One of the most remarkable successes of life is its ability to flourish in response to temporally and spatially varying environments. Fungi occupy diverse ecosystems, and their sensitivity to these environmental changes often drives major fungal life history decisions, including the major switch from vegetative growth to asexual or sexual reproduction. Spore germination comprises the first and simplest stage of vegetative growth. We examined the dependence of this early life history on the nutritional environment using genome-wide transcriptomics. We demonstrated that for developmental regulatory genes, expression was generally conserved across nutritional environments, whereas metabolic gene expression was highly labile. The level of activation of developmental genes did depend on current nutrient conditions, as did the modularity of metabolic and developmental response network interactions. This knowledge is critical to the development of future technologies that could manipulate fungal growth for medical, agricultural, or industrial purposes.
KEYWORDS: artificial medium, asexual development, asexual-sexual switch, conidiospore, filamentous fungi, germination, natural medium
ABSTRACT
Fungal spores germinate and undergo vegetative growth, leading to either asexual or sexual reproductive dispersal. Previous research has indicated that among developmental regulatory genes, expression is conserved across nutritional environments, whereas pathways for carbon and nitrogen metabolism appear highly responsive—perhaps to accommodate differential nutritive processing. To comprehensively investigate conidial germination and the adaptive life history decision-making underlying these two modes of reproduction, we profiled transcription of Neurospora crassa germinating on two media: synthetic Bird medium, designed to promote asexual reproduction; and a natural maple sap medium, on which both asexual reproduction and sexual reproduction manifest. A later start to germination but faster development was observed on synthetic medium. Metabolic genes exhibited altered expression in response to nutrients—at least 34% of the genes in the genome were significantly downregulated during the first two stages of conidial germination on synthetic medium. Knockouts of genes exhibiting differential expression across development altered germination and growth rates, as well as in one case causing abnormal germination. A consensus Bayesian network of these genes indicated especially tight integration of environmental sensing, asexual and sexual development, and nitrogen metabolism on a natural medium, suggesting that in natural environments, a more dynamic and tentative balance of asexual and sexual development may be typical of N. crassa colonies.
INTRODUCTION
Fungi exhibit a diversity of morphology and natural history characteristics and can be found in nearly every environment inhabited by living organisms. Their dispersal via spores—and, in some cases, via hyphal fragments—spawns new opportunities over long distances but also creates unexpected environmental challenges for the initial growth of individual fungi (1–4). Many ascomycetes can produce resistant meiotic spores (ascospores) via sexual reproduction and/or multiple bouts of large numbers of mitotic spores (conidia; 5) via asexual reproduction. Conidia usually lack the thick cell walls or dark pigments that provide resistance against radiation or drought conditions that are characteristic of ascospores. In the typical life cycle of ascomycete fungi, vegetative growth is an adaptive mechanism functioning to maintain asexual reproduction via rapid hyphal growth and production of conidia versus reproducing sexually via production of resistant meiotic spores that survive harsh changes in the environment. Insight into the mechanisms of responses to environmental signals in fungi requires illumination of the genetics and biology of conidial germination—especially illumination of the mechanisms of response that are active during early vegetative growth leading to asexual and/or sexual reproduction.
Neurospora crassa, a model filamentous fungus that flourishes in postfire environments, has long been studied to understand fungal biology and ecology (6, 7). Morphological development during asexual growth in N. crassa has been characterized mainly via gene-by-gene study of specific developmental stages, and genes have been identified that are responsive to external environmental as well as internal environmental factors during N. crassa growth (8–17). Recently, methods of computational annotation of metabolic pathways associated with the N. crassa genome have improved (8, 18–21), incorporating the extensive history of biochemical genetics performed on N. crassa metabolism.
Regulation in response to properties of the environment plays a key role in the fundamental life cycle fork governed by the classic autoregulatory asexual-sexual switch (9, 10). As a general rule for fungi, nitrogen starvation inhibits conidiation and induces sexual development, resulting in slow, robust dispersal, and carbon starvation leads to conidiation and sexual development (11, 12). Nitrogen starvation has long been known to induce or upregulate synthesis of “sexual development genes” (sdv), most of which are responsive to mating-type expression, suggesting that fixed nitrogen is one of the key environmental regulators in N. crassa sexual development (13). The effects of carbon starvation on induction of reproduction are also associated with the specific downregulation of expression of a large set of genes (termed “carbon catabolite repression”) (14). Abundant carbon and nitrogen, in contrast, promote asexual growth, resulting in rapid dispersal.
