Abstract
Background
The incidence of atrial fibrillation (AF) is increasing, conferring a major health-care issue in Asia. No risk score for predicting incident AF has been specifically developed in Asian subjects. Our aim was to investigate risk factors for incident AF in Asian subjects and to combine them into a simple clinical risk score.
Methods
Risk factors for incident AF were analyzed in 471,446 subjects from the Chinese Yunnan Insurance Database (internal derivation cohort) and then combined into a simple clinical risk score. External application of the new score was performed in 451,199 subjects from the Korean National Health Insurance Service (external cohort).
Results
In the internal cohort, structural heart disease (SHD), heart failure (HF), age ≥ 75 years, coronary artery disease (CAD), hyperthyroidism, COPD, and hypertension were associated with incident AF. Given the low prevalence and the strong association of SHD with incident AF (hazard ratio, 26.07; 95% CI, 18.22-37.30; P < .001), these patients should be independently considered as high risk for AF and were excluded from the analysis. The remaining predictors were combined into the new simple C2HEST score: C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point). The C2HEST score showed good discrimination with the area under the curve (AUC) of 0.75 (95% CI, 0.73-0.77) and had good calibration (P = .774). The score was internally validated by bootstrap sampling procedure, giving an AUC of 0.75 (95% CI, 0.73-0.77). External application gave an AUC of 0.65 (95% CI, 0.65-0.66). The C2HEST score was superior to CHADS2 and CHA2DS2-VASc scores in both cohorts in predicting incident AF.
Conclusions
We have developed and validated the C2HEST score as a simple clinical tool to assess the individual risk of developing AF in the Asian population without SHD.
Key Words: Asian, atrial fibrillation, cohort study, prediction model, risk factors, risk score
Abbreviations: AF, atrial fibrillation; ARIC, Atherosclerosis Risk In Communities Study; AUC, area under the curve; CAD, coronary artery disease; CHARGE-AF, Cohorts for Heart and Aging Research in Genomic Epidemiology-Atrial Fibrillation; FHS, Framingham Heart Study; HF, heart failure; HR, hazard ratio; ICD-10, International Classification of Diseases, 10th Revision; IR, incidence rate; IS, ischemic stroke; NHIS, National Health Insurance Service; SHD, structural heart disease
FOR EDITORIAL COMMENT, SEE PAGE 458
Atrial fibrillation (AF) is the most prevalent arrhythmia worldwide and is associated with an increased risk of ischemic stroke (IS), heart failure (HF), coronary artery disease (CAD), and mortality.1, 2, 3 The 2010 Global Burden of Disease Study4 demonstrated that the worldwide age-adjusted prevalence of AF is 596 per 100,000 men and 373 per 100,000 women. The incidence of AF increases dramatically with the development of incident risk factors for AF, such as ageing, hypertension, HF, CAD, and COPD. Indeed, this arrhythmia confers a major health-care burden in Asia. For example, Japan will have 1 million patients with AF and China will have 9 million patients with AF by 2050.5, 6
Given that many patients with incident AF are asymptomatic, a considerable number of patients are only diagnosed with AF when presenting with AF-related complications. Because of this underdiagnosis, the actual prevalence of AF could be considerably higher.7, 8 Hence, risk evaluation and early identification would be important for the targeting of early prevention strategies and improve prognosis.9, 10 Therefore, effective and cost-effective strategies should focus on identifying patients who are at higher risk of incident AF.
Several clinical risk scores have been developed to predict incident AF, including the Framingham Heart Study (FHS) score,11 the Atherosclerosis Risk In Communities Study (ARIC) score,12 and the Cohorts for Heart and Aging Research in Genomic Epidemiology-Atrial Fibrillation (CHARGE-AF) score.13 These three scoring systems were all derived from large follow-up cohorts and have good predictive ability for incident AF.11, 12, 13 However, they require many instrumental and laboratory variables to be calculated, being generally too complicated for everyday clinical application. Moreover, many of the previous scores were derived in Western populations, and a predictive score for incident AF in an Asian population has been never been developed. This is relevant, given that risk factors for AF may be different between Western and Asian populations.14, 15
Our primary objective was to analyze risk factors for incident AF in a large cohort of Asian subjects, which were combined into a simple clinical risk stratification score, which would be easy and practical to apply in everyday practice.
