Abstract
Objective
Enhanced odor sensitivity, particularly toward threat-related cues, may be adaptive during periods of danger. Research also suggests that chronic psychological distress may lead to functional changes in the olfactory system that cause heightened sensitivity to odors. Yet, the association between self-reported odor sensitivity, objective odor detection, and affective psychopathology is currently unclear, and research suggests that persons with affective problems may only be sensitive to specific, threat-related odors.
Methods
The current study compared adults with self-reported odor sensitivity that was described as functionally impairing (OSI; n = 32) to those who reported odor sensitivity that was non-impairing (OS; n = 17) on affective variables as well as quantitative odor detection.
Results
Increased anxiety sensitivity, trait anxiety, depression, and life stress, even while controlling for comorbid anxiety and depressive disorders, was found for OSI compared to OS. While OSI, compared to OS, demonstrated only a trend increase in objective odor detection of a smoke-like, but not rose-like, odor, further analysis revealed that increased detection of that smoke-like odor was positively correlated with anxiety sensitivity.
Conclusion
These findings suggest that persons with various forms of psychological distress may find themselves significantly impaired by an intolerance of odors, but that self-reported odor sensitivity does not necessarily relate to enhanced odor detection ability. However, increased sensitivity to a smoke-like odor appears to be associated with sensitivity to aversive anxiogenic stimuli. Implications for the pathophysiology of fear- and anxiety-related disorders are discussed.
Keywords: Odor Sensitivity, Anxiety Sensitivity, Somatization, Anxiety, Stress
Despite the popular misconceptions that humans have poor olfactory ability and do not rely on smell for navigating the environment, olfaction is an ancient chemical sense that provides important sensory information across many different contexts.1 In fact, olfaction plays a role in number of everyday, routine processes such as eating, social communication, and attaching emotional attributes to autobiographical memories.2 Smell also has a primary role for survival in the detection of environmental threats and signaling of potential danger (e.g. identifying spoiled/rotten food, an unseen fire, or nearby chemical spill or gas leak). Thus, enhanced olfactory function during periods when increased threat detection is required, or when perceived life threat or emotional stress is high, would likely aid in survival by alerting one to the presence of danger and facilitating avoidance behaviors.
Evidence from animal studies suggests that odor sensitivity is heightened during periods when increased threat detection is required, such as in the vicinity of a predator3 or in response to immobilization stress4. Moreover, structural changes within the olfactory system of rodents in response to fear/threat support those changes in function.5, 6 The data in humans are less clear however, with some studies reporting fear/threat-related enhanced odor detection,6–11 and others demonstrating impaired function.12, 13 Odor detection/sensitivity across stress, anxiety, and other fear-related disorders is equally mixed.14–19 Taken together, these findings suggest that additional variables may moderate the relationship between fear/threat and enhanced odor detection/sensitivity in humans. For instance, our research suggests that situations of real, life-threatening danger may shift olfactory system functions toward the more sensitive detection of specific, threat-related, odor cues, including burning odors.20 Accordingly, sensitivity to certain odors, particularly those linked to real danger, may be associated with anxiety proneness and/or history of negative affective experiences. Another possible explanation for mixed evidence in the anxiety-odor sensitivity relationship may be due to differences between those with and without clinically significant odor sensitivity. It may be that persons who are impaired by their odor sensitivity (e.g. endorse unpleasant physical/emotional reactions to certain odors) are indeed highly anxious, but that no relationship exists between odor sensitivity and anxiety in persons that are not impaired by their odor sensitivity (e.g. endorse a keen, but non-problematic, sense of smell).
In fact, the proposed linkage between odor sensitivity impairment and psychological distress has particular relevance to individuals who describe themselves as “chemically sensitive,” or having an enhanced ability to detect and experience distress from noxious smells, particularly harsh chemicals and environmental pollutants. Studies have found that between 11–33% of individuals in the general population consider themselves “chemically sensitive”21, 22 and between 1–6% are impaired by the condition23. Impaired individuals endorse the ability to detect very faint concentrations of certain smells (i.e., much lower than harmful levels), and that the presence of such odors causes intense irritation and symptoms akin to panic attacks (e.g., lightheadedness, fatigue, difficulty breathing, headaches, concentration difficulties).24–26 However, it is currently unclear whether individuals who describe themselves as being chemically sensitive have physiological differences in olfactory function or if their sensitivity is more reflective of fear-based psychopathology. Preliminary objective testing has revealed mixed results regarding whether true differences in odor detection exist in persons with chemical sensitivity,27, 28 whereas data consistently suggest that persons who endorse chemical sensitivity have increased anxiety, depression, and psychological distress.29–31 Yet, while this evidence provides useful information on those who endorse “chemical sensitivity,” it is unclear whether these findings would also hold true for those who consider themselves to be “odor sensitive,” which reflects a more general sensitivity to odors, rather than specific sensitivity to harsh chemicals. It stands to reason that persons with greater fear-based psychopathology may be particularly vigilant of harsh chemicals and environmental pollutants that could potentially be harmful if inhaled in large volumes. Yet, the extent to which the purported effects of stress and anxiety on olfaction would associate to greater perceptual sensitivity to both potentially dangerous and neutral odors is currently unknown.
To more clearly elucidate the relationship between odor sensitivity and psychiatric symptoms, we recruited a sample of adults who endorsed increased sensitivity to odors. To extend previous findings, we sought to determine the relationship between odor sensitivity and various indices of stress, anxiety, and depression in those impaired and not impaired by their odor sensitivity. We also sought to examine whether self-reported odor sensitivity impairment would correspond to an objective measure of odor detection, including increased differential detection of an odor potentially related to danger (smoke-like) compared to an odor typically not associated with danger (rose-like). Finally, in order to link affective variables to possible physiological differences in odor detection, we explored whether various indices of stress, anxiety, and depression were associated with objective measures of odor detection.
