Abstract
Myelin is made by highly specialized glial cells and enables fast axonal impulse propagation. Recent studies show that oligodendrocytes in the central nervous system are, in addition to myelination, required for the integrity and survival of axons, independent of the presence or absence of myelin itself. The underlying mechanism of this support is given by glycolytic oligodendrocytes which provide axons with energy-rich metabolites. These findings represent a paradigm shift for the physiological function of axon-associated glia, and open the intriguing possibility that oligodendrocytes are important contributors to neurodegenerative diseases in which myelinated axons are lost, such as in Alzheimer disease, amyotrophic lateral sclerosis, and multiple system atrophy. Understanding the role of axon-oligodendrocyte coupling in neurodegenerative diseases may pave the way for the development of metabolism-based therapeutic approaches.
Keywords: Alzheimer disease; α-synuclein; amyloid-β, amyotrophic lateral sclerosis; axonal trophic support; multiple system atrophy; myelin; neurodegeneration, oligodendrocyte; superoxide dismutase 1 (SOD1)
Abstract
La mielina es producida por clulas gliales altamente especializadas y permite la propagacin rpida del impulso axonal. Estudios recientes muestran que los oligodendrocitos en el sistema nervioso central son, junto con la mielinizacin, necesarios para la integridad y sobrevida de los axones, independientemente de la presencia o ausencia de mielina. El mecanismo que subyace a este soporte est dado por oligodendrocitos glicolticos que aportan a los axones metabolitos ricos en energa. Estos hallazgos representan un cambio de paradigma para la funcin fisiolgica de la gla asociada a los axones, y abre la intrigante posibilidad que los oligodendrocitos sean importantes contribuyentes a las enfermedades neurodegenerativas en que se pierden los axones mielinizados, como la Enfermedad de Alzheimer, la esclerosis lateral amiotrfica y la atrofia de mltiples sistemas. La comprensin del papel del acoplamiento del oligodendrocito con el axn en las enfermedades neurodegenerativas puede abrir la va para el desarrollo de aproximaciones teraputicas basadas en el metabolismo.
Abstract
Fabrique par des cellules gliales trs spcialises, la myline permet la propagation rapide de l'influx axonal. D'aprs des tudes rcentes, les oligodendrocytes du systme nerveux central sont ncessaires, outre la mylinisation, l'intgrit et la survie des axones, indpendamment de la prsence ou non de myline. Les oligodendrocytes glycolytiques, apportant aux axones des mtabolites riches en nergie, en forment le mcanisme sous-jacent. Ces rsultats reprsentent un changement de paradigme pour la fonction physiologique de la glie associe aux axones. De manire trs intressante, les oligodendrocytes pourraient participer de faon importante aux maladies neurodgnratives dans lesquelles il y a une perte d'axones myliniss comme la maladie d'Alzheimer, la sclrose latrale amyotrophique et les atrophies multiples de systme. Comprendre le rle du couplage axone-oligodendrocytes dans les maladies neurodgnratives peut ouvrir la voie du dveloppement de traitements bass sur le mtabolisme.
Introduction
Neurodegenerative diseases comprise a spectrum of illnesses characterized by progressive neuronal, synaptic, and axonal loss. Clinically they manifest as dementia with declining overall cognitive abilities (language, problem-solving, and memory) and/or movement impairments. The neurological symptoms manifested in each disease depends largely on the part of the nervous system that is primarily affected. Classical neurodegenerative diseases in which a large proportion of cases are sporadic include Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), and multiple system atrophy (MSA). A common risk factor for all of these sporadic neurodegenerative diseases is aging. Life expectancy is increasing globally, leading to a steady rise in disease prevalence that is putting an enormous financial burden on the healthcare system. Why aging constitutes the greatest risk factor for neurodegenerative diseases remains poorly understood. Intriguingly, myelinated white matter integrity is significantly affected by aging, more so than gray matter.1 Recent studies have shown that, in addition to insulating axons to allow faster impulse propagation, oligodendrocytes also play an important role in providing axonal trophic support independent of the presence or absence of myelin itself.2,3 With this in view, we aim to discuss the emerging role of dysfunctional axon-oligodendrocyte coupling in neurodegenerative diseases.
Axon-oligodendrocyte coupling
Myelination of axons in the nervous system of vertebrates is essential for rapid impulse propagation. During development, portions of the peripheral nervous system mature first, followed by the spinal cord and the brain. Glial cells, notably oligodendrocytes in white matter tracts and Schwann cells in peripheral nerves, enwrap segments (internodes) of axons with their plasma membranes. Most of the longitudinal expansion of internodes coincides with secondary axon elongation during body growth, a process which in humans takes decades.4 In the neocortex, myelination continues at least through to the end of the second decade.
White matter tracts comprise about 50% of the human brain and constitute the unusual cellular underpinning of long-range “connectivity.” Many intracortical axons are also myelinated. Cortical projection neurons and spinal motor neurons can have a cellular volume, >99% which consists of long axonal projections. These axons are far away from their neuronal somata, where genetic information is read out, presynaptic proteins are synthesized, and output of a spiking neuron is determined at the axon hillock. However, axons are always physically close to glial cells.
