Abstract
The cloning of the renal NaPi-2a (SLC34A1) and NaPi-2c (SLC34A3) phosphate transporters has made it possible to characterize the molecular and biophysical regulation of renal proximal tubular reabsorption of inorganic phosphate (Pi). Dietary factors, such as Pi and K, and several hormones and phosphatonins, including parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and glucocorticoids, regulate the transporters through various transcriptional, translational, and posttranslational mechanisms that involve acute trafficking via endocytosis or exocytosis, interactions with PDZ domain proteins, lipid microdomains, and diffusion and clustering in the apical brush border membrane. The visualization of these trafficking events by means of novel microscopy techniques that includes Fluorescence Lifetime Imaging Microscopy (FLIM), Forster Resonance Energy Transfer (FRET), Fluctuation Correlation Spectroscopy (FCS), and Modulation Tracking (MT), is the primary focus of this review.
Keywords: NaPi-PDZ protein interactions, NaPi transporter diffusion and clustering, NaPi-lipid interactions, Fluorescence Lifetime Imaging Microscopy (FLIM), Förster Resonance Energy Transfer (FRET), Fluctuation Correlation Spectroscopy (FCS)
INTRODUCTION
Prior to cloning of the renal phosphate transporters extensive work involving micropuncture studies, isolated perfused tubules, isolated apical brush border membrane (BBM) vesicles, and cultured opossum kidney (OK) cells, a model of the renal proximal tubule, had identified regulation of sodium gradient-dependent phosphate (NaPi) transport by dietary, hormonal and metabolic factors. However, the molecular nature and molecular mechanisms of the NaPi transporters was unknown. The cloning of the renal NaPi-2a (SLC34A1) and NaPi-2c (SLC34A3) phosphate transporters [13, 48, 61, 70] and generation of highly specific antibodies has made it possible to decipher the cellular and molecular mechanisms for the regulation of renal proximal tubular Pi reabsorption. It is now known that NaPi-2a is responsible for approximately 70% of the Pi reabsorbed in the adult kidney of mice and rats, and NaPi-2c handles the remaining 30%. The kidney also expresses PiT-2 (SLC20A2) which is upregulated by dietary Pi deprivation [80], however the contribution of this transporter to overall renal proximal tubular transport remains debated. This review will focus on the regulation of SLC34 proteins by acute changes in dietary Pi, parathyroid hormone (PTH); interactions with PDZ domains; and membrane lipid composition using a variety of novel microscopy techniques developed largely in the authors’ laboratories.
Regulation of NaPi activity by apical BBM trafficking by acute changes in dietary phosphate
Dietary Pi restriction results in increase of proximal tubular NaPi transport [2, 11, 35, 41, 76]. This regulation is in part independent of extrarenal factors such as parathyroid hormone (PTH), 1,25 dihydroxyvitamin D3, plasma calcium, or growth hormone [57]. The increase in NaPi transport also occurs in OK cells grown in culture containing a low Pi medium. In response to in vivo or cell culture Pi restriction, the increase in Na-Pi cotransport is mediated by an increase in the maximal capacity of transport, Vmax, whereas there are no alterations in the affinities (Km) for Pi or Na ions [57].
Following the cloning of NaPi-2a, the first set of studies determined that in response to chronic (days) low Pi diet there were increases in BBM NaPi cotransport activity, NaPi-2a mRNA, and BBM NaPi-2a protein abundance as demonstrated by western blotting and confocal immunofluorescence microscopy. On the other hand, in response to acute (2 hour) feeding of a low Pi diet there were increases in BBM NaPi cotransport activity and NaPi-2a protein abundance, with no alterations in NaPi-2a mRNA. In addition, in response to acute (2 hour) feeding of a high Pi diet there were decreases in BBM NaPi cotransport activity and NaPi-2a protein abundance, with no alterations in NaPi-2a mRNA [43, 45, 59, 84].
To further characterize the mechanisms of the acute adaptation to a low Pi diet, and determine the roles of transcription and translation, animals were treated with actinomycin D, which is an inhibitor of transcription, or cycloheximide, which is an inhibitor of translation. The results indicated that in response to acute feeding of a low Pi diet the increase in apical membrane NaPi-2a protein was independent of de novo transcription and translation or de novo protein synthesis. These findings were also confirmed by absence of alterations in cortical homogenate NaPi-2a protein content.
Interestingly treatment of animals with colchicine, which disrupts the microtubules, prevented the increases in BBM NaPi cotransport activity and NaPi-2a protein content. In contrast, in response to acute feeding of a high Pi diet, colchicine did not prevent the rapid decreases in NaPi cotransport activity or BBM NaPi-2a protein content. These studies provided evidence that the rapid increase sin renal proximal tubular apical BBM NaPi cotransport activity and NaPi-2a protein content is most likely induced by microtubule-dependent trafficking of existing NaPi-2a transporters to the apical BBM [46].
PTH-mediated regulation of NaPi-2a and NaPi-2c activity by apical BBM trafficking
Parathyroid hormone (PTH) is an important regulator of Pi reabsorption by the kidney. Parathyroidectomy (PTX) decreases urinary Pi excretion, whereas infusion of PTH increases Pi excretion [6]. The phosphaturic action of PTH is mediated by decrease in NaPi transport activity in the apical BBM of the renal proximal tubule [58]. The decrease in NaPi transport is characterized by a decrease in Vmax with no alteration in the Km for Pi [27].
Following the cloning of NaPi-2a, the studies indicated that chronic parathyroidectomy in rats resulted in an increase in BBM Na-Pi cotransport activity and BBM NaPi-2a protein abundance. Surprisingly, there was no alteration in NaPi-2a mRNA abundance. Acute (2 hour or less) administration of PTH resulted in significant decreases in BBM NaPi cotransport activity and NaPi-2a protein content. Once again and surprisingly there was only a minor decrease in NaPi-2a mRNA [36, 59].
Further studies in rats showed that PTH induced a decrease in NaPi-2a protein abundance in BBM within 15 minutes of administration. In parallel, NaPi-2a transporter accumulated in intracellular vesicles. After 60 minutes of PTH administration, there were marked decrease in BBM and cellular NaPi-2a protein. Pretreatment of the animals with paclitaxel (Taxol) which inhibits microtubule dynamics resulted in subapical accumulation of NaPi-2a after 15 minutes. In addition, there was a delay in depletion of intracellular transporters after 60 minutes of PTH administration. Colchicine administration similarly caused a delay in intracellular NaPi-2a depletion. These results indicated that PTH downregulates NaPi-2a through rapid endocytosis by (a) first internalization of the transporter into subapical vesicles, and (b) then delivery to lysosomes by a process that depends on microtubules [47].
For further investigation of the molecular regulation of the NaPi transporters the authors used several novel microscopy techniques, largely developed in their laboratories. These included applications of apical membrane total internal reflection fluorescence (TIRF) microscopy, which allowed for the imaging of the GFP-tagged NaPi transporters within 100 nm of the coverslip. For apical TIRF the OK cells were grown on filters and then inverted upside down on the inverted Zeiss microscope stage [5]. This technique was then used to examine how PTH induces the movement of NaPi-2a out of brush border microvilli in living OK cells in real time. These studies showed that PTH induces rapid removal of NaPi-2a from the apical brush border microvilli and that a dynamic actin cytoskeleton is required for that process. Moreover, myosin VI is also required for PTH-induced removal of NaPi-2a from BBM microvilli [5].