Methods
Derivation Cohort
Details of the derivation database, the Yunnan Medical Insurance Database, have been described previously.16 In brief, this is a medical insurance database in Yunnan Province, China, including > 10 million individuals from January 1, 2001, through December 30, 2012.16 This medical insurance scheme covers urban residents in Yunnan Province, located in the far southwest of China, spanning approximately 394,000 km2 and with a population of 46.3 million (2011 population statistics), representing 3% of the total Chinese population. It was part of a governmental medical insurance plan, ensuring all participants had a permanent and personal registration number, through which the information of medical history, drugs, and mortality data recorded could be collected. All medical information of participants was obtained from local 2A- and 3A-grade hospitals, which guaranteed the reliability of records regarding any medical service. Random sampling was performed on the enrolled individuals biennially, based on the periods of 2001 to 2002, 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010, and 2011 to 2012. A total of 1,228,539 people was selected. After excluding people with incomplete data (n = 2,611), readmission (n = 754,582), and prevalent AF at baseline (n = 30), 471,446 cases were entered in the final analysis.16
This derivation study was approved by the medical ethics committee of Chinese People’s Liberation Army General Hospital (approval No. 13BJZ40). The definitions of AF and other risk factors were according to the International Classification of Diseases, Ninth Revision/International Classification of Diseases, 10th Revision (ICD-10) (e-Appendix 1). AF was defined based on an ECG or Holter recording. The inclusion criteria for an AF case were limited to inpatients with AF diagnosis confirmed on admission and discharge. The accuracy of AF diagnosis was validated previously using sensitivity analysis.16
Patients with paroxysmal AF were also included in the incident analysis. Patients with rheumatic heart disease were those who suffered from rheumatic fever and concomitant valvular heart disease (ie, mitral stenosis). Patients with structural heart disease (SHD) included those patients with rheumatic heart disease and dilated cardiomyopathy.
External Application
The external cohort study was based on the Korean National Health Insurance Service (NHIS)-Health Screening cohort released in 2015, including subjects who participated in health screening programs provided by the NHIS in the Republic of Korea in 2002 and 2003. This external cohort consisted of 514,764 Korean subjects 40 to 80 years of age, who comprised a 10% simple random sample of all health screening participants. A total of 55.3% of the participants lived in nonmetropolitan areas, which covers some urban areas and all rural areas. The follow-up started from 2002 through 2013, with a mean duration of 87.3 ± 17.6 months. Detailed information and a profile of the NHIS-Health Screening cohort have been described in a previous report.17 The baseline demographics for this external cohort are summarized in e-Table 1.
Table 1.
Baseline Characteristics of 471,446 Subjects Included in the Internal Validation Cohort
| Characteristics | Subjects Without AF (n = 470,525) | Subjects With Incident AF (n = 921) | P Value |
|---|---|---|---|
| Age, y | 47 ± 16 | 62 ± 12 | < .001 |
| Male sex | 247,752 (52.7) | 574 (62.3) | < .001 |
| Medical history | |||
| Hypertension | 45,444 (9.7) | 236 (25.6) | < .001 |
| Diabetes | 18,900 (4.0) | 29 (3.1) | .180 |
| CAD | 14,813 (3.1) | 187 (20.3) | < .001 |
| Hyperlipidemia | 6,610 (1.4) | 17 (1.9) | .198 |
| Vascular disease | 6,003 (1.3) | 9 (1.0) | .486 |
| COPD | 1,687 (0.4) | 25 (2.7) | < .001 |
| Previous IS | 1,608 (0.3) | 7 (0.8) | .023 |
| Renal dysfunction | 1,423 (0.3) | 8 (0.9) | .002 |
| Hyperthyroidism | 971 (0.2) | 5 (0.5) | .025 |
| HF | 700 (0.1) | 43 (4.7) | < .001 |
| SHD | 401 (0.1) | 33 (3.6) | < .001 |
| Hypothyroidism | 203 (0.0) | 1 (0.1) | .340 |
| CHA2DS2VASc score | 1 (0-1) | 2 (1-3) | < .001 |
Values are mean ± SD, No. (%), median (interquartile range), or as otherwise indicated. AF = atrial fibrillation; CAD = coronary artery disease; HF = heart failure; IS = ischemic stroke; SHD = structural heart disease.
