Abstract
Objective:
To study the predictive relationship among persons with traumatic brain injury (TBI) between an objective indicator of injury severity (the adapted Marshall computed tomography [CT] classification scheme) and clinical indicators of injury severity in the acute phase; functional outcomes at inpatient rehabilitation discharge; and functional and participation outcomes at 1 year after injury, including death.
Participants:
The sample involved 4,895 individuals who received inpatient rehabilitation following acute hospitalization for TBI and were enrolled in the Traumatic Brain Injury Model System National Data Base between 1989 and 2014.
Design:
Head CT variables for each person were fit into adapted Marshall CT classification categories I through IV.
Main Measures:
Prediction models were developed to determine the amount of variability explained by the CT classification categories compared with commonly used predictors, including a clinical indicator of injury severity.
Results:
The adapted Marshall classification categories aided only in the prediction of craniotomy or craniectomy during acute hospitalization, otherwise making no meaningful contribution to variance in the multivariable models predicting outcomes at any time point after injury.
Conclusion:
Results suggest that head CT findings classified in this manner do not inform clinical discussions related to functional prognosis or rehabilitation planning after TBI.
Keywords: clinical decision making, computed tomography, craniocerebral trauma, forecasting, neuroimaging
Introduction
Computed tomography (CT) of the head is the standard initial brain imaging technique used to evaluate persons with suspected acute traumatic brain injury (TBI). It also is the only neuroimaging method accepted by the US Food and Drug Administration for use in TBI-related drug and device trials. The sensitivity of head CT to acute life-threatening intracranial hemorrhage, contusion, mass effect, and fracture—as well as its broad availability, relatively low cost, and rapid image acquisition—contributes to its widespread acceptance in TBI clinical practice and research (1, 2). In addition to information about injury type, location, and severity, head CT findings provide information that directs medical triage and surgical decision making, and they are commonly used in the acute postinjury phase during discussions with patients and their families about prognosis for recovery. Beyond the contribution that head CT makes to mortality prediction, its findings may predict longer-term functional outcomes, to inform clinical dialogue and the transitional care planning that occur in the early stages of recovery after TBI.
Marshall et al (3) developed an injury severity classification scheme using findings on the initial head CT of patients with severe TBI. Their classification focused on the status of the basal cisterns, degree of midline shift, presence of a mass lesion, and criteria for surgical evacuation. The Marshall classification scheme has independently predicted categories of mortality rate and broad clinical outcome categories in the acute postinjury period (3). A collapsed Marshall classification scheme used in multiple clinical trials after moderate-severe TBI also predicts mortality and clinical outcomes 6 months after injury, with Marshall classification IV contributing most strongly to these predictions (4, 5). The Marshall classification categories were used as the basis for developing head CT variables contained in the longitudinal Traumatic Brain Injury Model Systems (TBIMS) National Data Base (NDB) (6).
Analyses predicting functional outcomes after TBI in the NDB that used the available head CT variables have yielded conflicting results. Midline shift >5 mm, subcortical contusions, and bilateral cortical contusions have been associated with increased need for assistance with ambulation, activities of daily living, and supervision at rehabilitation discharge (7). However, when head CT variables were used in a recursive partitioning analysis in this data set, no additional value was gained with respect to prediction of outcomes at rehabilitation discharge or at 1 year postinjury (8). In cohorts of persons with severe head injury, use of a CT-based classification system in outcome prediction models has been supported by class 1 and class 2 evidence, with ≥70% positive predictive value for 6-month outcomes (9). Of particular interest is whether a head CT classification scheme derived from the TBIMS NDB variables for CT is useful in predicting clinical measures of injury severity in the acute phase and in clinically relevant postacute and long-term outcomes after moderate-severe TBI in this large representative US data set.
The purpose of this study was 2-fold. First, its objective was to examine the predictive relationship between an adapted Marshall classification scheme derived from TBIMS NDB head CT data and the clinical measures of injury severity in the acute phase of recovery, including neurosurgical procedures. Second, its objective was to determine whether this CT classification scheme independently contributes to prediction of functional and participation outcomes at rehabilitation discharge and at 1 year after injury—after control for established clinical indicators of injury severity and outcome prediction.