METHODS
Participants
Participants were recruited over a 6-month time period from the Medical University of South Carolina campus and the greater Charleston, South Carolina community through advertisement seeking “adults who are sensitive to odors”. At screening, self-reported odor sensitivity was confirmed by affirmative answers to the questions, “are you sensitive to odors?” and “do you smell things other people do not or prior to other people?”. Next, impairment due to odor sensitivity was probed by the questions, “are you bothered by odors?”, including “do you have unpleasant physical/emotional reactions to odors and do you avoid odors?”. Participants were required to endorse “yes” to all questions to be categorized as being impaired by their odor sensitivity “OSI”. All others served as odor sensitive (OS) controls, as they self-reported odor sensitivity (keen sense of smell), but were not impaired by it. We chose this recruitment methodology based on our primary hypotheses, that impairment from odor sensitivity, not odor sensitivity per se, is the critical factor regarding psychological distress. Exclusion criteria were limited and included (a) history of head trauma/concussion, (b) heavy smoking, and (3) problems with nose/sense of smell (e.g. upper respiratory infection, chronic rhinosinusitis, polyps, etc.). All participants signed written informed consent approved by the Institutional Review Board (IRB) at the Medical University of South Carolina prior to participation.
Materials and Methods
Odor Detection
Objective odor detection was measured through administration of two versions of the Snap and Sniff ® Threshold Test (SSTT)32: the first containing phenyl-ethyl alcohol (PEA), a “rose-like” scent, and the second containing guaiacol (GUA), a “smoke-like” scent. Administration procedures were the same, regardless of odorant. The SSTT required the systematic presentation of a set of wands containing a serial dilution (half-log concentration steps ranging from the most intense, 10−2 to the least intense, 10−9) of odorant. In a single staircase method with forced choice regarding which wand smelled more strongly of the odorant, a wand containing a given concentration of odorant was presented under the nose in rapid succession with an odorless wand. Subsequent presentation of a higher or lower concentration of odorant was dependent on a correct or incorrect response from the previous trial. This method was repeated until 7 reversals (up and down the staircase) were made. Odor detection threshold score was determined by the average of the last 4 reversals.
Psychological Measures
The Anxiety Sensitivity Index-3 (ASI-3) is an 18-item, self-report measure of anxiety sensitivity, or the fear of experiencing anxiety and its related cognitive, physiological, and social consequences.33 Using a 5-point rating scale that ranged from 0 (very little) to 4 (very much), items were rated according to how much the respondent agreed to each statement. Total ASI-3 scores were calculated by the sum of all items and could range from 0 to 72, with higher scores indicating greater anxiety sensitivity. Three lower-order factors of anxiety sensitivity (i.e. cognitive, physical, and social) were comprised of 6 items each, allowing subscale scores to range from 0–24. The cognitive concerns subscale measured fear of the mental consequences of anxiety such as worry of “going crazy” or being “mentally ill”. The physical concerns subscale measured fear of anxiety-related physiological arousal including worry of a “heart attack” or “choking to death”. And finally, the social concerns subscale measured fear of the social aspects of anxiety such as worry of being evaluated negatively by others for blushing, sweating, or fainting. The psychometric properties of the ASI-3 are adequate to good on indices of reliability and validity.33, 34 Evidence indicates that a cut-off score of ≥ 17 reflects moderate-to-severe anxiety sensitivity versus mild-to-negligible anxiety sensitivity.35, 36
The State-Trait Anxiety Inventory (STAI)37 is a 40-item, self-report measure of two types of anxiety: state anxiety and trait anxiety.38 State anxiety is conceptualized as a transitory emotional state, whereas trait anxiety is thought to be an enduring personality dimension. Each type of anxiety is measured via 20 distinct items. On the STAI, participants are asked to rate a series of statements regarding how much the statement applies to them on a 4-point rating scale ranging from 1 (“Not at all”) to 4 (“Very much so”). Total scores for the state subscale (STAI-S) and trait subscale (STAI-T) are formed by summing all items, resulting in subscale scores ranging from 20–80. Studies have shown that the STAI has good reliability and validity.39–43 Cut-off scores for normative versus clinically significant symptoms on the STAI scales have been defined as 40 for the State scale44 and 46 for the Trait scale45.