This study was approved by the institutional review board of Yonsei University Health System, and the informed consents were waived. We excluded the following subjects: (1) those diagnosed with AF before conducting the health check-ups (n = 5,019); (2) those with valvular heart disease (n = 935; with a diagnosis of mitral stenosis [ICD-10: I05.0, I05.2, and I34.2] or prosthetic heart valves [ICD-10: Z95.2-Z95.4], and insurance claims for valve replacement or valvuloplasty); and (3) those with cardiomyopathy (n = 357) (ICD-10: I42.0-I42.2). Finally, a population cohort of 451,199 patients was included for this analysis. Patients were defined as having incident AF (1) if there was a discharge diagnosis of AF for inpatients with no prior history of AF or (2) when an episode of AF was detected in an outpatient and confirmed by a specialist. This strategy of AF diagnosis has previously been validated in the NHIS database with a positive predictive value of 94.1%.18, 19
Statistical Analysis
Continuous variables were reported as mean with SD, and categorical variables were expressed as number of patients (%). Annual incident rates were defined as the number of patients with incident AF divided by the number of person-years free of AF within a 1-year period.
Multivariable Cox regression analysis was used to identify independent risk factors for incident AF over 11 years of follow-up (mean ± SD, 4.1 ± 3.5 years) in the derivation cohort. The new predictive score was obtained with a stepwise model selection procedure based on Akaike information criterion. The pool of variables was also confirmed by a removal approach with a P value threshold of 10%. Risk factors in the final model were tested for interaction, but no further improvement was possible by including interactions.
The score was evaluated by means of the time-dependent (at follow-up at 11 years) area under the curve (AUC) for the receiver operating characteristic curve for predicting incident AF. Internal validation was obtained by means of 1,000 bootstrap replicates, and calibration was assessed by means of the Gronnesby and Borgan test.20 Performance of the external application was also evaluated as the AUC in the whole Korean cohort.
The Kaplan-Meier curves were computed to present the survival rates free from AF during follow-up, after dividing patients into 3 groups (low-, medium-, and high-risk) according to the new score.
Given that the CHADS2 (congestive HF, hypertension, age >75, diabetes mellitus, prior stroke or transient ischemic attack [2 points]), CHA2DS2-VASc (congestive HF, hypertension, age ≥75 [2 points], stroke/transient ischemic attack/thromboembolism [2 points], vascular disease, age 65-74, sex category), and HATCH (hypertension, age ≥75, transient ischemic attack or stroke [2 points], COPD, HF [2 point]) scores have been previously reported to be associated with incident AF,21, 22, 23 we also performed an exploratory analysis to investigate how the C2HEST score compares against these scores.
All tests were two-tailed, and a value of P < .05 was considered as statistically significant. Analyses were performed using SPSS Statistics version 23.0 (IBM) and R version 3.4.2 (The R Project for Statistical Computing).
Results
Baseline characteristics of the derivation cohort are presented in Table 1. A total of 471,446 subjects were included in the final analysis, of whom 921 subjects developed AF during 11 years of follow-up (mean ± SD, 4.1 ± 3.5 years), with an incidence of 0.5 per 1,000 person-years. Compared with subjects without AF, patients who developed AF were older (P < .001), more frequently men (P < .001), and with higher rates of hypertension, CAD, COPD, previous IS, renal dysfunction, hyperthyroidism, HF, and SHD (P < .001, for all).
Risk Factors for Incident AF and Score Development
Univariate Cox regression analysis is shown in Table 2. On multivariable analysis, SHD, HF, age ≥ 75 years, CAD, hyperthyroidism, COPD, and hypertension were independent risk factors for incident AF (Table 2). Given the low prevalence and very strong association of SHD with incident AF (hazard ratio [HR], 26.07; 95% CI, 18.22-37.30; P < .001), this subgroup of patients should be considered as being at (very) high risk for incident AF independently from other risk factors. Therefore, this variable was not considered to build the score. The HRs of other independent risk factors for incident AF did not change after removal of SHD from the multivariable model (not shown).
Table 2.