Methods
Patient Sample
The study participants are individuals enrolled in the TBIMS NDB from 1989 to 2014. The TBIMS NDB—a multicenter, prospective, longitudinal investigation—analyzes a patient’s recovery progression and outcomes after receipt of coordinated inpatient rehabilitation for acute neurotrauma (6). The NDB includes self-reported outcomes from up to 25 years postinjury for >15,000 patients, with 16 currently funded and 3 previously funded follow-up TBIMS centers contributing new data of patients and follow-up quarterly. For the present study, the patient cohort was composed only of those participants with CT scan data, a documented time interval from injury to the ability to accurately follow simple motor commands as a clinical indicator of injury severity, and outcomes of interest at rehabilitation admission, rehabilitation discharge, and at 1 year.
Head CT Variables and Classification
The head CT variables of the TBIMS NDB were developed using the Marshall classification model (3). All but 2 elements of the original Marshall classification scheme are included in these variables. High- or mixed-density lesions with volumes >25 cc and those lesions that were surgically evacuated were excluded as head CT variables in the data set because they are not described consistently in clinical neuroradiology reports, which are the source of CT data for the NDB. When established in 1988, the TBIMS NDB head CT variables were extended to include cortical or subcortical, or both, contusion locations, extra-axial blood and its locations, and intracranial fragments. However, those specific data elements were not included in the adapted Marshall scheme used in this analysis because the lesion volumes could not be reliably or consistently quantified. Head CT data in the NDB are abstracted by the medical director or other personnel trained on the criteria established by the TBIMS Data Committee at each TBIMS center. The data are from clinical reports produced by radiologists at each acute care hospital from which referred participants are entered into the TBIMS NDB. Radiology reports of all head CT scans obtained for each TBIMS NDB participant during the 7 days following injury are reviewed and recorded as an aggregate on the CT case report form. This approach avoids reliance on the initial or “worst” CT scans, which may differ in predictive power (10, 11). All investigators abstracting and entering head CT data into the TBIMS NDB are certified initially to a standard of ≥80% accuracy against consensus scoring in a training set of head CT reports. They must recertify to that standard at a frequency of every 10 years. The head CT data in the TBIMS NDB have been shown to have acceptable interrater reliability (7).
Because the TBIMS NDB head CT variables do not include data required to establish a complete Marshall classification (ie, high- or mixed-density lesions with volumes >25 cc and whether they were surgically evacuated), each study participant’s head CT variables were classified into only the original Marshall classification categories I through IV (Table 1), referred to as adapted categories. These categories indicate progressive severity of TBI-related structural abnormality.
Table 1.
Adapted Marshall Head Computed Tomography Scan Classification Categories
| Category | Definition |
|---|---|
| I | No visible pathologic condition exists; no visible intracranial compression is seen |
| II | Intracranial pathologic condition exists; cisterns are present but midline shift of 1–5 mm is noted |
| III | Intracranial pathologic condition exists; cisterns are compressed or absent, with midline shift of 0–5 mm |
| IV | Intracranial pathologic condition exists; midline shift is >5 mm |
Adapted from Marshall et al (3). Used with permission.
Clinical Indicators of Injury Severity
We studied the predictive relationship between an objective indicator of injury severity provided by the adapted Marshall classification and the clinical indicators of injury severity in the data set. These indicators included time to recovery of command following, defined by the first date that the patient was able to follow simple motor commands accurately at least 2 times consecutively in 24 hours. They also included duration of posttraumatic amnesia (PTA), defined as the number of days between the TBI and the first of 2 occasions within 72 hours that the participant was fully oriented), and the onset days, defined as the time from TBI to rehabilitation admission. The Glasgow Coma Scale score was not used in this analysis because this variable was missing for one-half of the participant sample.
Continuous and Pseudocontinuous Outcomes Measures
Functional outcome measures at rehabilitation discharge were rehabilitation length of stay (RLOS) in days, Functional Independence Measure (FIM) (UB Foundation Activities, Inc) total score, FIM motor score, FIM cognitive score, and the Disability Rating Scale (DRS) score. The FIM instrument and the DRS are measures of activity limitations and functional outcome that have been validated in this patient population (12–17) and are widely used.
Outcomes at 1 year postinjury were FIM total score, FIM cognitive score, DRS, and Extended Glasgow Outcome Scale (EGOS) (18). The outcome scale is a global scale that has been validated in this patient population (18–21) and is widely used.
Dichotomous Outcomes
We examined the contribution that this CT classification scheme made to predicting 8 outcomes. The outcomes were 1) intracranial hypertension (defined as intracranial pressure ≥20 mm Hg) during acute hospitalization; 2) craniotomy or craniectomy during acute hospitalization; 3) interruption in care during inpatient rehabilitation; 4) rehabilitation discharge to a private residence (vs any other location); 5) rehospitalization during the year after rehabilitation discharge; 6) living in a private residence at 1 year; 7) productive activity at 1 year (defined as being a full-time student, part-time student, or competitively employed or taking care of house or family) (22), and 8) death during the year after rehabilitation discharge.