In recent years, our understanding of oligodendrocyte and Schwann cell function has seen a paradigm shift that will be relevant for human brain disease and possibly for the development of new therapies. It has become clear that myelinating glial cells maintain the long-term integrity and survival of the axons that they ensheath, independent of the presence or absence of myelin itself.5-7 Specifically, oligodendroglial energy metabolism switches after myelination to aerobic glycolysis, where glycolysis products (ie, pyruvate or lactate) are transferred by diffusion into the myelinated axon to support mitochondrial respiration.8,9 Diffusion of metabolites through the myelin sheath occurs through minute cytosolic (20 to 300 nanometers wide) channels that coalesce into a continuous system of tubes, collectively termed “myelinic channels.”10 Indeed, it is the nearly complete encapsulation of axons by myelin itself, which deprives the neuronal compartment of rapid access to extracellular metabolites (ie, glucose), and which makes oligodendroglial metabolic support so important. Unmyelinated axons can also be supported by astrocytes. Thus, similar to astrocytes that drive the “lactate shuttle” at cortical synapses, oligodendrocytes serve the additional function of metabolically supporting the axons they ensheath.
The integrity of myelinic channels is maintained in part by the antagonizing roles of two widely studied myelin structural proteins; myelin basic protein (MBP) which acts as an adhesive zipper in the formation of compact myelin,11 and 2',3'-cyclic nucleotide phosphodiesterase (CNP) which prevents “complete” myelin compaction.12 CNP knockout mice show axonal degeneration in myelinated fibers in the presence of functional myelin, demonstrating that oligodendrocytes play an important role in axonal support that is independent of myelination.7 Conversely, MBP knockout mice (known as “shiver er” mice), are able to support axons and show no axonal loss in the absence of myelination.5 Recently it has been proposed that oligodendroglial support of axons is controlled by activity-dependent glutamate release that increases oligodendroglial glucose uptake and glycolytic support of fast-spiking axons.13 In this way, glutamate released from axons activates oligodendroglial N-methyl-D-aspartate (NMDA) receptors which in turn increases the expression of oligodendroglial cell surface glucose transporter 1 (GLUT1). Lactate is subsequently released towards the axon via monocarboxylate transporter 1 (MCT1) and taken up from the periaxonal space by axonal monocarboxylate transporter 2 (MCT2),8 as illustrated in Figure 1. Physiological experiments with the myelinated optic nerve from mice with FRET-based ATP sensors in the axonal compartment suggest that lactate support is more critical at higher spiking frequency.14 However, oligodendrocytes also deliver glucose to the myelinated axons, at least in the subcortical white matter.15
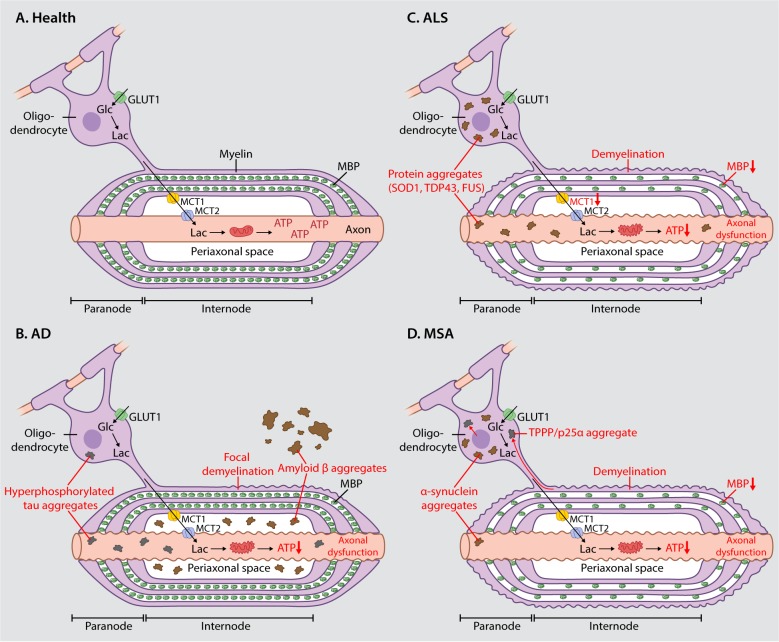
Figure 1. Axon-oligodendrocyte coupling in health and neurodegenerative disease. (A) In the healthy central nervous system, oligodendrocytes provide trophic support to axons by the transfer of energy-rich molecules (ie, lactate) from the oligodendroglial compartment to the axonal compartment. Lactate is released into the periaxonal space via MCT1 and is taken up by axons via MCT2, where it is used to fuel mitochondrial respiration. MBP acts as a zipper in the formation of compact myelin. In the neurodegenerative diseases AD (B), ALS (C), and MSA (D), pathological protein aggregates accumulate in the oligodendroglial cytoplasm and in the periaxonal space which could physically disrupt motor-driven transport and the free diffusion of metabolites (ie, lactate) from the oligodendroglial to the axonal compartment. In ALS, decreased expression of MCT1 would directly impair the ability of oligodendrocytes to provide metabolic support to axons. Demyelination is observed in all three diseases and it is associated with decreased expression of MBP in ALS and MSA. Impaired metabolic support together with mitochondrial dysfunction leads to decreased ATP supply and subsequent axonal dysfunction. Abbreviations: AD; Alzheimer disease, ALS; amyotrophic lateral sclerosis, FUS; fused in sarcoma, Glc; glucose, GLUT1; glucose transporter 1, Lac; lactate, MBP; myelin basic protein, MCT1; monocarboxylate transporter 1, MCT2; monocarboxylate transporter 2, MSA; multiple system atrophy, SOD1; superoxide dismutase 1, TDP43; transactive response DNA binding protein 43,TPPP/p25α; tubulin polymerization-promoting protein/p25α.