The Holmes-Rahe Social Readjustment Scale (HRSS)46 is a 43-item, self-report measure of recent individual experiences of stressful life events. Participants are asked to indicate whether a series of stressful life events (e.g., death of a spouse, change in living conditions, vacation) have occurred to them in the past year. Each life event is assigned an empirically derived point value (or “Life Change Units”) ranging from 11–100,47 with greater values indicating greater stress impact. The total number of Life Change Units are added together from all items endorsed by the participant, resulting in a total score that reflects one’s risk for illness or maladjustment as a result of life stress. Scores are interpreted as follows: scores < 150 reflect reduced risk of illness, scores between 150–299 reflect moderate risk of illness, and scores ≥ 300 reflect high risk of illness. Evidence suggests that the HRSS has good criterion validity.48
Similarly, the Life Events Checklist (LEC)49 is a 17-item, self-report measure of exposure to potentially traumatizing events. The measure lists 16 potentially traumatizing events, and participants are asked to rate whether (a) the event happened to them, (b) they witnessed the event, (c) they learned about the event, (d) the event happened as part of their job, (e) they are not sure, or (f) the event did not happen to them. In addition, an additional item allows respondents to enter any other extraordinary stressful life event. A total score is derived by adding up the number of instances and/or contexts in which someone has endorsed that they experienced a traumatic event. The LEC has adequate test-retest reliability and good convergent validity.49
The Penn State Worry Questionnaire (PSWQ)50 is a 16-item, self-report measure of chronic and persistent worrying. Participants rate how a series of statements apply to them on a 5-point rating scale ranging from 1 (“Not at all typical of me”) to 5 (“Very typical of me”), and the total score consists of a sum of all item scores ranging from 16–80. Higher scores indicate greater trait worrying. Evidence indicates that the PSWQ possesses good reliability and excellent validity.51
The Patient Health Questionnaire – depression scale (PHQ-9)52 is a 9-item, self-report measure of the nine diagnostic criteria for major depression from DSM-IV. Respondents rate the frequency each of the diagnostic criteria on a 4-point rating scale ranging from 0 (“Not at all”) to 3 (“Nearly every day”), resulting in total scores ranging from 0–27. A cutoff score of ≥10 is generally employed to detect significant depressive symptoms.53 Evidence generally supports the construct validity of the PHQ-9.54
The Pittsburg Sleep Quality Index (PSQI)55 is a 19-item, self-report measure designed to measure sleep quality in persons over a 1-month interval. Item content covers seven content areas: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Items are rated on a 4-point rating scale ranging from 0 (“Not during the past month”) to 3 (“Three or more times a week”). The total score is computed by summing all items, which can range from 0–57. Evidence indicates that the PSQI possesses acceptable reliability and good validity.55, 56
Procedure
Interested participants who responded to study advertisements and met the study criteria were mailed a comprehensive packet of questionnaires related to stress, anxiety, and mood and scheduled for a 30-minute study visit. At the visit, informed consent procedures were conducted, followed by the collection of the participants’ completed packet of questionnaires, determination of whether participants were functionally impaired by their odor sensitivity, and finally by objective odor detection testing (SSTT for PEA and GUA).
RESULTS
Participant characteristics
Participants in the overall sample (N = 49) were mostly middle-aged (M = 37.43, SD = 13.20, Range = 20–68) and predominantly white (73.5%), female (89.8%), and college-educated (73.5%). While all participants endorsed odor sensitivity, further probing revealed that 32 of the 49 participants (65.3%) reported that their odor sensitivity was impairing (OSI) and that 17 of the 49 participants (34.7%) were not impaired by their odor sensitivity (OS).
Independent t-test and chi-square analyses revealed significant differences in demographic and clinical characteristics between the OSI and OS groups (see Table 1). Most notably, persons in the OSI group were significantly more likely to be females than males. This result is likely based on the findings that only 10% of those who answered the study advertisement were male, and that only 1 (20%) of the males in the study were included in the OSI group, while 31 (70%) of the females in the sample were included in the OSI group. Additionally, the OSI participants were significantly more likely to endorse a previous diagnosis and/or treatment for anxiety or depression. In fact, of the 16 participants who endorsed a diagnosis, 15 of them were in the OSI group, meaning that of the 17 participants in the OS group, just one endorsed being previously treated for depression.
Table 1.
Demographic and Clinical Characteristics
| Measure | OSI | OS | t or χ2 | p-value | OR/g |
|---|---|---|---|---|---|
| (n=32) | (n=17) | ||||
| Age in years – Mean (SD) | 37.00 (13.79) | 38.24 (12.37) | 0.31 | 0.76 | 0.09 |
| Sex - N (%) male | 1 (3.1) | 4 (23.5) | 5.04 | 0.025 | −6.91 |
| Race - N (%) minority | 7 (21.9) | 6 (35.3) | 1.03 | 0.31 | 2.10 |
| Education – N (%) college degree | 24 (82.8) | 12 (70.6) | 0.93 | 0.33 | 1.89 |
| Mood/Anxiety Diagnosis - N (%) endorsed | 15 (46.9) | 1 (5.9) | 8.48 | 0.004 | 14.04 |
| Psychotropic Medication - N (%) endorsed | 7 (21.9) | 1 (5.9) | 2.08 | 0.15 | 4.49 |
OSI = participants impaired by their odor sensitivity; OS = participants not impaired by their odor sensitivity; OR = Odds Ratio; g = Hedge’s g
Influence of Odor Sensitivity Impairment on Depression, Anxiety, and Stress
Given the high rate of mood and anxiety disorders in the OSI group, ANCOVA with history of diagnosis/treatment added as a categorical covariate was used to determine the influence of odor sensitivity impairment on the severity of psychiatric symptoms and life stress (see Table 2). Consistent with our hypotheses, the OSI group had significantly greater overall AS, as well as greater scores on the physical, cognitive, and social ASI-3 subscales. Additionally, the OSI group reported significantly greater symptoms of depression, greater frequencies of stressful life events on the HRSS, higher trait anxiety, but state anxiety was not elevated. Marginally higher frequencies of traumatic life events and worry severity were reported by the OSI group compared to the OS group. However, impairment from odor sensitivity did not affect sleep quality, as there were no significant differences between groups on the PSQI.
Table 2.
