Abstract
Inflammatory cytokines are necessary for an acute response to injury and the progressive healing process. However, when this acute response does not resolve and becomes chronic, the same proteins that once promoted healing then contribute to chronic inflammatory pathologies, such as atherosclerosis. Osteopontin (OPN) is a secreted matricellular cytokine that signals through integrin and CD44 receptors, is highly upregulated in acute and chronic inflammatory settings, and has been implicated in physiologic and pathophysiologic processes. Evidence from the literature suggests that OPN may fit within the “Goldilocks” paradigm with respect to cardiovascular disease, where acute increases are protective, attenuate vascular calcification, and promote post-ischemic neovascularization. In contrast, chronic increases in OPN are clinically associated with an increased risk for a major adverse cardiovascular event and OPN expression is a strong predictor of cardiovascular disease independent of traditional risk factors. With the recent finding that humans express multiple OPN isoforms as the result of alternative splicing and that these isoforms have distinct biologic functions, future studies are required to determine what OPN isoform(s) are expressed in the setting of vascular disease and what role each of these isoforms plays in vascular disease progression. This review aims to discuss our current understanding of the role(s) of OPN in vascular disease pathologies using evidence from in vitro, animal and clinical studies. Where possible, we discuss what is known about OPN isoform expression and our understanding of OPN isoform contributions to cardiovascular disease pathologies.
Keywords: Vascular Disease, Extracellular Matrix, Inflammation, Osteopontin, OPNa, OPNb, OPNc, Peripheral Vascular Disease, Atherosclerosis, Biomarkers, Inflammation
1. Introduction
Osteopontin (OPN) is a secreted multifunctional glyco-phosphoprotein that plays important roles in physiological and pathophysiological processes. As the name implies, OPN is produced by cells involved in bone morphogenesis and one major physiological role of OPN is in the control of biomineralization and calcification.1 In this brief review, we aim to examine OPN functions specifically in the vasculature and additional physiological functions of OPN have been reviewed previously. Under physiologic conditions, circulating and tissue OPN expression levels in the vasculature are quite low, but are important for normal arterial physiology.2 Indeed, these low OPN expression levels were shown to be necessary for normal arterial mechanics and it is also know that OPN acts as a physiological inhibitor of vascular calcification. In response to injury, OPN is acutely upregulated and promotes cell adhesion, proliferation, migration and survival to aid in the healing process, but expression typically resolves over time.3, 4 Vascular cell types that upregulate and secrete OPN include endothelial cells, vascular smooth muscle cells (VSMCs), and macrophages.3, 5 In contrast, OPN expression levels remain elevated in several disease pathologies with a chronic inflammatory component including Crohn’s disease, multiple sclerosis and other autoimmune disorders, wound healing, various cancer types and cardiovascular disease (CVD) pathologies.
More recently, there has been considerable interest in OPN as a biomarker for various pathological conditions. Peripheral blood and cerebrospinal fluid concentrations of OPN are elevated in multiple sclerosis patients6 and neurodegenerative diseases like Alzheimer’s.7 There is also interest in OPN as a prognostic and diagnostic marker for diseases including, but not limited to, multiple sclerosis,6 coronary artery disease,8 and several cancer types9 with a recent specific focus on individual OPN isoforms for this purpose.10, 11 Increased OPN expression is also a strong predictor of outcomes in patients with calcific aortic valve disease12, 13 and ischemic vascular pathologies including stroke,14, 15 myocardial infarction,16–18 and peripheral artery disease.19, 20 While interest in OPN as a potential biomarker increases, there is a large body of evidence that clearly establishes that OPN is specific driver of cellular functions that impact physiologic and pathophysiologic processes in the vascular setting, as discussed in more detail below, and ultimately point to OPN as a potential therapeutic target.21 In this review, we will examine our current understanding of OPN protein structure and function, regulation of OPN expression, and role(s) of OPN in vascular disease pathologies while introducing what is currently understood about OPN isoform contributions.
2. Osteopontin Structure and Function
Osteopontin was first described in 1985 by Franzen, et al. as one of two sialoproteins derived from bovine bone matrix.22 Osteopontin was previously identified as secreted phosphoprotein 1 (SPP1), bone sialoprotein 1 (BSP-1), and early T-lymphocyte activation-1 (Eta-1). This plurality of names reflects the range of functions attributable to OPN. As a matricellular protein, OPN differs from the structural extracellular matrix proteins, such as collagen, in that it does not serve a primary structural role.23 Matricellular proteins do, however, function as modulators of cell-matrix interactions, often achieved by binding to cell-surface receptors, growth factors, proteases, and structural matrix proteins, making them important components of the extracellular matrix environment.24 Indeed, matricellular proteins like OPN are often induced during tissue remodeling and repair, as well as in disease states.23 The original members of the matricellular protein family included secreted protein acidic and rich in cysteine (SPARC), thrombospondin 1 (TSP-1), and tenascin-C and has expanded to include CCN proteins (Cyr61, CCN2, CCN3) and OPN.23
Osteopontin Receptor Binding Domains
OPN contains several functional domains that allow for receptor binding to promote various biological functions and include: 1) Arg-Gly-Asp (RGD) binding domain that allows interaction with integrin receptors including: αvβ1, αvβ3, αvβ5, αvβ6, α5β1,25 2) SVVYGLR domain that interacts with α9β1, α4β1, and α4β, 3) ELVTDFTDLPAT domain reported to bind to α4β1,26 4) calcium binding domain (aa 216–228) and 5) heparin binding domain. Furthermore, OPN has been shown to interact with several splice variants of the hyaluronic acid receptor, known as CD44, via the C-terminal calcium binding domain including: CD44v3 and CD44v6–7. Katagiri et al. suggested that multiple CD44 binding domains are present in the N- and C-terminal regions of OPN.27 This same study proposed a potential complex between CD44 variants and integrin receptors, since the binding of CD44 variants to OPN was inhibited by anti-β1 antibodies.27 OPN has primarily been described as a secreted protein; however, an intracellular form of OPN, made possible by a non-AUG alternative start codon that omits the N-terminal secretion peptide,28 has been reported in rodents and localizes to the cell membrane where it binds to CD44 to regulate cell migration.29, 30
Post-translational Modifications of Osteopontin
OPN is a complex protein that is aspartic-acid rich, contains long stretches of negatively charged sequences that bind calcium, and can be cleaved by thrombin and matrix metalloproteinases (MMPs). OPN is subjected to numerous post-translational modifications (PTMs) including serine/threonine phosphorylation, glycosylation, and tyrosine sulfation, which increase the monomeric molecular weight from the predicted ~35 kDa to 41–75 kDa.31 A polymeric form of OPN with a mass of >200 kDa can also be generated upon protein transglutamination.32 Importantly, it has been shown that many of these PTMs regulate and alter OPN function.