Data Analyses
All analyses were performed using statistical software (SAS version 9.4; SAS Institute Inc). These analyses were to determine the distinct proportion of variance explained by the adapted Marshall classification categories I through IV as 1) predictors of clinical indicators of injury severity (ie, time to recovery of command following, PTA duration, and onset days) among covariates and 2) predictors of functional and participation outcomes of interest at rehabilitation discharge and at 1 year after injury. Covariates at all time points included age, sex, race/ethnicity, and level of education. Additional covariates of the initial time to follow motor commands and onset days were included in predictive models for outcomes at rehabilitation discharge and at 1 year, and RLOS was included as a covariate for 1-year outcomes. A backwards selection procedure (with an α criterion of .05) was used to identify candidate predictors because it allows all variables to be considered in the model reduction process. After reduced models were identified for continuous and pseudocontinuous outcomes, a semipartial omega squared statistic (SPOS) was used to assess the amount of variability explained by the CT classification categories and to evaluate the contribution of CT classification categories to outcome prediction after controlling for other predictors. The SPOS is an unbiased estimate of the amount of variability explained by a predictor after controlling for the other model predictors (23).
An analogous SPOS value does not exist when the outcome variable is dichotomous for models predicting intracranial hypertension, craniotomy or craniectomy during acute hospitalization, rehabilitation interruption, rehabilitation discharge location, rehospitalization during the year following rehabilitation discharge, private residence at 1 year, productive activity and death at 1 year. We therefore compared the difference in the sensitivity (true positive), specificity (true negative), and percent correct classification for the reduced model containing the adapted Marshall classification categories with the reduced model in which these classification categories were removed.
Results
The sample was composed of 4,895 patients who had TBI with characteristics and outcomes at rehabilitation admission, rehabilitation discharge, and 1 year after TBI (Tables 2, 3, and 4, respectively). The average participant was almost 44 years old at the time of injury, spent 3 weeks in an acute care hospital before rehabilitation admission, and was admitted to rehabilitation with moderate-severe activity limitations (mean [SD] total admission FIM score, 49.5 [22.4]). On average, the patient was hospitalized for rehabilitation for about 26 days and discharged after substantial functional improvement (mean [SD] total discharge FIM score, 89 [21.9]), most often toa private residence (81%). The mean time to return of ability to follow command among the participants was 7.6 days postinjury; the mean PTA duration was 22 days. About 18% of the sample were lost to follow-up at 1 year. Mean (SD) total FIM score at 1 year increased to 113.8 (18.2), one-third of participants were classified in the good recovery EGOS categories, and one-third of the sample reported participation in productive activity. By 1 year after TBI, 140 deaths had occurred.
Table 2.
Sample Participant Characteristics at Rehabilitation Admission
| Characteristic | Valuea (N=4,895) |
|---|---|
| Age, mean (SD), y | 43.78 (20.15) |
| Female sex | 1,307 (26.7) |
| Race/ethnicity | |
| White | 3,354 (68.5) |
| Black | 790 (16.1) |
| Hispanic | 535 (10.9) |
| Asian/Pacific Islander, Native American | 160 (3.3) |
| Missing | 56 (1.1) |
| Education | |
| Less than high school diploma | 1,048 (21.4) |
| High school or GED diploma | 1,748 (35.7) |
| More than high school diploma | 2,060 (42.1) |
| Missing | 39 (0.8) |
| FIM motor score admit, mean (SD) | 34.64 (17.17) |
| Missing | 42 (0.9) |
| FIM cognitive score admit, mean (SD) | 14.86 (7.48) |
| Missing | 9 (0.2) |
| DRS score at rehabilitation admit, mean (SD) | 11.72 (5.16) |
| Missing | 63 (1.3) |
Abbreviations: admit, admission; DRS, Disability Rating Scale; FIM, Functional Independence Measure instrument; GED, General Educational Development; missing, data unavailable.
Values are presented as number and percentage of patients unless specified otherwise.
Table 3.