Differences Between Odor Sensitive Groups on Self-Report Measures
| Measure | OSI | OS | F | p-value | ηp2 |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| ASI-Total | 23.69 (14.25) | 6.76 (4.71) | 12.95 | .001 | .22 |
| ASI-Physical | 7.63 (5.46) | 1.94 (2.11) | 10.14 | .003 | .18 |
| ASI-Cognitive | 5.06 (5.17) | 0.82 (1.33) | 4.22 | .046 | .08 |
| ASI-Social | 11.00 (6.12) | 4.00 (3.35) | 12.38 | .001 | .21 |
| STAI-State | 38.38 (12.48) | 31.65 (8.28) | 1.76 | .19 | .04 |
| STAI-Trait | 42.78 (11.17) | 31.76 (8.85) | 4.93 | .031 | .10 |
| HRSS | 175.72 (99.55) | 100.24 (101.93) | 4.42 | .041 | .09 |
| LEC | 11.25 (5.76) | 8.24 (6.08) | 3.44 | .070 | .07 |
| PSWQ | 51.81 (17.50) | 38.13 (15.32) | 3.42 | .071 | .07 |
| PHQ-9 | 6.06 (4.55) | 1.88 (2.00) | 7.59 | .008 | .14 |
| PSQI | 6.78 (4.07) | 4.53 (3.06) | 2.00 | .16 | .04 |
OSI = participants impaired by their odor sensitivity; OS = participants not impaired by their odor sensitivity; ASI = Anxiety Sensitivity Index; STAI = State/Trait Anxiety Inventory; HRSS = Holmes-Rahe Stress Readjustment Scale; LEC = Life Events Checklist; PSWQ = Penn State Worry Questionnaire; PHQ-9 = Patient Health Questionnaire; PSQI = Pittsburg Sleep Quality Index
We also examined whether persons who were impaired by their odor sensitivity would be more likely to be classified as having clinically significant symptoms of depression, trait anxiety, life stress, and anxiety sensitivity, as evidenced by scores greater than the cut-offs on the PHQ-9, STAI Trait Scale, HRSS, and the total score of the ASI. Results of chi-square analyses revealed that the OSI group was significantly more likely to report clinically significant symptoms of depression on the PHQ-9 (χ2 [1, 49] = 6.07, p = .014, OR = 313.99). Indeed, while 9 (29.0%) of the OSI group scored 10 or higher on the PHQ-9, none of the OS group scored at or above that cut-off. The same pattern of results was evident on the ASI, wherein the OSI group was more likely to report clinically significant symptoms of AS with scores greater than or equal to 17 (χ2 [1, 49] = 19.52, p < .001, OR = 4630.67). While 21 (65.6%) of the OSI group scored in the clinically significant range for AS, none of the OS group did. However, individuals in the OSI group were only marginally more likely to score above the clinical cut-off on the STAI Trait Anxiety Scale (χ2 [1, 49] = 3.60, p = .058, OR = 5.89), and were not more likely to score above the clinical cut-off for life stress on the HRSS (χ2 [1, 49] = 1.92, p = .17, OR = 2.40).
Influence of Odor Sensitivity Impairment on Objectively-measured Odor Detection
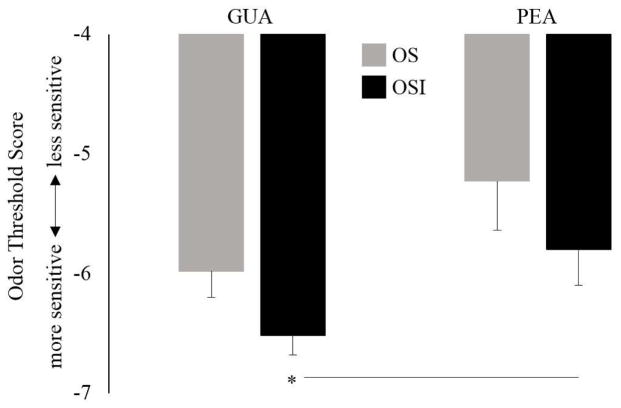
A 2 × 2 (group x odor) mixed ANOVA was used to test objectively-measured odor detection of GUA and PEA between the OSI and OS groups. There was a significant main effect of odor (F[1, 47] = 7.34, p = .009, ηp2 = .14), revealing that, for the entire sample, mean odor detection threshold was lower (enhanced detection) for GUA (M = −6.34, SD = 0.94, range = −2 to −8.125) than for PEA (M = −5.60, SD = 1.70, range = −2 to −8.5). While the group by odor interaction was non-significant (F[1, 47] = .003, p = .95), paired samples t-tests revealed that the main effect of odor was mainly driven by the OSI group, who demonstrated significantly enhanced detection of GUA (M = −6.52, SD = 0.60, range = −4.75 to −8.125) compared to PEA (M = −5.80, SD = 1.57, range = −2.375 to −8.5; t(31) = 2.53, p = .02; d = .45). In contrast, the OS group did not show differential detection of GUA (M = −5.98, SD = 1.33, range = −2 to −7.375) compared to PEA (M = −5.23, SD = 1.91, range = −2 to −8.25; t(16) = 1.45, p = .17, d = .35). Independent samples t-tests showed that GUA threshold trended lower (enhanced detection) in the OSI group compared to the OS group (t(47) = 1.96, p = .057, g = .59), while detection thresholds for PEA did not differ between groups (t(47) = 1.12, p = .27, g = .34) (See Figure 1).
Figure 1.
Odor threshold scores for guaiacol (GUA), a smoke-like odorant, and phenyl ethyl alcohol (PEA), a rose-like odorant, in adults impaired and not impaired by their odor sensitivity (OSI and OS, respectively). Odor detection was determined with the Snap and Sniff ® Threshold Test (SSTT), wherein a lower number (more negative) indicated a lower concentration of odorant and enhanced detection. Overall, the entire sample showed enhanced detection of GUA compared to PEA. This effect was driven mainly by the OSI group who demonstrated significantly enhanced detection for GUA compared PEA. * = p < .05.