Additional microscopy techniques were applied by the authors including nanometer-scale imaging by the modulation tracking method [38], also known as nano-scale precise imaging by rapid beam oscillation (nSPIRO) [12]. This yields 20 nm resolved 3D images of GFP-tagged NaPi in the microvilli of live OK cells (Fig 1A). An additional technique, raster image correlation spectroscopy (RICS), together with cross correlation RICS, and number and brightness (N&B) analysis [8, 17, 18, 68] further permitted the measurement of the lateral movement (diffusion coefficient) and clustering/aggregation of GFP-tagged NaPi-2a/c in the microvilli live OK cells (Fig 1B). These techniques made it possible to determine the molecular mechanisms of the differential modulation of NaPi-2a versus NaPi-2c by PTH. In the absence of PTH the two NaPi cotransporters have similar diffusion coefficients within the membrane. After PTH addition NaPi-2a moves faster than NaPi-2c (Fig 1B). PTH treatment of OK cells causes endocytosis of NaPi-2a from the apical BBM within 1 h. In contrast PTH induced NaPi-2c endocytosis is markedly delayed. Interestingly, myosin VI is also required for PTH-induced internalization of NaPi-2c. These studies indicate that NaPi-2c is present in the apical membrane for longer periods of time after PTH treatment, which can explain, at least in part, for the difference in kinetics of NaPi-2a versus NaPi-2c endocytosis following PTH [39].
Figure 1.
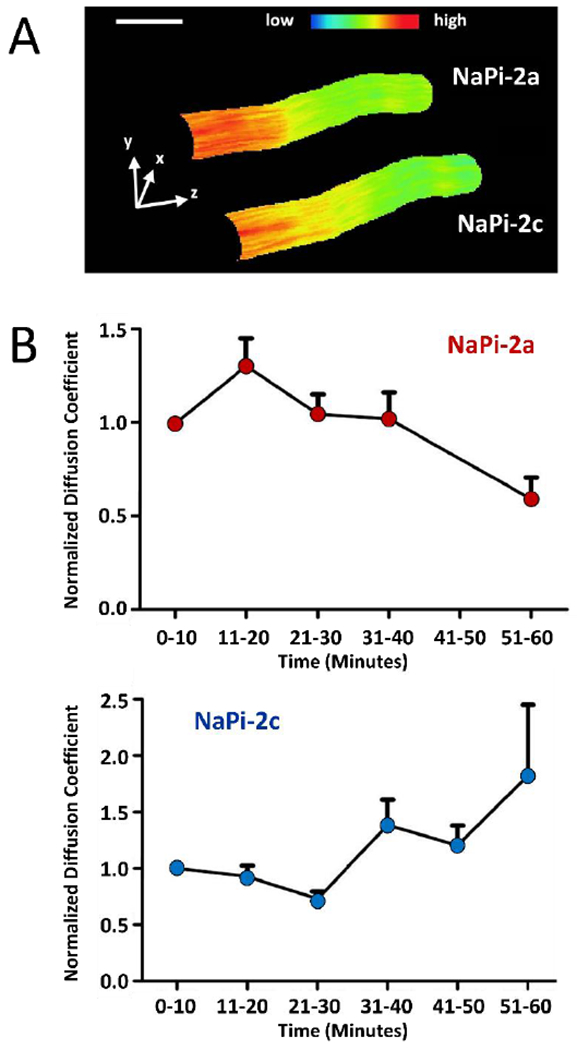
Molecular dynamics of NaPi-2a and NaPi-2c at the apical membrane of OK cells. (A) Three-dimensional representation of a microvillus expressing NaPi-2a and NaPi-2c carrying different tags, Cerulean and mCherry respectively. Expression levels are color coded, scale bar, 200 nm. (B). Normalized diffusion coefficients of NaPi-2a (upper panel) and NaPi-2c (lower panel) upon PTH addition. Whereas diffusion of NaPi2a peaked within 20 minutes and then decreased markedly, NaPi2c showed delayed reaction and diffusion only increased significantly after almost an hour after PTH application. Figure adapted from ref 39.
Parallel studies in several other laboratories determined the signaling processes involved in PTH-induced regulation of NaPi-2a. PTH interacts with a G protein-coupled receptor (PTH1R) expressed at the apical and basolateral membranes of proximal tubules [53, 56, 78]. PTH via activating apical or basolateral PTH receptors induces marked decrease in NaPi-2a protein due to endocytosis from the BBM, followed by trafficking to the lysosomes for degradation [37, 65, 77]. Several PTH isoforms that selectively activate apical and/or basolateral PTH receptors have been developed [53, 78]. PTH-(1–34) activates both apical and basolateral PTH receptors, whereas PTH-(3–34) activates only apical PTH receptors [56, 78]. Apical PTH receptors couple to the phospholipase C (PLC)-protein kinase C (PKC) signaling pathways. In contrast, basolateral PTH receptors activate the cAMP- and protein kinase A (PKA)-dependent signaling pathways [56, 78]. Activation of both signaling pathways result in PTH induced NaPi-2a endocytosis from the BBM and degradation in the lysosome [60].
Regulation of NaPi activity and NaPi-2 protein membrane abundance by NaPi-2-PDZ interactions
The three COOH-terminal amino acid residues of the NaPi-2a protein interact with several PDZ (PSD-95, discs-large, and ZO-1) motif containing proteins, some of which colocalize in the BBM with NaPi-2a [23, 24]. These proteins include the Na/H exchanger regulatory factor 1 (NHERF1) and PDZ-Domain Containing 1 (PDZK1) [3, 29]. In addition to NHERF1, which is also known as ezrin-binding protein 50 (EBP50) [73, 83], there is a second isoform, NHERF2, which resides in a different compartment as compared to NHERF1 [81, 82].
NHERF1 regulates NaPi transporter activity and NaPi-2a protein abundance in the BBM. BBM NaPi-2a abundance is reduced in in Nherfl-deficient mice [74], and OK cells [28].
While NHERF1 contains two PDZ domains, PDZ1 and PDZ2, only PDZ1 is required for interaction with NaPi-2a [49, 50]. NHERF1 forms part of a signaling complex in OK cells that contains PTH1 receptor, phospholipase C (PLCβ), and the actin cytoskeleton [50]. Studies suggest that both NHERF1 and NHERF2 functional roles in the coupling of PTH1 receptor to PLC [49, 50].
PTH-(1–34) induces the endocytosis of NaPi-2a in wild-type and Nherfl-deficient mice. In contrast, PTH-(3–34) does not cause NaPi-2a endocytosis in Nherfl-deficient mice, which may be mediated by decreased activation of PLC activity [9].