Phosphorylation
The OPN sequence contains 36 predicted phosphorylation sites that include serine, threonine and tyrosine residues.33 The serine/threonine phosphorylation of OPN has been primarily attributed to a golgi apparatus casein kinase over casein kinases-1 and −2,34 identified recently as FAM20C, which phosphorylates numerous secreted proteins with S-x-E motifs.33, 35 The distinct functional properties of OPN are dependent, in part, on the nature and extent of phosphorylation. Interestingly, Christensen et al. demonstrated that the degree of OPN phosphorylation can be cell-type specific, where they showed a osteoblast cell line (MC3T3-E1) added 21 phosphates, while only ~4 phosphates were added by a ras-transformed fibroblast cell line (275–3-2).36 This same study demonstrated that fibroblast-derived OPN (low phosphorylation) mediated greater adherence of human breast cancer cells than the highly phosphorylated osteoblast-derived OPN.36 Therefore, OPN generated by different cell types and, thus, differentially phosphorylated may exhibit different biologic effects. Indeed, phosphorylated OPN can inhibit calcification in cultured human VSMCs, which was not observed with recombinant bacterial OPN that lacks PTMs.37 Additionally, OPN phosphorylation is necessary for interleukin-12 expression in macrophages, while dephosphorylation abolishes this effect.38 The differences in PTMs made by vascular wall cell types, such as endothelial cells and VSMCs, remains to be established. Furthermore, there is a lack of understanding with respect to how OPN phosphorylation patterns change in physiologic vs. pathophysiologic settings and the few studies that have explored this in the setting of CVD are discussed below.
Glycosylation
Exon 6 of human OPN contains five O-glycosylation sites.31, 33 Glycosylation influences the folding structure, proteolytic cleavage and, subsequently, the functional properties of OPN.39 Indeed, deletion of multiple O-glycosylation sites in OPN affects cell adhesion activity and phosphorylation state.40,41 Similarly, lung cancer cells that stably expressed an OPN mutant lacking three O-glycosylation sites exhibit a reduction in cell growth and migration.42 To our knowledge, N-glycosylation has so far only been reported in rat and human bone OPN43, 44 and no apparent N-linked oligosaccharides were found in human milk OPN, suggesting that this form of glycosylation may be tissue specific.33
Transglutamination
Osteopontin can serve as a substrate for transglutaminase 2 (TG2).45 TG2 is a ubiquitously expressed calcium-dependent enzyme that catalyzes cross-linking of glutamine and lysine residues.45, 46 TG2 cross-linking of OPN can be both inter- and intra-molecular.47 TG2-mediated OPN polymerization alters both conformation and function, which is attributed to the exposure a new integrin binding site that allows polymeric OPN to bind the α9β1 receptor independent of the SVVYGLR sequence.48 Other in vitro studies show that polymeric OPN displays increased collagen type I binding affinity49 and promotes enhanced cell adhesion and migration compared to monomeric OPN.45, 50 The significance of OPN polymerization in vivo was highlighted by Nishimichi et al., who demonstrated OPN polymerization is required for neutrophil recruitment.32 Originally, two TG2 reactive glutamine (Gln) residues were described in bovine exon 4, Gln34 and Gln36,51 which correspond to Gln50 and Gln52 in OPNa when designating the initial methionine (Met) residue as aa 1*; however, OPN was recently reported to have 12 TG2 reactive residues, with Gln34, Gln42, Gln193 and Gln248 (corresponding to Gln50, Gln58, Gln209, and Gln264 in OPNa*) exhibiting the highest reactivity.52 This could explain why polymerization of OPN-c, a splice variant lacking exon 4 and two glutamine residues, could still be observed with high TG2 concentrations.32
Cleavage
In addition to PTMs, OPN can also undergo proteolytic cleavage by thrombin and MMPs. A conserved thrombin cleavage site at Arg168-Ser169 can be found seven amino acids downstream of the RGD integrin receptor binding site. OPN function is altered when cleaved by thrombin, which generates N-terminal and C-terminal fragments. The cryptic 162SVVYGLR168 motif, just c-terminal to the RGD site, also allows OPN to interact integrin receptors, including: α4β1, α9β1 and α4β7 integrin receptors.53 Recently, it was shown that phosphorylation of the C-terminal fragment could inhibit OPN binding to αvβ3.54 This could potentially explain the conflicting findings by various studies suggesting that thrombin cleavage was not a prerequisite for adhesion to αvβ3, αvβ5 and αvβ6 as they could bind equally well to full-length OPN or to the RGD sequence at its N-terminal end. 25 Several members of the MMP family have also been reported to cleave OPN at various sites. These include MMP-2, MMP-3, MMP-7, MMP-9 and MMP-12. Interestingly, cleavage of OPN by MMP-3 and MMP-7 within the SVVGLR motif sequence interferes with α4β1 and α9β1 binding.25, 55 Evidence points to OPN fragments generated by thrombin and MMP cleavage having important pathophysiological functions. For instance, the N-terminal OPN fragment is associated greater degrees of inflammation in carotid plaques in patients with hypertension (HTN).56 Additionally, the thrombin-cleaved N–terminal fragment with SVVYGLR motif promotes synthesis of collagen type III in cardiac fibrosis.57 And finally, several in vitro studies have shown that the N-terminal fragment of OPN, rather than full length OPN, promotes adhesion due to a conformational change that increases its binding activity.1
Osteopontin Isoforms
Humans express five OPN isoforms due to alternative splicing of a single SPP1 mRNA transcript to generate: 1) full length OPN, known as OPNa (NP_001035147.1), 2) OPNb, which lacks exon 5 (NP_000573.1), 3) OPNc, which lacks exon 4 (NP_001035149.1), 4) OPN4, which lacks exons 4 and 5 (NP_001238758.1), and 5) OPN5, which contains an extra exon due to the retention of a portion of intron 3 (NP_001238759.1). Whether OPN4 and OPN5 are translated to protein remains to be determined; therefore, in this review we focus on OPNa, OPNb and OPNc. OPN splice variants were first described in glioma cells58, are differentially expressed, and display isoform-specific biologic functions. The RGD and SVVYGLR integrin receptor binding domains and the CD44 binding domain are conserved across all three isoforms. Despite intact receptor binding domains, OPN isoforms clearly exhibit different intrinsic biological functions.59, 60 Isoforms differ in that OPNb lacks three serine/threonine residues and one glutamine residue due to deletion of exon 5, whereas OPNc lacks two tyrosine, two serine/threonine, and three glutamine residues due to deletion of exon 4. Whether these missing exons lend to changes in protein folding, as suggested by predictive modeling,61 and/or receptor binding remains to be investigated and additional studies are required to define the molecular mechanisms underlying OPN isoform-specific biological effects. With a growing interest in the roles OPN isoforms may play in cardiovascular physiology and pathophysiology, we will discuss what is established thus far in this review.
