Abstract
OBJECTIVES:
Obstetrical levator avulsion may be an important risk factor for prolapse. This study compares the size of the levator hiatus, the width of the genital hiatus, and pelvic muscle strength between vaginally parous women with or without levator avulsion, 5–15 years after delivery.
METHODS:
Parous women were assessed for levator ani avulsion, using 3dimensional transperineal ultrasound. Women with and without levator ani avulsion were compared with respect to levator hiatus areas (measured on ultrasound), genital hiatus (measured on examination), and pelvic muscle strength (measured with perineometry). Further analysis also considered the association of forceps-assisted birth.
RESULTS:
At a median interval of 11 years from first delivery, levator avulsion was identified in 15% (66/453). A history of forceps-assisted delivery was strongly associated with levator avulsion (45% vs 8%; p<0.001). Levator avulsion was also associated with a larger levator hiatus area (+7.3 cm2; 95% confidence interval (CI) 4.1, 10.4 with Valsalva), wider genital hiatus (+0.6 cm; 95%CI 0.3, 0.9 with Valsalva), and poorer muscle strength (−14.5 cm H20; 95%CI −20.4, −8.7 peak pressure). Among those with levator avulsion, forceps-assisted birth was associated with a marginal increase in levator hiatus size but not genital hiatus size or muscle strength.
CONCLUSIONS:
Obstetrical levator avulsion is associated with a larger levator hiatus, wider genital hiatus, and poorer pelvic muscle strength. Forceps-assisted birth is an important marker for levator avulsion, but may not be an independent risk factor for the development of pelvic muscle weakness or changes in hiatus size in the absence of levator avulsion.
INTRODUCTION:
Pelvic organ prolapse is significantly more common among vaginally parous women versus nulliparas or those who have delivered exclusively by cesarean [1]. However, the specific biological mechanisms that might link vaginal childbirth to prolapse are unknown. An important clue may be provided by studies suggesting a strong association between obstetrical avulsions of the levator ani muscle, diagnosed via magnetic resonance imaging or 3D transperineal ultrasound, and pelvic organ prolapse [2–3]. Avulsions of the levator ani muscle are detachments of the puborectalis muscle from its insertion on the pubis [2,4]. Delancey and colleagues found levator avulsion among 55% of women with prolapse compared to 16% of controls [3]. Thus, levator ani avulsion, which is detected among 10–30% of women who have had a vaginal delivery [4–6], may be an important step in the biological pathway that links vaginal childbirth to prolapse.
The long term consequences of levator avulsion are not understood. The aim of this study is to compare levator ani muscle structure (e.g., size of the levator hiatus) and function (e.g., contraction strength), 5–15 years from childbirth, between women with and without levator avulsion, as identified on 3D ultrasound. We hypothesize that women with levator avulsion have a larger levator hiatus area and weaker pelvic muscles. Both of these long term changes could plausibly contribute to the development of pelvic organ prolapse among parous women. This study is intended to explore the long term structural and functional consequences of levator avulsion, years after delivery, in order to provide a clinically meaningful picture of the sequelae of levator avulsion among parous women.
MATERIALS AND METHODS:
Participants for this research were recruited from a longitudinal cohort study of parous women, the Mothers’ Outcomes after Delivery study (MOAD) [7–8]. Participants were community volunteers, recruited 5–10 years after delivery of their first child and followed annually [8]. Although the entire cohort for the MOAD study included women who delivered by either cesarean or vaginal birth, the present study focused on women who had experienced vaginal birth. Institutional review board approval was obtained and all participants provided written informed consent.
Participants were enrolled in this supplementary study, which included the implementation of a three dimensional ultrasound, between May 2015 and April 2017. During an annual study visit, all vaginally parous participants were invited to join this sub-study. We also included a small number of women who had delivered all their children by cesarean; they were included only if their study examination demonstrated prolapse to or beyond the hymen. The inclusion of these cesarean-only participants blinded the investigators performing and interpreting the ultrasound volumes to women’s obstetrical history. In addition, they served as negative controls in the ultrasound protocol, as they were not expected to have any levator trauma.