In addition to NaPi-2a, PDZ interactions also play an important role in regulation of NaPi-2c expression and trafficking. NaPi-2a and NaPi-2c exhibit several physiological differences: A) NaPi-2a is electrogenic [21], and NaPi-2c is electroneutral [70]; B) both NaPi-2a and NaPi-2c are decreased in response to feeding a high Pi diet. However NaPi-2a endocytosis is rapid (less than 1 h) and occurs along the entire proximal tubule, endocytosis is independent of microtubules, and NaPi-2a is delivered to the lysosome NaPi-2c internalization is observed in the S1 segment only, internalization takes place via a microtubule-dependent process, but however, and then NaPi-2c proteins accumulate in a subapical compartment rather than being degraded in the lysosome [71]; C) As discussed in the preceding section in detail, similar differences also occur for PTH induced NaPi-2a and NaPi-2c internalization from the apical BBM [72].
The differential regulatory mechanisms of dietary PI and PTH can also be explained by the type and number of PDZ proteins with which NaPi-2a and NaPi-2c interact. [4, 30]. PDZ proteins that interact with NaPi-2a and NaPi-2c were identified by means of two-hybrid systems, co-immunoprecipitation, confocal microscopy and apical TIRF microscopy. These studies determined that both NaPi-2c and NaPi-2a interact with the PDZ domain-containing proteins NHERF1 and NHERF3 (PDZK1) [79].
The functional significance of the NaPi-2c and PDZK1 interaction was established in Pdzk1 knock-out mice. In Pdzk1 −/− mice fed low Pi diet, there was impaired upregulation of NaPi-2c but normal upregulation of NaPi-2a protein in the apical BBM. These results indicated an important role for PDZK1 in localization of NaPi-2c in the BBM.
Additional studies determined the specific protein-protein interactions of NaPi transporters with PDZK1 (Fig 2) and NHERF-1 (Fig 3) by Förster Resonance Energy Transfer via Fluorescence Lifetime Imaging Microscopy (FLIM-FRET). The FLIM-FRET technique is more precise and relatively less error prone compared to the classical intensity-based FRET technique. For this purpose, the fit-free phasor approach to fluorescence lifetime imaging analysis was applied [16, 31, 32, 51, 66, 67]. The phasor representation allows for the determination of the presence of different molecular species that result in fluorescence decay (lifetime), as well as the occurrence of fluorescence resonance energy transfer (FRET), which allows for the determination of molecular interactions that occur within 10 nm intermolecular spacing (see insets, Figs 2A-D and 3A-D). NaPi-2c had slightly increased FRET efficiency (indicative of increased interaction) with PDZK1 when compared to NaPi-2a (Fig 2E and Fig 2F). In contrast, FRET measurements showed a much stronger interaction of NHERF-1 with NaPi-2a than with NaPi-2c (Fig 3E and Fig 3F). The differential affinity of NaPi-2a and NaPi-2c transporters for NHERF-1 and PDZK1 proteins could partially explain their differential regulation by dietary Pi and/or PTH [22].
Figure 2.
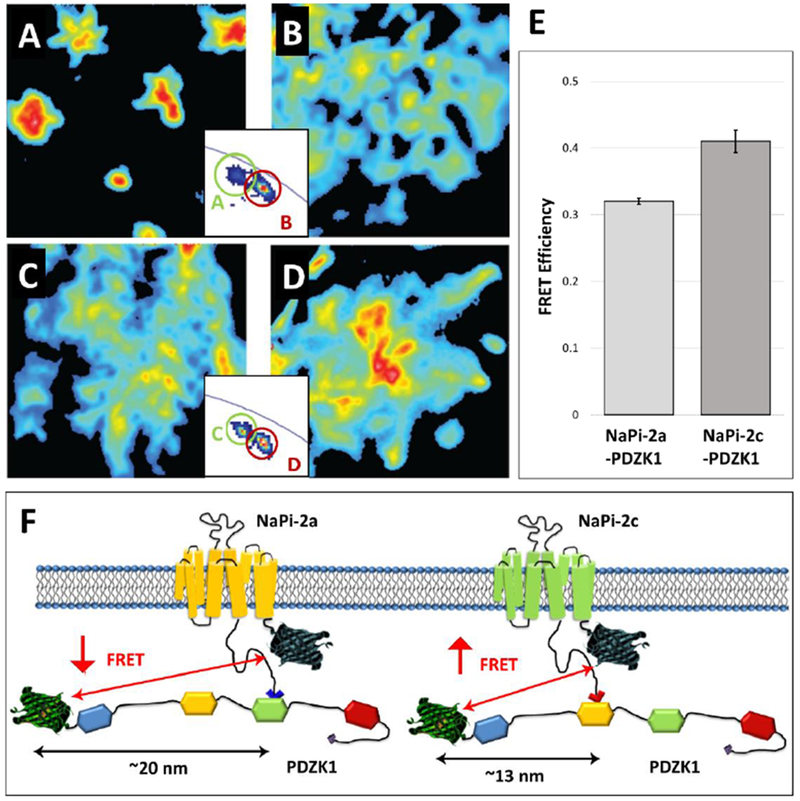
Interaction of NaPi-2a and NaPi-2c with PDZK1. (A-D) Intensity images of OK cells transfected with Cerulaen-NaPi-2a (A), Cerulean -NaPi-2a / EYFP-PDZK1 (enhanced yellow fluorescent protein, B), Cerulean -NaPi-2c (C) and Cerulean -NaPi-2c / EYFP-PDZK1 (D).The two insets exemplify the complex quantification of FRET: The relative spatial separation of fluorescent signals from controls (A,C) and cells co-expressing the FRET partners (B,C) is indicative for FRET. (E) Quantification of the FRET signal between NaPi-2a/2c and PDCK1. For experimental and methodological details concerning the quantification method refer to ref 22. (F) Schematic representation of NaPi-2a and NaPi-2c interactions with PDZK1. The two NaPi isoforms are depicted with the Cerulean tags; PDZK1 contains four PDZ domains and has an N-terminal EYFP tag. NaPi-2a binds to the third PDZ domain of PDZK1 whereas NaPi-2c interacts with the second one. FRET intensity depends crucially on the distance between the two fluorophores (but also on the number of interacting molecules. Figure adapted from ref 22.
Figure 3.