3. Osteopontin in Vascular Physiology and Pathophysiology
Osteopontin in Acute and Chronic Ischemia
Inflammation is a central component of numerous diseases and can promote tissue damage and inhibit healing if unresolved; however, it is also well understood that immune suppression can limit successful tissue regeneration and recovery of homeostasis. Osteopontin is a secreted protein that is highly upregulated in settings of both acute and chronic ischemia and functions, in part, as an inflammatory cytokine that can promote recruitment of multiple inflammatory cell types and, thus, modulate the inflammatory response. In this section, we will discuss OPN and the “Goldilocks” principle using ischemia-mediated neovascularization as a model, since OPN expression is upregulated in response to ischemia in stroke,14, 15 myocardial infarction,16, 17 and peripheral artery disease.19, 20
OPN expression is often upregulated 20–50 fold in response to ischemic insult, but typically resolves over time in murine models of stroke,62 myocardial infarction,63 and hindlimb ischemia.3, 64 Studies have established that OPN is a clear driver of the immune response in ischemic conditions, demonstrating that OPN is necessary for macrophage infiltration in vivo.59, 65 Indeed, OPN is required for post-ischemic neovascularization3, 65 and ischemia-induced OPN expression is reactive oxygen species (ROS)-dependent.3 More recently, it was established that OPN isoforms have differential effects on macrophage migration and accumulation and arteriogenesis in vivo, where OPNc was the most potent mediator of these processes.59 With respect to macrophage function, phagocytosis is diminished in OPN−/− macrophages, but can be rescued by recombinant OPN.66 Pharmacological inhibition or genetic ablation of OPN has also been found to greatly impair macrophage infiltration in various models of acute inflammation.67 Furthermore, OPN induces macrophage migration via interaction of C-terminal fragment with CD44 surface receptors68 and more recently, via SLAYGLR domain (SVVYGRL in human OPN) with α4 and α9-integrin receptors.69 Effects of OPN on macrophage polarization remain controversial. While one group reported OPN knockdown polarizes macrophages toward an M2c subtype, recently accepted as a pro-regenerative phenotype,70 another study using OPN−/− macrophages showed no effects on polarization.71 Similarly, we did not observe differences in OPN−/− macrophage polarization, nor did macrophage polarization shift in response to stimulation with purified recombinant human OPN isoforms, suggesting OPN isoforms do not differentially effect polarization of macrophages.59 One recent study reports that CD206+ macrophages strongly express OPN and suggests that specific macrophage subtypes involved in tissue repair may differentially express OPN,63 which requires further investigation. Another point that requires further investigation is whether macrophages at different points within the polarization spectrum respond differently to OPN.
Many of the stimuli known to promote OPN expression including ROS, angiotensin II (Ang II), high glucose and low oxygen tension also contribute to chronic vascular inflammation that, when unresolved, promote long-term, chronic expression of OPN. Recently, oxidized low-density lipoprotein was also shown to promote proliferation and migration of human coronary artery SMCs via upregulation of OPN and MMP-9.72 Additionally, hypoxia and hyperglycemia synergistically increase OPN expression in VSMCs.73 This is in line with clinical findings that show that OPN is highly and chronically upregulated in patients with peripheral artery disease and in patients with type 1 and type 2 diabetes mellitus and several studies establish OPN as a clear predictor of cardiovascular outcomes in these patient populations.74–77 Interestingly, a recent study demonstrated that high glucose-mediated OPN expression is mediated, in part, through changes in histone acetylation and methylation regulated by histone deacetylase and histone methyltransferase, respectively, which was reversed by histone methyltransferase inhibition.78 This suggests that high glucose-mediated OPN expression is regulated in some cells through epigenetic mechanisms; however, further studies are required to determine if this is true in vascular cells. Collectively, these findings point towards OPN as an integral part of the first line immune response to tissue injury and the tissue remodeling processes required for healing. However, in disease states in which the inflammatory process fails to resolve and becomes chronic, OPN may be detrimental, as discussed further below.
Osteopontin in Atherosclerosis and Neo-intimal Hyperplasia
Atherosclerosis is characterized by a persistent inflammatory response in the vascular wall in response to noxious stimuli such as hypoxia, endothelial injury and hyperglycemia. The role of OPN in atherosclerotic plaque progression has been shown in human and murine vascular diseases and was reviewed in detail by Wolak.79 High concentrations of OPN are observed in human atherosclerotic plaques in the aorta, carotid and coronary arteries, and are primarily expressed in endothelial cells, macrophages and VSMCs.5, 80 In a high fat diet-induced mouse model of atherosclerosis, OPN overexpression significantly increased fatty-streak and mononuclear cell rich lesion formation, as well as decreased levels of interleukin-10, an anti-inflammatory atheroprotective cytokine.81 Likewise, Matsui et al. reported that OPN−/− mice had significantly smaller atherosclerotic lesions, but this was only true in female OPN−/− mice and the authors predominantly attributed this to higher triglyceride and total cholesterol levels in male OPN−/− mice.82 Worth noting, estrogen has been shown to induce OPN expression83 while testosterone was shown to suppress it.84 However, there are conflicting reports in the literature of sex differences in OPN expression, which may be disease and/or context specific and this requires further investigation.85–87 In an Ang II-accelerated model of atherosclerosis and abdominal aortic aneurysm (AAA) formation, Bruemmer et al. showed that a partial or complete lack of OPN protects against atherosclerosis, partially due to reduced inflammatory macrophage accumulation and viability in atherosclerotic lesions.88 When Zheng et al. used a microarray to compare gene expression profiles in normal aortas and tissues from abdominal aortic aneurysm patients, they found that OPN mRNA is upregulated as much as 125 fold.89 In this same study, the authors demonstrate that stimulation with high doses of OPN (200 – 500 ng/mL) promotes increased autophagy,89 whereas lower doses of OPN have pro-survival effects59, 69, 90; however, the source of OPN used in these studies was not disclosed, suggesting that further studies are required to determine if cellular source, dose, PTMs, and/or isoforms are important for the survival vs. autophagy effects of OPN. In clinical studies, increased plasma OPN expression levels are associated with the presence and severity of coronary artery disease.91, 92 Interestingly, coronary revascularization and Ang II receptor blockers reduce plasma OPN levels,93–95 which is in line with in vitro studies that demonstrate that Ang II induces OPN expression.96, 97 Several clinical studies have also independently demonstrated that the use of plasma OPN expression levels alone can predict CVD events and all-cause mortality commensurate with other atherosclerosis prognostic markers including lipid profile and hsCRP in patients with HTN, type 1 diabetes, and type 2 diabetes.8, 98, 99