There have been few genetics-of-development studies that have investigated the early stages in conidial germination in N. crassa and none that compared the effects of carbon supply and nitrogen supply (15–17, 20, 22). Conidial germination is rapid and dramatic, constituting a suite of morphological changes that must represent a challenge to regulation in the face of the sparsity of nutrients within the environments that conidia of N. crassa often encounter. Most studies on conidial germination of N. crassa have used standard artificial media, such as Vogel’s medium (23) and Bird medium (24), which contain an abundance of carbon and nitrogen sources that repress sexual development. With results determined on artificial media available as a basis for comparison, it is becoming increasingly feasible to design experiments that illuminate fungal ecology (25). Conclusions based on analysis of cultures on artificial media need to be investigated with nutritional resources approximating the natural environments in which the fungus grows and for which metabolic and developmental pathways have been naturally selected. These natural environments likely contain low concentrations of simple carbohydrates and organic acids. Using artificial Bird medium (BM), which promotes asexual development, as well as a more natural medium, i.e., maple-sap medium (MSM), which supports both asexual and sexual reproduction, we investigated the synchronous metabolic and developmental processes that occur during the germination of N. crassa conidia.
RESULTS
We collected and compared genome-wide gene expression data associated with fine-scaled morphological differentiation during conidial germination in N. crassa cultured on artificial BM and natural MSM. Developmental differences between conidial germination on BM and conidial germination of MSM were quantified, and differential regulation in gene expression was observed for genes affecting histones and genomic methylation, hyphal development, transcription factors (TFs), and responses to environmental signals. The expression profiles of these genes enabled reconstruction and comparison of regulatory networks and metabolic pathways relevant to development on BM and MSM. Expression profiling led to the identification of genes with knockout (KO) phenotypes relevant to conidial germination and demonstrated differential expression of predicted isoforms in N. crassa, including isoforms of key regulators in the asexual-sexual switch.
Conidial germination on different media.
Nearly 50% of wild-type conidia germinated within 3 h after plating onto BM and MSM, which are similar in carbohydrate content but dissimilar in nitrogen and mineral content (Fig. 1; see also Table S1 in the supplemental material). Nitrogen content in MSM is too low to be detectable (Table S1), and it is known that low-nitrogen conditions promote sexual development in N. crassa (26). Germination commenced earlier on MSM (20% germination within the first 60 min) when compared to BM (almost no germination within the first 60 min). In contrast, extension of the germ tube and hyphal development were slower on MSM than on BM (Fig. 2). In plate tests, more conidia failed to germinate on MSM (6/60 = 10%) than on BM (3/60 = 5%), but the difference was not statistically significant (Fisher’s exact test, P = 0.4906). Cultures on MSM started to produce protoperithecia and then perithecia within 10 days after inoculation and yet did so at a visibly lower density than is typically observed on synthetic crossing medium (SCM; 27), a low-nitrogen medium frequently used to induce sexual development in N. crassa (see Fig. S1 in the supplemental material).
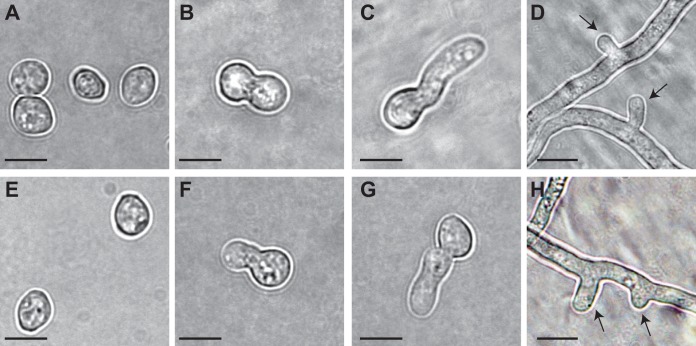
FIG 1.
