia, hypotonia, dysmorphic features | Stability versus mild improvement, they mimic NEURODEV disorders, although long-term outcomes have not been reported | 48 | |
| 7-dehydrocholesterol reductase | Congenital microcephaly may be present | Microcephaly | Smith Lemli Opitz syndrome: Facial dysmorphism, poly malformations Mild forms may mimic NDEV disorders: ID, NPSY signs including ASD | 48 | |
| Sterol Delta8 Delta7 isomerase Hemizygous males | Dandy-Walker malformations CC agenesis | Microcephaly | Facial dysmorphism. ID and NPSY signs mimicking a NEURODEV disease | 48 | |
| NSDHL (X-linked) Hemizygous males | Cortical malformations, congenital microcephaly | The same abnormalities as at prenatal stages | CK syndrome: facial dysmorphism. Poly malformations, mild to severe ID, seizures beginning in infancy, NPSY signs including aggression, and ADHD | 48 | |
| III PEROXISOME DISORDERS | Many peroxisomal disorders interfere with fetal neurodevelopment | Cerebellum and white matter are often affected | Severe early-onset encephalopathies with multisystem involvement and late onset presentations | In general they have a neurodegenerative outcome | 49 |
| Plasmalogen synthesis defects: Of the 5 types known so far only the fatty acyl-CoA oxido reductase 1 that involves the fatty cohol cycle presents with isolated severe neurological dysfunction | |||||
| RCDP types 1-4 | Cerebellar atrophy | The same abnormalities as at prenatal stages | Mostly severe skeletal dysplasia, (RCDP), facial dysmorphism Severe ID, cerebellar atrophy, seizures | May mimic NDEV disorders as complex malformative syndromes but may evolve towards NDEG | 49 |
| Fatty acyl-CoA oxido-reductase | Dandy Walker variant | The same abnormalities as at prenatal stages | No RCDP. Severe ID, earlyonset epilepsy, microcephaly, congenital cataracts, growth retardation, and spasticity | 49 | |
| Peroxisomal β-oxidation: PZO plays an indispensable role in the oxidation of VLCFA, pristanic, bile acids, and eisosanoids the deficit of which causes severe neurological deficits | |||||
| Peroxisome biogenesis defects (>12 PEX defects | Cortical dysplasia, neuronal heterotopias, polymicrogyria pachygyria, periventricular cysts | Dysmyelination, demyelination, cerebellar atrophy | ZW spectrum disorders: 1) Classic ZW: Severe psychomotor retardation, profound hypotonia, seizures, deafness, RP 2) Many variants forms as NALD or IRD with overlapping symptoms; 3) Very mild forms with unspecific ID or cerebellar ataxia | Stability later on evolving towards NDEG | 49 |
| Isolated FA oxidation enzyme defects: Of these only the D-bifunctional protein (DBP) may interfere with antenatal development and mimics classic ZW | |||||
| X-ALD (male) AMN (male and female) | Not reported | Demyelination | Childhood cerebral form leading to vegetative state and early death Adult progressive spastic paraparesis | X-ALD may mimic initially ADHD and other NPSY signs later on evolving towards NDEG | 49 |
| 1. DBP 2. Acyl CoA oxidase 3. Racemase 4. Phytanyl-CoA hydroxylase | Not reported | Demyelination, Cerebellar atrophy | 1 - ZW like, Perrault syndrome or late onset neurodegeneration 2 - Neonatal adrenoleukodystrophy phenotype, mild ZW 3 - Mild forms mimic Refsum disease relapsing encephalopathy 4 - Adult Refsum disease: progressive polyneuropathy with RP and deafness | These disorders evolve towards NDEG | 49 |
| IV CONGENITAL DISORDERS OF GLYCOSYLATION | Many disorders interfere with neurodevelopment in fetal life | Cerebellar involvement is very common and may be progressive at the brain MRI with no clinical translation51 | CDG should be considered in any unexplained clinical condition particularly in multiorgan disease with neurological involvement but also in nonspecific ID | Some forms (O-glycosylation and COG6) lead to early death. Most forms have stable/mild improvement outcome | 50 |
| Congenital disorders of protein O-Glycosylation: Major symptoms involve brain, eye, skin, skeleton, cartilage and skeletal muscles in variable combination. Among the >10 defects involving the brain two are responsible for neuronal migration disorders. Screening methods: serum apolipoprotein C-lll isoelectrofocusing | |||||
| Cerebro-ocular -muscular dystrophy syndromes (POMT1/2 -CDG) | Cobblestone lissencephaly, cerebellar hypoplasia. Hydrocephaly, encephalocele CC agenesis | The same abnormalities as at prenatal stages | Absent psychomotor development Congenital muscular dystrophy Brain, eye dysgenesis | Early death (<1y) | 50 |
| Muscle -eye- brain disease POMGNT1 CDG | Same as above | Same as above | Same as above but less severe with longer survival | 50 | |
| Congenital disorders of protein N-glycosylation: Cerebellar involvement is an important feature of PMM2-CDG. It has also been reported in some patients with ALG1-CDG, ALG3-CDG, ALG9-CDG, ALG6-CDG, ALG8-CDG, SLC35A2-CDG (UDP-galactose transporter), B4GALT1-CDG (GM2 synthase). Screening methods are limited to serum transferrin isoelectrofocusing and molecular analysis. | |||||
| PMM2-CDG | Olivopontocerebellar hypoplasia | Cerebellar atrophy may progress | 1) Alternating internal strabismus, axial hypotonia, developmental disability, ataxia RP. Dysmorphism, fat pads 2) Mild forms: isolated slight ID | Stability versus mild improvement | 50, 51 |
| 1. ALG6-CDG 2.ALG1-CDG 3. DPAGT1-CDG 4. MAN1B1CDG | Stroke, brain atrophy Periventricular lesions CC agenesis (rare) | 1- Same as above with skeletal abnormalities. No RP. NPSY signs 2- Severe neurological dysfunction: severe ID, hypotonia, intractable seizures, tremor, ataxia, visual disturbances 3- Moderate/severe ID, hypotonia, epilepsy 4- Mild to severe ID, hypotonia. Abnormal speech development. NPSY signs | Stability versus mild improvement | 50 | |
| Dolichol synthesis utilization/recycling: Cerebellar involvement is a frequent finding in SRD5A3-CDG, DPM1-CDG, DPM2-CDG, COG1-CDG, COG5-CDG, COG7-CDG, and COGS-CDG. | |||||
| SRD5A3-CDG | Vermis atrophy | Vermis atrophy | ID, hypotonia, spasticity, cerebellar ataxia, with ophtalmological symptoms (coloboma,optic atrophy) Diagnosis on serum transferrin (IEF type 1 pattern) | Stability versus mild improvement | 50 |
| COG6-CDG (CDG+) | Cerebellar atrophy | Microcephaly | Developmental/ID. Liver involvement | Early death | 50 |
| Lipid glycosylation/GPI synthesis PIGA-CDG. Multiple brain antenatal abnormalities. Multiple congenital anomalies “hypotoniaseizures syndrome” or “Ferro-cerebro-cutaneous syndrome.” Facial dysmorphism. Diagnosis on hyperphosphatasia. | |||||
| N-Glycanase deficiency: IUGR, developmental disability, microcephaly, movement disorder, hypotonia, seizures, alacrimia, liver involvement. | |||||
| V INTRACELLULAR TRAFFICKING/ PROCESSING DISORDERS | Frequent abnormalities, in particular if the defect is localized at the ER/Golgi/Cytoskeleton | Very diverse abnormalities | From severe encephalopathies with multisystem involvement to the “synaptopathy” spectrum signs | Trafficking and autophagy defects are often related to neurodegeneration | 52, 53, 55 |