Sample Characteristics of Participants at Rehabilitation Discharge
| Characteristic | Valuea (N=4,895) |
|---|---|
| Rehabilitation length of stay, mean (SD), d | 25.50 (22.67) |
| Missing | 10 (0.2) |
| FIM total score, mean (SD) | 89.03 (21.92) |
| Missing | 72 (1.5) |
| FIM motor score, mean (SD) | 65.45 (17.73) |
| Missing | 66 (1.3) |
| FIM cognitive score, mean (SD) | 23.62 (6.56) |
| Missing | 15 (0.3) |
| Days to follow motor commands, mean (SD) | 7.58 (13.51) |
| Duration of PTA, mean (SD), d | 22.42 (21.46) |
| PTA still apparent | 840 (17.2) |
| Rehabilitation interruption | 295 (6.0) |
| Transfer to private residence | 3,978 (81.3) |
| Missing | 8 (0.2) |
Abbreviations: FIM, Functional Independence Measure instrument; missing, data unavailable; PTA, posttraumatic amnesia.
Presented as number and percentage of patients unless specified otherwise.
Table 4.
Characteristics of Participants at 1 Year
| Characteristic | Valuea (N=4,895) |
|---|---|
| FIM total score, mean (SD) | 113.79 (18.21) |
| Missing | 900 (18.4) |
| FIM motor score, mean (SD) | 83.13 (14.42) |
| Missing | 897 (18.3) |
| FIM cognitive score, mean (SD) | 30.64 (5.15) |
| Missing | 863 (17.6) |
| DRS score, mean (SD) | 4.17 (6.69) |
| Missing | 1,837 (37.5) |
| Education | |
| Less than high school diploma | 756 (15.4) |
| High school or GED diploma | 1,435 (29.3) |
| More than high school diploma | 1,983 (40.5) |
| Missing | 721 (14.7) |
| EGOS upper and lower good recovery | 1,436 (29.3) |
| Missing | 854 (17.5) |
| Living in a private residence | 3,888 (79.4) |
| Missing | 647 (13.2) |
| Productive activity | 1,420 (29.0) |
| Missing | 806 (16.5) |
| Rehospitalization | 1,120 (22.9) |
| Missing | 738 (15.1) |
| Death | 140 (2.9) |
Abbreviations: DRS, Disability Rating Scale; EGOS, Extended Glasgow Outcome Scale; FIM, Functional Independence Measure instrument; GED, General Educational Development; missing, data unavailable.
Values are presented as number and percentage of patients unless specified otherwise.
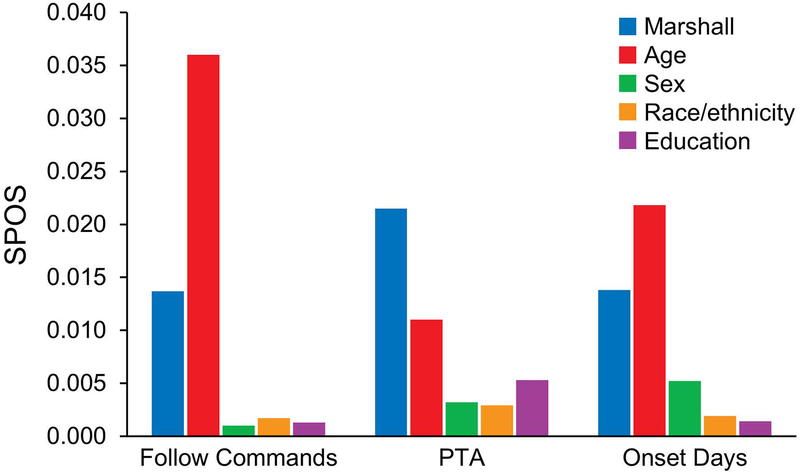
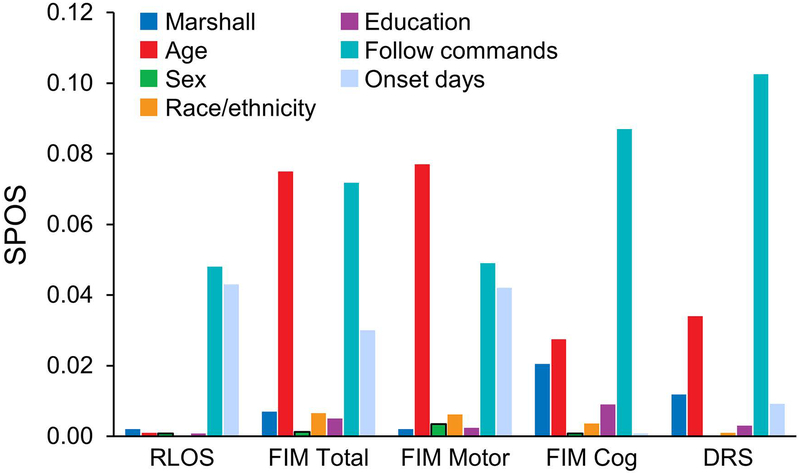
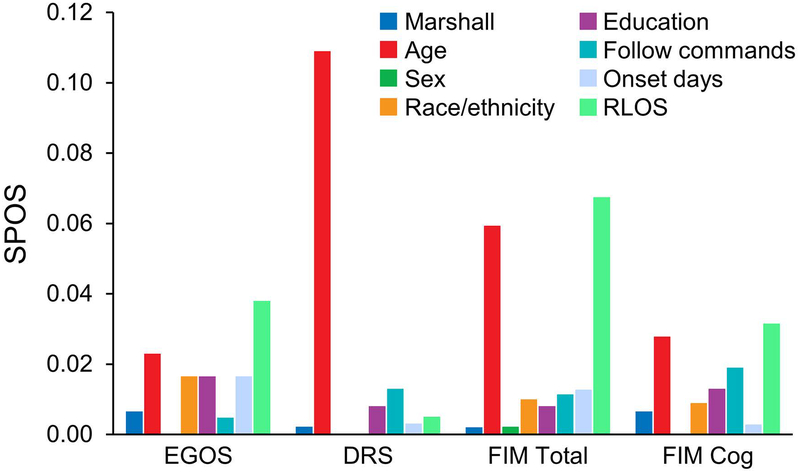
The adapted Marshall classification categories did not meaningfully contribute to the multivariable models predicting outcomes at any time point after injury. Compared with the adapted Marshall classification, participant age contributed the most variance among covariates in prediction of clinical measures of injury severity in the acute phase after TBI, although the extent of this contribution was trivial (Figure 1). Initial time to follow commands—a clinical indicator of injury severity—and age made the strongest contributions to prediction of outcome at rehabilitation discharge (Figure 2). By comparison, age and RLOS contributed the most variance among outcomes at 1 year (Figure 3).
Figure 1.