Three-dimensional transperineal ultrasound acquisition and interpretation was based on published protocols [9–10]. We used a GE Voluson s6 system with a RAB2–6-RS convex transducer (General Electric Corporate USA). The ultrasound transducer, covered with a sheath, was applied to the perineum in the midsagittal plane. Three-dimensional ultrasound volumes were captured as cine loops. Each participant was imaged at rest, with maximum Valsalva and with pelvic floor muscle contraction. Imaging was performed by one of three trained sonographers who remained blinded to each participant’s obstetrical history and current symptoms.
The ultrasound volumes were stored for later analysis, which was performed with GE 4Dview (GE Healthcare). Two examiners reviewed the ultrasound volumes; each was masked to obstetrical history, to the physical examination, to any symptoms, and to the interpretation of the other examiner. Validated methods were used to identify levator avulsions [10]. Specifically, tomographic ultrasound images were prepared from contraction volumes at 2.5 mm slice intervals, from 5 mm below to 12.5 mm above the plane of minimal hiatal dimension. Complete levator ani avulsion was diagnosed during maximal pelvic floor contraction for volumes demonstrating complete discontinuity between the levator muscle and the inferior pubis ramus at the plane of minimal hiatal dimension and for at least 5 mm above that level [10]. The diagnosis of levator avulsion, therefore, required that three contiguous tomographic images demonstrated evidence of a separation between the muscle and the pubic ramus. All suspected avulsions were confirmed by two investigators. If diagnosis of levator avulsion was questionable, we used the levator-urethra gap to confirm the presence of avulsion [11]. The levator-urethra gap is the distance from the urethral lumen to the most medial insertion of the levator on the inferior pubic ramus; a distance greater than 2.5 cm is highly specific for the diagnosis of levator avulsion [11].
The area of the levator hiatus, the open space between the two arms of the levator muscle, was measured at rest, with voluntary contraction, and with Valsalva, using the area tool provided by GE 4Dview. Hiatal areas under each condition were assessed at the plane of minimal hiatal dimension [12–13]. The change in hiatal area from rest to pelvic floor muscle contraction was calculated by subtracting area at pelvic floor muscle contraction from area at rest. This change in area represents the woman’s ability to close the levator hiatus voluntarily and reflects muscle strength [14].
Immediately following the ultrasound procedure, a manometric assessment of pelvic muscle strength was performed using the Peritron perineometer (CardioDesign, Oakleigh, Australia). Women with allergy to Latex did not participate in perineometry. The team member performing the perineometry did not observe the ultrasound and was masked to obstetrical history and participant symptoms. Methods for perineometry were previously described [15]. The peak pressure was measured in cm H2O and averaged over two contractions.
Additional study data was obtained from the electronic data base of the parent study. This included self-reported race, age at the time of ultrasound, parity, and body mass index (kg/m2) measured at the time of ultrasound. Obstetrical data included maternal age at first vaginal delivery, any forceps-assisted birth, any deliveries with second stage of labor greater than 120 minutes, any deliveries with birth weight greater than 4 kilograms, history of any episiotomy, and history of obstetrical anal sphincter laceration. Obstetric information was obtained from a review of hospital records; if unavailable, (<5% of deliveries), the woman’s description of her birth was used to classify her obstetrical exposures.
Characteristics of women with and without levator ani avulsion were compared using Pearson’s chi-squared tests (for categorical variables) and Wilcoxon rank sum tests (for continuous variables). The primary interest was to compare the dimensions of the levator hiatus, the size of the genital hiatus, and levator ani strength in women with and without levator ani avulsion. Given the known association between levator ani avulsion and forceps-assisted birth [5, 16–17], we also considered the independent effect of forceps-assisted birth. In these analyses, we estimated difference in outcomes according to (a) only a history of forceps-assisted birth, (b) only the presence or absence of levator ani avulsion, and (c) a combination of both forceps history and avulsion. Percentile plots were generated to depict the distributions of the outcomes across these groups [18]. Linear regression models were used to quantify and test differences between groups, adjusting by confounders. Given that some of the dependent variables showed right skewness, we repeated the analyses by transforming the outcomes logarithmically.To convey both the magnitude of associations as well as their precisions, we report 95% confidence intervals.