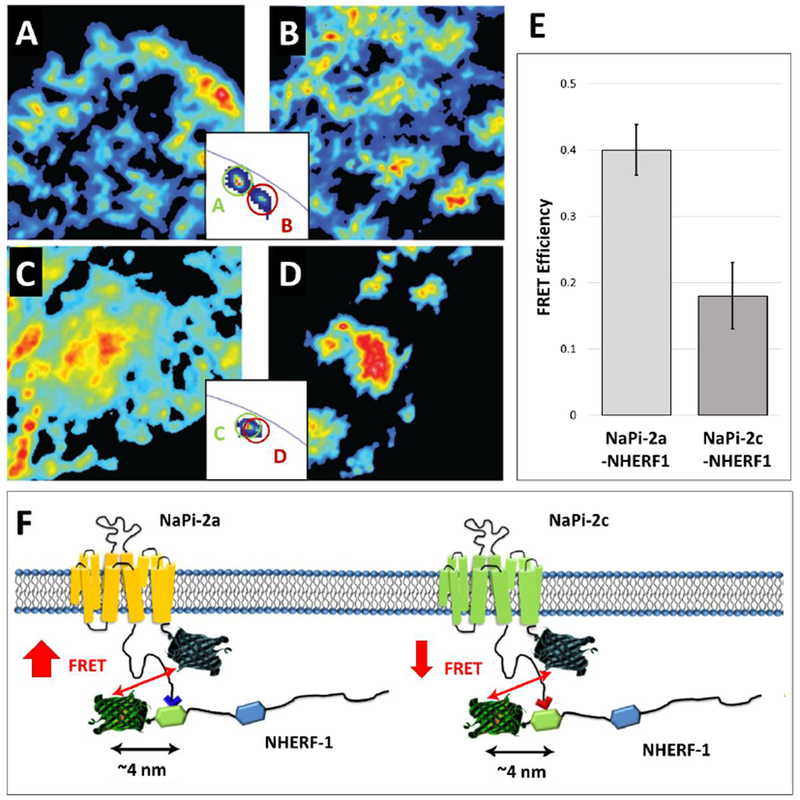
Interaction of NaPi-2a and NaPi-2c with NHERF1. (A-D) Intensity images of OK cells transfected with Cerulaen-NaPi-2a (A), Cerulean -NaPi-2a plus EYFP-PDZK1 (B), Cerulean -NaPi-2c (C) and Cerulean -NaPi-2c plus EYFP-PDZK1 (D).The two insets exemplify the complex quantification of FRET. (E) Quantification of the FRET signal between NaPi-2a/2c and NHERF1. (F) Schematic representation of NaPi-2a and NaPi-2c interactions with NHERF1. The two NaPi isoforms are depicted with the Cerulean tags; NHERF1 contains two PDZ domains and an N-terminal EYFP tag. Both NaPi-2a and NaPi-2c bind to the first PDZ domain of NHERF1,although NaPi-2c binds with a lower prevalence. Figure adapted from ref 22.
Applying similar techniques Shank2E has also been shown to interact with NaPi-2a in native BBM isolated from rat and in OK cells [19, 20, 54]. In rats and in OK cells maintained in low Pi conditions Shank 2E plays an important role in the apical retention of NaPi-2a whereas under high Pi conditions Shank2E remains associated with NaPi-2a and both are endocytosed from the apical membrane [19, 20, 54].
Regulation of NaPi activity by perturbations in apical BBM lipid composition and lipid rafts
The first evidence for lipids playing a role in modulating NaPi cotransport activity became apparent in studies in aging rat kidneys where tubular Pi transport was found to be impaired [40]. The studies revealed two potentially complementary mechanisms that mediate the decrease in NaPi transport: a) a parallel decrease in NaPi-2a mRNA and BBM NaPi-2a protein abundance, which indicates transcriptional and translational regulation of the age-dependent decrease in NaPi transport [1, 75] and b) post-transcriptional and post-translational regulation via alterations in BBM lipid composition, especially cholesterol, that can regulate NaPi-2a by multiple mechanisms, including trafficking [7] and modulation of NaPi-2a protein diffusion and clustering.
In the aged rat BBM there was a significant increase in BBM cholesterol and sphingomyelin content and a decrease in BBM fluidity (lipid dynamics) as determined by measuring anisotropy of diphenylhexatriene (DPH) by fluorescence spectroscopy. Indeed previous studies had indicated that cholesterol, sphingomyelin and saturated fatty acids are important regulators of BBM fluidity [55]. The alteration of BBM lipid composition and fluidity may also mediate the impaired renal response to a low Pi diet in the aged rat. In the adult rat the renal adaptation to a low Pi diet is accompanied with a decrease in BBM cholesterol content and an increase in BBM fluidity. In contrast, in aged rats there is impaired renal tubular NaPi transport in response to a low Pi diet which may be caused by higher BBM cholesterol content and decreased BBM fluidity. The study therefore suggested that in aged rats the increase in BBM cholesterol content and the concomitant decrease in BBM fluidity may mediated the decreased renal tubular Pi transport and impaired adaptation to a low-Pi diet [40].
To further determine a role for BBM cholesterol per se in regulation of Na-Pi transport activity, follow-up studies determined whether in vitro enrichment of renal BBM with cholesterol had a direct modulating effect on Na-Pi cotransport. Increases in BBM cholesterol content caused a dose-dependent decreases in NaPi cotransport activity, which was paralleled by dose-dependent increases in fluorescence anisotropy of diphenylhexatriene (DPH), i.e. a decrease in BBM fluidity. When BBM NaPi cotransport activity was analyzed as a function of BBM fluidity, a direct correlation was established between BBM fluidity and BBM NaPi cotransport activity. In addition, in BBMs isolated from rats fed a low Pi diet, in vitro enrichment with cholesterol completely reversed the increase in BBM NaPi cotransport activity and BBM fluidity. These findings indicated that cholesterol directly modulates renal BBM NaPi cotransport activity with potential consequences for the regulation of Pi homeostasis [41].
The findings in vivo were complemented with additional studies using OK cells. To determine the effects of acute decreases in cholesterol content, OK cells were treated with beta-methyl cyclodextrin (beta-MCD). To determine the effects of acute increases in cholesterol content, OK cells were treated with cholesterol complexed to beta-MCD. The results indicated that acute cholesterol depletion resulted in increases in NaPi cotransport and NaPi-2a protein abundance. In contrast, acute cholesterol enrichment caused decreases in NaPi cotransport and NaPi-2a protein abundance.
In additional studies, to determine the effects of chronic decrease in cholesterol content, OK cells were cultured in lipoprotein deficient serum (LPDS). To determine the effects of chronic increase in cholesterol content, OK cells were grown in the presence of LPDS followed by loading with LDL. Chronic decrease in cholesterol content resulted in increases in NaPi cotransport protein and apical membrane as well as total cell NaPi-2a protein abundance. The converse occurred with chronic increase in cholesterol content.
Cholesterol depletion in OK cells also resulted in a significant increase in membrane lipid fluidity and alterations in lipid microdomains or lipid rafts as determined by Laurdan (6-Dodecanoyl-N, N-dimethyl-2-naphthylamine) fluorescence spectroscopy and multiphoton excitation (MPE) fluorescence confocal microscopy imaging. Laurdan is a fluorescent probe [34] that has been widely used in the authors’ laboratories to probe lipid dynamics and lipid nanodomains (also known as lipid rafts) in living cells and in isolated membranes using two-photon excitation microscopy as well as spectroscopy [10, 14, 25, 26, 42, 52, 62, 63].
These results with OK cells corroborated the observations in vivo in rats fed a low Pi diet and in ageing kidney and suggested that acute and chronic alterations in cholesterol content per se modulate NaPi cotransport activity and apical membrane NaPi-2a protein abundance. In addition cholesterol has marked effects on BBM fluidity and regulation of lipid nanodomains which may have important regulatory effects in regulation of NaPi activity [7].