| Synaptic vesicle (SV) disorders: Synaptic vesicle disorders have been recently defined as a group of diseases which involve defects in the biogenesis, transport and synaptic vesicle cycle. Clinical signs of synaptic dysfunction include intellectual disability, neuropsychiatric symptoms, epilepsy and movement disorders. Many disorders are involved that overlap many IEM categories. The complexity of this group of diseases is beyond the scope of this article11,32,44 | |||||
| SNARE proteins: SNARE proteins compose a large group of proteins involved in membrane fusion between vesicles and target membranes. Most SNARE deficiencies disturb neurotransmission at the synaptic vesicle level | |||||
| SNAP29 (CEDNIK syndrome) | Cortical dysplasia. Pachygyria, polymicrogyria CC hypoplasia | The same than antenatal | CEDNIK syndrome: neurocutaneous syndrome characterized by cerebral dysgenesis, early severe ID, microcephaly, ichthyosis, and keratoderma | Stability | 54 |
| SNARES involved in the SV cycle: NAPB, PRRT, SNAP25, STXBP1, S GOSR2, TX1B... | In general, they do not present antenatal brain malformations | Microcephaly, hypomyelination, cerebellar/cortical atrophy, may be present | Most present with various types of early onset epileptic encephalopathy with ID, NPSY signs and movement disorders | These diseases are NDEV disorders. Long-term outcomes have not been reported | 53 |
| SV proteins involved in other SV cycle functions: trafficking, endocytosis. Some of the SNARE proteins participate also in these functions. Patients present with the clinical spectrum of the “synaptopathies” but multisystem diseases with complex neurological disorders including antenatal brain malformations are more likely to appear in trafficking defects between the ER and Golgi. Cortical migration defects are more common if the SV trafficking defect is localized at the axonal and/or dendritic level (involving cytoskeleton) | |||||
| Rabenosyn-5 | Not reported | Microcephaly | Complex phenotype: early infantile spasms, epilepsy, ID, Vitamin B12 deficiency with MMA accumulation | Stability | 32 |
| VPS 15 | Localized cortical dysplasia (hippocampal) | The same as antenatal | Severe cortical and optic nerve atrophy, ID, spasticity, ataxia, psychomotor delay, muscle wasting, pseudobulbar palsy, mild hearing deficit and late-onset epilepsy | Stability | 32 |
| Autophagy disorders: Clinically, these disorders prominently affect the central nervous system at various stages of development, leading to brain malformations, developmental delay, intellectual disability, epilepsy, movement disorders, and neurodegeneration, among others | |||||
| AP-5 (SPG 48) | Thin corpus callosum | The same as antenatal | HSP (SPG 48): ID and white matter lesions.. Accumulation of storage material in endoLSD | These disorders may present initially as NDEV diseases but evolve towards NDEG | 55 |
| EPG5 (VICI syndrome) | Non lissencephalic cortical or cerebellar vermis, pons dysplasia, hypoplasia, CC agenesis (constant) often with colpocephaly | The same as antenatal | Vici syndrome: multisystem disease. The neurological phenotype is broad and includes progressive postnatal microcephaly, profound developmental delay and ID, motor impairment, nystagmus, sensorineuronal deafness, and seizures | NDEG | 55 |
| WDR45 (BPAN: SENDA syndrome) | Not reported | May present NBIA features | BPAN: Childhood: ID, seizures, spastic paraplegia, Rett-like stereotypies, NPSY signs (ASD) Adolescence: progressive dementia, parkinsonism, dystonia, optic atrophy, sensorineural hearing loss | These disorders may present initially as NDEV diseases but evolve towards NDEG | 55 |
| SNX14 (Childhood ARCA and ID syndrome) | Not reported | Cerebellar atrophy | Globally delayed development, ID, ASD, hypotonia, absent speech, progressive cerebellar atrophy and ataxia, seizures, and a storage disease phenotype | 55 | |
| VI t-RNA SYNTHETASES | Brain antenatal abnormalities may appear | White matter, cerebellum and brain stem are particularly affected | Great variety of neurological manifestations often associated with high lactate levels | Severe diseases, mostly evolving towards neurodegeneration | |
| Mitochondrial t-RNA: Pathogenic variants in ARS genes encoding a mitochondrial enzyme tend to cause phenotypes in tissues with a high metabolic demand. Leukoencephalopathies, myopathies, and liver disease are all common features of mitochondrial ARS disease phenotypes. Additionally, epilepsy, developmental delay, ID, ovarian failure, and sensorineural hearing loss are frequently observed in patients with mitochondrial ARS mutations56-58 | |||||
| RARS2 (mt arginyl-tRNA synthetase) | Pontocerebellar hypoplasia, brain stem thinning | The same abnormalities than antenatal findings | Perinatal. Encephalopathy with lethargy, hypotonia, epilepsy, and microcephaly | In general these diseases evolve towards NDEG | 56, 58 |
| DARS2 (mt aspartyl-tRNA synthetase) | Not reported | Leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation (LBSL) | Childhood to adulthood. Cerebellar ataxia, spasticity, dorsal column dysfunction, cognitive impairment | 56, 58 | |
| FARS2 (mt phenylalanine-tRNA synthetase) | Cerebral and cerebellar, brain stem and basal ganglia atrophy | The same abnormalities than antenatal findings | Perinatal. Epileptic encephalopathy, liver disease, and lactic acidosis | 56, 58 | |
| MARS2 (mt methionyl -tRNA synthetase) | Not reported | Cerebellar atrophy and white matter alterations, thin corpus callosum | Childhood to adulthood: autosomal recessive spastic ataxia | 56, 58 | |
| EARS2 (mt glutamyl-tRNA synthetase) | Not reported | Leukoencephalopathy with thalamus and brain stem involvement and high lactate (LTBL) | Early childhood: Global developmental delay or arrest, epilepsy, dystonia, spasticity, and high lactate | 56, 58 | |
| TARS2 (mt threonyl-tRNA synthetase) | Thin corpus callosum, bilateral lesion of the pallidum | The same abnormalities than antenatal findings | Perinatal to early childhood: psychomotor delay, hypotonia | 56, 58 | |
| VARS2 (mt valyl-tRNA synthetase) | Not reported | Hyperintense lesions in the insula and frontotemporal right cortex | Childhood. Psychomotor delay, seizures, facial dysmorphism, lactic acidosis | 56, 58 | |
| Cytoplasmic t-RNA | The recessive neurological phenotypes associated with cytoplasmic ARSs include hypomyelination, microcephaly, seizures, sensorineural hearing loss, and developmental delay. Some multisystem, cytoplasmic ARS-linked disorders also include liver dysfunction and lung disease. Dominant ARS-mediated disorders have, to date, a limited phenotypic range. Mutations in five ARS loci have been implicated in dominant Charcot-Marie-Tooth (CMT) disease and related neuropathic phenotypes: glycyl-(GARS), tyrosyl-(VA/ 5), alanyl-(AA/ 5), histidyl-(HARS), and tryptophanyl-tRNA synthetase (WARS). ARS-mediated CMT disease is predominantly caused by a defect in peripheral nerve axons (CMT Type 2).57 | ||||
| VII Purines and Pyrimidines | Diagnosis is based on P, U biomarkers (GCMS and HPLC), enzyme assays. Many enzymatic defects affecting the de novo synthesis, catabolic and salvage metabolic pathways of purines and pyrimidine involve brain development.59 A few disorders are potentially treatable like CAD deficiency that presents with an early severe epileptic encephalopathy responsive to uridine.60 | ||||