Based on the size of the parent study, we anticipated we would identify 598 eligible women for this supplementary study. The sample size calculations for this research were based on this pool of eligible women. We anticipated that 75–80% would participate in this supplementary study (n=449 to 478) and that 15–20% of the participants would demonstrate levator avulsion. We designed the study to have 80% power to detect a difference of ≥2.65 cm2 in the levator hiatus area. This threshold was selected because it was expected to represent three times the standard deviation of the measure. All statistical analysis was completed using SAS version 9.4.
RESULTS:
Of 598 women eligible for this study (Figure 1), 10 (2%) declined to participate and 93 (16%) did not return for a study visit during the recruitment period. Thus, 495 women were included in this study. Of those, 41 were recruited as cesarean controls. No levator ani avulsions were identified among these 41 cesarean controls; these women did not contribute further data to this analysis. One ultrasound was uninterpretable and therefore excluded from the analysis. Thus, this report includes data for 453 vaginally parous women. At the time of ultrasound, these women were a median of 11 years from first vaginal delivery (range 6–17 years). There was no difference in this interval between those with and without levator ani avulsion (median (interquartile range) = 11.2 years (9.4, 13.4) versus 11.1 years (9.3, 13.7), p=0.973).
Figure 1:
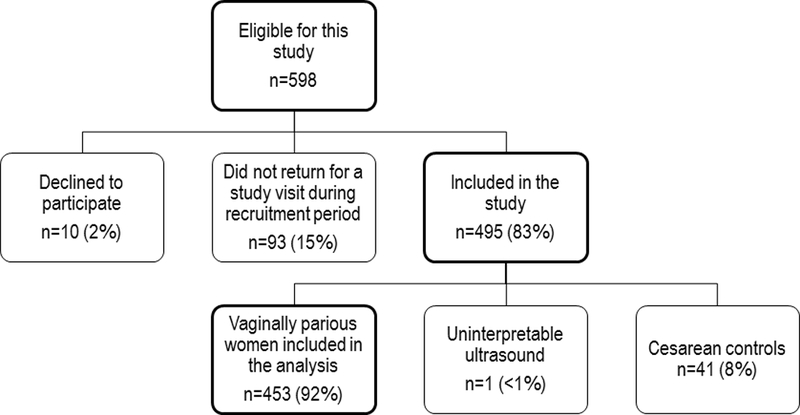
Enrollment summary
Levator ani avulsion was identified in 66/453 participants (15%). Table 1 compares the characteristics of women with and without levator avulsion. Women with levator avulsion were older at the time of first vaginal birth. They were also more likely to have delivered a macrosomic baby, to have had a second stage longer than 2 hours and to have had an obstetrical anal sphincter laceration. Notable was the very strong association of levator ani avulsion with a history of forceps delivery: 45% (30/66) with levator avulsion had a history of at least one forceps-assisted birth versus only 8% (32/387) without levator avulsion (p<0.001).
Table 1:
Characteristics of 453 vaginally parous women, by levator ani avulsion.