HRs of Risk Factors for Incident Atrial Fibrillation
| Risk Factors | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| SHD | 39.00 | 27.6-55.2 | < .001 | 26.1 | 18.2-37.3 | < .001 |
| HF | 34.66 | 25.5-47.1 | < .001 | 7.95 | 5.76-11.0 | < .001 |
| Age ≥ 75 y | 8.24 | 6.80-9.98 | < .001 | 5.83 | 4.80-7.09 | < .001 |
| CAD | 7.01 | 5.97-8.23 | < .001 | 4.14 | 3.50-4.90 | < .001 |
| Hyperthyroidism | 2.06 | 0.85-4.96 | .107 | 3.20 | 1.33-7.71 | .010 |
| COPD | 6.81 | 4.57-10.1 | < .001 | 3.01 | 1.33-7.71 | < .001 |
| Hypertension | 4.63 | 4.05-5.29 | < .001 | 3.24 | 2.82-3.73 | < .001 |
| Renal dysfunction | 2.91 | 1.45-5.83 | .003 | … | … | … |
| Previous IS | 2.13 | 1.01-4.49 | .046 | … | … | … |
| Hyperlipidemia | 1.12 | 0.71-1.79 | .620 | … | … | … |
| Male | 1.22 | 1.07-1.40 | .003 | … | … | … |
| Hypothyroidism | 1.79 | 0.25-12.7 | .561 | … | … | … |
| Diabetes | 0.61 | 0.42-0.89 | .009 | … | … | … |
| Vascular disease | 1.36 | 0.38-1.41 | .355 | … | … | … |
HR = hazard ratio. See Table 1 legend for expansion of other abbreviations.
We combined these risk factors into the new simple C2HEST score (Table 3): C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point). Total score ranged from 0 to 8 points.
Table 3.
C2HEST Score for Incident Atrial Fibrillation
| Acronym | Risk Factor | Points |
|---|---|---|
| C2 | CAD/COPD | 1-2 |
| H | Hypertension | 1 |
| E | Elderly (age ≥ 75 y) | 2 |
| S | Systolic HF | 2 |
| T | Thyroid disease (hyperthyroidism) | 1 |
| Total points | 0-8 | |
| AUC (C index) | 95% CI | |
| C2HEST score | 0.749 | 0.729-0.769 |
AUC = area under the curve. See Table 1 legend for expansion of other abbreviations.
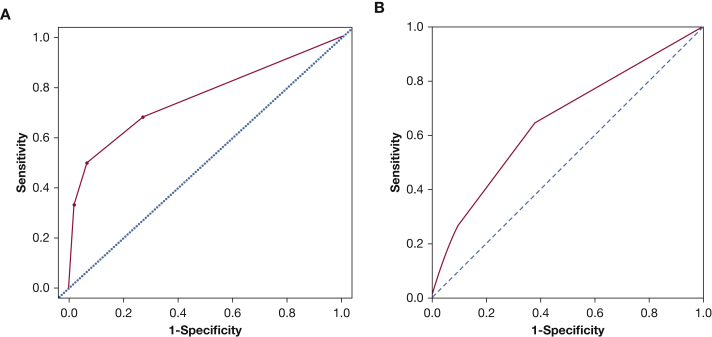
The score showed a good discrimination with an AUC of 0.750 (95% CI, 0.730-0.771) (Fig 1A) and a good calibration (P = .774). The score was then internally validated by a bootstrap sampling procedure, which gave an AUC of 0.749 (95% CI, 0.729-0.769).
Figure 1.
A, B, Receiver operating characteristic curves for the C2HEST score in predicting incident atrial fibrillation: (A) internal cohort and (B) external cohort.
When applied to the external cohort, the score showed moderate discrimination with an AUC of 0.654 (95% CI, 0.649-0.659) (Fig 1B).