| ABH12: a/3 hydrolase domain-containing protein 12APBD that releases arachidonic acid; ADHD; attention deficient hyperactivity disorder; APBD: adult polyglucosan body disease; ASMD: acid-sphingomyelinase deficiency; AA: amino acids; AP-5: adaptor proteins (AP 1-5) are ubiquitously expressed protein complexes that facilitate vesicle-mediated intracellular sorting and trafficking of selected transmembrane cargo proteins; AP-5 is part of a stable complex with two other proteins, spatacsin (SPG1 1) and spastizin (SPG1 5); ARCA: autosomal recessive cerebellar ataxia; ASD: autism spectrum disorder; BPAN: 3-propeller proteinassociated neurodegeneration; B4GALT1-CDG: GM2 synthetase; CAD: enzymatic complex responsible for the first three steps of the de novo pyrimidine synthesis; CER l/ll: ceramide synthetase l/ll; COG: conserved oligomeric Golgi complex (plays a major role in Golgi trafficking and positioning of glycosylation enzymes; CP: cerebral palsy; DDHD1/DDHD2: encode an A1 phospholipase; EPG-5 protein is critically involved in the late stages of the autophagy cascade such as autophagosome- lysosome fusion or proteolysis within autolysosomes; FAHN: fatty acid hydroxylase associated neurodegeneration; GBA2: Non lysosomal glucosylcerebrosidase; GCMS: gas chromatography mass spectrometry; GD2: disialoganglioside; GM1/2 monosialoganglioside; GOSR2: Golgi SNAP that indirectly regulates exocytosis; receptor GPI: Glycosylphosphatidylinositol; HSP: Hereditary spastic paraplegia; ID: intellectual disability; FA: Fatty acids; HM: hypomyelination; MKD-MA: mevalonate kinase-mevalonic aciduria; MMA Methylmalonic acid; NABP: N-ethylmaleimide-sensitive factor attachment protein beta implicated in synaptic vesicle docking; NALD: Neonatal adrenoleukodystrophy; NBIA: Neurodegeneration with brain iron accumulation; NDEG: neurodegeneration; NR: not reported; NDEV: neurodevelopmental; NPSY: neuropsychiatric; NSDHL: NAD(P) dependent steroid dehydrogenase-like; PEX: peroxin integral proteins of PZO membrane; PIGA: phosphatidylinositol glucosaminyltransferase (one of the 7 proteins require for the 1st step of the GPI synthesis); PMM2: phosphomannomutase 2; PNPLA6: phospholipase B; PLA2G6: phospholipase A2; PPRT: proline rich transmembrane protein 2 that regulates exocytosis; PZO: Peroxysome; Rabenosyn-5: multidomain protein implicated in receptor-mediated endocytosis and recycling; RCDP: rhizomelic chondrodysplasia punctata; RP: retinitis pigmentosa; SENDA: static encephalopathy of childhood with neurodegeneration in adulthood; SERAC1: protein with a lipase domain involved in the remodeling of phosphatidylglycerol, bis monoacyl glycerol phosphate and cardiolipin; SNAP 25: synaptosomal associated protein that regulates the neurotransmitter release; SNAP29: encodes for a SNARE protein involved in vesicle fusion; SRD5A3-CDG: steroid 5-alpha reductase; STXBP1:syntaxin binding protein 1 that regulates exocytosis; STX1B: syntaxin 1B that regulates exocytosis; VLCFA: very long chain fatty acids; VPS15 mutations perturb endosomal-lysosomal trafficking and autophagy; WDR45 interacts with autophagy proteins Atg2 and Atg9 to regulate autophagosome formation and elongation; WM: white matter; ZW: Zellwegger | |||||
| (*) This variation of clinical manifestations may be common in many other disorders. Severe defects affect early developmental stages and behave as brain malformations whereas mild forms may present as “synaptopathies.” |
Neurodevelopment versus neurodegeneration in IEM: opposites or a continuum
Due to the fact that brain function is still one of the most unknown mysteries of biology, concepts and approaches that could have been well-established in the past, can be seen as a matter of controversy nowadays. This is the case for neurodevelopment and neurodegeneration. Initially considered as opposite ends of the spectrum, an accumulating body of evidence shows significant similarities between cellular processes involved in both of them.2,3,17 For that matter, an important question includes the extent to which rare neurodevelopmental diseases have progressive pathology across the lifespan into adulthood, and how these potentially neurodegenerative mechanisms may inform more common diseases. For trisomy 21 and Rett syndrome, the existence of both neurodevelopmental and neurodegenerative pathology is well-known.18 Interestingly, IEMs provide many examples of these apparently paradoxical clinical outcomes. In this sense we could consider the following situations:
IEMs that disrupt biological programs at any stage of neurodevelopment but then remain stable over time. In fact they tend to have a constant improvement as long as they follow a proper treatment (in those treatable disorders). This is the case of treatable disorders of small molecules such as urea cycle defects, homocystinurias, and other aminoacidopathies. A major constraint in this group of diseases is the lack of knowledge about long-term outcomes. There are few reports on neurological and mental health status at old age, even in those IEMs that have been studied for a long time. Recent articles point towards a significant percentage of neuropsychiatric problems in adolescents and adults in diseases such as urea cycle defects and organic acidurias.19,20
IEMs that mimic neurodevelopmental disorders such as learning difficulties, ID, neuropsychiatric signs (ADHD, autism) and cerebral palsy because they present with similar symptoms and onset age, and remain apparently stable. However, this stability evolves towards clinical impairment and then to a degenerative course.17,21 This is the case of some lysosomal disorders such as Sanfilippo disease, that may start with symptoms mimicking ADHD, and BPAN (an autopaghy disorder) that often presents initially as autism and Rett-like phenotype.22 The time span between stability and progressive symptoms is variable and represents a major trait that characterizes every disease. Additionally, the interaction between genetic and environmental factors probably has an important role and modulates the mixed neurodevelopmental and neurodegenerative coexistence.
IEMs that present as pure neurodegenerative diseases because of the rapid and dramatic progressive outcome or due to adult presentations.
These are just descriptions of clinical scenarios without the confirmation of appropriate pathological studies. This is the case for patients that after a number of years of being asymptomatic, present with psychiatric, motor, or cognitive decline. Lysosomal disorders are amongst the most frequent in this group.23,24 Neurodevelopment is linked to reduced neuronal arborization, abnormal dendritic morphology, and failures in synapse development.25 Neurodegeneration appears as gliosis, neuronal loss, and other specific markers such as apoptosis and protein aggregation. It is unknown if even those neurodegenerative diseases starting in adulthood may have pathology changes linked to neurodevelopmental dysfunction that are clinically silent.26 The lack of necropsy studies, animal models, and high-resolution brain image techniques prevents delineation of the hypothetical continuum of neurobiological disturbances.
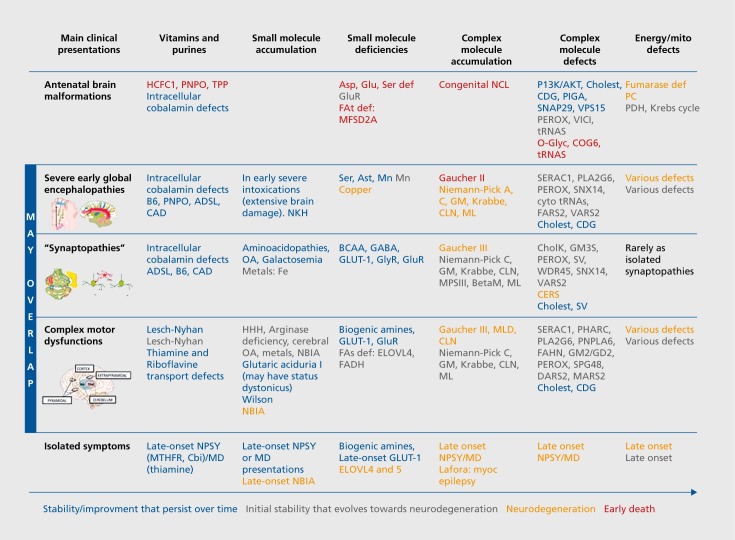
Figure 1 is a tentative overview to connect neurological presentations with pathophysiological IEM categories and neurodevelopmental to neurodegenerative features.