N. crassa asexual spores cultured on BM (A to D) and MSM (E to H) at four distinctive morphological stages of germination, corresponding to (A and E) fresh conidia, (B and F) polar growth, (C and G) doubling of the longest axis, and (D and H) the time of the first hyphal branching. Arrows indicate first hyphal branches. Scale bar, 5 μm.
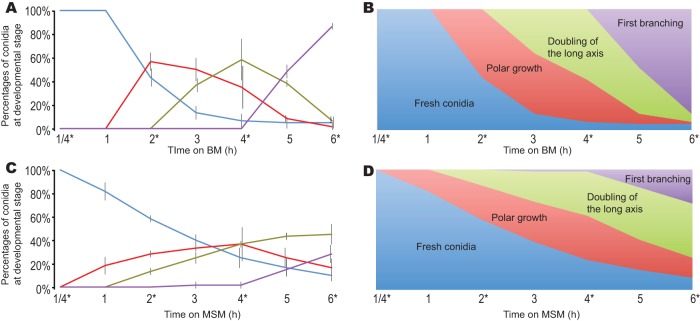
FIG 2.
Temporal analysis of growth and development of conidia of N. crassa cultured on BM and MSM. Plated conidia were examined at six time points across the process, enabling sector counts that revealed the (A) proportions and (B) stacked proportions of conidia at serial stages of germination cultured on BM and the (C) proportions and (D) stacked proportions of conidia at serial stages of germination cultured on MSM. Measurements for conidia were color-coded at each stage of germination, including those corresponding to fresh conidia (blue), polar growth (red), doubling of the longest axis (green), and first hyphal branching (purple). An asterisk (*) indicates time points when RNAs were sampled. For staging, germination of 20 randomly selected conidia per plate was monitored; the error bars delineate 1 standard deviation of the mean for three such plates.
Morphological development of N. crassa in (A to C) 3-day cultures and (D to F) 10-day cultures on (A and D) Bird medium (BM), (MSM) (B and E) maple sap medium, and (C and F) synthetic crossing medium (SCM). Abundant conidia were produced in the 3-day old cultures on (A) BM and (C) SCM. (D) No protoperithecia were detected in the 10-day cultures on BM. (E and F) Protoperithecia were produced in the 10-day cultures on MSM and SCM media (inset). Download FIG S1, PDF file, 2.3 MB (2.4MB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The analytical compositions of BM and MSM. Download Table S1, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sequencing mRNA during germination of conidia.
Culturing on both BM and MSM, we sampled RNAs from four conidial germination stages: fresh conidia, first polar growth of the germ tube, doubling of the germ tube length, and appearance of the first branch. A total of 40.6 to 94.5 million 76-bp paired-end reads were obtained from each sample (GEO accession no. GSE101412). Levels of total reads and mapped reads from RNA extracted from cultures on BM were slightly higher (6.8% and 7.3%, respectively, on average) than those from cultures on MSM (Table S2A). The average coverage depths were calculated following Illumina guidelines (C = LN/G [C, coverage; G, haploid genome length; L, read length; N, number of reads]), yielding 70× to 120× coverage, and the rate at which reads mapped to the genome ranged from 93.7% to 96.6% (Table S2A). The number of genes for which at least one read mapped to the gene for at least one time point on BM or MSM ranged from 9,167 to 9,202 (Table S2B and C).
Analyses of RNAseq data. (A) Summary of transcriptome sequencing (RNA-seq) data and mapping quality. (B) Gene expression levels during conidial germination on BM. (C) Gene expression levels during conidial germination on MSM. Download Table S2, XLSX file, 3.9 MB (3.9MB, xlsx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Transcriptomics profiles during germination of conidia under different nutrient conditions.
Two major expression patterns—downregulation across all four stages and upregulation across stages after the second stage of germ tube appearance—can be recognized during conidial germination on BM. In contrast, expression patterns on MSM were more multifarious, with many genes upregulated from germination across stages until first branching event (Table S2B and C). Between the initial two stages (fresh conidia and polar growth), a large portion (1,818 genes) of the genome was significantly (Bonferroni adjusted P < 0.01) downregulated, and these genes are associated with regulation of transcription, DNA binding RNA polymerase II transcription factor activities, and zinc ion binding (Table S2B and C). Significant functional enrichment (P < 0.05) was identified for signaling pathways, cell cycle control, and carbon and nitrogen metabolism as well as for biosynthesis of amino acids both between BM and MSM cultures and across different morphological stages (Table S3A). Interestingly, mitogen-activated protein kinase (MAPK) signaling pathways were enriched at the last conidial germination stage but showed contrasting regulation patterns between BM and MSM.