Influence of Psychological Variables on Objectively-measured Odor Detection
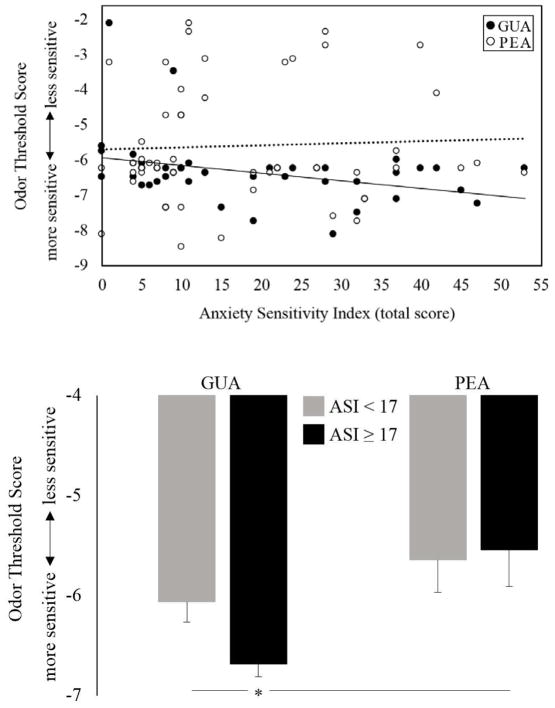
Pearson bivariate correlations were used to determine potential associations between odor threshold scores and clinical variables in the overall sample of odor sensitive adults. A significant relationship between AS and enhanced odor detection of GUA, but not PEA (r = − .33, p < .05, r = .05, p =.74, respectively) was found. Thus, detection for the smoke-like odor was best in those with the highest level of AS (see Figure 2a). Participants were then grouped by those with clinically significant symptoms of AS (ASI-3 total score ≥ 17; n = 21) and those without clinically significant AS (ASI-3 total score < 17; n = 28). Mixed ANOVA revealed a main effect of odor (F[1, 47] = 9.37, p = .004, ηp2 = .17) and a trend-level group by odor interaction (F[1, 47] = 2.01, p = .16, ηp2 = .04). T-tests showed that the high AS group was significantly more sensitive to only GUA, detecting it at lower concentrations than PEA (t(20) = 3.56, p = .002, d = .77), as well as GUA in the low AS group (t(47) = 2.40, p = .02, g = .69) (see Figure 2b). No other significant relationships between odor detection thresholds and psychological variables were noted.
Figure 2a and 2b.
Odor threshold scores for guaiacol (GUA), a smoke-like odorant, and phenyl ethyl alcohol (PEA), a rose-like odorant, as a function of anxiety sensitivity (AS). Odor detection was determined with the Snap and Sniff ® Threshold Test (SSTT), wherein a lower number (more negative) indicated a lower concentration of odorant and enhanced detection. 2a. Total AS was significantly correlated with enhanced odor detection of GUA (r = −.33, p < .05), but not PEA (r = .05, p > .1). 2b. Participants who scored above the cutoff for clinically significant AS (ASI score ≥ 17; n = 21) detected GUA at lower concentrations (enhanced detection) than PEA and also compared to detection of GUA in those who scored below the clinical cutoff (ASI score < 17; n = 28). * = p < .05.
DISCUSSION
Results of the current study were consistent with our predictions. We showed that self-reported odor sensitivity impairment was associated with increased anxiety, depression, and life stress, as well as with objectively-measured enhanced detection for GUA, the smoke-like, but not PEA, the rose-like, odorant. These findings suggest that self-reported impairing odor sensitivity and objective detection of an odorant related to potential danger are linked and both relate to anxiety and psychological distress.
Self-reported odor sensitivity impairment was related to a host of negative affective experiences, even when controlling for history of anxiety or depression, which was more prominent in that group. In fact, persons impaired by their odor sensitivity reported significantly increased trait anxiety, life stress, and depression, as well as a marginally greater total number of traumatic life events and marginally increased worry. Previous research has shown that anxious individuals are more perceptive of threatening stimuli than non-anxious individuals.57 Indeed, persons with high social anxiety are more reactive to emotional facial expressions in others,58 and victims of sexual assault can experience chronic aversion to physical contact.59 Together with the present findings, these results suggest that chronic fear and psychological distress may be associated with increased attention to, and intolerance of, potential danger cues across different sensory modalities, including olfaction.
Odor sensitivity impairment related to objectively-measured odor detection as well, as only those that were impaired by their odor sensitivity demonstrated significantly better detection of GUA than PEA. Moreover, there was a trend for better detection of GUA in the impaired, compared to non-impaired, odor sensitive group. These results provide support for our hypothesis that persons with odor sensitivity impairment would be more perceptive of an odor potentially related to danger, but not more perceptive of a neutral odor. Additional findings showing that enhanced detection of GUA, but not PEA, was linked to elevated anxiety sensitivity, as well as the strong association between anxiety sensitivity and odor sensitivity impairment, suggest that perhaps anxiety sensitivity is the mechanism through which persons who are impaired by odor sensitivity are more perceptive of odors potentially related to danger. Indeed, theoretical distinctions between anxiety and anxiety sensitivity60 suggest that odor sensitivity, regardless of impairment, may be more closely associated with fear of aversive anxiety-related sensations rather than fear of aversive stimuli, per say. Perhaps a disposition of hyper-vigilance to aversive sensations could reflect increased sensitivity to aversive/noxious odors, leading such individuals to report impairment due to their odor sensitivity.