| Characteristica | No levator ani avulsion (n=387) |
Levator ani avulsion (n=66) |
P value |
|---|---|---|---|
| Age at ultrasound, years | 42.9 [39.5, 47.2] | 45.9 [42.4, 48.9] | <.001 |
| Age at first vaginal birth | 31.2 [28.6, 35.0] | 34.8 [31.2, 36.9] | <.001 |
| Years from first vaginal birth to ultrasound visit | 11.1 [9.3, 13.7] | 11.2 [9.4, 13.4] | 0.973 |
| Black race (vs. nonblack) | 12% (47) | 5% (3) | 0.069 |
| Multiparous at ultrasound | 79% (305) | 73% (48) | 0.271 |
| Vaginal births prior to ultrasound > 1 | 65% (251) | 67% (44) | 0.776 |
| BMI ≥ 30kg/m2 at ultrasound | 24% (91) | 17% (11) | 0.218 |
| Anyb vaginal delivery with macrosomia (> 4 kg) | 13% (51) | 26% (17) | 0.008 |
| Anyb vaginal delivery with second stage > 2 hours | 24% (94) | 55% (36) | <.001 |
| Anyb episiotomy | 53% (204) | 62% (41) | 0.156 |
| Anyb obstetric anal sphincter laceration | 14% (56) | 30% (20) | 0.002 |
| Anyb vacuum-assisted vaginal delivery | 12% (45/387) | 11% (7/66) | 0.810 |
| Anyb forceps-assisted vaginal delivery | 8% (32) | 45% (30) | <.001 |
Abbreviations: BMI=body mass index
Categorical variables reported as percent (n); continuous variables reported as median [interquartile range].
“Any” refers to an occurrence across all deliveries.
Table 2 compares levator hiatus area, genital hiatus, and pelvic muscle strength for women with to those without levator ani avulsion. Women with levator avulsion had a wider levator hiatus area on ultrasound, a wider genital hiatus on pelvic examination, and reduced levator strength (i.e., peak pressure) on perineometry. Notably, the median size of the levator hiatus at maximum Valsalva was 34.6 cm2 for women with an avulsion versus 25.1 cm2 for those without avulsion. Moreover, the median size of the genital hiatus with Valsalva was 1cm larger for those with levator avulsion (4.0 cm versus 3.0 cm). There was no significant effect of levator ani avulsion on the change in levator hiatus area with a voluntary contraction, although the trend was in the expected direction.
Table 2:
Hiatus area measurements and strength outcomes (median [interquartile range]) among 453 vaginally parous women, by levator ani avulsion.
| Outcomes | No levator ani avulsion (n=387) |
Levator ani avulsion (n=66) |
P value |
|---|---|---|---|
| Hiatus area measurements (cm2) | |||
| at rest | 19.0 [16.0, 22.3] | 25.1 [20.3, 28.9] | <.001 |
| at maximum voluntary contraction | 13.9 [11.9, 16.5] | 20.6 [16.8, 23.5] | <.001 |
| at maximum Valsalva | 25.1 [19.6, 31.2] | 34.6 [27.7, 40.5] | <.001 |
| Genital hiatus (cm), measured on exam | |||
| at rest | 2.0 [1.5, 2.5] | 2.5 [2.0, 3.0] | <.001 |
| strain | 3.0 [2.5, 4.0] | 4.0 [3.0, 4.5] | <.001 |
| Strength | |||
| Change in hiatus area with voluntary contraction (rest minus contraction, cm2) | 4.2 [2.2, 6.2] | 3.7 [1.2, 6.2] | 0.171 |
| Peak pressurea, cm H2O (perineometry, averaged over 2 contractions) | 28.5 [19.0, 42.5] | 13.5 [9.8, 21.3] | <.001 |
Missing peak pressure in n=21 (15 due to latex allergy, 6 other reasons).
Given the strong associations between levator ani avulsion and forceps-assisted birth, as previously noted in Table 1, further analyses considered the outcomes across four groups, according to presence or absence of levator ani avulsion and forceps-assisted birth. The four groups therefore include those with neither levator avulsion nor a history of forceps-assisted birth (n=355), those with only forceps-assisted birth (n=32), those with only levator avulsion (n=36), and those with both levator avulsion and a history of forceps-assisted birth (n=30).
In Figure 2, these four groups are compared for levator hiatus area, genital hiatus, and pelvic muscle strength. The results of a corresponding multivariable regression is shown in table 3. Because the pattern of differences across groups was similar for differences in the measures at rest, with Valsalva and with contraction, table 3 includes hiatus measures only with Valsalva, as well as strength measures with contraction. An important finding was that forceps-assisted birth, in the absence of levator avulsion, was not associated with any significant difference in hiatus size or pelvic muscle strength.