Figure 1. Main neurological presentations, pathophysiological categories, and neurodevelopmental to neurodegenerative features. Main clinical presentations are presented depending on the severity degree, from antenatal malformations to isolated symptoms. It is not rare that the same disease could have different types of presentation. Severe global encephalopathies include genetic defects that lead to early disruption of fundamental biological processes that are required for a proper brain development and affect motor, cognitive and behavioural aspects. Synaptopathies correspond to diseases impairing synaptic communication and often have epilepsy, intellectual disability, behavioural abnormalities (including autism) and movement disorders in any combination. Synaptic diseases produce connectivity impairments (abnormal brain circuitries' organization). Complex motor presentations correspond to diseases leading to abnormal motor symptoms; these are related to brain structures and circuits that regulate voluntary and passive movements, strength, and muscle tone. In pediatrics' IEMs it is not rare to detect combinations of different motor signs (dyskinetic movements, pyramidal signs, hypotonia, ataxia...). Some additional explanations: COG6 plays a major role in Golgi trafficking and positioning of glycosylation enzymes. HCFC1: gene responsible for X-linked cobalamin deficiency. PHARC: Acronym for a neuropathy syndrome due to a phospholipid remodeling defect that mimics Refsum disease. PIGA is one of the 7 proteins required for the 1st step of the GPI synthesis. SERAC1: protein with a lipase domain involved in the remodeling of phosphatidylglycerol, bis monoacyl glycerol phosphate and cardiolipin. SNAP29: encodes for a SNARE protein involved in vesicle fusion. SNX14: gene mutations responsible for childhood ARCA syndrome (Autosomic Recessive Cerebellar Ataxia). VPS15: is implicated in endosomal-lysosomal trafficking and autophagy. WDR45: interacts with autophagy proteins Atg2 and Atg9 to regulate autophagosome formation and elongation. Ast: aspartate transporter; Asp: asparagine synthetase; ADSL: adenylosuccinate lyase deficiency: BCAA: branched chain amino acid; BetaM: β mannosidosis; CAD: enzymatic complex responsible for the first 3 steps of the de novo pyrimidine synthesis; Cbl: cobalamin; Cholest: cholesterol; CholK: choline kinase; COG6: conserved oligomeric Golgi complex; CDG: Congenital disorder of glycosylation; CERS: ceramide synthetase; DARS2: mt aspartyl-tRNA synthetases; EARS2: mt glutamyl-tRNA synthetases; ELOVL4: fatty acid elongase 4; FAt: fatty acid transporter; FAs: fatty acid synthetase; FADH: fatty alcohol deshydrogenase; FAHN: fatty acid hydroxylase associated neurodegeneration; FARS 2: mt phenylalanine-tRNA synthetases; GABA:y aminobutyric acid; Glu: glutamine synthetase; GLUT1: glucose transporter; GluR: glutamate receptor; GlyR: glycine receptor; GM: ganglioside; HCFC1: host cell factor C1; HHH: hyperammonemia, hyperornithinemia, homocitrullinemia syndrome; MARS2: mt methionyl-tRNA synthetases. MTHFR: methyltetrahydrofolate reductase; MD: movement disorders; ML: mucolipidosis; MLD: metachromatic leucodystrophy; MPS III: mucopolysaccharidosis type III (sanfilippo disease); NBIA: neurodegeneration with brain iron accumulation; NCL: neuronal ceroid lipofuschinosis; NPSY: neuropsychiatric symptoms; OA: organic aciduriasO-gly: O-glycosylation; PC: pyruvate carboxylase; PDH: pyruvate deshydrogenase; perox: peroxysome biogenesis defects; PHARC: polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataracts; PIGA: phosphatidylinositol glucosaminyltransferase; P13K/AKT: phosphatidyl inositides kinase; PLA2G6: phospholipase A2; PNPLA6: phospholipase B; PNPO: pyridox(am)ine 5'-phosphate oxidase; Ser: serine synthesis defects; SERAC1: serine active site containing protein 1; SNAP29: synaptosomal associated protein 29 KD; SNX14: sorting nexin 14 SV: synaptic vesicle; tRNAs: tRNA synthetases; TPP: thiamine pyrophosphate; VARS2 mt valyl-tRNA synthetases; VPS15 is also known as PHOSPHATIDYLINOSITOL 3-KINASE, REGULATORY SUBUNIT 4: PIK3R4; WDR45: WD repeat containing protein 45.
Classification of IEM with implications in neurodevelopment
Metabolism involves thousands of proteins: mostly enzymes, receptors, and transporters of which the deficits cause IEM. Deficits can affect small or complex molecules. Regardless of their size, metabolites involved in IEMs can behave in the brain as signaling molecules, structural components, and fuels, and many metabolites have more than one role. According to Morava, the “classification of a disorder as an IMD requires only that impairment of specific enzymes or biochemical pathways is intrinsic to the pathomechanism.”27 Using this extended definition the more recent tentative nosology of IEM encompasses more than 1100 IEMs currently identified and provisionally classified into 130 groups.28
A “simplified” classification of IEMs mixes practical diagnostic approach and pathophysiological considerations according to 3 large categories based on the size of molecules (“small and simple” or “large and complex”) and their implication in energy metabolism.29 Only some very specific references focused on the more recent and significant defects will be cited while the more classic and well-known IEM will be referred to in the recent clinical textbook Inborn Metabolic Diseases: Diagnosis and Treatment.1
Small molecule defects
Almost all these IEM have plasma and urine metabolic marker(s) (small diffusible water-soluble molecules) that can be easily and rapidly measured in emergency, in one run (such as amino acids, organic acids, acylcarnitine chromatography), or by using specific methods (such as metals or galactose metabolites).
There are two subcategories in small-molecule disorders defined by whether the phenotype primarily results from an accumulation or a deficiency (Table I).
Small molecule diseases linked to an accumulation of compounds
These cause acute or progressive “intoxication” disorders. Signs and symptoms result primarily in the abnormal accumulation of the compound(s) proximal to the block and can potentially reverse as soon as it is removed. They do not interfere with embryo and fetal neurodevelopment and they present after a symptom-free interval (days to years: late onset) with clinical signs of “intoxication” (acute, intermittent, chronic, and even progressive: neurodegeneration) provoked by fasting, catabolism, fever, intercurrent illness, and food intake. Most of these disorders are treatable and require the removal of the “toxin” by special diets, scavengers, and cofactors.
This group encompasses IEMs of amino acid catabolism (like phenylketonuria, maple syrup urine disease, or homocystinurias), urea cycle defects, organic acidurias (such as methylmalonic, or glutaric aciduria type 1), galactosemias, metals accumulation (such as Wilson disease, neuroferritinopathies and brain iron accumulation syndromes, or cirrhosis dystonia syndrome with hypermanganesemia).30 Some purines/pyrimidines and metabolite repair defects (D/L-2-OH-glutaric, NAXE mutations) are also included in this group. Hyperglycinemias behave more so as neurotransmitters disorders than as an intoxication (see following section). Vitamins in metabolism (transport and intracellular processing) interfere with many different metabolic pathways where they act as enzymatic cofactor, chaperone, or signalling molecules. Therefore IEM of vitamins may turn out to be an intoxication treatable disorder or a complex severe congenital encephalopathy.