Alzheimer disease
AD is the most common form of dementia, currently affecting more than 47 million people worldwide. Symptoms include memory loss, problems with language, disorientation, and mood swings, all of which progressively decline during the disease course. Morphologically, this correlates with synapse and neuronal loss apparent as widespread brain atrophy. On a molecular level, AD is characterized by extracellular deposits of amyloid-β (Aβ) and intracellular accumulation of hyperphosphorylated tau. A number of hypotheses have been proposed to explain the mechanism by which these pathophysiological protein aggregates lead to neuronal and synaptic loss in AD. The most common of these, is the amyloid cascade hypothesis which postulates that the build-up of Aβ in various forms acts as an initial disease trigger.16 Aβ is generated from the amyloid precursor protein (APP) by sequential cleavage of β-secretase (BACE1) and the γ-secretase complex (containing presenilin 1 or PSEN1), mainly in the endolysosomal compartment. While most cases are sporadic, the discovery of inherited forms of the disease has fueled the development of AD mouse models by introducing APP and PSEN1 transgenes harboring human/humanized sequences with familial AD mutations.
Although AD is classically considered a gray-matter disease, white-matter alterations are also common. Several neuroimaging studies have found an exacerbated decline of white matter integrity in AD patients. Intriguingly, this was also shown for presymptomatic adults that were at an increased risk of developing AD.17-20 These findings indicate that myelin changes are an early feature of AD and thus could play a key role in disease etiology. Of note, early neuropathological observations by Braak and Braak (1996)21 suggested an inverse temporal relationship between AD pathology and myelination: late and thinly myelinated regions of the cortex that suffer most from age-dependent myelin breakdown are susceptible to AD pathology, while heavily and early myelinated tracts such as the spinal cord remain unaffected until the late phases of the disease. Such findings prompted Bartzokis to hypothesize that age-related myelin breakdown which is observed in the cortex and the accompanying repair processes are crucial factors initiating AD.22 This myelin hypothesis centers on the unique vulnerability of oligodendrocytes and myelin to become damaged upon aging due to their high cholesterol and iron content, slow protein, and lipid turnover, the elaborated production and maintenance of the myelin sheath and their continuous proliferation/differentiation throughout life. This hypothesis is supported by electron microscopic analyses of aged brains in non-human primates,23 and by recent “omic” approaches that show oligodendrocyte/myelin gene and protein modules to be significantly altered in AD patients.24,25 Intriguingly, genome-wide association studies also identified bridging integrator 1 (BIN1), a protein which is primarily expressed by oligodendrocytes, as the second greatest genetic risk factor for AD after ApoE4.26
However, only a few patient and animal studies have examined the role of oligodendrocytes and myelin breakdown in AD in more detail.27-31 These studies describe focal demyelination around Aβ deposits (as illustrated in Figure 1) and a loss of oligodendrocyte lineage cells in AD patients.27 Intriguingly, this is met by an increased proliferation response of oligodendrocyte precursor cells in an AD mouse model, a process which seems to fail in AD patients.27
Inherently, animal studies aimed at describing oligodendrocyte alterations in AD mouse models are limited to observing myelin changes secondary to AD (neuronal) protein aggregation and do not test Bartzokis' hypothesis that oligodendrocyte dysfunction drives Aβ production. The neurocentric view of AD has established the dogma that neurons are the primary producers of Aβ and accordingly animal models have been developed in which the transgenes are driven by neuronal promotors, for example the Thy1-driven APP/PSEN1 transgene in the widely used 5xFAD mouse model.32 However, the Aβ producing machinery, including processing enzymes, is expressed in many non-neuronal cell types including oligodendrocytes.33 Interestingly, the selective inactivation of Aβ production from excitatory neurons does not affect amyloid burden in an AD mouse model with ubiquitous expression of a mutant APP transgene, which raises a question regarding the extent to which glial cells contribute to Aβ production.34 The established AD animal models also suffer from artefacts caused by the use of non-endogenous promotors to drive the expression of the APP and PSEN1 transgenes. These include overexpression of transgenes and an atypical region specificity of amyloid burden. Recently, Saito and colleagues generated several APP knock-in mice in which a humanized and mutated APP version is driven by the endogenous promotor.35 Therefore, these animal models should recapitulate human amyloidosis more closely and will allow for exploring the contribution of different cell types to cerebral Aβ load.