There is support for the notion that odor sensitivity impairment and anxiety sensitivity are associated with intolerance of aversive odors when considering differences in the neurophysiology of sensory processing between different types of odorants. The aesthetic properties of odors are processed in the primary olfactory (piriform) cortex, which is closely intertwined with the neural structures supporting emotion in the limbic cortex (i.e., the amygdala, hippocampus and surrounding cortex, anterior insula, and orbitofrontal cortices).61, 62 This suggests that persons with affective psychopathology, who often demonstrate limbic hyperactivity,63 may often attach strong emotional valence to odors and thus perceive odors as more intense due to affective conflation. Some research supports this notion, as odors have a remarkable ability to elicit emotionally-charged, distant memories,64, 65 and individuals with PTSD often report that certain odors trigger re-experiencing of traumatic events.66 In contrast, the nociceptive properties (i.e., burning, tingling) of odors are processed via the intranasal trigeminal system,67 thereby conveying information about potentially dangerous odorant chemicals. The degree to which odorants are processed more so through the trigeminal pathway than through the primary olfactory pathway varies depending on the odorant.67 Although PEA has some trigeminal properties, GUA and many other odors potentially related to danger have significant trigeminal properties. Indeed, GUA is still recognized as an intense odor in persons with anosmia, who cannot recognize many odors due to damage or dysfunction specific to the olfactory circuit.68 Our laboratory has previously suggested that chronic fear may lead to a shift in central processing of odorants, from olfactory to trigeminal, such that affected individuals lose sensitivity to the hedonic qualities and perhaps intensity of many odorants, while at the same time becoming more sensitive to the danger-related aspects of odorants that possess greater trigeminal properties.20 In fact, we recently reported that combat veterans with chronic post-traumatic stress disorder (PTSD) demonstrated decreased odor detection for PEA, but increased odor intensity ratings and brain activation in somatosensory, but not olfactory, cortex in response to a burning-like odor cue.17 Results of the current study are consistent with those findings; chronic sensitivity to feared stimuli (i.e., anxiety sensitivity) may result in functional changes to both the olfactory and intranasal trigeminal systems, leading to odor-specific sensitivities.
CONCLUSION
Findings from the current study offer insights into how odor sensitivity and odor sensitivity impairment, defined subjectively or objectively, may be a marker of certain types of anxiety and psychological distress. However, these results should be viewed as preliminary given certain limitations to the study design. Certainly, the sample size between groups was relatively small, and it could be argued that a separate group of adults with average smell ability may show differences from the current groups of odor sensitive adults. The study was also constrained by the use of just two odorants that were not specifically tested for trigeminal activation. It would be beneficial for future studies to test a variety of pleasant and unpleasant odors that range on their ability to activate the intranasal trigeminal system. Regardless of these limitations, the present study adds to the growing literature regarding anxiety-related changes in odor processing. Taken together with previous work in this area, the present results suggest that self-reported odor sensitivity impairment and objectively-measured enhanced detection of potentially dangerous odors may help identify clinically-relevant levels of anxiety and psychological distress in the general population.
Acknowledgments
Funding for this study was provided by NIMH Grant K01 MH090548 (BMC).
Footnotes
Disclosure information:
David Houghton, Samuel Howard, Thomas Uhde, Caitlin Paquet, Rodney Schlosser and Bernadette Cortese have the following disclosure: NIMH Grant K01 MH090548 (BMC).
All authors declare that they have no conflicts of interest.
References
- 1.McGann JP. Poor human olfaction is a 19th-century myth. Science. 2017;356(6338) doi: 10.1126/science.aam7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarafoleanu C, Mella C, Georgescu M, et al. The importance of the olfactory sense in the human behavior and evolution. Journal of Medicine and Life. 2009;2(2):196–198. [PMC free article] [PubMed] [Google Scholar]
- 3.Lukowiak K, Martens K, Rosenegger D, et al. The perception of stress alters adaptive behaviors in Lymnaea stagnalis. J Exp Biol. 2008;211:1747–1756. doi: 10.1242/jeb.014886. [DOI] [PubMed] [Google Scholar]
- 4.Sung KK, Jang DP, Lee S, et al. Neural responses in rat brain during acute immobilization stress: A [F-18]FDG micro PET imaging study. Neuroimage. 2009;44(3):1074–1080. doi: 10.1016/j.neuroimage.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Jones SV, Choi DC, Davis M, et al. Learning-dependent structural plasticity in the adult olfactory pathway. J Neurosci. 2008;28(49):13106–13111. doi: 10.1523/JNEUROSCI.4465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kass MD, Rosenthal MC, Pottackal J, et al. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013;342(6164):1389–1392. doi: 10.1126/science.1244916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahs F, Miller SS, Gordon AR, et al. Aversive learning increases sensory detection sensitivity. Biol Psychology. 2013;92(2):135–141. doi: 10.1016/j.biopsycho.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Hoenen M, Wolf OT, Pause BM. The impact of stress on odor perception. Perception. 2017;46(3–4):366–376. doi: 10.1177/0301006616688707. [DOI] [PubMed] [Google Scholar]