In the brain, molecules that accumulate can behave as neurotransmitters in the case of amino acids or stimulate biological pathways related to impaired autophagy and nerve growth factors. Secondary disturbances of essential amino acid (AA) transport are commonly observed in case of an elevation of one specific neutral AA using the L-type AA carrier like phenylalanine or leucine in phenylketonuria (PKU) and maple syrup urine disease respectively. This observation has been used to treat PKU.31 Synaptic plasticity and excitability are almost constantly impaired and executive functions are especially vulnerable. Therefore, and in spite of a proper metabolic control, most of these patients display learning, behavioural, and emotional difficulties.32
Small molecule diseases linked to a deficiency
Symptoms result primarily from the defective synthesis of compounds that are distal from the block or from the defective transportation of an essential molecule through intestinal epithelium, blood-brain barrier, and cytoplasmic or organelle membranes. Clinical signs are, at least in theory, treatable by providing the missing compound. Most of these defects cause a neurodevelopmental disruption, have a congenital presentation (antenatal), and may present as birth defects (Table I). They share many characteristics with disorders in the complex molecules group (Table II).
This group encompasses all carrier defects of essential molecules that must be transported through cellular membranes, and inborn errors of non-essential amino acids, and fatty acid synthesis. The most paradigmatic IEM linked to brain carrier defects are SLC7A5 deficiency resulting in defective transport of the branched chain amino acids (BCAA),33 and MFSD2A deficiency resulting in defective transport of essential fatty acids such as docosahexanoic acid (DHA).34 Interestingly the rare disorder branched chain dehydrogenase kinase (BCDHK) deficiency, which overactivates irreversible BCAA oxidation, causing very low levels of BCAA as in SLC7A5 mutations, presents a similarly devastating neurological syndrome with neurodevelopmental disruption.35
Non-essential amino acid synthesis defects may present also in antenatal life in the form of neurodevelopmental disruption. All severe forms of serine synthesis defects cause Neu Laxova syndrome.36 Glutamine synthetase37 and asparagine synthetase38 deficiencies display an almost complete agyria and clinically present with a severe congenital epileptic encephalopathy. Less severe forms of these disorders present with ID and epilepsy. This variation of clinical manifestations may be common in many other disorders. Severe defects affect early developmental stages and behave as brain malformations whereas mild forms may present as “synaptopathies.”
Fatty acids either derived from dietary sources or synthesized de novo can be converted into longer-chain fatty acid either saturated, mono-, or polyunsaturated to be further incorporated in complex lipids.39 Elongase 4 deficiency40 may present early in infancy with neurodevelopmental arrest, intellectual disability, spasticity, seizures, and ichthyosis similar to the Sjogren Larsson syndrome (Table I). Fatty acid synthesis/elongation defects share many similarities with peroxysomal very-long-chain fatty (VLCFA) acid catabolic disorders and complex lipids synthesis and remodeling deficiencies.
In addition to amino acid and fatty acid metabolism defects, the small molecules group also encompasses the IEM of neurotransmitters and those causing metal deficiency30 such as the manganese transporter deficiency syndrome (SLC39A8),41,42 the copper transporter ATP 7B deficiency causing Menkes disease and the copper regulator AP1S1 deficiency causing the MEDNIK syndrome.43
Inborn errors of neurotransmitters encompass the monoamine transport and synthesis, the γ-amino butyric acid (GABA) and GABA receptor deficiency, the glycine cleavage and transport defects and the glutamate defects. The diagnosis of monoamine disorders is based on CSF monoamine and neopterin analysis of carefully collected samples. In general, glycine and GABA defects are detected by means of amino acid quantification in plasma and urine (and also in the CSF for glycine-related disorders).44
In summary, most small-molecule defect disorders produce major neurodevelopmental disruptions, thereby leading to severe global encephalopathies where almost all neurological functions are chronically altered. In early-onset presentations, patients display severe psychomotor delays affecting both motor and cognitive milestones. Microcephaly and hypomyelination are very common as are epilepsy and movement disorders, which may demonstrate distinct features specific to each particular disease. These defects mimic early “non-metabolic” genetic encephalopathies that affect crucial neurodevelopmental functions such as neuronal precursor proliferation, migration, pruning, and dendrite development. This is because these small molecules contribute to antenatal brain “construction” in terms of signaling, cytoskeleton guidance, synapse formation later on in experience-dependent synapse remodelling.32
Complex molecule defects
This expanding group encompasses diseases that disturb the metabolism of complex molecules that are neither water soluble or diffusible. The main chemical categories of such complex molecules encompass glycogen, sphingolipids, phospholipids, cholesterol and bile acids, glycosaminoglycans, oligosaccharides, glycolipids, and nucleic acids. Similar to the small molecules, there are also two subcategories in complex molecule disorders, defined by whether the phenotype primarily results from an accumulation or a deficiency.
Complex molecule diseases linked to an accumulation
Catabolism defects lead to storage of a visible accumulated compound like classical lysosome defects. They are the most typical and historical group (such as the sphingolipidoses or mucopolysaccharidoses) in which signs and symptoms primarily result from the abnormal accumulation of compound(s) proximal to the block and potentially reverse as soon as the accumulation is removed. In general there are no antenatal manifestations. Neurological presentation signs display progressive disorders with late onset neurodegeneration with or without obvious “storage” signs. Diagnosis is mostly based on urine screening and leukocyte enzyme analysis.45
Complex molecule diseases linked to a deficiency
Phospholipids (PL) and sphingolipids (SPL) synthesis/remodeling defects compose a new rapidly expanding group of disorders without storage signs.39,46 Many SPL/PL remodeling disorders present as late-onset neurodegenerative diseases (hereditary spastic paraplegia: HSP, HSP+). Phosphatidylinositides (P-ins) are PL synthesized from cytidyl diphosphate diacylglycerol and inositol, and are highly regulated by a set of kinases and phosphatases. The P-ins 3 kinases (PIK3) are a family of signaling enzymes that regulate a wide range of processes including cell growth, proliferation, migration, metabolism, and brain development. Several disorders in this enzymatic system lead to megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndromes.47 In general, there is no metabolic marker and diagnosis is mostly based on molecular genetic techniques (eg, targeted next generation sequencing or whole exome sequencing).
Most cholesterol synthesis disorders may present with various multiple congenital and morphogenic anomalies including brain, internal organ, skeletal, or skin anomalies, and/or a marked delay in psychomotor development. Accumulation of substrate and consequent toxicity, with or without cholesterol deficiency, also explains the diversity of phenotypes observed. Alternatively, the anomalies might be attributable, at least in part, to deficient hedgehog signaling as has been suggested in CK syndrome and Smith Lemli Opitz syndrome.48 Diagnosis is based on plasma oxysterols analysis by gas chromatography/ mass spectrometry. Several bile acid synthesis defects present as late-onset neurodegenerative disorders after a symptom-free period following a transient episode of neonatal cholestatic jaundice.29
Glycosaminoglycan and oligosaccharide synthesis disorders do not present with preponderant neurological symptoms (therefore not presented here).
Peroxisomal disorders
Deficits may affect a specific matrix enzyme or a peroxin involved in the peroxysome membrane biogenesis. Many peroxysome biogenesis disorders interfere with neurodevelopment in the fetal life with a broad spectrum of phenotypes ranging from the severe Zellweger syndrome with cortical migration defect to mild ID. Peroxisomes play an indispensable role in the oxidation of VLCFA, pristanic, bile acids, and eisosanoids, the deficit of which causes severe neurological consequences. Out of all of them the D-bifunctional protein is the only one that may interfere with antenatal development and can mimic classic Zellweger syndrome. In both, diagnosis is based upon VLCFA, phytanic acid, and plasmalogen determinations in blood, RBC, and fibroblasts.49 Peroxisomal disorders are examples of early neurodevelopmental disruptions leading later on to neurodegeneration.