In summary, there is strong suggestive evidence for oligodendrocyte and myelin disturbances in AD. Bartzokis' hypothesis of myelin breakdown initiating AD was published before the role of oligodendroglia in metabolic support of axons was known. Could age-dependent pathological changes in oligodendrocytes compromise this trophic support function rather than myelination status per se? In support of this extended hypothesis, it was shown that myelinic channels, which are necessary for trophic support, are themselves vulnerable to aging, as evidenced by the striking downregulation of CNP in aged mice.36 It is tempting to speculate about the role of dysfunctional metabolic coupling of oligodendrocytes and axons in the manifestation of AD: one plausible mechanism is a decline of trophic support in aged and structurally perturbed myelin leading to axonal energy deprivation manifesting in impairments of axonal transport, reduced lysosomal acidification, and lysosomal degradation of BACE1 which then exacerbates Aβ production. Such a mechanism would be in accordance with different lines of evidence demonstrating enhancement of Aβ production by pharmacological energy deprivation,37 reduction of axonal transport rates38 as well as impairments of lysosomal trafficking.39 Metabolic support decline could affect tau phosphorylation and accumulation by very similar mechanisms.40-42 Intriguingly, a recent study from the Stys lab identified Aβ threads in the periaxonal space underneath the myelin sheath in white-matter regions in an AD mouse model.43 While difficult to see microscopically, it seems likely that such periaxonal Aβ aggregates are a burden on both communication and metabolite shuttling between the axon and the oligodendrocyte/myelin compartment. This could for example represent an early (premorbid) disease state of the white matter, which is too densely packed to develop “mature” Aβ plaques as observed in the gray matter. However, Aβ plaques do exist in the white matter in both animal models and patients.44 In the context of our hypothesis of reduced metabolic support driving Aβ production, such findings could even argue for a vicious circle between disruptive Aβ accumulation and functional loss of oligodendrocyte-axon coupling.
Amyotrophic lateral sclerosis
ALS, also known as Lou Gehrig's disease, is the most common form of motor neuron disease. Characterized by the progressive failure of the motor neurons required for normal muscle function, ALS is a very debilitating and ultimately fatal condition. The majority of ALS cases are sporadic, while only 10% of cases are caused by inherited genetic mutations. To date, mutations in over 20 different genes have been identified as causative of familial ALS.45 Of these, mutations in the genes encoding superoxide dismutase 1 (SOD1), fused in sarcoma (FUS), transactive response DNA binding protein 43 (TDP43), and hexanucleotide repeat expansions in chromosome 9 open reading frame 72 (C9ORF72), have been the most widely studied. The discovery of these disease-causing mutations has led to the development of several ALS animal models, of which the mutant SOD1 mouse model has been the most widely used.46 Both sporadic and familial forms of the disease are clinically and pathologically similar, therefore investigation into the pathogenesis of familial ALS is likely to provide insight into both forms of the disease.47 Most intriguing is the fact that, although mutant forms of these proteins are ubiquitously expressed, one cell type (the motor neuron) is predominantly affected. While the reasons for the heightened susceptibility of motor neurons to familial ALS-causing mutations remain unknown, the high energetic demand of motor neurons is likely to be an important factor.
More recently it has been recognized that ALS is a non-cell autonomous disease in which glial cells, including astrocytes, microglia, and oligodendrocytes, play an important role in disease pathogenesis.48 Specifically, the role of oligodendrocytes in supporting axonal energy metabolism, raises the possibility that oligodendrocytes may play a hitherto underestimated role in ALS that is characterized by the deterioration of energy-hungry motor neurons. Some motor neurons can be significantly long (>3 feet) and are therefore particularly dependent on the metabolic support of oligodendrocytes.48 Indeed, there is a wealth of emerging evidence demonstrating oligodendroglial dysfunction in ALS, as reviewed in ref 49. Multiple recent studies have demonstrated the presence of inclusions that contain ALS-linked proteins within oligodendrocytes. For example, several studies observed the presence of TDP-43-containing oligodendroglial cytoplasmic inclusions in the motor cortex and spinal cord of ALS patients50-52 and TDP-43 mutant mice.53 Another study found FUS-containing oligodendroglial cytoplasmic inclusions in the CNS of ALS patients with mutations in the FUS gene, and the abundance of these inclusions correlated with disease onset.54 SOD1-containing cytoplasmic inclusions were also recently observed in cultured oligodendrocytes derived from sporadic or familial ALS patients with mutations in the SOD1 or TDP-43 genes.55 Another study detected mutant SOD1 via immuno-electron microscopy in the periaxonal oligodendroglial cytoplasm of SOD1-G93A mutant mice.56 Taken together, these studies indicate that the presence of oligodendroglial inclusions containing ALS-linked proteins is a common pathological feature of multiple sporadic and familial forms of ALS. This leads to the intriguing hypothesis that aberrant protein aggregates in ALS could physically disrupt motor driven transport and the free diffusion of metabolites (ie, lactate) from the oligodendroglial to the axonal compartment by “clogging” myelinic channels.
Progressive gray-matter demyelination and reactive changes in NG2+ oligodendrocyte progenitor cells (OPCs) were observed in the motor cortex and spinal cord of both sporadic and familial ALS patients as well as in SOD1-G93A mutant mice.57 Of particular interest is the fact that oligodendrocytes in the spinal cord of SOD1-G93A mutant mice start to degenerate before the first clinical symptoms of motor neuron degeneration are detectable.51,57 Presumably, in an attempt to compensate for this loss, OPCs exhibit increased proliferation and differentiation in the spinal cord of SOD1-G93A mutant mice, essentially replacing the lost oligodendrocytes such that there is no overall change in oligodendrocyte numbers.58,59 However, the newly formed oligodendrocytes fail to mature, resulting in progressive demyelination. Failure to mature is evidenced by decreased expression of proteins essential to oligodendrocyte function, including MBP and MCT1,9,51 as illustrated in Figure 1. Decreased MCT1 expression is thought to decrease the ability of oligodendrocytes to export lactate/pyruvate and would therefore limit their ability to provide metabolic support to the axons they ensheath. This is consistent with the fact that decreased MCT1 expression is only seen in affected ALS tissues including the spinal cord and motor cortex while unaffected brain regions show normal MCT1 levels. Indeed the fact that the newly generated oligodendrocytes in the SOD1-G93A mutant mouse spinal cord are often associated with degenerating axons,57 indicates that the expression of mutant SOD1-G93A could impair the ability of these oligodendrocytes to provide the necessary trophic support to axons.