In addition to atherogenesis, OPN also contributes to the development of neo-intimal hyperplasia following vessel revascularization. Clinical studies have shown that higher baseline OPN levels are associated with rapid coronary plaque progress and in stent restenosis.100 OPN mRNA and protein were highly expressed during neo-intimal formation in mouse and human carotid vessels.5, 101 In a rat carotid artery injury model, blockade of OPN before endothelial denudation by balloon catheter decreases neo-intimal thickening of the artery.102 Similarly, a study by Isoda et al. demonstrated increased neo-intimal thickening after femoral artery cuffing in OPN transgenic mice.103 More recently, OPN was found to be significantly upregulated over the first postoperative week in the porcine venous wall after saphenous-vein artery interposition graft.104 OPN expression also correlated well with the number of PCNA-positive cells and MMP expression, suggesting OPN is a key regulator of VSMC proliferation and migration.104 It is well-established that mature, differentiated VSMCs can dedifferentiate into a more proliferative, synthetic phenotype during vascular remodeling and OPN appears to downregulate two differentiation markers, α-SM actin and calponin in VSMCs. However, neither SM22-α nor tropomyosin marker expression was altered with overexpression of OPN in vitro hence, further studies will be required to understand the mechanism by which these genes are regulated by OPN.105
Osteopontin in Vascular Calcification
Vascular calcification was once considered an end-stage, degenerative process of aging. However, calcification is now recognized as an active, tightly-regulated biomineralization process that may be treatable.106 One proposed major mechanism that drives vascular calcification is the loss of mineral inhibiting factors, such as matrix G1a (MGP) and osteoprotegerin.107 OPN is also an important inhibitor of mineral deposition in the vascular wall and in cardiac valves. In vitro, OPN−/− VSMCs calcify significantly more than wild-type VSMCs in the presence of elevated phosphate.108 Furthermore, the aortas of MGP−/−OPN−/− mice exhibit 2 and 3 fold more calcification at 2 and 4 weeks, respectively, compared to mice only lacking MGP.109 Several in vivo subcutaneous implantation models using OPN−/− mice have demonstrated an inhibitory role for OPN in vascular calcification. A study by Steitz et al. clearly demonstrated that aortic valve leaflets subcutaneously implanted in OPN−/− mice showed accelerated calcification (4 – 5 fold greater) compared to wild-type mice and, conversely, that increased OPN and carbonic anhydrase II accumulation correlates with calcification regression.110 These data were corroborated by another study that showed increased calcification of subcutaneously implanted bovine pericardium tissue in OPN−/− mice, which was mitigated by the administration of histidine-fused OPN at the implant site or adsorption of the OPN onto the implant materials.111 Interestingly, calcification was only reversed by OPN containing a functional RGD-motif and that was adequately phosphorylated.111 A separate, independent study showed that only phosphorylated OPN can inhibit calcification in cultured human VSMCs.37 More recently, OPN levels were shown to be elevated in asymptomatic calcific aortic valve disease patients;12 however, a separate study from the same group showed this circulating OPN is de-phosphorylated.13 Importantly, OPN isoforms were differentially expressed during calcific aortic valve disease progression and functioned to inhibit bio-mineralization, but only when phosphorylated.12 Altogether, these data support that OPN inhibits calcification and promotes dissolution, however, OPN phosphorylation is critical for these effects.
Osteopontin in Hypertension
The vessel wall undergoes remodeling in response to elevated pressure and pulsatile flow. OPN expression is elevated in HTN,98 which is mediated, in part, by increased aortic strain and ROS production.112 Indeed, overexpression of catalase in VSMC in vivo reduced mechanical strain mediated OPN expression in hypertensive animals.112, 113 One of the mechanism by which mechanical strain increases OPN expression in VSMCs is through activation of the phosphatidylinositol-3 kinase/Akt1 signaling pathway.113 Ang II, a peptide hormone that causes vasoconstriction and increases blood pressure, promotes HTN and has been shown to upregulate OPN expression,114 in part, via Ang II-mediated increases in ROS production.97, 112, 115 Vessel remodeling in HTN involves inflammatory cell infiltration, as well as MMP-mediated degradation and reorganization of the extracellular matrix.116 OPN has been linked to HTN-related vascular remodeling and inflammatory cell recruitment. Indeed, early macrophage infiltration into the vascular wall in response to HTN is blunted in OPN−/− mice compared to wild-type animals.112 Finally, OPN expression levels are significantly higher in plasma and aortic tissues in hypertensive rodents and expression positively correlates with systolic blood pressure,113 suggesting that OPN could be used as a clinical marker for HTN-induced vascular remodeling. One such study showed that treatment with Ang II blocker and statins significantly reduces plasma OPN level.94
Clinical Implications and Conclusions
Inflammation is a central component of numerous diseases and the discovery of inflammatory biomarkers highly predictive of CVD has the potential to improve targeted treatment strategies. Biomarkers are also of great interest because they can be utilized for diagnostic, prognostic and/or therapeutic purposes in the clinical setting.117, 118 As a secreted protein, OPN is of particular interest as a biomarker because of its detectability in body fluids that include plasma, urine, breast milk, and cerebrospinal fluid; thus, OPN is measurable by minimally invasive means and this allows for rapid repeated measures over time. Recent clinical studies have demonstrated that OPN expression levels are a strong predictor of CVD events and mortality in several patient populations and may prove to be a useful prognostic for disease activity and severity.8, 74, 76, 77, 92, 100 The recent finding that humans express multiple isoforms that have different functional effects has added a new layer of complexity to this protein and much remains to be discovered regarding the function of the individual OPN isoforms in vascular physiology and pathophysiology, what mediates OPN splicing, and if specific isoforms are possible therapeutic targets. Indeed, it was shown recently that OPN isoforms are differentially upregulated in patients with end-stage heart failure, where OPNa was significantly upregulated in patients with dilated cardiomyopathy, while expression of OPNb and OPNc were only detected in patients with ischemic cardiomyopathy.119 Therefore, additional studies are required to determine if individual OPN isoforms are better diagnostic and/or prognostic biomarkers of CVD severity than total OPN levels.
Substantial progress has been made recently toward our understanding of the biological functions of OPN in several vascular disease pathologies. The cellular sources of OPN have been identified, which has led to the discovery of many of its important cell- and tissue-type specific functions. Evidence presented in this review suggest that the role of OPN in vascular disease may follow the Goldilocks principle, with too little OPN impeding the tissue injury and wound healing responses, while too much OPN leads to deleterious vascular remodeling. We also require a better understanding of how specific vascular cell types differentially post-translationally modify OPN and if and how these modifications vary with disease state. Additional work is also necessary to determine the underlying molecular mechanisms of OPN isoform-specific biologic functions. The future development of “humanized” transgenic animals and isoform specific tools should greatly facilitate this work and will further our understanding of the physiologic and pathophysiologic roles of these newly defined OPN splice variants.
Supplementary Material
Figure 1. OPN isoform primary domain structure.