Congenital disorders of glycosylation
Congenital disorders of glycosylation (CDG) should be considered in any unexplained clinical condition, particularly in multiorgan disease with neurological involvement but also in nonspecific developmental disability.50 Many CDG interfere with neurodevelopment in the fetal life, particularly the protein O-glycosylation and lipid O glycosylation and glycosyl phosphatidyl inositol disorders. Cerebellar and olivopontocerebellar atrophy are a frequent finding in many protein N-glycosylation defects.51 Screening methods are limited to serum transferrin isoelectrofocusing (for N-glycosylation disorders with sialic acid deficiency) and serum apolipoprotein C-III isoelectrofocusing (for core 1 mucin-type O-glycosylation disorders). Exome/genome sequencing is increasingly used in the diagnostic workup of patients with CDG-X.50
Intracellular trafficking and processing disorders
Many defects affecting systems involved in intracellular trafficking, vesiculation, processing, and quality control of complex molecules, such as protein folding and autophagy52 have been recently discovered using next-generation sequencing. These diseases produce a wide repertoire of neurological symptoms including neurodevelopmental defects at various stages, progressive, and neurodegenerative disorders.32 Significant examples that involve either a category of molecules (like SNARE proteins),53,54 a metabolic process (like autophagy)55 or the synaptic vesicle cycle11 are presented in Table II In general there is no metabolic marker and diagnosis relies on molecular investigations.
t-RNA synthetases
Aminoacyl-tRNA synthetases (aaRSs) catalyze the specific attachment of each of the 20 amino acids to a cognate tRNA. With the exception of GARS and KARS, mitochondrial and cytoplasmic aaRSs are encoded by distinct nuclear genes.56,57 Aminoacyl-tRNA synthetases deficiencies present with a broad clinical spectrum. Interestingly, the neurological phenotypes range from later onset peripheral neuropathy (the so-called Charcot Marie Tooth type II) to severe, multisystem developmental syndromes. Mitochondrial aaRSs deficiencies may mimic an OXPHOS disorder with hyperlactatemia.58 Diagnosis relies on molecular technics. Only those with preponderant neurological signs are presented in Table II.
Nucleic acid disorders
Purine and pyrimidine synthesis and metabolism play major roles in controlling embryonic and fetal development and organogenesis. Any defect altering the tight regulation of purinergic transmission and purine and pyrimidine metabolism during pre- and post-natal brain development may translate into functional deficits, which could be at the basis of severe pathologies characterized by mental retardation or other disturbances.15 This can occur either at the level of the recruitment and/or signaling of specific nucleotide or nucleoside receptors or through genetic alterations in key steps of the purine salvage pathway. The pathophysiology of neurological signs remains badly known in many deficits such as in Lesch Nyhan syndrome.59 Some deficits are potentially treatable, like the uridine-responsive epileptic encephalopathy linked to CAD deficit, a protein complex involved in the first three steps of pyrimidine synthesis.60 Diagnosis relies on plasma/urines purines and pyrimidines profiles, specific enzymes assays and molecular studies.59
Disorders involving primarily energy metabolism
These consist of IEM with symptoms due, at least in part, to a deficiency in energy production or utilization within the brain, and other tissues. Inadequate ATP synthesis is an obvious issue for cells with constant or intermittently high energy demands such as neurons, which need to propagate action potentials. Astrocytes must recycle neurotransmitters released by neurons, a process that likely accounts for a large proportion of the brain's energy budget. Mitochondria are also key components of metabolic signaling pathways and a range of metabolites play key roles in influencing cellular function.
Membrane carriers of small energetic molecules
These molecules (glucose, fatty acids, ketone bodies, monocarboxylic acids) display many tissue specific isozymes such as the glucose cerebral transporter (GLUT1) and the monocarboxlic acid transporter (MCT). Glucose is the obligatory fuel for adult brain, but lactate produced from glucose by astrocytes within brain during activation has been proposed to serve as neuronal fuel. This simple and seductive hypothesis has been recently severely questioned.61 Clinically, and disregarding whatever the source of ATP may be, only essential fatty acid carrier deficiency presents with antenatal manifestations while GLUT162 and MCT163 deficiencies present only with relatively mild postnatal and late-onset manifestations.
Mitochondrial defects
These are the most severe and difficult to treat. The 289 genes categorized as causing mitochondrial disease can be divided into those that have a primary role specific to OXPHOS biogenesis, and those whose impact on OXPHOS is indirect or involve other cellular functions.64 Based on their function, the encoded proteins can be roughly grouped into
OXPHOS subunits, assembly factors and electron carriers
mtDNA maintenance
mtDNA expression
enzyme cofactors
mitochondrial homeostasis and quality control
general metabolism.
Brain manifestations of mitochondrial diseases are very frequent and cover a large variety of early postnatal to late-onset adult clinical features including seizures, encephalopathy, ataxia, spasticity, dystonia, movement disorders, parkinsonism, stroke-like episodes, developmental delay and/or regression, and cognitive decline.65 Aerobic glucose oxidation defects presenting with congenital lactic acidemias may display antenatal manifestations such as cerebral dysgenesis, corpus callosum atrophy, cerebral and cerebellar heterotopy, microcephaly, (pyruvate dehydrogenase system, pyruvate transporter, pyruvate carboxylase, fumarase and other Krebs cycle defects).66 Most of them have a progressive course. Some patients with severe forms of electron transfer (ETF) flavo protein and ETF ubiquinone oxidoreductase,67 and carnitine palmitoyl transferase II68 may have congenital anomalies with polycystic kidneys and neuronal migration defects that can be detected prenatally by fetal MRI, and facial dysmorphism.
Cytoplasmic energy defects
These are generally less severe. They include systemic glycolysis disorders, inborn errors of pentose phosphate pathways (untreatable), and creatine metabolism disorders (partially treatable).
Conclusions
Most, if not all, IEMs that involve the nervous system result in major neurodevelopmental disruptions. This is true not only for the “classic” historical group of “intoxication” disorders, with markers allowing a metabolic screening, but also for the vast new group of complex molecules disorders without easy-to-reach metabolic marker so far. This new, extended definition of IEM includes new categories and mechanisms, and as a general trend goes beyond a single biochemical pathway and/or organelle, and appears as a connection of multiple crossroads in a systems biology approach.32
Clinical features are very diverse and may present as a neurodevelopmental disorder (antenatal or late onset), as well as an intermittent, a fixed chronic, or a progressive neurodegenerative disorder. This is observed also within the same disorder in which a continuum spectrum of severity is frequently seen. However, little is still known about the biological mechanisms that potentially connect both neurodevelopment disorders and neurodegeneration. Therefore this linkage should be viewed as a hypothesis rather than a conclusion. An important issue to be addressed is the lack of natural history descriptions in most of these rare disorders. A deeper and detailed knowledge about how these disorders evolve over time, which symptoms will have a trend to improve, and which others will appear or worsen, is badly needed in order to understand the impact of these IEM through the lifespan. Moreover, these studies will be useful to monitor the effect of upcoming new treatments.
Recent molecular techniques have considerably contributed to the description of many new diseases and many new unexpected phenotypes. However metabolomics and lipidomics stand out among -omics due to the fact that they study the end products of cellular processes and therefore are more likely to be representative of clinical phenotypes than genetic variants or changes in gene expression.69 A greater understanding of phenotypic variability will be critical to personalize, or at least weigh, the initiation of long-term, sometimes burdensome, therapies in diseases detected by expanded newborn screening but that can manifest only in adulthood and with milder phenotypes.70
Acknowledgments
Jean-Marie Saudubray and Angela Garcia Cazorla declare that they have no conflict of interest. AGC is funded by FIS: PI1 5/01082 (Institute de Salud Carlos III: ISCIII and “Fondo Europeo de desarrollo regional” FEDER).
Contributor Information
Jean-Marie Saudubray, Department of Neurology, Neurometabolic Unit, Hopital Pitié Salpétrière, Paris, France.