An additional role for alterations in BBM lipid composition in the regulation of NaPi cotransport activity became apparent in glucocorticoid treated mice. Glucocorticoids inhibit renal Pi transport. Treatment of rats with dexamethasone decreases BBM NaPi cotransport activity which is mediated by decreased abundance of BBM NaPi-2a protein and renal cortical NaPi-2a mRNA. At the same time, there was a significant increase in BBM glucosylceramide content. Rats were then treated with the glucosylceramide synthase inhibitor, D-threo-1-phenyl-2-decanoyl-amino-3-morpholino-1-propanol (PDMP), to determine whether the increase in glucosylceramide content per se played a role in the decrease in NaPi cotransport activity and NaPi-2a protein abundance. The results indicated that decreasing BBM glucosylceramide content resulted in an increase in NaPi cotransport activity and an increase in BBM NaPi-2a protein abundance. Hence, BBM glucosylceramide content could at least in part play a regulatory role in the inhibitory effect of glucocorticoids on NaPi cotransport activity [44].
Dietary potassium (K) deficiency has been associated with phosphaturia and hypophosphatemia. Dietary K deficiency in rats resulted in a marked increase in urinary Pi excretion and a decrease in BBM NaPi cotransport activity. Surprisingly, the decrease in NaPi cotransport activity was associated with an increase in the abundance of NaPi-2a protein (Fig 4). The decrease in NaPi transport was also associated with significant alterations in BBM lipid composition, including increases in sphingomyelin, glucosylceramide, and ganglioside GM3 content and a decrease in BBM lipid fluidity (Fig 4A). Treatment with the glucosylceramide synthase inhibitor, D-threo-1-phenyl-2-decanoyl-amino-3-morpholino-1-propanol (PDMP), normalized BBM NaPi cotransport activity in K-deficient rats to the same level as in control rats. The increase in transport activity occurred despite no alterations in BBM NaPi-2a protein or renal cortical NaPi-2a mRNA [85].
Figure 4.
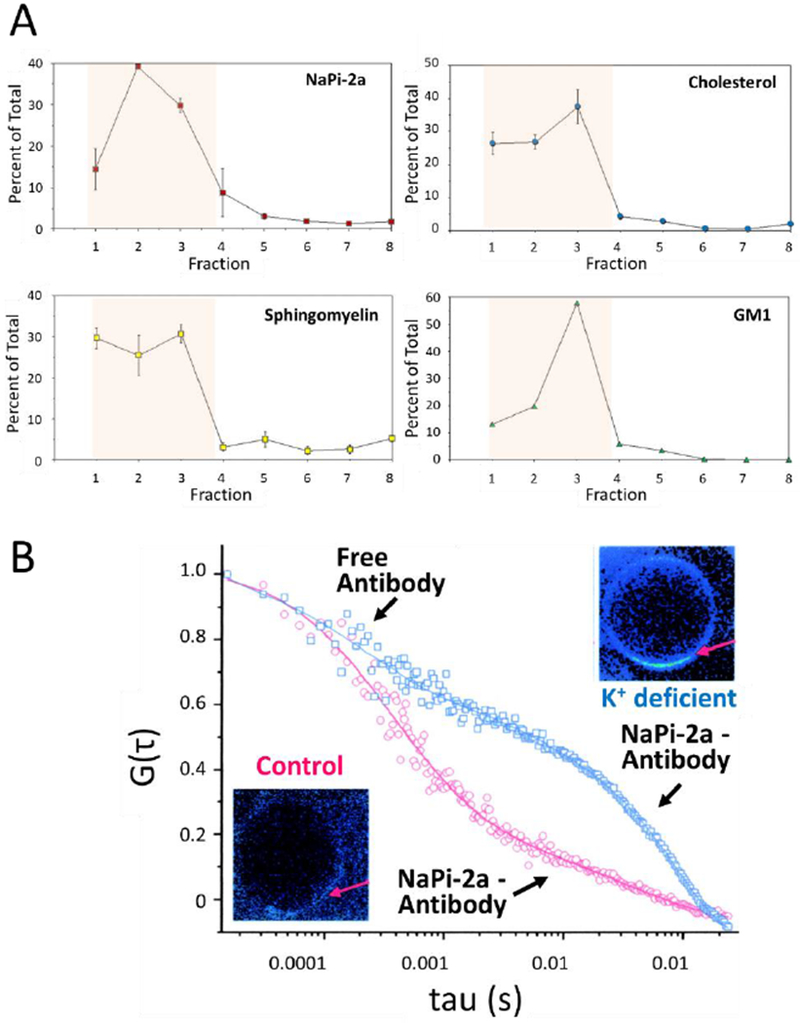
Association of NaPi-2a with lipid rafts. (A) BBM were gradient separated and quantified for NaPi-2a and lipids associated with lipid rafts, cholesterol, sphingomyelin and GM1 (monosialotetrahexosylganglioside) as indicated in the respective panels. NaPi-2a is preferentially partitioned into cholesterol-, sphingomyelin-, and glycosphingolipid-enriched BBM domains, i.e. fractions 1-4 (shaded area). (B) Diffusion of NaPi-2a in giant unilamellar vesicles extracted from K+ deficient (blue squares) and control rats (red squares). In preparations from K+ deficient animals NaPi-2a shows significantly reduced diffusion coefficient as compared to controls. Gt represents the autocorrelation function reflecting fluorescence fluctuation, i.e. fluorophore diffusion; tau represents the time delay. For experimental details see ref 33. The insets are fluorescent representations of giant unilamellar vesicles and the arrow depicts the area that where diffusion was measured. Fluorescence is increased in membranes from K+ deficient animals reflecting an accumulation of transporter at the membrane. Figure adapted from [33].
By using a detergent-free density gradient flotation technique, it was found that there is a highly significant amount of the apical membrane NaPi-2a protein which partitions into cholesterol-, sphingomyelin-, and GM1-enriched membrane fractions characterized as “lipid raft” fractions (Fig 4A). Dietary K deficiency results in a further increase of NaPi-2a protein localized in the lipid raft fractions. The molecular mechanisms mediating the regulation of NaPi cotransport activity by lipid rafts were investigated using fluorescence spectroscopy and multiphoton excitation (MPE) fluorescence microscopy with the lipid probe Laurdan. These studies provided evidence for presence of lipid rafts in BBM [14, 15, 42, 64]. To determine how lipids and lipid rafts modulate NaPi protein dynamics, as discussed in the preceding section, a novel technique scanning fluorescence correlation spectroscopy (SFCS) and photon counting histogram (PCH), also known as molecular brightness, was developed in the authors’ laboratory [69]. Giant unilamellar vesicles (GUVs) were then isolated from control BBM and K deficient BBM to determine the potential biophysical consequences of increased NaPi-2a portioning into lipid raft fractions. The results indicated a significant 2-fold decrease in lateral diffusion of NaPi-2a protein in GUVs from K deficiency despite a 2-fold increase in NaPi-2a protein abundance. In addition, compared to controls where NaPi-2a protein was present in dimers, in K deficiency the NaPi-2a protein was present in pentamers. These results agreed with western blots showing higher molecular weight complexes in K deficiency. These results indicated that the regulatory mechanism that modulates NaPi transport activity because of increased presence in lipid raft fractions involves decrease in NaPi-2a diffusion and increase in NaPi-2a clustering (Fig 4B) [33].