Figure 2:
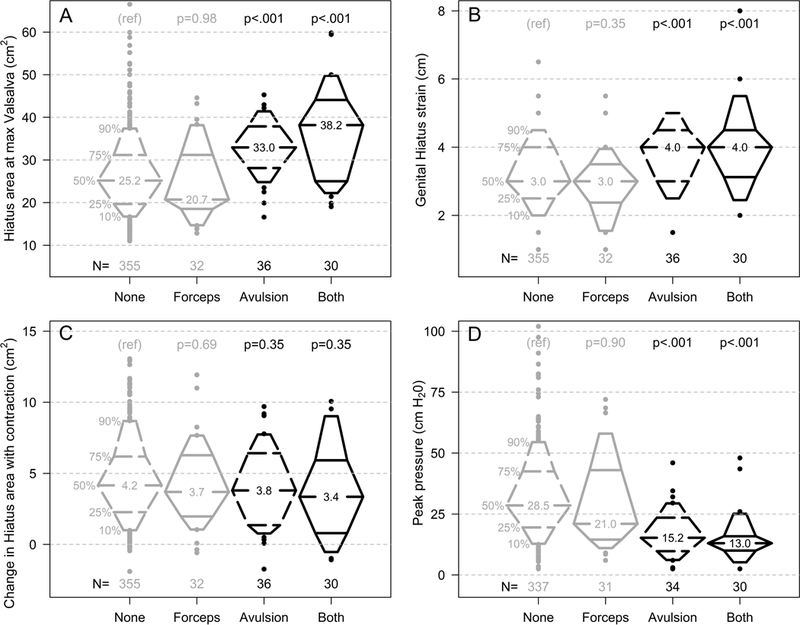
Percentile plots illustrating the distributions of four outcomes (A-D), each comparing women classified according to the presence or absence of levator ani avulsion and history of forceps-assisted birth: (A) levator hiatus area with Valsalva; (B) genital hiatus with Valsalva; (C) change in levator hiatus area with voluntary contraction (rest minus contraction); (D) pelvic muscle strength (peak pressure) with voluntary contraction. P values derived from linear regression.
Table 3:
Mean differencesa (95% confidence intervals) in levator hiatus area, genital hiatus, and strength outcomes.
| Group | Hiatus area at max Valsalva (cm2) | Genital hiatus (GH) with strain (cm) | Change in hiatus area with contraction (cm2) | peak pressure (cmH2O)b |
|---|---|---|---|---|
| Neither levator ani avulsion nor Forceps (n=355) | Ref | Ref | Ref | ref |
| Only Forceps delivery (n=32) | −0.7 (−4.1, 2.6) |
−0.3 (−0.6, 0.1) |
−0.3 (−1.5, 0.9) |
−2.7 (−8.9, 3.5) |
| Only Levator ani avulsion (n=36) |
7.3 (4.1, 10.4) |
0.6 (0.3, 0.9) |
−0.6 (−1.7, 0.6) |
−14.5 (−20.4, −8.7) |
| Levator ani avulsion and forceps delivery (n=30) |
11.4 (7.9, 14.9) |
0.7 (0.4, 1.1) |
−0.6 (−1.9, 0.6) |
−17.0 (−23.4, −10.6) |
Mean differences correspond to beta coefficients (increase or decrease for each measurement) derived from multivariable linear regression, adjusted for age, race, macrosomia, and prolonged second stage of labor. Statistically significant results are shown in bold font.
In addition, compared to women without levator avulsion, women with levator ani avulsion had a wider levator hiatus area with Valsalva (+7.3 cm2; 95%CI 4.1 to 10.4), a wider genital hiatus (+0.6 cm; 95%CI 0.3 to 0.9 for strain), and poorer muscle strength (−14.5 H20; 95%CI −20.4 to −8.7 for peak pressure). Table 3 also demonstrates that women with both levator avulsion and a history of forceps-assisted birth had the largest hiatus area and the poorest strength.