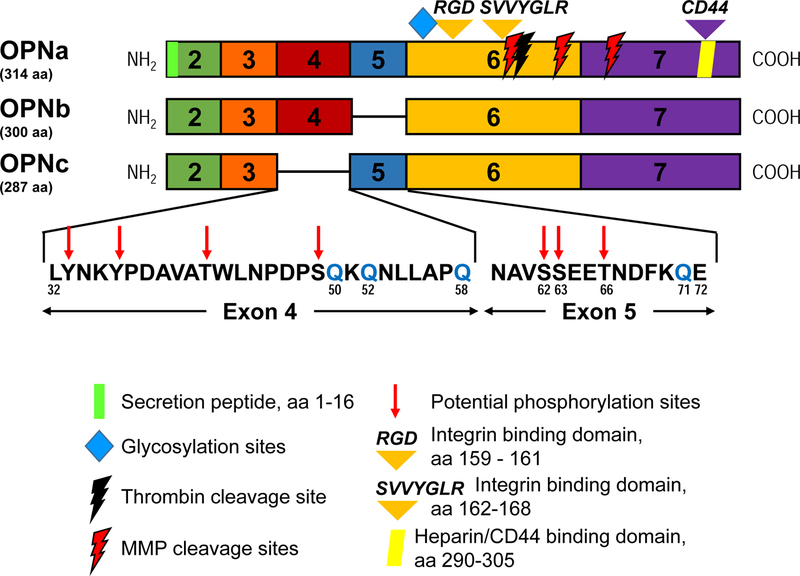
Each block corresponds to an exon (numbered). OPNa is full length (top; 314 aa), OPNb lacks exon 5 (middle; 300 aa), and OPNc lacks exon 4 (bottom; 287 aa). Expanded amino acid sequences of exons 4 and 5, absent in OPNc and OPNb, respectively, are included and glutamine residues (Q) that are potential sites for transglutamination are indicated in blue. Also indicated are OPN structural features and cleavage sites present within each exon. Corresponding amino acid numbers listed are for the OPNa isoform (with Met as aa 1).
Highlights.
Osteopontin (OPN) is a strong predictor of outcomes in patients with calcific aortic valve disease and ischemic vascular pathologies including stroke, myocardial infarction, and peripheral artery disease.
Evidence clearly establishes that OPN is driver of cellular functions that impact physiologic and pathophysiologic processes in the vascular setting including cell survival, adhesion, migration and proliferation.
Humans express multiple OPN isoforms, which have distinct biologic functions, and further investigation is required to determine what OPN isoform(s) are expressed in the setting of vascular disease and what role each isoform plays in vascular disease progression.
Acknowledgments
Sources of Funding
Supported by National Institutes of Health K99/R00 HL119567 (ANL), a Georgia Clinical and Translational Science Alliance (Georgia CTSA) pilot award through the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 (ANL), and funds from Emory University’s Department of Medicine and Division of Cardiology (to ANL).
Non-Standard Abbreviations and Acronyms
- Ang II
Angiotensin II
- CVD
Cardiovascular Disease
- HTN
Hypertension
- MMP
Matrix Metalloproteinases
- OPN
Osteopontin
- PTM
Post-translational Modification
- RGD
Arginine-Glycine-Aspartate
- ROS
Reactive Oxygen Species
- TG2
Transglutaminase 2
- VSMC
Vascular Smooth Muscle Cells
Footnotes
Note: Amino acid numbers identified in this review are identified using the initial methionine (Met) residue as aa 1 and, therefore, differ from some publications in which the first residue after the 16 aa signal peptide is cleaved off, aa 17 Isoleucine, as aa 1.
Disclosures
None.
REFERENCES
- 1.Scatena M, Liaw L and Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arteriosclerosis, thrombosis, and vascular biology 2007;27:2302–9. [DOI] [PubMed] [Google Scholar]
- 2.Myers DL, Harmon KJ, Lindner V and Liaw L. Alterations of arterial physiology in osteopontin-null mice. Arterioscler Thromb Vasc Biol 2003;23:1021–8. [DOI] [PubMed] [Google Scholar]
- 3.Lyle AN, Joseph G, Fan AE, Weiss D, Landazuri N and Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol 2012;32:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Louden C, Yue TL, Ellison JA, Barone FC, Solleveld HA and Feuerstein GZ. Delayed expression of osteopontin after focal stroke in the rat. J Neurosci 1998;18:2075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM and Giachelli CM. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb 1994;14:1648–56. [DOI] [PubMed] [Google Scholar]
- 6.Agah E, Zardoui A, Saghazadeh A, Ahmadi M, Tafakhori A and Rezaei N. Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: A systematic review and meta-analysis. PLoS One 2018;13:e0190252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heywood WE, Galimberti D, Bliss E, Sirka E, Paterson RW, Magdalinou NK, Carecchio M, Reid E, Heslegrave A, Fenoglio C, Scarpini E, Schott JM, Fox NC, Hardy J, Bhatia K, Heales S, Sebire NJ, Zetterberg H and Mills K. Identification of novel CSF biomarkers for neurodegeneration and their validation by a high-throughput multiplexed targeted proteomic assay. Mol Neurodegener 2015;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Azeez HA and Al-Zaky M. Plasma osteopontin as a predictor of coronary artery disease: association with echocardiographic characteristics of atherosclerosis. J Clin Lab Anal 2010;24:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevde LA and Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol 2014;37:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briones-Orta MA, Avendano-Vazquez SE, Aparicio-Bautista DI, Coombes JD, Weber GF and Syn WK. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim Biophys Acta Rev Cancer 2017;1868:93–108 A. [DOI] [PubMed] [Google Scholar]
- 11.Hao C, Cui Y, Owen S, Li W, Cheng S and Jiang WG. Human osteopontin: Potential clinical applications in cancer (Review). Int J Mol Med 2017;39:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau JB, Poggio P, Sainger R, Vernick WJ, Seefried WF, Branchetti E, Field BC, Bavaria JE, Acker MA and Ferrari G. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg 2012;93:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sainger R, Grau JB, Poggio P, Branchetti E, Bavaria JE, Gorman JH 3rd, Gorman RC and Ferrari G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers 2012;17:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Q, Luo X, Liu Y, Zhang J, Luo H, Huang Q, Cheng Y and Xie Z. Osteopontin as a potential therapeutic target for ischemic stroke. Curr Drug Deliv 2016. [DOI] [PubMed]
- 15.Li Y, Dammer EB, Zhang-Brotzge X, Chen S, Duong DM, Seyfried NT, Kuan CY and Sun YY. Osteopontin Is a Blood Biomarker for Microglial Activation and Brain Injury in Experimental Hypoxic-Ischemic Encephalopathy. eNeuro 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjerre M, Pedersen SH, Mogelvang R, Lindberg S, Jensen JS, Galatius S and Flyvbjerg A. High osteopontin levels predict long-term outcome after STEMI and primary percutaneous coronary intervention. Eur J Prev Cardiol 2013;20:922–9. [DOI] [PubMed] [Google Scholar]
- 17.Muller O, Delrue L, Hamilos M, Vercauteren S, Ntalianis A, Trana C, Mangiacapra F, Dierickx K, De Bruyne B, Wijns W, Behfar A, Barbato E, Terzic A, Vanderheyden M and Bartunek J. Transcriptional fingerprint of human whole blood at the site of coronary occlusion in acute myocardial infarction. EuroIntervention 2011;7:458–66. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Foster CR, Dalal S and Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 2010;48:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshikawa M, Aizawa K, Kasai H, Izawa A, Tomita T, Kumazaki S, Tsutsui H, Koyama J, Shimodaira S, Takahashi M and Ikeda U. Elevated osteopontin levels in patients with peripheral arterial disease. Angiology 2009;60:42–5. [DOI] [PubMed] [Google Scholar]
- 20.Kapetanios D, Karkos C, Giagtzidis I, Papazoglou K, Kiroplastis K and Spyridis C. Vascular calcification biomarkers and peripheral arterial disease. Int Angiol 2016;35:455–9. [PubMed] [Google Scholar]