Incident Rates of AF and the C2HEST Score
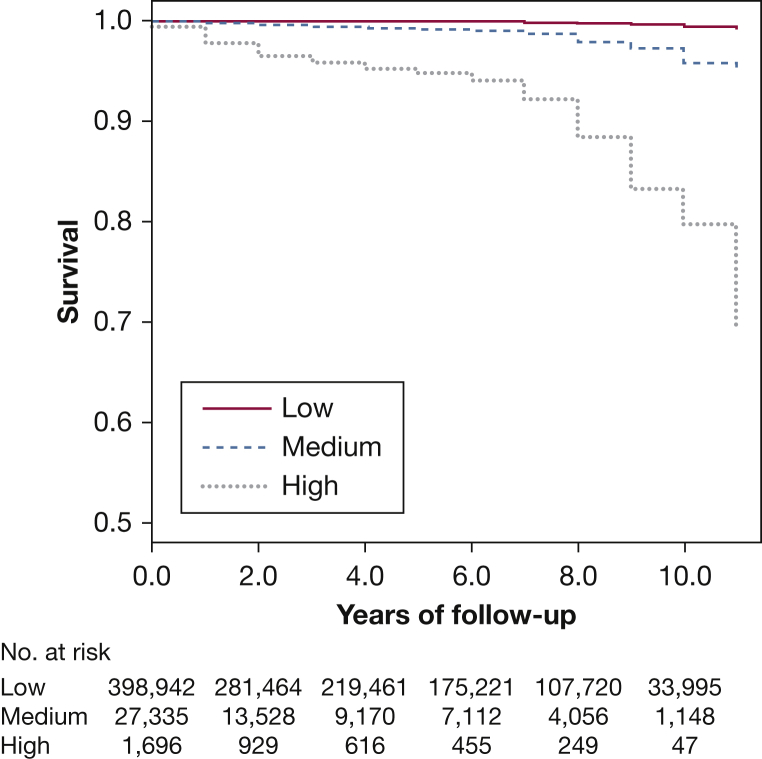
Table 4 shows incidence rates (IRs) and HRs at each point of the C2HEST score. We divided patients in the derivation cohort into three groups according to the C2HEST score: low (0-1 points, IR 0.34%/year), medium (2-3 points, IR 2.60%/year), and high risk (> 3 points, IR 15.98%/year). Kaplan-Meier curves for risk categories (Fig 2) showed an increased risk of AF across the three groups (log-rank test P < .001).
Table 4.
Annual Incidence of AF by C2HEST Score
| Score | No. of Subjects | No. of Incidents of AF | Incidence of AFa | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|
| 0 | 310,117 | 246 | 0.18 | 1.00 | … |
| 1 | 88,825 | 378 | 0.82 | 4.31 | 3.67-5.06 |
| 2 | 19,270 | 148 | 2.31 | 12.8 | 10.4-15.6 |
| 3 | 8,253 | 68 | 3.73 | 22.6 | 17.2-29.6 |
| 4 | 1,373 | 68 | 16.1 | 97.0 | 74.1-127.0 |
| 5 | 90 | 6 | 28.7 | 187.4 | 83.3-421.6 |
| ≥ 6 | 45 | 7 | 59.8 | 332.0 | 156.6-704.0 |
See Table 1 legend for expansion of abbreviation.
Per 1,000 person-years.
Figure 2.
Kaplan-Meier curves for risk categories according to the C2HEST score. Patients were divided into three groups: low (0-1 points), medium (2-3 points), and high risk (> 3 points).
Comparison of the C2HEST Score With the CHADS2, CHA2DS2-VASc, and HATCH Scores
Based on time-dependent prognostic models, the C2HEST score had significantly better predictivity for incident AF compared with the CHADS2, CHA2DS2-VASc, and HATCH scores in the derivation cohort from China (AUC CHADS2, 0.632; 95% CI, 0.604-0.660; P < .001 vs C2HEST; AUC CHA2DS2-VASc, 0.687; 95% CI, 0.659-0.716; P < .001 vs C2HEST; AUC HATCH, 0.633; 95% CI, 0.598-0.667; P < .001 vs C2HEST).
In the external cohort from Korea, the C2HEST score also had significantly better predictivity for incident AF compared with the CHADS2 and CHA2DS2-VASc scores (AUC CHADS2, 0.637; 95% CI, 0.632-0.642; P < .001 vs C2HEST; AUC CHA2DS2-VASc, 0.637; 95% CI, 0.632-0.642; P < .001 vs C2HEST).
However, this difference was only marginally significant for the HATCH score (AUC HATCH, 0.646; 95% CI, 0.641-0.651; P = .059 vs C2HEST).