Lastly, evidence pointing to a key role for oligodendrocytes in precipitating ALS disease onset, comes from a study which found that selective removal of mutant SOD1 from NG2+ OPCs via PDGFRα-creER driven recombination substantially delayed disease onset and prolonged survival in ALS mice.57 This was associated with a rescue in MCT1 expression at disease onset, indicating that oligodendroglial metabolic support of axons could play an important role at disease onset. Further evidence to support this comes from a recent study which showed that human-induced pluripotent stem cell-derived (iPSC-derived) oligodendrocytes obtained from both sporadic and familial (expressing either mutant SOD1 or mutant TDP43) ALS patients were significantly impaired in their ability to produce and release lactate into the extracellular environment, and this decreased motor neuron survival in co-culture.55 These studies indicate that in a human neurodegenerative disease oligodendroglial dysfunction likely accelerates disease progression by depriving motor neurons of essential metabolic support.
Multiple system atrophy
Multiple system atrophy (MSA) is a late-onset, progressive, neurodegenerative disease involving multiple brain regions and systems including motor impairment, behavioral alterations, and autonomic dysfunction.60 The majority of MSA cases are sporadic, although a loss-of-function mutation in the COQ2 gene which encodes the coenzyme Q10-synthesizing enzyme 4-hydroxybenzoate polyprenyltransferase has recently been linked to the disease in a Japanese cohort of patients.61 Alongside Parkinson disease and dementia with Lewy bodies, MSA is part of a large group of neurological disorders known as α-synucleinopathies that are characterized by the presence of α-synuclein protein aggregates.62 The postmortem diagnosis of MSA is based on the identification of α-synuclein-positive (oligodendro)glial cytoplasmic inclusions (GCIs), which are the distinctive pathological hallmark of the disease.63 The pathogenesis of MSA remains poorly understood, therefore replicating the disease in mouse models has been challenging.64 To date, the most widely used mouse models of MSA involve the expression of human α-synuclein from oligodendroglial promoters (PLP, CNP, or MBP), all of which replicate the α-synuclein-positive GCIs seen in MSA but fail to fully replicate the disease phenotype.64
Unlike Parkinson disease where α-synuclein inclusions are mostly seen in neurons, the presence of α-synuclein inclusions mostly in oligodendrocytes in MSA led to the classification of the disease as a primary oligodendrogliopathy.65 Although neuronal cytoplasmic inclusions (NCIs) containing α-synuclein are less frequently found in MSA, the fact that the distribution and severity of neurodegeneration reflects subregional GCI densities supports the view that oligodendrocytes play a central role in disease pathogenesis. Similar to Lewy bodies in Parkinson disease, α-synuclein in GCIs in MSA is also phosphorylated at residue Ser-129 and ubiquitinated.66-68 The gene for α-synuclein is not abundantly expressed in mature oligodendrocytes,33 but several studies have shown that α-synuclein oligomers can be released by neurons and then taken up by surrounding oligodendrocytes to form GCIs.68,69 Indeed, recent evidence suggests that misfolded α-synuclein may transcytose in a “prion-like” fashion to spread the disease throughout the nervous system. These studies also showed that MSA patient-derived brain biopsy homogenates containing α-synuclein, when injected into mice expressing human wild-type α-synuclein, triggered aggregation, and phosphorylation of the wild-type α-synuclein which initiated a neurodegenerative cascade.70,71 Another recent study found that oligodendroglial GCIs-derived α-synuclein was much more potent at inducing α-synuclein aggregation in injected mice when compared with neuronal Lewy body-derived α-synuclein.72 Furthermore, the same study also found that oligodendrocytes but not neurons transformed misfolded α-synuclein into a GCI-like strain, highlighting the fact that oligodendrocytes play a central role in disease pathogenesis.
Demyelination throughout the nervous system is frequently observed in MSA patients and is associated with a reduction in myelin lipids, including sphingomyelin, sulfatide, and galactosylceramide, all of which play an important role in myelin integrity and function.73 Furthermore, alterations in the subcellular localization of tubulin polymerization-promoting protein/p25α (TPPP/p25α) in MSA oligodendrocytes is significantly correlated with decreased MBP expression in degenerating tracts,74 as illustrated in Figure 1. TPPP/p25α is normally expressed in the nucleus, cytoplasm, and cellular processes of myelinating oligodendrocytes and functions in the stabilization of microtubules and the differentiation of oligodendrocytes.75,76 The relocation of TPPP/p25α from the nucleus and myelin sheath to the oligodendroglial cytoplasm followed by cytoplasmic accumulation of the protein prior to the formation of α-synuclein aggregates, is believed to be one of the earliest steps in the pathogenesis of MSA.77,78 Indeed, the interaction between TPPP/p25α, a potent stimulator of α-synuclein aggregation, and α-synuclein promotes its phosphorylation and aggregation into insoluble oligomers which later form GCIs.78-80 Although the cause of TPPP/p25α redistribution in MSA is unknown, one recent study found that TPPP/p25α co-localized with mitochondrial proteins in the oligodendroglial cytoplasm of both sporadic MSA patients and one familial MSA patient carrying mutations in the COQ2 gene.81 This indicates that mitochondrial dysfunction may lead to secondary TPPP/p25α relocation in both sporadic and familial forms of MSA.