- 9.Krusemark EA, Li W. Enhanced olfactory sensory perception of threat in anxiety: An event-related fMRI study. Chemosensory Perception. 2012;5(1):37–45. doi: 10.1007/s12078-011-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Buissonnière-Ariza V, Lepore F, Kojok KM, et al. Increased odor detection speed in highly anxious healthy adults. Chem Senses. 2013;38(7):577–584. doi: 10.1093/chemse/bjt028. [DOI] [PubMed] [Google Scholar]
- 11.Pacharra M, Schäper M, Kleinbeck S, et al. Olfactory acuity and automatic associations to odor words modulate adverse effects of ammonia. Chemosensory Perception. 2016;9(1):27–36. [Google Scholar]
- 12.Jovanovic H, Perski A, Berglund H, et al. Chronic stress is linked to 5-HT1A receptor changes and functional disintegration of the limbic networks. Neuroumage. 2011;55(3):1178–1188. doi: 10.1016/j.neuroimage.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Itoh H, Nishikawa Y, et al. Possible relation between olfaction and anxiety in healthy subjects. Psychiatry Clin Neurosci. 2015;69(7):431–438. doi: 10.1111/pcn.12277. [DOI] [PubMed] [Google Scholar]
- 14.Berlin HA, Stern ER, Ng J, et al. Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: An fMRI study. Psychiatry Research: Neuroimaging. 2017;262:15–24. doi: 10.1016/j.pscychresns.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burón E, Bulbena A, Bulbena-Cabré A. Olfactory functioning in panic disorder. J Affect Disord. 2015;175:292–298. doi: 10.1016/j.jad.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Clepce M, Reich K, Gossler A, et al. Olfactory abnormalities in anxiety disorders. Neurosci Lett. 2012;511(1):43–46. doi: 10.1016/j.neulet.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Cortese BM, Schumann AY, Howell AN, et al. Preliminary evidence for differential olfactory and trigeminal processing in combat veterans with and without PTSD. NeuroImage: Clinical. 2017;17:378–387. doi: 10.1016/j.nicl.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pause BM, Adolph D, Prehn-Kristensen A, et al. Startle response potentiation to chemosensory anxiety signals in socially anxious individuals. Int J Psychophysiol. 2009;74(2):88–92. doi: 10.1016/j.ijpsycho.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Segalàs C, Labad J, Alonso P, et al. Olfactory identification and discrimination in obsessive-compulsive disorder. Depress Anxiety. 2011;28(10):932–940. doi: 10.1002/da.20836. [DOI] [PubMed] [Google Scholar]
- 20.Cortese BM, Leslie K, Uhde TW. Differential odor sensitivity in PTSD: Implications for treatment and future research. J Affect Disord. 2015;179:23–30. doi: 10.1016/j.jad.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreutzer R, Neutra RR, Lashuay N. Prevalence of people reporting sensitivities to chemicals in a population based survey. American Journal of Epidemiology. 1999;150(1):1–12. doi: 10.1093/oxfordjournals.aje.a009908. [DOI] [PubMed] [Google Scholar]
- 22.Meggs WJ, Dunn KA, Dunn KA, et al. Prevalence and nature of allergy and chemical sensitivity in a general population. Arch Environ Health. 1996;51(4):275–282. doi: 10.1080/00039896.1996.9936026. [DOI] [PubMed] [Google Scholar]
- 23.Bailer J, Witthöft M, Rist F. The Chemical Odor Sensitivity Scale: Reliability and validity of a screening instrument for idiopathic environmental intolerance. J Psychosom Res. 2006;61(1):71–79. doi: 10.1016/j.jpsychores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Cullen MR. The worker with multiple chemical sensitivities: An overview. Occup Med. 1987;2:655–661. [PubMed] [Google Scholar]
- 25.Miller CA. Toxicant-induced loss of tolerance-an emerging theory of disease? Environ Health Perspect. 1997;105(2):445–453. doi: 10.1289/ehp.97105s2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks PJ, Daniell W, Black DW, et al. Multiple chemical sensitivity syndrome: A clinical perspective. I. Case definition, theories of pathogenesis, and research needs. Journal of Occupational Medicine. 1994;36(7):718–730. [PubMed] [Google Scholar]
- 27.Doty RL, Deems DA, Frye RE, et al. Olfactory sensitivity, nasal resistance, and autonomic function in patients with multiple chemical sensitivities. JAMA Otolaryngology-Head and Neck Surgery. 1988;114(12):1422–1427. doi: 10.1001/archotol.1988.01860240072027. [DOI] [PubMed] [Google Scholar]
- 28.Kärnekull SC, Jönsson FU, Larsson M, et al. Affected by smells? Environmental chemical responsivity predicts odor perception. Chem Senses. 2011;36:641–648. doi: 10.1093/chemse/bjr028. [DOI] [PubMed] [Google Scholar]
- 29.Bell IR, Miller CS, Schwartz GE, et al. Neuropsychiatric and somatic characteristics of young adults with and without self-reported chemical odor intolerance and chemical sensitivity. Arch Environ Health. 1996;51(1):9–21. doi: 10.1080/00039896.1996.9935987. [DOI] [PubMed] [Google Scholar]
- 30.Bell IR, Peterson JM, Schwartz GE, et al. Self-reported illness from chemical odors in young adults without clinical syndromes or occupational exposures. Arch Environ Health. 1994;48(1):6–13. doi: 10.1080/00039896.1993.9938387. [DOI] [PubMed] [Google Scholar]
- 31.Devriese S, Winters W, Stegen K, et al. Generalization of acquired somatic symptoms in response to odors: A pavlovian perspective on multiple chemical sensitivity. Psychosom Med. 2000;62(6):751–759. doi: 10.1097/00006842-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Doty RL. The Snap & Sniff® Threshold Test Administration Manual. Haddon Hts., NJ: Sensonics, Inc; 2015. [Google Scholar]
- 33.Taylor S, Koch WJ, McNally RJ, et al. Conceptualizations of anxiety sensitivity. Psychological Assessment. 1992;4(2):245–250. [Google Scholar]