Among women with levator avulsion (n=66), we compared those with a history of forceps-assisted delivery (n=30) to those without. Those with a history of forceps-assisted delivery had a larger levator hiatus area with Valsalva (an increase of 4.1 cm2, 95% CI −0.3 to 8.5). Among women with levator avulsion, those with and without a history of forceps-assisted delivery were similar with regard to genital hiatus size and muscle strength.
To account for skewness and high variability in some outcomes, additional analyses were performed after transformation of the outcomes to the logarithmic scale. The results (data not shown) were consistent with the results depicted in Table 3.
DISCUSSION:
These findings demonstrate that levator ani avulsion is associated with significant long term changes in the size of the levator hiatus, the size of the genital hiatus, and pelvic muscle strength. Specifically, we found that obstetrical levator avulsion is associated with a larger levator hiatus, wider genital hiatus, and poorer pelvic muscle strength. Prior studies have demonstrated short term changes [19–21]. Our results suggest that these differences are sustained or possibly magnified with time. For example, four months after delivery, Shek and Dietz [19] demonstrated that levator avulsion was associated with a wider hiatal area on Valsalva (25.5 cm2 versus 22.6 cm2). However, the difference in levator hiatus observed in the present study was much more dramatic (34.6 cm2 versus 25.1 cm2). This difference may reflect cumulative changes in levator hiatus in the years following childbirth. Indeed, the size of the levator hiatus appears to increase over time among parous women [22].
This study also provides new perspectives on forceps-assisted birth. As expected [5, 16–17], forceps-assisted birth was associated with levator ani avulsion in this population. Prior research suggests that forceps-assisted birth is associated with poorer muscle strength [15] and is a risk factor for the development of pelvic floor disorders, including organ prolapse [23]. In this study, we found that a history of forceps-delivery, among women without evidence of levator avulsion, had no association with the size of the levator hiatus, the size of the genital hiatus, or pelvic muscle strength. This is in contrast to the strong association we observed between levator avulsion and these outcomes. The impact of forceps history was limited to those with levator avulsion, in whom the history of forceps-assisted birth was associated with a small but significant additional increase in levator hiatus areas. These results suggest that forceps-assisted birth may be an important marker for (and cause of) levator avulsion, but may not have a critical independent impact on levator hiatus size or function in the absence of levator avulsion.
A weakness of the study was that we assessed the outcomes at one point in time. The relationship between levator avulsion and these outcomes might change over time. We also acknowledge that there may be other factors associated with levator avulsion, such as nerve injury, which could contribute to levator size and function that we were not able to assess in this study. The strengths of this study include the large sample, a rigorous assessment of levator avulsion, the quantitation of pelvic muscle strength via perineometry, and the confirmation of forceps-assisted delivery via review of obstetrical records. An additional strength is the unique opportunity to assess the outcomes of interest several years after delivery.
Given the results of this study, together with evidence suggesting that levator ani avulsion is a risk factor for prolapse later in life, we speculate that the observed chronic widening of the levator hiatus and weakness of the levator muscle may mediate the development of prolapse among vaginally parous women. This hypothesis is supported by computer simulation models suggesting that a wider levator hiatus can lead to the development of vaginal prolapse [24]. Additional evidence for this hypothesis is provided by our observation that a wide genital hiatus (on examination) is associated with more rapid worsening of uterovaginal support [8]. Further research to confirm the clinical significance of these findings is an important next step to improve our understanding of the biology of pelvic floor disorders.
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD082070 and R01HD056275).
Footnotes
No reprints will be available
Contributor Information
Victoria L. HANDA, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Joan L. BLOMQUIST, Greater Baltimore Medical Center, Baltimore, MD, USA.
Jennifer ROEM, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Alvaro MUÑOZ, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Hans Peter DIETZ, University of Sydney, Sydney, New South Wales, Australia.