Adapted Marshall Contribution to SPOS for Clinical Indicators of Injury Severity. Follow commands indicates days to recovery of command following after injury; Marshall, adapted Marshall classification categories I through IV; onset days, time from injury to rehabilitation admission; PTA, posttraumatic amnesia in days; SPOS, semipartial omega squared statistic.
Figure 2.
Adapted Marshall Contribution to SPOS at Rehabilitation Discharge. DRS indicates Disability Rating Scale; FIM, Functional Independence Measure; follow commands, days to recovery of command following after injury; Marshall, adapted Marshall classification categories I through IV; onset days, time from injury to rehabilitation admission; RLOS, rehabilitation length of stay, days; SPOS, semipartial omega squared statistic.
Figure 3.
Adapted Marshall Contribution to SPOS at 1 Year. DRS indicates Disability Rating Scale; EGOS, Extended Glasgow Outcome Scale, category dichotomized to good recovery or less than good recovery; FIM, Functional Independence Measure; follow commands, time in days to recovery of command following after injury; Marshall, adapted Marshall classification categories I through IV; onset days: time from injury to rehabilitation admission; RLOS, rehabilitation length of stay in days; SPOS, semipartial omega squared statistic.
Table 5 outlines the change in sensitivity, specificity, and overall classification rate for the predictive models, considering dichotomous outcome variables with and without the CT classification categories. The adapted Marshall classification categories aided only in prediction of whether a craniotomy or craniectomy was performed during acute hospitalization, reflected in its increase in percent correct classification.
Table 5.
Change in Sensitivity, Specificity, and Correct Classification Rate of the Predictive Models for Dichotomous Outcome Variables With and Without Inclusion of Adapted Marshall Classification Categories
| With Adapted Marshall Classification, % | Without Adapted Marshall Classification, % | |||||
|---|---|---|---|---|---|---|
| Outcome | Sensitivity | Specificity | Correct Classification | Sensitivity | Specificity | Correct Classification |
| Intracranial hypertension | 19.3 | 95.4 | 79.0 | 0.0 | 100.0 | 78.5 |
| Craniotomy or craniectomy | 52.4 | 93.7 | 83.1 | 0.0 | 100.0 | 74.2 |
| Rehabilitation interruption | 0.0 | 100.0 | 93.9 | 0.0 | 100.0 | 93.9 |
| Private residence at rehabilitation discharge | 99.2 | 2.0 | 81.1 | 99.2 | 1.9 | 81.1 |
| Private residence at 1 y after TBI | 99.8 | 3.2 | 91.7 | 99.8 | 3.4 | 91.7 |
| Rehospitalization during 1 y after TBI | 2.1 | 99.4 | 73.2 | 2.0 | 99.5 | 73.2 |
| Productive activity at 1 y after TBI | 52.9 | 85.5 | 74.2 | 51.8 | 85.6 | 73.9 |
| Death during 1 y after TBI | 0.0 | 100 | 97.1 | 0.0 | 100.0 | 97.1 |
Abbreviation: TBI, traumatic brain injury.