Discussion
To our knowledge, this is the largest cohort study from an Asian population aimed at developing a simple risk assessment tool for incident AF. We investigated risk factors for incident AF and derived and validated the new C2HEST score as a user-friendly clinical score to assess individual risk of developing incident AF.
In the multivariable analysis, we found that risk factors for incident AF were SHD, HF, ageing (≥ 75 years), CAD, hyperthyroidism, COPD, and hypertension. All these risk factors were also demonstrated to increase the risk of incident AF in previous studies.5, 24, 25, 26 Indeed, SHD dramatically increases the risk of incident AF such that patients with SHD per se are very high risk of incident AF.5 HF is also another significant and independent risk factor, with a two- to sixfold increase in the risk of incident AF.24, 25 The ARIC study showed that stable CAD was an independent risk factor for incident AF (HR, 2.21; 95% CI, 1.71-2.84).12
As previously highlighted, previous studies have proposed several predictive tools for incident AF, such as the FHS score, the ARIC score, and the CHARGE-AF score.11, 12, 13 All these scoring systems were derived from large cohorts and showed good predictive values (C statistic, 0.75-0.78). However, these scores require many instrumental and laboratory variables to be calculated. Recently, the Suita study27 in Japan has developed a risk score for incident AF with a good predictive power (C statistic, 0.75; 95% CI, 0.72-0.77). However, this score is complex (> 16 points), and we could not compare the C2HEST score with the Suita study because our dataset did not have reliable data on cardiac murmur, which could be regarded as a subjective parameter based on physician auscultation.
As an exploratory analysis, we do show that the C2HEST score had significantly better predictivity for incident AF compared with the CHADS2, CHA2DS2-VASc, and HATCH scores in the derivation cohort. Similarly, in the external cohort, the C2HEST confirmed higher predictivity for incident AF than the CHADS2 and CHA2DS2-VASc scores, whereas it was only marginally significant compared with the HATCH score. These comparisons are with the caveat that these scores were not derived nor designed for the prediction of incident AF, but they were elaborated for stroke risk stratification28, 29 or arrhythmia progression from paroxysmal to persistent AF.30
In contrast, the C2HEST was specifically designed to predict incident AF, and we used an established approach whereby a large Asian cohort was used to derive the score with internal validation using bootstrap and calibration methods. We then externally applied it in an independent Asian population sample for incident AF. The risk factors included in the C2HEST score may be promptly deduced by a careful clinical evaluation of a patient with no need for any laboratory evaluation to calculate the individual risk of incident AF.
Strengths and Limitations
This is the first study aimed at developing a simple, user-friendly clinical risk score, the C2HEST score, from a large (n = 471,446) community-based Asian population with long follow-up. Also, we provided an external application using a nationwide cohort derived from another large Asian population dataset, the Korean NIHS dataset (n = 451,199). The C2HEST score is easy to calculate and apply in clinical practice and allows for good identification of patients at risk for incident AF.
Nevertheless, this study has some limitations. There were some differences in the demographic characteristics and comorbidities between the Chinese and Korean cohorts, reflecting the different populations, the health-care system settings, and the nature of the cohorts per se (ie, health insurance scheme data from one single China province [Yunnan] from local 2A- and 3A-grade hospitals and a nationwide sample of Korean subjects).
The incidence of AF in the derivation cohort was also lower than that of Western populations, which may result from underdiagnosis and ethnic differences. Asymptomatic AF may be underrepresented, given that there was no opportunistic screening for AF performed in this study. Indeed, the lower incidence of AF in Asian populations compared with Western populations is in accordance with previous reports showing an incidence of AF of 0.50 to 1.37 per 1,000 person-years in Asians vs 3.04 to 3.68 per 1,000 person-years in Western populations.31, 32, 33, 34 The detection of asymptomatic AF is also problematic in large population studies considering the random pattern of AF onset. Nevertheless, it would generally be impossible to screen 0.5 million people for detecting asymptomatic AF, and such AF screening work could be done only in small highly selected samples (and possibly underpowered), which would again diminish the power of a large population-targeted study. Therefore, to do such a large population-based epidemiology study in developing countries, such as China and other Asian countries, we are using the most viable way, based on a simple clinical score-based approach. Another issue is that screening method selection could have a major impact on results.