- 21.Waller AH, Sanchez-Ross M, Kaluski E and Klapholz M. Osteopontin in cardiovascular disease: a potential therapeutic target. Cardiol Rev 2010;18:125–31. [DOI] [PubMed] [Google Scholar]
- 22.Franzen A and Heinegard D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. The Biochemical journal 1985;232:715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy-Ullrich JE and Sage EH. Revisiting the matricellular concept. Matrix Biol 2014;37:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bornstein P Matricellular proteins: an overview. Journal of cell communication and signaling 2009;3:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K and Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol 2005;24:418–27. [DOI] [PubMed] [Google Scholar]
- 26.Bayless KJ and Davis GE. Identification of dual alpha 4beta1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. The Journal of biological chemistry 2001;276:13483–9. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF and Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res 1999;59:219–26. [PubMed] [Google Scholar]
- 28.Inoue M and Shinohara ML. Intracellular osteopontin (iOPN) and immunity. Immunol Res 2011;49:160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber GF, Ashkar S, Glimcher MJ and Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996;271:509–12. [DOI] [PubMed] [Google Scholar]
- 30.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA and Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol 2000;184:118–30. [DOI] [PubMed] [Google Scholar]
- 31.Christensen B, Petersen TE and Sorensen ES. Post-translational modification and proteolytic processing of urinary osteopontin. The Biochemical journal 2008;411:53–61. [DOI] [PubMed] [Google Scholar]
- 32.Nishimichi N, Hayashita-Kinoh H, Chen C, Matsuda H, Sheppard D and Yokosaki Y. Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1. The Journal of biological chemistry 2011;286:11170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen B, Nielsen MS, Haselmann KF, Petersen TE and Sorensen ES. Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. The Biochemical journal 2005;390:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasa M, Chang PL, Prince CW and Pinna LA. Phosphorylation of osteopontin by Golgi apparatus casein kinase. Biochem Biophys Res Commun 1997;240:602–5. [DOI] [PubMed] [Google Scholar]
- 35.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV and Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 2012;336:1150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT and Sorensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. The Journal of biological chemistry 2007;282:19463–72. [DOI] [PubMed] [Google Scholar]
- 37.Jono S, Peinado C and Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. The Journal of biological chemistry 2000;275:20197–203. [DOI] [PubMed] [Google Scholar]
- 38.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ and Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000;287:860–4. [DOI] [PubMed] [Google Scholar]
- 39.Jayaprakash NG and Surolia A. Role of glycosylation in nucleating protein folding and stability. The Biochemical journal 2017;474:2333–2347. [DOI] [PubMed] [Google Scholar]
- 40.Kariya Y, Kanno M, Matsumoto-Morita K, Konno M, Yamaguchi Y and Hashimoto Y. Osteopontin O-glycosylation contributes to its phosphorylation and cell-adhesion properties. The Biochemical journal 2014;463:93–102. [DOI] [PubMed] [Google Scholar]
- 41.Oyama M, Kariya Y, Kariya Y, Matsumoto K, Kanno M, Yamaguchi Y and Hashimoto Y. Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin. The Biochemical journal 2018;475:1583–1595. [DOI] [PubMed] [Google Scholar]
- 42.Minai-Tehrani A, Chang SH, Park SB and Cho MH. The Oglycosylation mutant osteopontin alters lung cancer cell growth and migration in vitro and in vivo. Int J Mol Med 2013;32:1137–49. [DOI] [PubMed] [Google Scholar]
- 43.Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS, Bhown M and Schrohenloher RE. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. The Journal of biological chemistry 1987;262:2900–7. [PubMed] [Google Scholar]
- 44.Masuda K, Takahashi N, Tsukamoto Y, Honma H and Kohri K. N-Glycan structures of an osteopontin from human bone. Biochem Biophys Res Commun 2000;268:814–7. [DOI] [PubMed] [Google Scholar]
- 45.Higashikawa F, Eboshida A and Yokosaki Y. Enhanced biological activity of polymeric osteopontin. FEBS letters 2007;581:2697–701. [DOI] [PubMed] [Google Scholar]
- 46.Lorand L and Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003;4:140–56. [DOI] [PubMed] [Google Scholar]
- 47.Christensen B, Zachariae ED, Scavenius C, Kloverpris S, Oxvig C, Petersen SV, Enghild JJ and Sorensen ES. Transglutaminase 2-Catalyzed Intramolecular Cross-Linking of Osteopontin. Biochemistry 2016;55:294–303. [DOI] [PubMed] [Google Scholar]
- 48.Nishimichi N, Higashikawa F, Kinoh HH, Tateishi Y, Matsuda H and Yokosaki Y. Polymeric osteopontin employs integrin alpha9beta1 as a receptor and attracts neutrophils by presenting a de novo binding site. The Journal of biological chemistry 2009;284:14769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaartinen MT, Pirhonen A, Linnala-Kankkunen A and Maenpaa PH. Cross-linking of osteopontin by tissue transglutaminase increases its collagen binding properties. The Journal of biological chemistry 1999;274:1729–35. [DOI] [PubMed] [Google Scholar]
- 50.Forsprecher J, Wang Z, Goldberg HA and Kaartinen MT. Transglutaminase-mediated oligomerization promotes osteoblast adhesive properties of osteopontin and bone sialoprotein. Cell Adh Migr 2011;5:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorensen ES, Rasmussen LK, Moller L, Jensen PH, Hojrup P and Petersen TE. Localization of transglutaminase-reactive glutamine residues in bovine osteopontin. The Biochemical journal 1994;304 ( Pt 1):13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen B, Zachariae ED, Scavenius C, Thybo M, Callesen MM, Kloverpris S, Oxvig C, Enghild JJ and Sorensen ES. Identification of transglutaminase reactive residues in human osteopontin and their role in polymerization. PLoS One 2014;9:e113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y and Sheppard D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. The Journal of biological chemistry 1999;274:36328–34. [DOI] [PubMed] [Google Scholar]
- 54.Christensen B, Klaning E, Nielsen MS, Andersen MH and Sorensen ES. C-terminal modification of osteopontin inhibits interaction with the alphaVbeta3-integrin. The Journal of biological chemistry 2012;287:3788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC and Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). The Journal of biological chemistry 2001;276:28261–7. [DOI] [PubMed] [Google Scholar]