Discussion
The results of our analysis indicate that the adapted Marshall CT classification system developed for and used in this analysis neither meaningfully predicted acute clinical indicators of injury severity nor predicted functional or participation outcomes at rehabilitation discharge or 1 year after TBI. The large data set of assiduously characterized patients with TBI that was used in these analyses, the TBIMS NDB, provided the sample size needed to adequately evaluate the predictive models. This head CT classification system added meaningfully to variance only for the acute phase variable of whether a craniotomy or craniectomy was performed—an expected finding given the well-established utility of head CT in surgical decision making after TBI.
These results are consistent with a previous report using the TBIMS NDB, which showed that PTA, initial time to follow motor commands, and participant age dominated prediction of outcomes at rehabilitation discharge and at 1 year, with abnormal head CT findings adding no predictive value (8). The findings reported herein that used an adapted Marshall classification scheme are also consistent with those of a Scottish head injury registry, which showed that use of the complete Marshall classification of initial head CT findings did not contribute to a multivariable model for prediction of other outcomes, such as survival at 1 year after TBI in all severity levels (24). In addition, precise quantitative volumetric analysis of head CT lesions has failed to predict cognitive outcome during rehabilitation hospitalization beyond demographic factors and time to follow commands (25).
The results of this study stand in contrast to a previous study involving neuroimaging findings in the TBIMS NDB to predict outcome (7). That analysis reported that >5 mm of midline shift and presence of subcortical contusions were associated with increased need for assistance in mobility, self-care, and overall need for supervision at rehabilitation hospital discharge. At 1 year, midline shift of >5 mm was associated with need for supervision, and presence of subcortical or bilateral cortical contusions was associated with increased need for assistance with ambulation and stairs. Outcome measures for the analysis (eg, FIM score, DRS using its level of function subscale) were dichotomized. The differences between those findings and our results likely relate to the multivariable analytic methods used in this analysis. These differences also may reflect the utility of evaluation of head CT findings for neuroanatomic injury individually rather than as part of an adapted CT classification system that does not consider cerebral contusion or subdural hematoma.
The lack of an association between CT classification and outcome reported herein also differs from findings in other large data sets of acute care clinical trial samples (4, 5, 26). This difference is likely due to variation in cohort types and outcomes measured. The TBIMS NDB includes only the patients with TBI admitted for inpatient rehabilitation, who are likely to have different injury severity and head CT findings than acute clinical trial cohorts. The mean age of our TBIMS sample was also about 10 years greater than reported in the clinical trials, and age has a primary unmodifiable influence on outcome. In addition, the outcome measures used in our analysis described function and participation status in detail, compared with the 6-month mortality rate commonly reported in clinical trials. Our use of head CT findings grouped into an adapted classification system did not consider contusion, subdural or epidural hematoma, or subarachnoid or intraventricular hemorrhage, which have distinctly contributed to prediction of death and 6-month EGOS outcomes using acute data sets after moderate to severe injury (5, 26).
Multiple other factors likely influenced the results. Certain early abnormalities found on head CT are reversible and unassociated with lasting sequelae. Acute CT is often unable to identify the lesions (eg, punctate hemorrhages secondary to traumatic axonal injury) commonly seen after TBI that affect cognitive performance and are linked to outcome. Not surprisingly, static neuroimaging may have limitations as a predictor of something as complex as functional and participation outcomes, which are affected not only by structural injury but also by neuronal and functional compensation, psychosocial support systems, and motivation.
These findings have potential clinical relevance. Injured individuals and their families rely on their surgical and rehabilitation providers for estimates of prognosis and hope in the acute phase after injury. Our results, along with other reports showing limited prognostic utility of acute head CT findings for functional outcomes (8, 24, 25), suggest that this information is not useful when discussing an individual’s future function and participation. These results also may have meaning for research methodology and measurement selection because they indicate that the method of classifying head CT findings used in this study should not be used for long-term outcome prediction, for determination of treatment benefits, or for establishment of prognosis following such types of injuries as those of our study participants.