Functional analyses of selected genes during conidial germination in N. crassa. (A) Results of KEGG functional enrichment analysis for genes classified with stage-specific expression patterns. (B) Functional annotations of genes that were identified for the Bayesian network of asexual-sexual developmental responses to environmental factors during the conidial germination. Download Table S3, DOCX file, 0.05 MB (50.2KB, docx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of genes in hyphal development characterized for conidial germination.
Nutrition impacts during conidial germination were genome wide, affecting genes involved in development regulation (Fig. 3; see also Fig. S2 and S3). MAPK regulatory networks are known to regulate entry into serial morphological development stages as well as to activate the switch from the asexual phase to the sexual phase of the life cycle (28–31). For 59 genes that were able to be mapped to the MAPK signaling pathway of yeast (KEGG pathways) (32), expression exhibited much greater changes on MSM than on BM (Table S3; see also Table S4). Most (12/15) MAPK pathway genes expressed in response to starvation were significantly upregulated (P < 0.01) on MSM (Fig. S2).
FIG 3.
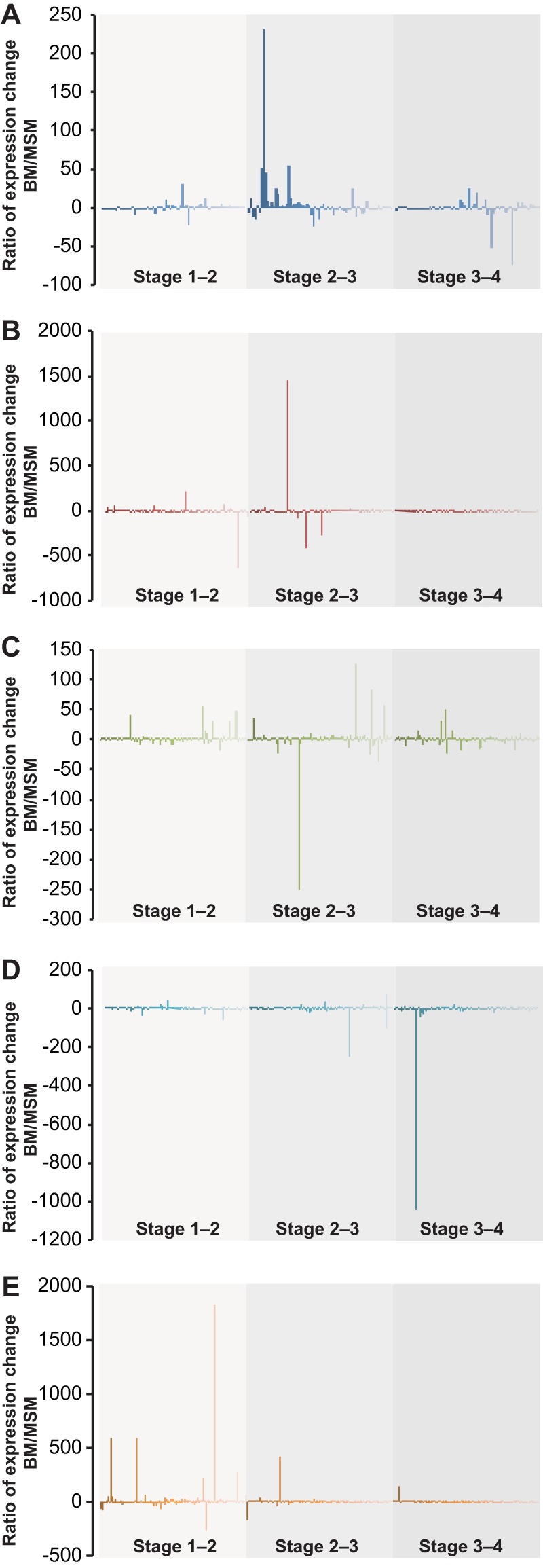
Effects of media on conidial germination are genome-wide, and functional groups differentially respond to BM and MSM during the process. Examples include (A) histone activities and genomic methylation, (B) hyphal development genes annotated as functioning in conidial germination, (C) transcription factors, (D) early light-responsive genes, and (E) late light-responsive genes. Genes within each panel are placed in a single order within each stage, with a diminishing color-shade corresponding to that order. Bars indicate ratios (BM/SM) of gene expression change from stage 1 to stage 2, stage 2 to stage 3, and stage 3 to stage 4.