CONCLUSIONS and PERSPECTIVES
Renal tubular Pi transport mediated by NaPi-2a and NaPi-2c is regulated by several molecular and cellular mechanisms that include transcriptional, translational, and posttranslational processes, such as acute internalization from the apical membrane (endocytosis), acute translocation to the apical membrane (exocytosis), NaPi-PDZ domain interactions, and NaPi-lipid interactions that can result in alterations in NaPi protein diffusion and clustering in the apical BBM.
Although the current studies have revealed several of the molecular and signaling mechanisms that regulate renal tubular NaPi transport and NaPi-2a protein, additional investigations are needed to elucidate the details of NaPi-2c regulation. These studies should include proximal tubular axial (i.e. convoluted versus straight proximal tubule) regulation of NaPi-2a and NaPi-2c in rodent models as well as in human proximal tubules as there may be differences in regulation of SLC34 transporters. Additional detailed molecular and cellular studies are also needed for the regulation of intestinal transporters and elucidation of transcellular transport versus paracellular mechanisms involved in Pi reabsorption, especially in the presence of chronic kidney disease.
Acknowledgements
The studies in this review were supported by the National Institute of General Medical Sciences (NIGMS) NIH grant 2P41GM103540 to Enrico Gratton and NIDDK National Institutes of Health grant R01 DK066029 to Moshe Levi. The authors also acknowledge the valuable contributions of several investigators in the Enrico Gratton, Moshe Levi, and Heini Murer labs that made these studies possible.
References
- 1.Alcalde AI, Sarasa M, Raldua D, Aramayona J, Morales R, Biber J, Murer H, Levi M, Sorribas V (1999) Role of thyroid hormone in regulation of renal phosphate transport in young and aged rats. Endocrinology 140:1544–51 DOI 10.1210/endo.140.4.6658 [DOI] [PubMed] [Google Scholar]
- 2.Barrett PQ, Gertner JM, Rasmussen H (1980) Effect of dietary phosphate on transport properties of pig renal microvillus vesicles. Am J Physiol 239:F352–F359 DOI [DOI] [PubMed] [Google Scholar]
- 3.Biber J, Gisler SM, Hernando N, Wagner CA, Murer H (2004) PDZ interactions and proximal tubular phosphate reabsorption. Am J Physiol Renal Physiol 287:F871–5 DOI 10.1152/ajprenal.00244.2004 [DOI] [PubMed] [Google Scholar]
- 4.Biber J, Gisler SM, Hernando N, Murer H (2005) Protein/protein interactions (PDZ) in proximal tubules. J Membr Biol 203:111–8 DOI 10.1007/s00232-005-0738-7 [DOI] [PubMed] [Google Scholar]
- 5.Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M (2009) PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Cell Physiol 297:C1339–46 DOI 10.1152/ajpcell.00260.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaine J, Chonchol M, Levi M (2015) Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 10:1257–72 DOI 10.2215/CJN.09750913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breusegem SY, Halaihel N, Inoue M, Zajicek H, Lederer E, Barry NP, Sorribas V, Levi M (2005) Acute and chronic changes in cholesterol modulate Na-Pi cotransport activity in OK cells. Am J Physiol Renal Physiol 289:F154–65 DOI 10.1152/ajprenal.00331.2004 [DOI] [PubMed] [Google Scholar]
- 8.CM Brown, Dalal RB, Hebert B, Digman MA, Horwitz AR, Gratton E (2008) Raster image correlation spectroscopy (RICS) for measuring fast protein dynamics and concentrations with a commercial laser scanning confocal microscope. J Microsc 229:78–91 DOI 10.1111/j.1365-2818.2007.01871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capuano P, Bacic D, Roos M, Gisler SM, Stange G, Biber J, Kaissling B, Weinman EJ, Shenolikar S, Wagner CA, Murer H (2007) Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol 292:C927–34 DOI 10.1152/ajpcell.00126.2006 [DOI] [PubMed] [Google Scholar]
- 10.Celli A, Gratton E (2010) Dynamics of lipid domain formation: fluctuation analysis. Biochim Biophys Acta 1798:1368–76 DOI 10.1016/j.bbamem.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Liang CT, Sacktor B (1983) Phosphate uptake by renal membrane vesicles of rabbits adapted to high and low phosphorus diets. Am J Physiol 245:F175–80 DOI 10.1152/ajprenal.1983.245.2.F175 [DOI] [PubMed] [Google Scholar]
- 12.Chiu CL, Aguilar JS, Tsai CY, Wu G, Gratton E, Digman MA (2014) Nanoimaging of focal adhesion dynamics in 3D. PLoS One 9:e99896 DOI 10.1371/journal.pone.0099896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Custer M, Lotscher M, Biber J, Murer H, Kaissling B (1994) Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol 266:F767–74 DOI 10.1152/ajprenal.1994.266.5.F767 [DOI] [PubMed] [Google Scholar]
- 14.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E (2001) Lipid rafts reconstituted in model membranes. Biophys J 80:1417–28 DOI 10.1016/S0006-3495(01)76114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K (2001) Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci U S A 98:10642–7 DOI 10.1073/pnas.191168698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Digman MA, Caiolfa VR, Zamai M, Gratton E (2008) The phasor approach to fluorescence lifetime imaging analysis. Biophys J 94:L14–6 DOI 10.1529/biophysj.107.120154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Digman MA, Wiseman PW, Horwitz AR, Gratton E (2009) Detecting protein complexes in living cells from laser scanning confocal image sequences by the cross correlation raster image spectroscopy method. Biophys J 96:707–16 DOI 10.1016/j.bpj.2008.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Digman MA, Stakic M, Gratton E (2013) Raster image correlation spectroscopy and number and brightness analysis. Methods Enzymol 518:121–44 DOI 10.1016/B978-0-12-388422-0.00006-6 [DOI] [PubMed] [Google Scholar]
- 19.Dobrinskikh E, Giral H, Caldas YA, Levi M, Doctor RB (2010) Shank2 redistributes with NaPilla during regulated endocytosis. Am J Physiol Cell Physiol 299:C1324–34 DOI 10.1152/ajpcell.00183.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobrinskikh E, Lanzano L, Rachelson J, Cranston D, Moldovan R, Lei T, Gratton E, Doctor RB (2013) Shank2 contributes to the apical retention and intracellular redistribution of NaPiIIa in OK cells. Am J Physiol Cell Physiol 304:C561–73 DOI 10.1152/ajpcell.00189.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster IC, Hernando N, Biber J, Murer H (2006) Proximal tubular handling of phosphate: A molecular perspective. Kidney Int 70:1548–59 DOI [DOI] [PubMed] [Google Scholar]
- 22.Giral H, Lanzano L, Caldas Y, Blaine J, Verlander JW, Lei T, Gratton E, Levi M (2011) Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J Biol Chem 286:15032–42 DOI 10.1074/jbc.M110.199752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H (2001) Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem 276:9206–13 DOI 10.1074/jbc.M008745200 [DOI] [PubMed] [Google Scholar]