More advanced and precise imaging modalities (eg, functional magnetic resonance imaging, diffusion tensor imaging, susceptibility weighted imaging) and analysis methods might be expected to contribute most to predicting functional outcomes from an imaging perspective because they have potential to provide more detailed anatomical, functional, and metabolic data (27–29). However, these technologies are more difficult to perform during the acute TBI period and are not broadly available in communities.
Limitations
All patients in the TBIMS NDB were admitted for inpatient rehabilitation, and these results may not generalize to people with TBI who do not receive acute inpatient rehabilitation or are not hospitalized. In addition, CT techniques and interpretations were not standardized, and our procedure lacks the precision that a single central interpretation process could provide.
However, all CT interpretations used to acquire the variables in the TBIMS NDB are read by radiologists with clinical privileges to perform this and other routine radiologic interpretations at each of the level 1 trauma centers where TBIMS database participants are treated. Accordingly, the CT interpretations are of a type and quality consistent with those available to clinicians in everyday clinical practice at any major medical center in the United States or comparable clinical locations. The information they yield therefore has clinical relevance and immediacy. Further, abstraction of data from the radiologist reports is performed by TBIMS investigators who are specifically trained and serially reexamined to criteria according to TBIMS protocols. Hence, the development of CT data that enter the TBIMS database uses a standardized abstraction process that is applied uniformly across all TBIMS sites. This method of abstracting CT data and transforming them into variables as used for this analysis is considerably more standardized than the methods of interpreting CT data applied to everyday clinical practice and could have real-world utility for purposes of prognosis.
The precision of these predictive models also may have been affected by measuring outcomes at rehabilitation admission and discharge, the timings of which are unique for each participant and do not occur at fixed time points after injury. Given these limitations, a definitive determination of the extent to which TBIMS NDB head CT variables in their current form contribute to outcome prediction would require a more detailed and sophisticated analysis, such as analyses with predictive modeling methodology used in other large international data sets (4, 26).
A structural indicator of injury severity based on an adapted head CT classification scheme did not predict clinical indicators of injury severity after moderate-severe TBI in this data set beyond its expected association with neurosurgical procedures. This head CT classification scheme also did not predict functional or participation outcomes in the postacute and long-term phases of recovery after TBI. This suggests that head CT findings classified in this manner do not inform clinical discussions related to functional prognosis or rehabilitation planning after TBI. Additional analyses considering the entire CT variable set beyond a classification scheme are required to determine whether the TBIMS NDB head CT variables in their current form can contribute to prediction of postacute outcomes.
Acknowledgment:
The authors thank Jessica McKinney Ketchum, PhD, for her assistance in completing the analysis.
Source of Funding: The contents of this report were developed under grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (grant numbers 90DP0030, 90DP0036, 90DP0039, 90DP0037, 90DP0038, 90DP0034, 90DP0060, 90DP0046, 90DP0045, and 90DP0033). NIDILRR is a center within the Administration for Community Living (ACL), US Department of Health and Human Services (DHHS). This report is solely the responsibility of the authors and does not necessarily represent the official views of the NIDILRR, the ACL, or the DHHS and should not be assumed an endorsement by the US Federal Government.
Abbreviations
- CT
computed tomography
- DRS
Disability Rating Scale
- EGOS
Extended Glasgow Outcome Scale
- FIM
Functional Independence Measure
- NDB
National Data Base
- PTA
Posttraumatic amnesia
- RLOS
rehabilitation length of stay
- SPOS
semipartial omega squared statistic
- TBI
traumatic brain injury
- TBIMS
Traumatic Brain Injury Model Systems
Footnotes
Disclosures of interest: No potential conflict of interest was reported by the authors.