Differential expression of MAPK genes between BM and MSM cultures. Download FIG S2, PDF file, 0.1 MB (126.7KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Differential expression of genes involved in hyphal growth between cultures on Bird medium and cultures on maple sap medium, including (A) genes involved in bud emergence and cell polarity, (B) genes involved in bud site selection, (C) genes involved in septins development and regulation, (D) genes involved in syntenic homolog actin-ring formation, (E) genes involved in syntenic homolog formation, (F) genes involved in transport and secretion during hyphal growth, (G) genes involved in morphological growth and hyphal polarity showing infrequent expression regulation in BM and MSM, and (H) genes involved in nitrogen metabolism showing differential expression regulation in BM and MSM. Download FIG S3, PDF file, 0.2 MB (262.5KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Significant expression changes between stages observed for isoforms in Neurospora crassa during germination of conidia on BM and MSM. (B) Isoforms whose expression results were opposite and significantly different during germination of conidia on BM and on MSM. Download Table S4, XLSX file, 0.1 MB (97.5KB, xlsx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of genes associated with hyphal growth exhibited different patterns between BM and MSM (Fig. 4; see also Fig. S3). Genes associated with carotenoid synthesis were upregulated 2-fold to 7-fold on MSM but experienced nearly a 2-h delay in upregulation on BM (Fig. 4A and B). cot genes—which have been shown to confer colonial-temperature-sensitive (cot) phenotypes (33–37)—were first downregulated and then upregulated during germing tube extension on BM. These genes, excluding cot-3, were generally upregulated throughout the process for MSM cultures (Fig. 4C and D). Similar contrasts in expression levels between BM and MSM were also observed for genes related to polarity establishment, chitin synthesis, septation, and budding (Fig. S3A to E). Expression of genes required for sexual development was generally downregulated across stages on BM but was upregulated across stages on MSM (Fig. 4E and F).
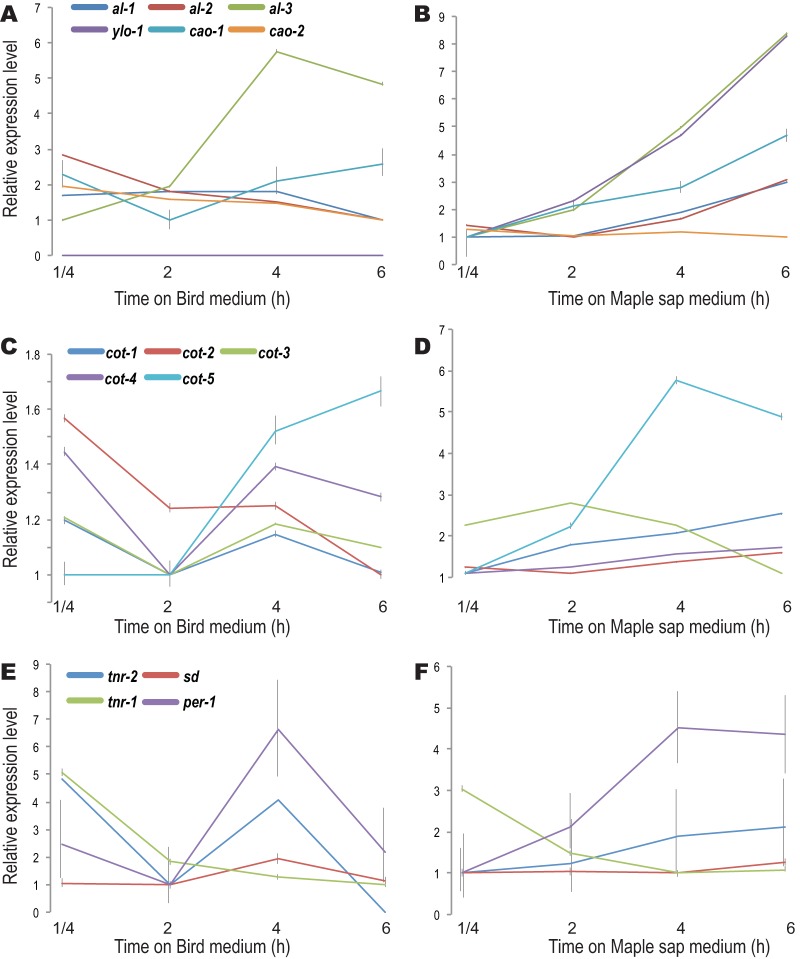
FIG 4.