Angela Garcia-Cazorla, Neurometabolic Unit and Synaptic Metabolism Lab (Department of Neurology), Institut Pediàtric de Recerca, Hospital Sant Joan de Déu and CIBERER (ISCIII), Barcelona, Spain.
REFERENCES
- 1.Saudubray JM., Baumgartner M., Walter J. 6th edition. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:1–658. [Google Scholar]
- 2.Shin J., Salameh JS., Richter JD. Impaired neurodevelopment by the low complexity domain of CPEB4 reveals a convergent pathway with neurodegeneration. Sci Rep. 2016;6:29395. doi: 10.1038/srep29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne S., Jansen L., U-King-Im JM., et al. EPG5-related Vici syndrome: a paradigm of neurodevelopmental disorders with defective autophagy. Brain. 2016;139(Pt 3):765–781. doi: 10.1093/brain/awv393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WF., Chahrour MH., Walsh CA. The diverse genetic landscape of neurodevelopmental disorders. Annu Rev Genomics Hum Genet. 2014;15:195–213. doi: 10.1146/annurev-genom-090413-025600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice D., Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Union. Draft Baseline Report on neurodevelopmental disorders in the framework of the European Environment and Hea Ith Strategy. Technical working group on priority diseases, subgroup neurodevelopmental disorders. 2003. [Google Scholar]
- 7.Dietrich KN., Eskenazi B., Schantz S., et al. Principles and practices of neurodevelopmental assessment in children: lessons learned from the Centers for Children's Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1437–1446. doi: 10.1289/ehp.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboitiz F., Zamorano F. Neural progenitors, patterning and ecology in neocortical origins. Front Neuroanat. 2013;7:38. doi: 10.3389/fnana.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertipaglia C., Gonçalves JC., Vallee RB. Nuclear migration in mammalian brain development. Semin Cell Dev Biol. 2018;82:57–66. doi: 10.1016/j.semcdb.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Farhy-Tselnicker I., Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13(1):7. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortès-Saladelafont E., Lipstein N., García-Cazorla A. Presynaptic disorders: a clinical and pathophysiological approach focused on the synaptic vesicle. J Inherit Metab Dis. 2018 Jul 18. doi: 10.1007/s10545-018-0230-z. [DOI] [PubMed] [Google Scholar]
- 12.Barateiro A., Brites D., Fernandes A. Oligodendrocyte development and myelination in neurodevelopment: molecular mechanisms in health and disease. Curr Pharm Des. 2016;22(6):656–679. doi: 10.2174/1381612822666151204000636. [DOI] [PubMed] [Google Scholar]
- 13.Lasser M., Tiber J., Lowery LA. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front Cell Neurosci. 2018;12:165. doi: 10.3389/fncel.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfeind AL., Babbitt CC. The appropriation of glucose through primate neurodevelopment. J Hum Evol. 2014;77:132–140. doi: 10.1016/j.jhevol.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Fumagalli M., Lecca D., Abbracchio MP., Ceruti S. Pathophysiological role of purines and pyrimidines in neurodevelopment: unveiling new pharmacological approaches to congenital brain diseases. Front Pharmacol. 2017;8:941. doi: 10.3389/fphar.2017.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh D., Dragich JM. Autophagy in mammalian neurodevelopment and implications for childhood neurological disorders. Neurosci Lett. 2018;pii:S0304-3940(18)30278-7. doi: 10.1016/j.neulet.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Fuller M., Futerman AH. The brain lipidome in neurodegenerative disorders. Biochem Biophys Res Commun. 2018;504:623–628. doi: 10.1016/j.bbrc.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Choong XY., Tosh JL., Pulford U., Fisher EM. Dissecting Alzheimer disease in Down syndrome using mouse models. Front Behav Neurosci. 2015;9:268. doi: 10.3389/fnbeh.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waisbren SE., Cuthbertson D., Burgard P., Holbert A., McCarter R., Cederbaum S. Members of the Urea Cycle Disorders Consortium. Biochemical markers and neuropsychological functioning in distal urea cycle disorders. J Inherit Metab Dis. 2018;41(4):657–667. doi: 10.1007/s10545-017-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizon M., Ottolenghi C., Valayannopoulos V., et al. Long-term neurological outcome of a cohort of 80 patients with classical organic acidurias. Orphanet J Rare Dis. 2013;8:148. doi: 10.1186/1750-1172-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza PVS., de Rezende Pinto WBV., de Rezende Batistella GN., Bortholin T., Oliveira ASB. Hereditary spastic paraplegia: clinical and genetic hallmarks. Cerebellum. 2017;16:525–551. doi: 10.1007/s12311-016-0803-z. [DOI] [PubMed] [Google Scholar]
- 22.Gregory A., Kurian MA., Haack T., Hayflick SJ., Hogarth P. Beta-propeller protein-associated neurodegeneration. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews®[lnternet]. Seattle (WA): University of Washington, Seattle; 1993-2018.2017 Feb 16. [PubMed] [Google Scholar]
- 23.Sedel F. [Inborn errors of metabolism in adult neurology]. Rev Neurol (Paris). 2013;169(Suppl 1):S63–69. doi: 10.1016/S0035-3787(13)70062-6. [DOI] [PubMed] [Google Scholar]
- 24.Maubert A., Hanon C., Sedel F. [Psychiatric disorders in adult form of Niemann-Pick disease type C]. Encephale. 2016;42(3):208–213. doi: 10.1016/j.encep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Harrill JA., Chen H., Streifel KM., Yang D., Mundy WR., Lein PJ. Ontogeny of biochemical, morphological and functional parameters of synaptogenesis in primary cultures of rat hippocampal and cortical neurons. Mol Brain. 2015;8:10. doi: 10.1186/s13041-015-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu TT., Ye XL., Zhang JP., et al. Increased adult neurogenesis associated with reactive astrocytosis occurs prior to neuron loss in a mouse model of neurodegenerative disease. CNS Neurosci Ther. 2017;23(11):885–893. doi: 10.1111/cns.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morava E., Rahman S., Peters V., Baumgartner MR., Patterson M., Zschocke J. Quo vadis: the re-definition of “inborn metabolic diseases”. J Inherit Metab Dis. 2015;38(6):1003–1006. doi: 10.1007/s10545-015-9893-x. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira CR., van Karnebeek CDM., Vockley J., Blau N. A proposed nosology of inborn errors of metabolism. Genet Med. In press. doi: 10.1038/s41436-018-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saudubray JM., Garcia-Cazorla A. Inborn errors of metabolism overview: pathophysiology, manifestations, evaluation, and management. Pediatr Clin North Am. 2018;65(2):179–208. doi: 10.1016/j.pcl.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Van Hasselt PM., Clayton P., Houwen RJ. Disorders in the transport of copper, iron, magnesium, manganese, selenium and zinc. In: Saudubray JM, Baumgartner M, Walter J Eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:531–546. [Google Scholar]