- 34.Osman A, Gutierrez PM, Smith K, et al. The Anxiety Sensitivity Index-3: Analyses of dimensions, reliability estimates, and correlates in nonclinical samples. J Pers Assess. 2010;92(1):45–52. doi: 10.1080/00223890903379332. [DOI] [PubMed] [Google Scholar]
- 35.Allan NP, Korte KJ, Capron DW, et al. Factor mixture modeling of anxiety sensitivity: A three-class structure. Psychological Assessment. 2014;26(4):1184–1195. doi: 10.1037/a0037436. [DOI] [PubMed] [Google Scholar]
- 36.Allan NP, Raines AM, Capron DW, et al. Identification of anxiety sensitivity classes and clinical cut-scores in a sample of adult smokers: Results from a factor mixture model. J Anx Disord. 2014;28(7):696–703. doi: 10.1016/j.janxdis.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 38.Kendall PC, Finch AJ, Auerbach SM, et al. The State-Trait Anxiety Inventory: A systematic evaluation. J Consul Clin Psychol. 1976;44(3):406–412. doi: 10.1037//0022-006x.44.3.406. [DOI] [PubMed] [Google Scholar]
- 39.Barnes LLB, Harp D, Jung WS. Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Educational and Psychological Measurement. 2002;62(4):603–618. [Google Scholar]
- 40.Hishinuma ES, Miyamoto RH, Nishimura ST, et al. Prediction of anxiety disorders using the State-Trait Anxiety Inventory for multiethnic adolescents. J Anx Disord. 2001;15(6):511–533. doi: 10.1016/s0887-6185(01)00079-2. [DOI] [PubMed] [Google Scholar]
- 41.Kabakoff RI, Segal DL, Hersen M, et al. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the state-trait anxiety inventory with older adult psychiatric inpatients. J Anx Disord. 1997;11(1):33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- 42.Spielberger CD. State-Trait Anxiety Inventory: Bibiography. 2. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- 43.Vautier S. A longitudinal SEM model approach to STAI data: Two comprehensive multitrait-multistate models. J Pers Assess. 2004;83(2):167–179. doi: 10.1207/s15327752jpa8302_11. [DOI] [PubMed] [Google Scholar]
- 44.Dennis CL, Coghlan M, Vigod S. Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory? J Affect Disord. 2013;150(3):1217–1220. doi: 10.1016/j.jad.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 45.Fisher PL, Durham RC. Recovery rates in generalized anxiety disorder following psychological therapy: An analysis of clinically significant change in the STAI-T across outcome studies since 1990. Psychol Med. 1999;29(6):1425–1434. doi: 10.1017/s0033291799001336. [DOI] [PubMed] [Google Scholar]
- 46.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 47.Masuda M, Holmes TH. Magnitude estimations of social readjustments. J Psychosom Res. 1967;11(2):219–225. doi: 10.1016/0022-3999(67)90011-6. [DOI] [PubMed] [Google Scholar]
- 48.Scully JA, Tosi H, Banning K. Life Events Checklists: Revisiting the Social Readjustment Rating Scale after 30 years. Educational and Psychological Measurement. 2000;60(6):864–876. [Google Scholar]
- 49.Gray MJ, Litz BT, Hsu JL, et al. Psychometric properties of the Life Events Checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 50.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 51.Brown TA, Antony MM, Barlow DH. Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behav Res Ther. 1992;30(1):33–37. doi: 10.1016/0005-7967(92)90093-v. [DOI] [PubMed] [Google Scholar]
- 52.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):509–515. [Google Scholar]
- 53.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ. 2012;184(3):191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin A, Rief W, Klaiberg A, et al. Validity of the brief Patient Health Questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71–77. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburg Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 56.Carpentera JS, Andrykowskia MA. Psychometric evaluation of the Pittsburg Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 57.Frenkel TI, Lamy D, Algom D, et al. Individual differences in perceptual sensitivity and response bias in anxiety: Evidence from emotional faces. Cognition and Emotion. 2009;23(4):688–700. [Google Scholar]
- 58.Gentili C, Cristea IA, Angstadt M, et al. Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Exp Biol Med. 2016;241(3):225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maltz W. Treating the sexual intimacy concerns of sexual abuse survivors. Sexual and Relationship Therapy. 2002;17(4):321–327. [Google Scholar]
- 60.McNally RJ. Is anxiety sensitivity distinguishable from trait anxiety? Reply to Lilienfeld, Jacob, and Turner (1989) J Abnorm Psychol. 1989;98(2):193–194. doi: 10.1037//0021-843x.98.2.193. [DOI] [PubMed] [Google Scholar]
- 61.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price JL. Olfactory system. In: Paxinos G, editor. The Human Nervous System. San Diego, CA: Academic Press; 1990. [Google Scholar]
- 63.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;160(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu S, Downes JJ. Proust nose best: Odors are better cues of autobiographical memories. Memory & Cognition. 2002;30(4):511–518. doi: 10.3758/bf03194952. [DOI] [PubMed] [Google Scholar]
- 65.Nickell PV, Uhde TW. Dose-response effects of intravenous caffeine in normal volunteers. Anxiety. 1994;1(4):161–168. doi: 10.1002/anxi.3070010403. [DOI] [PubMed] [Google Scholar]
- 66.Daniels JK, Vermetten E. Odor-induced recall of emotional memories in PTSD-Review and new paradigm for research. Exp Neurol. 2016;284:168–180. doi: 10.1016/j.expneurol.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Doty RL, Cometto-Muniz JE. Trigeminal Chemosensation. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2. New York, NY: Marcel Dekker; 2003. pp. 981–1000. [Google Scholar]
- 68.Doty RL, Brugger WE, Jurs PC, et al. Intranasal trigeminal stimulation from odorous volatiles: Psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]