Relative expression levels of genes involved in carotenoid synthesis (pigmentation) pathways in culture on (A) BM and (B) MSM, of colonial temperature-sensitive asexual development genes (Cot) in culture on (C) BM and (D) MSM, and of a selection of genes whose expression is required for protoperithecial development in culture on (E) BM and (F) MSM.
Expression of genes in metabolic pathways characterized for conidial germination.
G-proteins and coupled receptors (gpr)—which are known to be critical regulators of fungal responses to carbon and nitrogen nutrition and major regulators of N. crassa development (38–41)—were differentially expressed. Genes gna-1 and gpr-4—which regulate the responses to carbon sources (42)—were highly coordinately downregulated in cultures on BM but showed contrasting results in cultures on MSM, upon which gna-1 was upregulated (Fig. 5A). Expression levels of gpr-5 and gpr-6 and the corresponding potentially coupled gene gna-3 were highly coordinately downregulated on nitrogen-rich BM (Fig. 5B). Upregulated expression of gpr-6 and gna-3 in MSM with extremely low levels of nitrogen invites further investigation of their roles as potential nitrogen sensors in N. crassa.
FIG 5.
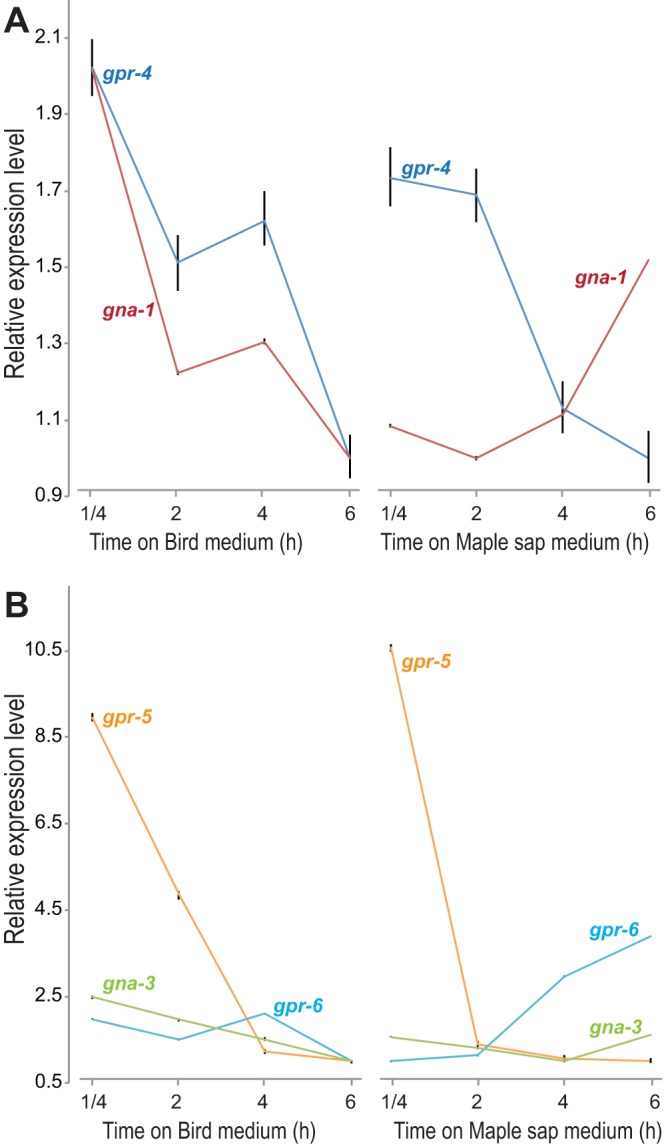
Relative expression levels of G-protein coding genes exhibited differential regulation during conidial germination on BM (left) and on MSM (right), including (A) cAMP signaling genes that regulate responses to carbon sources and (B) expression of genes, including genes gpr-5 and gpr-6 and corresponding potential coupled G-protein coding gene gna-3, that regulate responses to nitrogen starvation.