- 56.Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, Tarnovscki T, Nov O, Shelef I, Paran E and Rudich A. N-terminal rather than full-length osteopontin or its C-terminal fragment is associated with carotid-plaque inflammation in hypertensive patients. Am J Hypertens 2013;26:326–33. [DOI] [PubMed] [Google Scholar]
- 57.Uchinaka A, Hamada Y, Mori S, Miyagawa S, Saito A, Sawa Y, Matsuura N, Yamamoto H and Kawaguchi N. SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis. Molecular and cellular biochemistry 2015;408:191–203. [DOI] [PubMed] [Google Scholar]
- 58.Saitoh Y, Kuratsu J, Takeshima H, Yamamoto S and Ushio Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab Invest 1995;72:55–63. [PubMed] [Google Scholar]
- 59.Lee GS, Salazar HF, Joseph G, Lok ZSY, Caroti CM, Weiss D, Taylor WR and Lyle AN. Osteopontin isoforms differentially promote arteriogenesis in response to ischemia via macrophage accumulation and survival. Lab Invest 2018. [DOI] [PMC free article] [PubMed]
- 60.Gimba ER and Tilli TM. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Lett 2013;331:11–7. [DOI] [PubMed] [Google Scholar]
- 61.Sivakumar S and Niranjali Devaraj S. Tertiary structure prediction and identification of druggable pocket in the cancer biomarker - Osteopontin-c. J Diabetes Metab Disord 2014;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan JL, Reeves TM and Phillips LL. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp Neurol 2014;261:757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shirakawa K, Endo J, Kataoka M, Katsumata Y, Yoshida N, Yamamoto T, Isobe S, Moriyama H, Goto S, Kitakata H, Hiraide T, Fukuda K and Sano M. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation 2018;138:2021–2035. [DOI] [PubMed] [Google Scholar]
- 64.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE and Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol 2004;43:474–82. [DOI] [PubMed] [Google Scholar]
- 65.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE and Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arteriosclerosis, thrombosis, and vascular biology 2008;28:290–5. [DOI] [PubMed] [Google Scholar]
- 66.Heilmann K, Hoffmann U, Witte E, Loddenkemper C, Sina C, Schreiber S, Hayford C, Holzlohner P, Wolk K, Tchatchou E, Moos V, Zeitz M, Sabat R, Gunthert U and Wittig BM. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med 2009;13:1162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lund SA, Giachelli CM and Scatena M. The role of osteopontin in inflammatory processes. Journal of cell communication and signaling 2009;3:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H and Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol 2002;72:752–61. [PubMed] [Google Scholar]
- 69.Lund SA, Wilson CL, Raines EW, Tang J, Giachelli CM and Scatena M. Osteopontin mediates macrophage chemotaxis via alpha4 and alpha9 integrins and survival via the alpha4 integrin. J Cell Biochem 2013;114:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, Miceli MC and Spencer MJ. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J Cell Biol 2016;213:275–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuch K, Wanko B, Ambroz K, Castelo-Rosa A, Moreno-Viedma V, Grun NG, Leitner L, Staffler G, Zeyda M and Stulnig TM. Osteopontin affects macrophage polarization promoting endocytic but not inflammatory properties. Obesity (Silver Spring) 2016;24:1489–98. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Ren Y, Kang L and Zhang L. Oxidized low-density lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin. Int J Mol Med 2014;33:1341–7. [DOI] [PubMed] [Google Scholar]
- 73.Sodhi CP, Phadke SA, Batlle D and Sahai A. Hypoxia stimulates osteopontin expression and proliferation of cultured vascular smooth muscle cells: potentiation by high glucose. Diabetes 2001;50:1482–90. [DOI] [PubMed] [Google Scholar]
- 74.Barchetta I, Ceccarelli V, Cimini FA, Bertoccini L, Fraioli A, Alessandri C, Lenzi A, Baroni MG and Cavallo MG. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J Endocrinol Invest 2018. [DOI] [PubMed]
- 75.Kahles F, Findeisen HM and Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab 2014;3:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barchetta I, Alessandri C, Bertoccini L, Cimini FA, Taverniti L, Di Franco M, Fraioli A, Baroni MG and Cavallo MG. Increased circulating osteopontin levels in adult patients with type 1 diabetes mellitus and association with dysmetabolic profile. Eur J Endocrinol 2016;174:187–92. [DOI] [PubMed] [Google Scholar]
- 77.Gordin D, Forsblom C, Panduru NM, Thomas MC, Bjerre M, Soro-Paavonen A, Tolonen N, Sandholm N, Flyvbjerg A, Harjutsalo V, Groop PH and FinnDiane Study G. Osteopontin is a strong predictor of incipient diabetic nephropathy, cardiovascular disease, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2014;37:2593–600. [DOI] [PubMed] [Google Scholar]
- 78.Cai M, Bompada P, Atac D, Laakso M, Groop L and De Marinis Y. Epigenetic regulation of glucose-stimulated osteopontin (OPN) expression in diabetic kidney. Biochem Biophys Res Commun 2016;469:108–113. [DOI] [PubMed] [Google Scholar]
- 79.Wolak T Osteopontin - a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014;236:327–37. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S and Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest 1993;92:2814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isoda K, Kamezawa Y, Ayaori M, Kusuhara M, Tada N and Ohsuzu F. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 2003;107:679–81. [DOI] [PubMed] [Google Scholar]
- 82.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A and Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 2003;23:1029–34. [DOI] [PubMed] [Google Scholar]
- 83.Craig AM and Denhardt DT. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene 1991;100:163–71. [DOI] [PubMed] [Google Scholar]
- 84.Yagisawa T, Ito F, Osaka Y, Amano H, Kobayashi C and Toma H. The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J Urol 2001;166:1078–82. [PubMed] [Google Scholar]
- 85.Latoche JD, Ufelle AC, Fazzi F, Ganguly K, Leikauf GD and Fattman CL. Secreted Phosphoprotein 1 and Sex-Specific Differences in Silica-Induced Pulmonary Fibrosis in Mice. Environ Health Perspect 2016;124:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marek I, Canu M, Cordasic N, Rauh M, Volkert G, Fahlbusch FB, Rascher W, Hilgers KF, Hartner A and Menendez-Castro C. Sex differences in the development of vascular and renal lesions in mice with a simultaneous deficiency of Apoe and the integrin chain Itga8. Biol Sex Differ 2017;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO and Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun 2009;10:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE and Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest 2003;112:1318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng YH, Tian C, Meng Y, Qin YW, Du YH, Du J and Li HH. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol 2012;227:127–35. [DOI] [PubMed] [Google Scholar]