References
- 1.Lee B, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005;2(2):372–83 doi: 10.1602/neurorx.2.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, Workshop Scientific T, Advisory Panel M. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38 doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall LF, Marshall SB, Klauber MR. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(Special Supplements):S14–S20 [Google Scholar]
- 4.Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, Marshall LF, Maas AI. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22(10):1025–39 doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 5.Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A, Mushkudiani NA, McHugh GS, Murray GD. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):303–14 doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 6.Dijkers MP, Harrison-Felix C, Marwitz JH. The traumatic brain injury model systems: history and contributions to clinical service and research. J Head Trauma Rehabil. 2010;25(2):81–91 doi: 10.1097/HTR.0b013e3181cd3528. [DOI] [PubMed] [Google Scholar]
- 7.Englander J, Cifu DX, Wright JM, Black K. The association of early computed tomography scan findings and ambulation, self-care, and supervision needs at rehabilitation discharge and at 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(2):214–20 doi: 10.1053/apmr.2003.50094. [DOI] [PubMed] [Google Scholar]
- 8.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J, Cifu DX. Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma. 2005;22(10):1040–51 doi: 10.1089/neu.2005.22.1040. [DOI] [PubMed] [Google Scholar]
- 9.Chesnut R, Ghajar J, Maas A. Part 2: Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2009;17:555- doi: 10.1089/neu.2000.17.555. [DOI] [Google Scholar]
- 10.Lobato RD, Cordobes F, Rivas JJ, de la Fuente M, Montero A, Barcena A, Perez C, Cabrera A, Lamas E. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg. 1983;59(5):762–74 doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 11.Servadei F, Murray GD, Penny K, Teasdale GM, Dearden M, Iannotti F, Lapierre F, Maas AJ, Karimi A, Ohman J, et al. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. European Brain Injury Consortium. Neurosurgery. 2000;46(1):70–5; discussion 5–7 [DOI] [PubMed] [Google Scholar]
- 12.Heinemann AW, Linacre JM, Wright BD, Hamilton BB, Granger C. Prediction of rehabilitation outcomes with disability measures. Arch Phys Med Rehabil. 1994;75(2):133–43 [PubMed] [Google Scholar]
- 13.Hobart JC, Lamping DL, Freeman JA, Langdon DW, McLellan DL, Greenwood RJ, Thompson AJ. Evidence-based measurement: which disability scale for neurologic rehabilitation? Neurology. 2001;57(4):639–44 [DOI] [PubMed] [Google Scholar]
- 14.Malec JF, Hammond FM, Giacino JT, Whyte J, Wright J. Structured interview to improve the reliability and psychometric integrity of the Disability Rating Scale. Arch Phys Med Rehabil. 2012;93(9):1603–8 doi: 10.1016/j.apmr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118–23 [PubMed] [Google Scholar]
- 16.Testa JA, Malec JF, Moessner AM, Brown AW. Outcome after traumatic brain injury: effects of aging on recovery. Arch Phys Med Rehabil. 2005;86(9):1815–23 doi: 10.1016/j.apmr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Whyte J, Katz D, Long D, DiPasquale MC, Polansky M, Kalmar K, Giacino J, Childs N, Mercer W, Novak P, et al. Predictors of outcome in prolonged posttraumatic disorders of consciousness and assessment of medication effects: A multicenter study. Arch Phys Med Rehabil. 2005;86(3):453–62 doi: 10.1016/j.apmr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Hellawell DJ, Signorini DF. The Edinburgh Extended Glasgow Outcome Scale (EEGOS): rationale and pilot studies. Int J Rehabil Res. 1997;20(4):345–54 [DOI] [PubMed] [Google Scholar]
- 19.Levin HS, Boake C, Song J, McCauley S, Contant C, Diaz-Marchan P, Brundage S, Goodman H, Kotrla KJ. Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J Neurotrauma. 2001;18(6):575–84 doi: 10.1089/089771501750291819. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85 doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JT, Pettigrew LE, Teasdale GM. Emotional and cognitive consequences of head injury in relation to the glasgow outcome scale. J Neurol Neurosurg Psychiatry. 2000;69(2):204–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherer M, Sander AM, Nick TG, High WM Jr., Malec JF, Rosenthal M. Early cognitive status and productivity outcome after traumatic brain injury: findings from the TBI model systems. Arch Phys Med Rehabil. 2002;83(2):183–92 [DOI] [PubMed] [Google Scholar]
- 23.SAS. Effect Size Measures for F Tests in GLM (Experimental) [Internet] [Internet]. 2010. [updated 2010 Jun 22. Available from: https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_glm_sect032.htm.
- 24.Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry. 2002;72(2):188–92; discussion 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherer M, Stouter J, Hart T, Nakase-Richardson R, Olivier J, Manning E, Yablon SA. Computed tomography findings and early cognitive outcome after traumatic brain injury. Brain Inj. 2006;20(10):997–1005 doi: 10.1080/02699050600677055. [DOI] [PubMed] [Google Scholar]
- 26.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82; discussion -82 [DOI] [PubMed] [Google Scholar]
- 27.Yuh EL, Cooper SR, Ferguson AR, Manley GT. Quantitative CT improves outcome prediction in acute traumatic brain injury. J Neurotrauma. 2012;29(5):735–46 doi: 10.1089/neu.2011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, Valadka AB, Okonkwo DO, Schnyer DM, Vassar MJ, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2014;31(17):1457–77 doi: 10.1089/neu.2013.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73(2):224–35 doi: 10.1002/ana.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]