Both nitrogen resources and carbon resources are critical for hyphal growth. Consistent with our observation of morphological development differences between BM cultures and MSM cultures, genes regulating polar growth of hyphae exhibited homogeneous regulation under both growth conditions (Fig. S3G). However, the nature of nitrogen metabolism regulation was complex. On nitrogen-deficient MSM, 14 of 17 genes for which homologs are annotated in yeast during nitrogen metabolism (KEGG pathways) were dramatically upregulated, including 51-fold, 81-fold, and 147-fold increases for nitrate transporter-10 (nit-10), NCU02361 (formamidase), and nit-6, respectively (Fig. S3H). As sucrose is the dominant carbon source in MSM, we observed stably high expression of the invertase gene (inv; NCU04265) during the first two stages of conidial germination on MSM but abrupt downregulation in expression of this gene on BM.
Expression of transcription factors in conidial germination.
Two expression patterns were commonly observed for TFs in culture on BM; 48 TFs were steadily downregulated, exhibiting lowest expression at the last stage of development, and 36 exhibited their lowest expression at the second stage (polar growth). Expression of these TFs showed various expression patterns for MSM cultures. The TFs regulating both basal hyphal growth and asexual development showed similar downregulated expression patterns in RNAs obtained from cultures grown under both nutrient conditions. The exceptional transcription factors were tah-1 (tall aerial hyphae) and tah-4, which were both significantly upregulated in both BM and MSM cultures. Among the 100 TFs profiled (Table S2B and C), some regulate both asexual development and sexual development (30, 43–45). Expression of sub-1, a key regulator in the asexual-sexual switch (45, 46), exhibited contrasting patterns of regulation between nutrient conditions: significant upregulation across stages on sexual development inducing MSM and significant downregulation across stages on nitrogen-rich BM.
Expression of genes in response to environmental signals during conidial germination.
Many genes that exhibit early light-regulated responses (ELRGs [47]) were upregulated from germination to the first hyphal branching on MSM but were downregulated during the same stages on BM (Fig. S4A). For example, NCU01258 (cyn-1) encodes cyanate lyase in nitrogen metabolism and exhibited contrasting expression patterns between the cultures on nitrogen-rich BM and cultures on nitrogen-poor MSM. Upregulation of cyn-1 indicates that the fungus had turned to complex compounds such as cyanate as an alternative nitrogen supply in MSM. Late light-regulated genes (LLRGs [47]) were generally upregulated during germ tube extension and hyphal growth (Fig. S4B). However, there was a dramatic downregulation of these genes before the appearance of germ tubes in conidia germinated on BM, including a nearly 70-fold drop for the inv gene (invertase; NCU04265), which catalyzes degradation of sucrose to glucose.
(A) Expression of early light-responsive genes (ELRGs) was generally downregulated in cultures on BM and MSM during the first two stages of conidial germination. (B) Expression of late light-responsive genes (LLRGs) was generally downregulated in cultures on BM but upregulated in cultures on MSM. Download FIG S4, PDF file, 0.1 MB (143.4KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Coordinated metabolic and developmental networks during conidial germination.
Key genes annotated in nitrogen metabolism, conidial germination, and the asexual-sexual switch were investigated for their associations in Bayesian coexpression networks (Fig. 6; see also Table S3B). Associations among genes associated with asexual development, including conidiation genes con-8 and con-13 and genes cot-2, cot-1, and cot-5 regulating asexual growth and development, were conserved between cultures on BM and MSM. Associations among genes playing roles in the initiation of sexual development, including light sensor genes nop-1 and phy-2 as well as genes per-1, pp-1, sd, and tnr-1, were also conserved between the two cultural conditions. For cultures on nitrogen-rich BM, however, the asexual development subnetwork (cot genes) and nitrogen transport subnetwork (nit genes) were tightly modular. For cultures grown on nitrogen-poor MSM, which facilitates entry into both asexual development and sexual development, the asexual and sexual initiation modules were distinct but appeared less hierarchically organized, and the nitrogen metabolism genes were integrated with the developmental pathways much more extensively than with each other. For cultures on BM, the sexual development initiation subnetwork was positioned downstream, perhaps being responsive to expression regulation within the asexual development subnetwork—specifically, with respect to nitrogen metabolism. For cultures on MSM, the sexual development initiation subnetwork was located further upstream and was more integral to nitrogen transporter expression than on BM.