The fact that neurons degenerate in MSA, a disease in which α-synuclein aggregates are primarily found in oligodendrocytes and not in neurons, suggests that α-synuclein aggregates in oligodendrocytes perturb the ability of these cells to provide trophic support to neurons. This is supported by a recent study which found decreased expression of glial-derived neurotrophic factor (GDNF) in transgenic mice expressing human α-synuclein under the control of an oligodendrocytic (MBP) promoter, but no change in GDNF levels were observed in transgenic mice expressing human α-synuclein under the control of neuronal (PDGFβ or mThyl) promoters.82 Consistent with this, the same study also found decreased GDNF expression in frontal cortex white matter and in the cerebellum of MSA patients. Furthermore, intracerebroventricular infusion of GDNF improved behavioral deficits and ameliorated neurodegenerative pathology in MBP-human-α-synuclein transgenic mice. Likewise, another study treated PLP-human-α-synuclein transgenic mice with rasagiline, a monoamine oxidase-B inhibitor that is reported to increase expression of GDNF83, and found that the drug ameliorated motor deficits and significantly reduced neuronal loss in the striatum, substantia nigra pars compacta, cerebellar cortex, pontine nuclei, and inferior olives.84 Taken together, these studies indicate that α-synuclein expression in oligodendrocytes may impact the trophic support provided by oligodendrocytes to neurons, thereby contributing to neurodegeneration in MSA.
Conclusion
The emerging role of oligodendrocytes as key players that provide tropic support to neuronal axons has paved the way for a better understanding of the contribution of dysfunctional axon-oligodendrocyte coupling in neurodegenerative diseases. Here we have reviewed the growing evidence demonstrating that oligodendrocyte dysfunction plays an important role in several neurodegenerative diseases, including Alzheimer disease, amyotrophic lateral sclerosis, and multiple system atrophy. Given that aging is the single greatest risk factor for all of these diseases, age-related myelin deterioration could be an underlying neuropathological mechanism leading to neuronal dysfunction. Novel therapeutic approaches aimed at mimicking or increasing trophic support, ideally by restoring axon-oligodendrocyte coupling could be beneficial in a wide range of neurodegenerative diseases.
Acknowledgments
The authors declare that they have no conflicts of interest. Our research is supported by grants of the DFG (CNMPB and SPP1757), the Adelson Foundation, and an ERC Advanced grant to KAN. CD is supported by a PhD fellowship of the Boehringer Ingelheim Fonds.
Selected abbreviations and acronyms
- AD
Alzheimer disease
- Aβ
amyloid-β
- APP
amyloid precursor protein
- ALS
amyotrophic lateral sclerosis
- CNP
2',3'-cyclic nucleotide phosphodiesterase
- GCIs
α-synuclein-positive glial cytoplasmic inclusions
- GDNF
glial-derived neurotrophic factor
- MCT1
monocarboxylate transporter 1
- MSA
multiple system atrophy
- MBP
myelin basic protein
- SOD1
superoxide dismutase 1
- TPPP/p25α
tubulin polymerization-promoting protein/p25α
Contributor Information
Alexandra I. Mot, Department of Neurogenetics, Max Planck Institute of Experimental Medicine, Gottingen, Germany.
Constanze Depp, Department of Neurogenetics, Max Planck Institute of Experimental Medicine, Gottingen, Germany.
Klaus-Armin Nave, Department of Neurogenetics, Max Planck Institute of Experimental Medicine, Gottingen, Germany.
REFERENCES
- 1.Gunning-Dixon FM., Brickman AM., Cheng JC., Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison BM., Lee Y., Rothstein JD. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 2013;23(12):644–651. doi: 10.1016/j.tcb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saab AS., Tzvetanova ID., Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol. 2013;23(6):1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Nave KA., Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths I., Klugmann M., Anderson T., et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 6.Kassmann CM., Lappe-Siefke C., Baes M., et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007;39(8):969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 7.Lappe-Siefke C., Goebbels S., Gravel M., et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 8.Funfschilling U., Supplie LM., Mahad D., et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y., Morrison BM., Li Y., et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M., Nave KA. Oligodendrocytes: myelination and axonal support. Cold Spring Harb Perspect Biol. 2015;8(1):a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harauz G., Ladizhansky V., Boggs JM. Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry. 2009;48(34):8094–8104. doi: 10.1021/bi901005f. [DOI] [PubMed] [Google Scholar]
- 12.Snaidero N., Velte C., Myllykoski M., et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017;18(2):314–323. doi: 10.1016/j.celrep.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saab AS., Tzvetavona ID., Trevisiol A., et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91(1):119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevisiol A., Saab AS., Winkler U., et al. Monitoring ATP dynamics in electrically active white matter tracts. Elife. 2017;6 doi: 10.7554/eLife.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer N., Richter N., Fan Z., et al. Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep. 2018;22(9):2383–2394. doi: 10.1016/j.celrep.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean DC. 3rd, Hurley SA., Kecskemeti SR., et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer Disease. JAMA Neurol. 2017;74(1):41–49. doi: 10.1001/jamaneurol.2016.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold BT., Johnson NF., Powell DK., Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822(3):416–422. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoy AR., Ly M., Carlsson CM., et al. Microstructural white matter alterations in preclinical Alzheimer's disease detected using free water elimination diffusion tensor imaging. PLoS One. 2017;12(3):e0173982. doi: 10.1371/journal.pone.0173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringman JM., O'Neill J., Geschwind D., et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130(Pt 7):1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 21.Braak H., Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996;92(2):197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- 22.Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31(8-9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie AT., Moyon S., Wang M., et al. Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer's disease. Mol Neurodegener. 2017;12(1):82. doi: 10.1186/s13024-017-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyfried NT., Dammer EB., Swarup V., et al. A multi-network approach identifies protein-specific co-expression in asymptomatic and symptomatic Alzheimer's Disease. Cell Syst. 2017;4(1):60–72 e64. doi: 10.1016/j.cels.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Rossi P., Buggia-Prevot V., Clayton BL., et al. Predominant expression of Alzheimer's disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol Neurodegener. 2016;11(1):59. doi: 10.1186/s13024-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrendt G., Baer K., Buffo A., et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61(2):273–286. doi: 10.1002/glia.22432. [DOI] [PubMed] [Google Scholar]
- 28.Desai MK., Sudol KL., Janelsins MC., Mastrangelo MA., Frazer ME., Bowers WJ. Triple-transgenic Alzheimer's disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitew S., Kirkcaldie MT., Halliday GM., Shepherd CE., Vickers JC., Dickson TC. Focal demyelination in Alzheimer's disease and transgenic mouse models. Acta Neuropathol. 2010;119(5):567–577. doi: 10.1007/s00401-010-0657-2. [DOI] [PubMed] [Google Scholar]
- 30.Roher AE., Weiss N., Kokjohn TA., et al. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41(37):11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- 31.Schmued LC., Raymick J., Paule MG., Dumas M., Sarkar S. Characterization of myelin pathology in the hippocampal complex of a transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2013;10(1):30–37. doi: 10.2174/1567205011310010005. [DOI] [PubMed] [Google Scholar]
- 32.Oakley H., Cole SL., Logan S., et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Chen K., Sloan SA., et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veeraraghavalu K., Zhang C., Zhang X., Tanzi RE., Sisodia SS. Age-dependent, non-cell-autonomous deposition of amyloid from synthesis of beta-amyloid by cells other than excitatory neurons. J Neurosci. 2014;34(10):3668–3673. doi: 10.1523/JNEUROSCI.5079-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito T., Matsuba Y., Mihira N., et al. Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci. 2014;17(5):661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- 36.Hagemeyer N., Goebbels S., Papiol S., et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4(6):528–539. doi: 10.1002/emmm.201200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velliquette RA., O'Connor T., Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25(47):10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokin GB., Lillo C., Falzone TL., et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307(5713):1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 39.Gowrishankar S., Wu Y., Ferguson SM. Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. J Cell Biol. 2017;216(10):3291–3305. doi: 10.1083/jcb.201612148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohamed NV., Plouffe V., Remillard-Labrosse G., Planel E., Leclerc N. Starvation and inhibition of lysosomal function increased tau secretion by primary cortical neurons. Sci Rep. 2014;4:5715. doi: 10.1038/srep05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Harg JM., Nolle A., Zwart R., et al. The unfolded protein response mediates reversible tau phosphorylation induced by metabolic stress. Cell Death Dis. 2014;5:e1393. doi: 10.1038/cddis.2014.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagisawa M., Planel E., Ishiguro K., Fujita SC. Starvation induces tau hyperphosphorylation in mouse brain: implications for Alzheimer's disease. FEBS Lett. 1999;461(3):329–333. doi: 10.1016/s0014-5793(99)01480-5. [DOI] [PubMed] [Google Scholar]
- 43.Chu TH., Cummins K., Sparling JS., et al. Axonal and myelinic pathology in 5xFAD Alzheimer's mouse spinal cord. PLoS One. 2017;12(11):e0188218. doi: 10.1371/journal.pone.0188218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwamoto N., Nishiyama E., Ohwada J., Arai H. Distribution of amyloid deposits in the cerebral white matter of the Alzheimer's disease brain: relationship to blood vessels. Acta Neuropathol. 1997;93(4):334–340. doi: 10.1007/s004010050624. [DOI] [PubMed] [Google Scholar]
- 45.Chia R., Chio A., Traynor BJ. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17(1):94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurney ME., Pu H., Chiu AY., et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 47.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(suppl 1):S3–9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 48.Philips T., Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Exp Neurol. 2014;262 Pt B:111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nonneman A., Robberecht W., Van Den Bosch L. The role of oligodendroglial dysfunction in amyotrophic lateral sclerosis. Neurodegener Dis Manag. 2014;4(3):223–239. doi: 10.2217/nmt.14.21. [DOI] [PubMed] [Google Scholar]