- 24.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H (2003) PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int 64:1733–45 DOI 10.1046/j.1523-1755.2003.00266.x [DOI] [PubMed] [Google Scholar]
- 25.Golfetto O, Hinde E, Gratton E (2013) Laurdan fluorescence lifetime discriminates cholesterol content from changes in fluidity in living cell membranes. Biophys J 104:1238–47 DOI 10.1016/j.bpj.2012.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golfetto O, Hinde E, Gratton E (2015) The Laurdan spectral phasor method to explore membrane micro-heterogeneity and lipid domains in live cells. Methods Mol Biol 1232:273–90 DOI 10.1007/978-1-4939-1752-5_19 [DOI] [PubMed] [Google Scholar]
- 27.Hammerman MR, Karl IE, Hruska KA (1980) Regulation of canine renal vesicle Pi transport by growth hormone and parathyroid hormone. Biochim Biophys Acta 603:322–35 DOI [DOI] [PubMed] [Google Scholar]
- 28.Hernando N, Deliot N, Gisler SM, Lederer E, Weinman EJ, Biber J, Murer H (2002) PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc Natl Acad Sci U S A 99:11957–62 DOI 10.1073/pnas.182412699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernando N, Wagner CA, Gisler SM, Biber J, Murer H (2004) PDZ proteins and proximal ion transport. Curr Opin Nephrol Hypertens 13:569–74 DOI [DOI] [PubMed] [Google Scholar]
- 30.Hernando N, Gisler SM, Pribanic S, Deliot N, Capuano P, Wagner CA, Moe OW, Biber J, Murer H (2005) NaPi-IIa and interacting partners. J Physiol 567:21–6 DOI 10.1113/jphysiol.2005.087049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinde E, Digman MA, Welch C, Hahn KM, Gratton E (2012) Biosensor Forster resonance energy transfer detection by the phasor approach to fluorescence lifetime imaging microscopy. Microsc Res Tech 75:271–81 DOI 10.1002/jemt.21054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinde E, Digman MA, Hahn KM, Gratton E (2013) Millisecond spatiotemporal dynamics of FRET biosensors by the pair correlation function and the phasor approach to FLIM. Proc Natl Acad Sci U S A 110:135–40 DOI 10.1073/pnas.1211882110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue M, Digman MA, Cheng M, Breusegem SY, Halaihel N, Sorribas V, Mantulin WW, Gratton E, Barry NP, Levi M (2004) Partitioning of NaPi cotransporter in cholesterol-, sphingomyelin-, and glycosphingolipid-enriched membrane domains modulates NaPi protein diffusion, clustering, and activity. J Biol Chem 279:49160–71 DOI 10.1074/jbc.M408942200 [DOI] [PubMed] [Google Scholar]
- 34.Jameson DM (1998) Gregorio Weber, 1916-1997: a fluorescent lifetime. Biophys J 75:419–21 DOI 10.1016/S0006-3495(98)77528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempson SA, Dousa TP (1979) Phosphate transport across renal cortical brush border membrane vesicles from rats stabilized on a normal, high or low phosphate diet. Life Sci 24:881–7 DOI [DOI] [PubMed] [Google Scholar]
- 36.Kempson SA, Lotscher M, Kaissling B, Biber J, Murer H, Levi M (1995) Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol 268:F784–91 DOI 10.1152/ajprenal.1995.268.4.F784 [DOI] [PubMed] [Google Scholar]
- 37.Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J (1998) Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int 54:1224–32 DOI 10.1046/j.1523-1755.1998.00115.x [DOI] [PubMed] [Google Scholar]
- 38.Lanzano L, Digman MA, Fwu P, Giral H, Levi M, Gratton E (2011) Nanometer-scale imaging by the modulation tracking method. J Biophotonics 4:415–24 DOI 10.1002/jbio.201100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O, Gratton E, Levi M, Blaine J (2011) Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol 301 :C850–61 DOI 10.1152/ajpcell.00412.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levi M, Jameson DM, van der Meer BW (1989) Role of BBM lipid composition and fluidity in impaired renal Pi transport in aged rat. Am J Physiol 256:F85–94 DOI 10.1152/ajprenal.1989.256.1.F85 [DOI] [PubMed] [Google Scholar]
- 41.Levi M, Baird BM, Wilson PV (1990) Cholesterol modulates rat renal brush border membrane phosphate transport. J Clin Invest 85:231–7 DOI 10.1172/JCI114417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levi M, Wilson PV, Cooper OJ, Gratton E (1993) Lipid phases in renal brush border membranes revealed by Laurdan fluorescence. Photochem Photobiol 57:420–5 DOI [DOI] [PubMed] [Google Scholar]
- 43.Levi M, Lotscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J (1994) Cellular mechanisms of acute and chronic adaptation of rat renal P(i) transporter to alterations in dietary P(i). Am J Physiol 267:F900–8 DOI 10.1152/ajprenal.1994.267.5.F900 [DOI] [PubMed] [Google Scholar]
- 44.Levi M, Shayman JA, Abe A, Gross SK, McCluer RH, Biber J, Murer H, Lotscher M, Cronin RE (1995) Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J Clin Invest 96:207–16 DOI 10.1172/JCI118022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi M, Kempson SA, Lotscher M, Biber J, Murer H (1996) Molecular regulation of renal phosphate transport. J Membr Biol 154:1–9 DOI [DOI] [PubMed] [Google Scholar]
- 46.Lotscher M, Kaissling B, Biber J, Murer H, Levi M (1997) Role of microtubules in the rapid regulation of renal phosphate transport in response to acute alterations in dietary phosphate content. J Clin Invest 99:1302–12 DOI 10.1172/JCI119289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, Biber J, Murer H, Kaissling B (1999) Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest 104:483–94 DOI 10.1172/JCI3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H (1993) Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A 90:5979–83 DOI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahon MJ, Donowitz M, Yun CC, Segre GV (2002) Na(+)/H(+ ) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417:858–61 DOI 10.1038/nature00816 [DOI] [PubMed] [Google Scholar]
- 50.Mahon MJ, Cole JA, Lederer ED, Segre GV (2003) Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17:2355–64 DOI 10.1210/me.2003-0043 [DOI] [PubMed] [Google Scholar]
- 51.Malacrida L, Gratton E, Jameson DM (2015) Model-free methods to study membrane environmental probes: a comparison of the spectral phasor and generalized polarization approaches. Methods Appl Fluoresc 3:047001 DOI 10.1088/2050-6120/3/4/047001 [DOI] [PubMed] [Google Scholar]
- 52.Malacrida L, Jameson DM, Gratton E (2017) A multidimensional phasor approach reveals LAURDAN photophysics in NIH-3T3 cell membranes. Sci Rep 7:9215 DOI 10.1038/s41598-017-08564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannstadt M, Juppner H, Gardella TJ (1999) Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol 277:F665–75 DOI [DOI] [PubMed] [Google Scholar]