- 90.Cao Z, Dai J, Fan K, Wang H, Ji G, Li B, Zhang D, Hou S, Qian W, Zhao J, Wang H and Guo Y. A novel functional motif of osteopontin for human lymphocyte migration and survival. Mol Immunol 2008;45:3683–92. [DOI] [PubMed] [Google Scholar]
- 91.Ohmori R, Momiyama Y, Taniguchi H, Takahashi R, Kusuhara M, Nakamura H and Ohsuzu F. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis 2003;170:333–7. [DOI] [PubMed] [Google Scholar]
- 92.Momiyama Y, Ohmori R, Fayad ZA, Kihara T, Tanaka N, Kato R, Taniguchi H, Nagata M, Nakamura H and Ohsuzu F. Associations between plasma osteopontin levels and the severities of coronary and aortic atherosclerosis. Atherosclerosis 2010;210:668–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sbarouni E, Georgiadou P, Mihas C, Chaidaroglou A, Degiannis D and Voudris V. Significant peri-operative reduction in plasma osteopontin levels after coronary artery by-pass grafting. Clin Biochem 2012;45:1513–5. [DOI] [PubMed] [Google Scholar]
- 94.Lorenzen JM, Neunhoffer H, David S, Kielstein JT, Haller H and Fliser D. Angiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension--results from the EUTOPIA trial. Atherosclerosis 2010;209:184–8. [DOI] [PubMed] [Google Scholar]
- 95.Kurata M, Okura T, Irita J, Enomoto D, Nagao T, Jotoku M, Miyoshi K, Desilva VR and Higaki J. Angiotensin II receptor blockade with valsartan decreases plasma osteopontin levels in patients with essential hypertension. J Hum Hypertens 2011;25:334–9. [DOI] [PubMed] [Google Scholar]
- 96.deBlois D, Lombardi DM, Su EJ, Clowes AW, Schwartz SM and Giachelli CM. Angiotensin II induction of osteopontin expression and DNA replication in rat arteries. Hypertension 1996;28:1055–63. [DOI] [PubMed] [Google Scholar]
- 97.Remus EW, Lyle AN, Weiss D, Landazuri N, Weber M, Searles C and Taylor WR. miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells. Atherosclerosis 2013;228:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stepien E, Wypasek E, Stopyra K, Konieczynska M, Przybylo M and Pasowicz M. Increased levels of bone remodeling biomarkers (osteoprotegerin and osteopontin) in hypertensive individuals. Clin Biochem 2011;44:826–31. [DOI] [PubMed] [Google Scholar]
- 99.Berezin AE and Kremzer AA. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis 2013;229:475–81. [DOI] [PubMed] [Google Scholar]
- 100.Mazzone A, Parri MS, Giannessi D, Ravani M, Vaghetti M, Altieri P, Casalino L, Maltinti M, Balbi M, Barsotti A and Berti S. Osteopontin plasma levels and accelerated atherosclerosis in patients with CAD undergoing PCI: a prospective clinical study. Coron Artery Dis 2011;22:179–87. [DOI] [PubMed] [Google Scholar]
- 101.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE and Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest 1993;92:1686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liaw L, Lombardi DM, Almeida MM, Schwartz SM, deBlois D and Giachelli CM. Neutralizing antibodies directed against osteopontin inhibit rat carotid neointimal thickening after endothelial denudation. Arteriosclerosis, thrombosis, and vascular biology 1997;17:188–93. [DOI] [PubMed] [Google Scholar]
- 103.Isoda K, Nishikawa K, Kamezawa Y, Yoshida M, Kusuhara M, Moroi M, Tada N and Ohsuzu F. Osteopontin plays an important role in the development of medial thickening and neointimal formation. Circ Res 2002;91:77–82. [DOI] [PubMed] [Google Scholar]
- 104.Kang N, Ng CS, Hu J, Qiu ZB, Underwood MJ, Jeremy JY and Wan S. Role of osteopontin in the development of neointimal hyperplasia in vein grafts. Eur J Cardiothorac Surg 2012;41:1384–9. [DOI] [PubMed] [Google Scholar]
- 105.Gao H, Steffen MC and Ramos KS. Osteopontin regulates alpha-smooth muscle actin and calponin in vascular smooth muscle cells. Cell Biol Int 2012;36:155–61. [DOI] [PubMed] [Google Scholar]
- 106.Wu M, Rementer C and Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 2013;93:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Demer LL and Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 2008;117:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Speer MY, Chien YC, Quan M, Yang HY, Vali H, McKee MD and Giachelli CM. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res 2005;66:324–33. [DOI] [PubMed] [Google Scholar]
- 109.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G and Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. The Journal of experimental medicine 2002;196:1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H and Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol 2002;161:2035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohri R, Tung E, Rajachar R and Giachelli CM. Mitigation of ectopic calcification in osteopontin-deficient mice by exogenous osteopontin. Calcif Tissue Int 2005;76:307–15. [DOI] [PubMed] [Google Scholar]
- 112.Caesar C, Lyle AN, Joseph G, Weiss D, Alameddine FMF, Lassegue B, Griendling KK and Taylor WR. Cyclic Strain and Hypertension Increase Osteopontin Expression in the Aorta. Cell Mol Bioeng 2017;10:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo KW, Lee SJ, Ye BH, Kim YW, Bae SS and Kim CD. Mechanical stretch enhances the expression and activity of osteopontin and MMP-2 via the Akt1/AP-1 pathways in VSMC. J Mol Cell Cardiol 2015;85:13–24. [DOI] [PubMed] [Google Scholar]
- 114.Giachelli CM, Pichler R, Lombardi D, Denhardt DT, Alpers CE, Schwartz SM and Johnson RJ. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int 1994;45:515–24. [DOI] [PubMed] [Google Scholar]
- 115.Abe K, Nakashima H, Ishida M, Miho N, Sawano M, Soe NN, Kurabayashi M, Chayama K, Yoshizumi M and Ishida T. Angiotensin II-induced osteopontin expression in vascular smooth muscle cells involves Gq/11, Ras, ERK, Src and Ets-1. Hypertens Res 2008;31:987–98. [DOI] [PubMed] [Google Scholar]
- 116.Raffetto JD and Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008;75:346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burke HB. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark Cancer 2016;8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Selleck MJ, Senthil M and Wall NR. Making Meaningful Clinical Use of Biomarkers. Biomark Insights 2017;12:1177271917715236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cabiati M, Svezia B, Matteucci M, Botta L, Pucci A, Rinaldi M, Caselli C, Lionetti V and Del Ry S. Myocardial Expression Analysis of Osteopontin and Its Splice Variants in Patients Affected by End-Stage Idiopathic or Ischemic Dilated Cardiomyopathy. PLoS One 2016;11:e0160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