- 50.Murray ME., DeJesus-Hernandez M., Rutherford NJ., et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122(6):673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philips T., Bento-Abreu A., Nonneman A., et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136(Pt 2):471–482. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan CF., Eguchi H., Tagawa A., et al. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113(5):535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 53.Ditsworth D., Maldonado M., McAlonis-Downes M., et al. Mutant TDP-43 within motor neurons drives disease onset but not progression in amyotrophic lateral sclerosis. Acta Neuropathol. 2017;133(6):907–922. doi: 10.1007/s00401-017-1698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackenzie IR., Ansorge O., Strong M., et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011;122(1):87–98. doi: 10.1007/s00401-011-0838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferraiuolo L., Meyer K., Sherwood TW., et al. Oligodendrocytes contribute to motor neuron death in ALS via SOD1 -dependent mechanism. Proc Natl Acad Sci U S A. 2016;113(42):E6496–E6505. doi: 10.1073/pnas.1607496113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stieber A., Gonatas JO., Gonatas NK. Aggregates of mutant protein appear progressively in dendrites, in periaxonal processes of oligodendrocytes, and in neuronal and astrocytic perikarya of mice expressing the SOD1(G93A) mutation of familial amyotrophic lateral sclerosis. J Neurol Sci. 2000;177(2):114–123. doi: 10.1016/s0022-510x(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 57.Kang SH., Li Y., Fukaya M., et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16(5):571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang SH., Fukaya M., Yang JK., Rothstein JD., Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magnus T., Carmen J., Deleon J., et al. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56(2):200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- 60.Pfeiffer RF. Multiple system atrophy. Handb Clin Neurol. 2007;84:305–326. doi: 10.1016/S0072-9752(07)84046-2. [DOI] [PubMed] [Google Scholar]
- 61.Multiple-System Atrophy Research C Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 62.Fellner L., Jellinger KA., Wenning GK., Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol. 2011 ;121(6):675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefanova N., Bucke P., Duerr S., Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;S(12):1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 64.Overk C., Rockenstein E., Valera E., Stefanova N., Wenning G., Masliah E. Multiple system atrophy: experimental models and reality. Acta Neuropathol. 2018;135(1):33–47. doi: 10.1007/s00401-017-1772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jellinger KA. Multiple System Atrophy: An oligodendroglioneural synucleinopathy1. J Alzheimers Dis. 2018;62(3):1141–1179. doi: 10.3233/JAD-170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujiwara H., Hasegawa M., Dohmae N., et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 67.Hasegawa M., Fujiwara H., Nonaka T., et al. Phosphorylated alphasynuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 68.Rey NL., George S., Brundin P. Review: Spreading the word: precise animal models and validated methods are vital when evaluating prion-like behaviour of alpha-synuclein. Neuropathol Appl Neurobiol. 2016;42(1):51–76. doi: 10.1111/nan.12299. [DOI] [PubMed] [Google Scholar]
- 69.Reyes JF., Rey NL., Bousset L., Melki R., Brundin P., Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62(3):387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- 70.Bernis ME., Babila JT., Breid S., Wusten KA., Wullner U., Tamguney G. Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun. 2015;3:75. doi: 10.1186/s40478-015-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prusiner SB., Woerman AL, Mordes DA., et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112(38):E5308–5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng C., Gathagan RJ., Covell DJ., et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature. 2018. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Don AS., Hsiao JH., Bleasel JM., Couttas TA., Halliday GM., Kim WS. Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathol Commun. 2014;2:150. doi: 10.1186/s40478-014-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rohan Z., Milenkovic I., Lutz MI., Matej R., Kovacs GG. Shared and Distinct Patterns of Oligodendroglial Response in alpha-Synucleinopathies and Tauopathies. J Neuropathol Exp Neurol. 2016;75(12):1100–1109. doi: 10.1093/jnen/nlw087. [DOI] [PubMed] [Google Scholar]
- 75.Lehotzky A., Lau P., Tokesi N., Muja N., Hudson LD., Ovadi J. Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia. 2010;58(2):157–168. doi: 10.1002/glia.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vincze O., Tokesi N., Olah J., et al. Tubulin polymerization promoting proteins (TPPPs): members of a new family with distinct structures and functions. Biochemistry. 2006;45(46):13818–13826. doi: 10.1021/bi061305e. [DOI] [PubMed] [Google Scholar]
- 77.Kovacs GG., Laszlo L., Kovacs J., et al. Natively unfolded tubulin polymerization promoting protein TPPP/p25 is a common marker of alphasynucleinopathies. Neurobiol Dis. 2004;17(2):155–162. doi: 10.1016/j.nbd.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Song YJ., Lundvig DM., Huang Y., et al. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171(4):1291–1303. doi: 10.2353/ajpath.2007.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hlavanda E., Kovacs J., Olah J., Orosz F., Medzihradszky KF., Ovadi J. Brain-specific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry. 2002;41(27):8657–8664. doi: 10.1021/bi020140g. [DOI] [PubMed] [Google Scholar]
- 80.Lindersson E., Lundvig D., Petersen C., et al. p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies. J Biol Chem. 2005;280(7):5703–5715. doi: 10.1074/jbc.M410409200. [DOI] [PubMed] [Google Scholar]
- 81.Ota K., Obayashi M., Ozaki K., et al. Relocation of p25alpha/tubulin polymerization promoting protein from the nucleus to the perinuclear cytoplasm in the oligodendroglia of sporadic and COQ2 mutant multiple system atrophy. Acta Neuropathol Commun. 2014;2:136. doi: 10.1186/s40478-014-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ubhi K., Rockenstein E., Mante M., et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30(18):6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weinreb O., Amit T., Bar-Am O., Youdim MB. Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann N Y Acad Sci. 2007;1122:155–168. doi: 10.1196/annals.1403.011. [DOI] [PubMed] [Google Scholar]
- 84.Stefanova N., Poewe W., Wenning GK. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210(2):421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]