Although we have excluded those patients with known AF at baseline according to available medical records, there may have been some patients with unknown AF (or transient AF) for which we could not confirm thorough AF screening at the patient level. However, this would not defeat the meaning of evaluating the subject’s risk of developing incident AF during follow-up. Indeed, patients with AF occurring transiently are associated with high incidence of another AF episode in upcoming days,35 and these patients usually have poor AF-related outcomes.36, 37
The derivation cohort subjects of this study came from Yunnan Provence in the southwest of China and may not represent the whole scenario of China and Asia. We could not compare the C2HEST score with other risk scores for incident AF, such as FHS and CHARGE-AF, because some variables from these scores were not collected for this dataset. However, the use of such complicated scoring systems relies on easily accessible computing and information systems, which are not applicable in the relatively underdeveloped Asia area. The simplicity of the C2HEST score, which could be calculated by every clinical practitioner without relying on advanced information technology, may address the current unmet medical needs in Asia and help address the burden of AF in this part of the world. Although we have performed external application in another very large Asian cohort from Korea, further validation of the C2HEST score is needed, especially in non-Asian cohorts (currently ongoing). Cultural health and environmental variations may be other factors which would have increased the complexity of any derived score (defeating the purpose of a simple clinical score for everyday practice), and are generally subordinated factors compared with clinical situations or disease conditions when making clinical decisions for risk assessment of incident AF.
Conclusions
We have developed and validated the C2HEST score as a simple clinical tool to assess the individual risk of developing AF in the Asian population. This novel score may help identify patients without SHD who are at risk of incident AF and may be targeted for prevention strategies and screening programs.
Acknowledgments
Author contributions: Y.-G. L., D. P., A. F., and G. Y. H. L. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Y.-G. L. was the principal author. Y.-G. L., D. P., A. F., P.-S. Y., E. J., B. J., and G. Y. H. L. contributed to the study concept and data analyses. Y.-G. L., D. P., Y.-T. W., Y.-T. G., and G. Y. H. L. contributed to drafting and revisions of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. Y. H. L. is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo; and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo, but no fees are directly received personally. None declared (Y.-G. L., D. P., A. F., P.-S. Y., E. J., B. J., Y.-T. W., Y.-T. G.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Li and Pastori contributed equally to this manuscript.
Drs Wang, Guo, and Lip are joint senior authors.
FUNDING/SUPPORT: This study was funded by the China Scholarship Council [Grant 201708110232].
Supplementary Data
References
- 1.Pastori D., Pignatelli P., Angelico F. Incidence of myocardial infarction and vascular death in elderly patients with atrial fibrillation taking anticoagulants: relation to atherosclerotic risk factors. Chest. 2015;147(6):1644–1650. doi: 10.1378/chest.14-2414. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.Y., Yang P.S., Kim T.H. Atrial fibrillation and the risk of myocardial infarction: a nation-wide propensity-matched study. Sci Rep. 2017;7(1):12716. doi: 10.1038/s41598-017-13061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Outes A., Lagunar-Ruiz J., Terleira-Fernandez A.I., Calvo-Rojas G., Suarez-Gea M.L., Vargas-Castrillon E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68(23):2508–2521. doi: 10.1016/j.jacc.2016.09.944. [DOI] [PubMed] [Google Scholar]
- 4.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z., Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18(5):209–216. doi: 10.2188/jea.JE2008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohsawa M., Okayama A., Sakata K. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular diseases in 1980, 1990 and 2000. J Epidemiol. 2005;15(5):194–196. doi: 10.2188/jea.15.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friberg L., Engdahl J., Frykman V., Svennberg E., Levin L.A., Rosenqvist M. Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP) Europace. 2013;15(1):135–140. doi: 10.1093/europace/eus217. [DOI] [PubMed] [Google Scholar]
- 8.Lowres N., Neubeck L., Redfern J., Freedman S.B. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 9.Cosio F.G., Aliot E., Botto G.L. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10(1):21–27. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S., Guasch E., Savelieva I. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J. 2014;35(22):1448–1456. doi: 10.1093/eurheartj/ehu028. [DOI] [PubMed] [Google Scholar]