REFERENCES
- 1.Hallock JL, Handa VL. The Epidemiology of Pelvic Floor Disorders and Childbirth: An Update. Obstet Gynecol Clin North Am 2016;43:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115:979–84. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 2007;109:295–302 [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 2003;101:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caudwell-Hall J, Kamisan Atan I, Martin A, et al. Intrapartum predictors of maternal levator ani injury. Acta Obstet Gynecol Scand 2017;96:426–431. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol 2005;106:707–12. [DOI] [PubMed] [Google Scholar]
- 7.Handa VL, Blomquist JL, Knoepp LR, et al. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol 2011;118:777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handa VL, Blomquist JL, Roem J, et al. Longitudinal study of quantitative changes in pelvic organ support among parous women. Am J Obstet Gynecol. 2018;218:320.e1–320.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz HP. Ultrasound imaging of the pelvic floor: Part II Three-dimensional or volume imaging. Ultrasound Obstet Gynecol 2004;23:615–625. [DOI] [PubMed] [Google Scholar]
- 10.Dietz H, Bernardo M, Kirby A, et al. Minimal criteria for the diagnosis of avulsion of the puborectalis muscle by tomographic ultrasound Int Urogynecol J 2011;22:699–704 [DOI] [PubMed] [Google Scholar]
- 11.Dietz HP, Abbu A, Shek KL. The levator-urethra gap measurement: a more objective means of determining levator avulsion? Ultrasound Obstet Gynecol 2008;32:941–5. [DOI] [PubMed] [Google Scholar]
- 12.Dietz HP, Shek C, De Leon J, et al. Ballooning of the levator hiatus. Ultrasound Obstet Gynecol 2008;31:676–680. [DOI] [PubMed] [Google Scholar]
- 13.Abdool Z, Shek KL, Dietz HP. The effect of levator avulsion on hiatal dimension and function. Am J Obstet Gynecol 2009;201:89. [DOI] [PubMed] [Google Scholar]
- 14.Siff LN, Hill AJ, Walters SJ, et al. The Effect of Commonly Performed Exercises on the Levator Hiatus Area and the Length and Strength of Pelvic Floor Muscles in Postpartum Women. Female Pelvic Med Reconstr Surg 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Friedman S, Blomquist JL, Nugent JM, et al. Pelvic muscle strength after childbirth. Obstet Gynecol 2012;120:1021–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memon HU, Blomquist JL, Dietz HP, et al. Comparison of levator ani muscle avulsion injury after forceps-assisted and vacuum-assisted vaginal childbirth. Obstet Gynecol 2015;125:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney R, Fitzpatrick M, Brennan S, et al. Levator ani injury in primiparous women with forceps delivery for fetal distress, forceps for second stage arrest, and spontaneous delivery. Int J Gynaecol Obstet 2010;111:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esty WW, Banfield JD. The box-percentile plot. Journal of Statistical Software 2003; 8:1–14. [Google Scholar]
- 19.Shek KL, Dietz HP. The effect of childbirth on hiatal dimensions. Obstet Gynecol 2009;113:1272–8. [DOI] [PubMed] [Google Scholar]
- 20.Cyr MP, Kruger J, Wong V, et al. Pelvic floor morphometry and function in women with and without puborectalis avulsion in the early postpartum period. Am J Obstet Gynecol. 2017; 216:274.e1–274.e8. [DOI] [PubMed] [Google Scholar]
- 21.van Delft KW, Thakar R, Sultan AH, et al. The natural history of levator avulsion one year following childbirth: a prospective study. BJOG. 2015;122(9):1266–73. [DOI] [PubMed] [Google Scholar]
- 22.Chan SS, Cheung RY, Lee LL, et al. A longitudinal follow-up of pelvic floor biometry: what are the factors affecting it? Ultrasound Obstet Gynecol. 2017. doi: 10.1002/uog.17446. [DOI] [Google Scholar]
- 23.Lisonkova S, Lavery JA, Ananth CV, et al. Temporal trends in obstetric trauma and inpatient surgery for pelvic organ prolapse: an age-period-cohort analysis. Am J Obstet Gynecol. 2016;215:208.e1–208.e12. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Ashton-Miller JA, Hsu Y, et al. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol 2006;108:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