- 31.Matalon R., Michals-Matalon K., Bhatia G., Grechanina E., Novikov P., McDonald JD., Grady J., Tyring SK., Guttler F. Large neutral amino acids in the treatment of phenylketonuria (PKU). J Inherit Metab Dis. 2006;29(6):732–738. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Cazorla A., Saudubray JM. Cellular neurometabolism: a tentative to connect cell biology and metabolism in neurology. J Inherit Metab Dis. 2018 Jul 16. doi: 10.1007/s10545-018-0226-8. [Epub ahead of print]. doi: 10.1007/s10545-018-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tärlungeanu DC., Deliu E., Dotter CP., et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell. 2016;167(6):1481–1494.e18. doi: 10.1016/j.cell.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guemez-Gamboa A., Nguyen LN., Yang H., et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novarino G., El-Fishawy P., Kayserili H., et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338(6105):394–397. doi: 10.1126/science.1224631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acuna-Hidalgo R., Schanze D., Kariminejad A., et al. Neu-Laxova syndrome is a heterogeneous metabolic disorder caused by defects in enzymes of the L-serine biosynthesis pathway. Am J Hum Genet. 2014;95(3):285–293. doi: 10.1016/j.ajhg.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haberle J., Görg B., Rutsch F., et al. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353(18):1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- 38.Ruzzo EK., Capo-Chichi JM., Ben-Zeev B., et al. Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron. 2013;80(2):429–441. doi: 10.1016/j.neuron.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamari F., Mochel F., Saudubray JM. An overview of inborn errors of complex lipid biosynthesis and remodelling. J Inherit Metab Dis. 2015;38(1):3–18. doi: 10.1007/s10545-014-9764-x. [DOI] [PubMed] [Google Scholar]
- 40.Aldahmesh MA., Mohamed JY., Alkuraya HS., et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89(6):745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boycott KM., Beaulieu CL., Kernohan KD., et al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am J Hum Genet. 2015;97(6):886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH., Hogrebe M., Gruneberg M., et al. SLC39A8 Deficiency: A disorder of manganese transport and glycosylation. Am J Hum Genet. 2015;97(6):894–903. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinelli D., Travaglini L., Drouin CA., et al. MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain. 2013;136(Pt 3):872–81. doi: 10.1093/brain/awt012. [DOI] [PubMed] [Google Scholar]
- 44.Tristan-Noguero A., García-Cazorla A. Synaptic metabolism: a new approach to inborn errors of neurotransmission. J Inherit Metab Dis. 2018 Jul 16. doi: 10.1007/s10545-018-0235-7. [Epub ahead of print]. doi: 10.1007/s10545-018-0235-7. [DOI] [PubMed] [Google Scholar]
- 45.Vanier MT., Caillaud C., Levade T. Disorders of sphingolipid synthesis, sphingolipidoses, Niemann-Pick disease type C and neuronal ceroid-lipofuscinoses. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:551–575. [Google Scholar]
- 46.García-Cazorla A., Mochel F., Lamari F., Saudubray JM. The clinical spectrum of inherited diseases involved in the synthesis and remodeling of complex lipids. A tentative overview. J Inherit Metab Dis. 2015;38(1):19–40. doi: 10.1007/s10545-014-9776-6. [DOI] [PubMed] [Google Scholar]
- 47.Waugh MG. PIPs in neurological diseases. Biochim Biophys Acta. 2015;1851(8):1066–1082. doi: 10.1016/j.bbalip.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Waterham HR., Clayton PT. Disorders of isoprenoids and cholesterol synthesis. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:455–464. [Google Scholar]
- 49.Wanders RJA., Aubourg P., Poll-The BW. Inborn errors of non-mitochondrial fatty acid metabolism including peroxysomal disorders. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:591–606. [Google Scholar]
- 50.Jaeken J., Morava E. Congenital disorders of glycosylation, dolichol and glycophosphatidyl-inositol metabolism. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:609–622. [Google Scholar]
- 51.Barone R., Fiumara A., Jaeken J. Congenital disorders of glycosylation with emphasis on cerebellar involvement. Semin Neurol. 2014;34:357–366. doi: 10.1055/s-0034-1387197. [DOI] [PubMed] [Google Scholar]
- 52.Tokarev AA., Alfonso A., Segev N. Overview of Intracellular Compartments and Trafficking Pathways. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available at: https:// www.ncbi.nlm.nih.gov/books/NBK7286/. Accessed November 2018. [Google Scholar]
- 53.Kimura T., Jia J., Kumar S., et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017;36(1):42–60. doi: 10.15252/embj.201695081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sprecher E., Ishida-Yamamoto A., Mizrahi-Koren M., et al. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am J Hum Genet. 2005;77(2):242–251. doi: 10.1086/432556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebrahimi-Fakhari D., Saffari A., Wahlster L., et al. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Brain. 2016;139(Pt 2):317–337. doi: 10.1093/brain/awv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sissler M., González-Serrano LE., Westhof E. Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol Med. 2017;23(8):693–708. doi: 10.1016/j.molmed.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Meyer-Schuman R., Antonellis A. Emerging mechanisms of aminoacyl tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet. 2017;26(R2):R114–R127. doi: 10.1093/hmg/ddx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diodato D., Ghezzi D., Tiranti V. The mitochondrial aminoacyl tRNA synthetases: genes and syndromes. Int J Cell Biol. 2014;2014:787–956. doi: 10.1155/2014/787956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marie S., van den Berghe G., Vincent MF. Disorders of purines and pyrimidines metabolism. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:433–449. [Google Scholar]
- 60.Koch J., Mayr JA., Alhaddad B., et al. CAD mutations and uridine-responsive epileptic encephalopathy. Brain. 2017;140(2):279–286. doi: 10.1093/brain/aww300. [DOI] [PubMed] [Google Scholar]
- 61.Dienel GA. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain. J Neuroso Res. 2017;95(11):2103–2125. doi: 10.1002/jnr.24015. [DOI] [PubMed] [Google Scholar]
- 62.Pons R., Collins A., Rotstein M., Engelstad K., De Vivo DC. The spectrum of movement disorders in Glut-1 deficiency. Mov Disord. 2010;25(3):275–281. doi: 10.1002/mds.22808. [DOI] [PubMed] [Google Scholar]
- 63.van Hasselt PM., Ferdinandusse S., Monroe GR., et al. Monocarboxylate transporter 1 deficiency and ketone utilization. N Engl J Med. 2014;371(20):1900–1907. doi: 10.1056/NEJMoa1407778. [DOI] [PubMed] [Google Scholar]
- 64.Frazier AE., Thorburn DR., Compton AG. Mitochondrial energy generation disorders: genes, mechanisms and clues to pathology. J Biol Chem. 2017 Dec 12. doi: 10.1074/jbc.R117.809194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahman S., Mayr JA. Disorders of oxidative phophorylation. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. . 2016:223–242. [Google Scholar]
- 66.De Merleir LJ., Garcia-Cazorla A., Brivet M. Disorders of pyruvate metabolism and the tricarboxylic acid cycle. In: Saudubray JM, Baumgartner M, Walter J, eds. Inborn Metabolic Diseases: Diagnosis and Treatment. Heidelberg, Germany: Springer. 2016:185–199. [Google Scholar]
- 67.Lehnert W., Wendel U., Lindenmaier S., Böhm N. Multiple acyl-CoA dehydrogenation deficiency (glutaric aciduria type II), congenital polycystic kidneys, and symmetric warty dysplasia of the cerebral cortex in two brothers. I. Clinical, metabolical, and biochemical findings. Eur J Pediatr. 1982;139(1):56–59. doi: 10.1007/BF00442081. [DOI] [PubMed] [Google Scholar]
- 68.North KN., Hoppel CL., De Girolami U., Kozakewich HP., Korson MS. Lethal neonatal deficiency of carnitine palmitoyltransferase II associated with dysgenesis of the brain and kidneys. J Pediatr. 1995;127(3):414–420. doi: 10.1016/s0022-3476(95)70073-0. [DOI] [PubMed] [Google Scholar]
- 69.Wevers R., Blau N. Think big-think omics. J Inherit Metab Dis. 2018;41(3):281–283. doi: 10.1007/s10545-018-0165-4. [DOI] [PubMed] [Google Scholar]
- 70.Saudubray JM., Mochel F. The phenotype of adult versus pediatric patients with inborn errors of metabolism. J Inherit Metab Dis. 2018 Jun 6. doi: 10.1007/s10545-018-0209-9. [Epub ahead of print]. doi: 10.1007/s10545-018-0209-9. [DOI] [PubMed] [Google Scholar]