- 11.Schnabel R.B., Sullivan L.M., Levy D. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain A.M., Agarwal S.K., Folsom A.R. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107(1):85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A., Krijthe B.P., Aspelund T. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tse H.F., Wang Y.J., Ahmed Ai-Abdullah M. Stroke prevention in atrial fibrillation--an Asian stroke perspective. Heart Rhythm. 2013;10(7):1082–1088. doi: 10.1016/j.hrthm.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Pastori D., Guo Y., Wang Y., Lip G.Y.H. Risk factors for new-onset atrial fibrillation: a focus on Asian populations. Int J Cardiol. 2018;261(2018):92–98. doi: 10.1016/j.ijcard.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y., Tian Y., Wang H., Si Q., Wang Y., Lip G.Y.H. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147(1):109–119. doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 17.Seong S.C., Kim Y.Y., Park S.K. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7(9):e016640. doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T.H., Yang P.S., Uhm J.S. CHA2DS2-VASc score (congestive heart failure, hypertension, age >/=75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65-74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48(6):1524–1530. doi: 10.1161/STROKEAHA.117.016926. [DOI] [PubMed] [Google Scholar]
- 19.Kim T.H., Yang P.S., Kim D. CHA2DS2-VASc score for identifying truly low-risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48(11):2984–2990. doi: 10.1161/STROKEAHA.117.018551. [DOI] [PubMed] [Google Scholar]
- 20.Gronnesby J.K., Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2(4):315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 21.Suenari K., Chao T.F., Liu C.J., Kihara Y., Chen T.J., Chen S.A. Usefulness of HATCH score in the prediction of new-onset atrial fibrillation for Asians. Medicine. 2017;96(1):e5597. doi: 10.1097/MD.0000000000005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao T.F., Liu C.J., Chen S.J. CHADS2 score and risk of new-onset atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. 2013;168(2):1360–1363. doi: 10.1016/j.ijcard.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh C.Y., Lee C.H., Wu D.P., Sung S.F. Prediction of new-onset atrial fibrillation after first-ever ischemic stroke: a comparison of CHADS2, CHA2DS2-VASc and HATCH scores and the added value of stroke severity. Atherosclerosis. 2018;272(2018):73–79. doi: 10.1016/j.atherosclerosis.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Bordignon S., Corti M.C., Bilato C. Atrial fibrillation associated with heart failure, stroke and mortality. J Atr Fibrillation. 2012;5(1):467. doi: 10.4022/jafib.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin E.J., Levy D., Vaziri S.M., D'Agostino R.B. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(1994):840–844. [PubMed] [Google Scholar]
- 26.Heeringa J., van der Kuip D.A., Hofman A. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 27.Kokubo Y., Watanabe M., Higashiyama A., Nakao Y.M., Kusano K., Miyamoto Y. Development of a basic risk score for incident atrial fibrillation in a Japanese general population - The Suita Study. Circ J. 2017;81(11):1580–1588. doi: 10.1253/circj.CJ-17-0277. [DOI] [PubMed] [Google Scholar]
- 28.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 29.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 30.de Vos C.B., Pisters R., Nieuwlaat R. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55(8):725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 31.Miyasaka Y., Barnes M.E., Gersh B.J. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 32.Chien K.L., Su T.C., Hsu H.C. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010;139(2):173–180. doi: 10.1016/j.ijcard.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 33.Ruigomez A., Johansson S., Wallander M.A., Rodriguez L.A. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol. 2002;55(4):358–363. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 34.Stewart S., Hart C.L., Hole D.J., McMurray J.J. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86(5):516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubitz S.A., Yin X., Rienstra M. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131(19):1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adderley N.J., Nirantharakumar K., Marshall T. Risk of stroke and transient ischaemic attack in patients with a diagnosis of resolved atrial fibrillation: retrospective cohort studies. BMJ. 2018;361:k1717. doi: 10.1136/bmj.k1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauchier L., Clementy N., Bisson A. Prognosis in patients with atrial fibrillation and a presumed “temporary cause” in a community-based cohort study. Clin Res Cardiol. 2017;106(3):202–210. doi: 10.1007/s00392-016-1040-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.