- 54.McWilliams RR, Breusegem SY, Brodsky KF, Kim E, Levi M, Doctor RB (2005) Shank2E binds NaP(i) cotransporter at the apical membrane of proximal tubule cells. Am J Physiol Cell Physiol 289:C1042–51 DOI 10.1152/ajpcell.00568.2004 [DOI] [PubMed] [Google Scholar]
- 55.Molitoris BA, Alfrey AC, Harris RA, Simon FR (1985) Renal apical membrane cholesterol and fluidity in regulation of phosphate transport. Am J Physiol 249:F12–9 DOI 10.1152/ajprenal.1985.249.1.F12 [DOI] [PubMed] [Google Scholar]
- 56.Muff R, Fischer JA, Biber J, Murer H (1992) Parathyroid hormone receptors in control of proximal tubule function. Annu Rev Physiol 54:67–79 DOI 10.1146/annurev.ph.54.030192.000435 [DOI] [PubMed] [Google Scholar]
- 57.Murer H, Werner A, Reshkin S, Wuarin F, Biber J (1991) Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol 260:C885–99 DOI 10.1152/ajpcell.1991.260.5.C885 [DOI] [PubMed] [Google Scholar]
- 58.Murer H (1992) Homer Smith Award. Cellular mechanisms in proximal tubular Pi reabsorption: some answers and more questions. J Am Soc Nephrol 2:1649–65 DOI [DOI] [PubMed] [Google Scholar]
- 59.Murer H, Lotscher M, Kaissling B, Levi M, Kempson SA, Biber J (1996) Renal brush border membrane Na/Pi-cotransport: molecular aspects in PTH-dependent and dietary regulation. Kidney Int 49:1769–73 DOI [DOI] [PubMed] [Google Scholar]
- 60.Murer H, Hernando N, Forster I, Biber J (2000) Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80:1373–409 DOI 10.1152/physrev.2000.80.4.1373 [DOI] [PubMed] [Google Scholar]
- 61.Ohkido I, Segawa H, Yanagida R, Nakamura M, Miyamoto K (2003) Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch 446:106–15 DOI 10.1007/s00424-003-1010-6 [DOI] [PubMed] [Google Scholar]
- 62.Parasassi T, Conti F, Gratton E (1986) Time-resolved fluorescence emission spectra of Laurdan in phospholipid vesicles by multifrequency phase and modulation fluorometry. Cell Mol Biol 32:103–8 DOI [PubMed] [Google Scholar]
- 63.Parasassi T, Gratton E, Yu WM, Wilson P, Levi M (1997) Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys J 72:2413–29 DOI 10.1016/S0006-3495(97)78887-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parasassi T, Gratton E, Zajicek H, Levi M, Yu W (1999) Detecting membrane lipid microdomains by two-photon fluorescence microscopy. IEEE Eng Med Biol Mag 18:92–9 DOI [DOI] [PubMed] [Google Scholar]
- 65.Pfister MF, Ruf I, Stange G, Ziegler U, Lederer E, Biber J, Murer H (1998) Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter. Proc Natl Acad Sci U S A 95:1909–14 DOI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranjit S, Lanzano L, Gratton E (2014) Mapping diffusion in a living cell via the phasor approach. Biophys J 107:2775–2785 DOI 10.1016/j.bpj.2014.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranjit S, Malacrida L, Jameson DM, Gratton E (2018) Fit-free analysis of fluorescence lifetime imaging data using the phasor approach. Nat Protoc 13:1979–2004 DOI 10.1038/s41596-018-0026-5 [DOI] [PubMed] [Google Scholar]
- 68.Rossow MJ, Sasaki JM, Digman MA, Gratton E (2010) Raster image correlation spectroscopy in live cells. Nat Protoc 5:1761–74 DOI 10.1038/nprot.2010.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruan Q, Cheng MA, Levi M, Gratton E, Mantulin WW (2004) Spatial-temporal studies of membrane dynamics: scanning fluorescence correlation spectroscopy (SFCS). Biophys J 87:1260–7 DOI 10.1529/biophysj.103.036483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K (2002) Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277:19665–72 DOI 10.1074/jbc.M200943200 [DOI] [PubMed] [Google Scholar]
- 71.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K (2005) Internalization of renal type Ilc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol 288:F587–96 DOI 10.1152/ajprenal.00097.2004 [DOI] [PubMed] [Google Scholar]
- 72.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K (2007) Parathyroid hormone-dependent endocytosis of renal type Ilc Na-Pi cotransporter. Am J Physiol Renal Physiol 292:F395–403 DOI 10.1152/ajprenal.00100.2006 [DOI] [PubMed] [Google Scholar]
- 73.Shenolikar S, Weinman EJ (2001) NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280:F389–95 DOI 10.1152/ajprenal.2001.280.3.F389 [DOI] [PubMed] [Google Scholar]
- 74.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ (2002) Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci U S A 99:11470–5 DOI 10.1073/pnas.162232699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorribas V, Lotscher M, Loffing J, Biber J, Kaissling B, Murer H, Levi M (1996) Cellular mechanisms of the age-related decrease in renal phosphate reabsorption. Kidney Int 50:855–63 DOI [DOI] [PubMed] [Google Scholar]
- 76.Stoll R, Kinne R, Murer H (1979) Effect of dietary phosphate intake on phosphate transport by isolated rat renal brush-border vesicles. Biochem J 180:465–70 DOI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Traebert M, Roth J, Biber J, Murer H, Kaissling B (2000) Internalization of proximal tubular type II Na-P(i) cotransporter by PTH: immunogold electron microscopy. Am J Physiol Renal Physiol 278:F148–54 DOI 10.1152/ajprenal.2000.278.1.F148 [DOI] [PubMed] [Google Scholar]
- 78.Traebert M, Volkl H, Biber J, Murer H, Kaissling B (2000) Luminal and contraluminal action of 1-34 and 3-34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol 278:F792–8 DOI 10.1152/ajprenal.2000.278.5.F792 [DOI] [PubMed] [Google Scholar]
- 79.Villa-Bellosta R, Barac-Nieto M, Breusegem SY, Barry NP, Levi M, Sorribas V (2008) Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int 73:456–64 DOI 10.1038/sj.ki.5002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC (2009) The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol 296:F691–9 DOI 10.1152/ajprenal.90623.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ (2001) Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280:C192–8 DOI 10.1152/ajpcell.2001.280.1.C192 [DOI] [PubMed] [Google Scholar]
- 82.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ (2003) Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285:C1494–503 DOI 10.1152/ajpcell.00092.2003 [DOI] [PubMed] [Google Scholar]
- 83.EJ Weinman, Minkoff C, Shenolikar S (2000) Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am J Physiol Renal Physiol 279:F393–9 DOI 10.1152/ajprenal.2000.279.3.F393 [DOI] [PubMed] [Google Scholar]
- 84.Werner A, Kempson SA, Biber J, Murer H (1994) Increase of Na/Pi-cotransport encoding mRNA in response to low Pi diet in rat kidney cortex. J Biol Chem 269:6637–9 DOI [PubMed] [Google Scholar]
- 85.Zajicek HK, Wang H, Puttaparthi K, Halaihel N, Markovich D, Shayman J, Beliveau R, Wilson P, Rogers T, Levi M (2001) Glycosphingolipids modulate renal phosphate transport in potassium deficiency. Kidney Int 60:694–704 DOI 10.1046/j.1523-1755.2001.060002694.x [DOI] [PubMed] [Google Scholar]