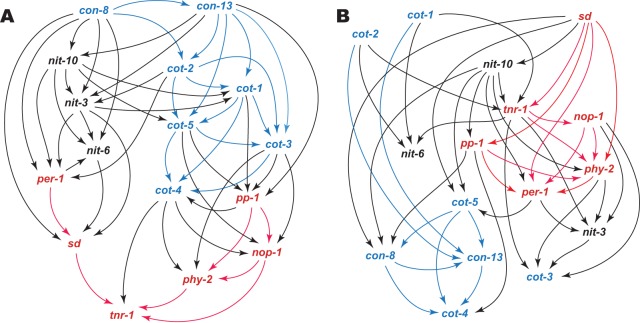
FIG 6.
Bayesian networks of expression regulation show associations (corresponding to arrows indicating directed edges) among genes involved in nitrogen metabolism (black), asexual growth and reproduction (blue), and light-regulated sexual development (red) during conidial germination and early hyphal growth (A) on BM and (B) on MSM.
Alternative splicing.
By analysis of our paired-end sequencing data, we differentiated among the expression results determined for isoforms of 898 predominantly metabolic genes that have been annotated with at least two isoforms (http://genome.jgi.doe.gov/Neucr2/) (Table S4A). Isoforms of 21 genes exhibited differential expression patterns between BM and MSM cultures, especially during early germination from stage 1 to stage 2 (Table S4B). Among the 17 isoforms that exhibited a significant (P < 0.01) expression change for cultures on both media, isoforms of essential sexual reproduction gene sub-3 (submerged protoperithecia-3; NCU01154T1) exhibited 2-fold downregulation on BM but 2.5-fold upregulation on MSM.
Knockout phenotypes during conidial germination.
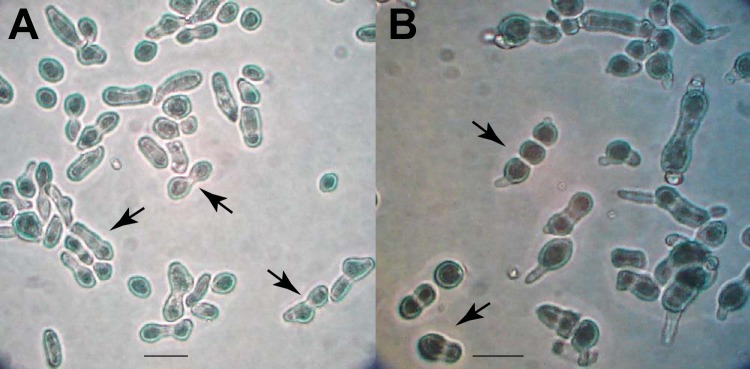
A total of 195 genes exhibited statistically (P < 0.05) and biologically (>5-fold) significant differences between the two media in comparisons of data obtained from similar time points or across the conidial germination process. Among these genes, 144 knockout strains (43) were available from the Fungal Genetic Stock Center (FGSC [48]) for phenotypic investigation. In comparisons of wild-type strains with matched mating-type gene knockouts, we observed altered phenotypes in 22 of the knockout strains (Fig. 7; see also Fig. S5). Of the 22, only 13 genes are functionally annotated (Table 1). Generally, upregulation of expression was consistent with the time of function. For example, expression of a hypothetical protein was detected only in the early stages of germination, and the knockout of the corresponding gene—NCU07801 (idler)—resulted in significantly delayed germination and slower hyphal growth. In another example, one knockout exhibited a novel phenotype of spore elongation, in which conidiophores extended their long axis to form a dumbbell-like structure (NCU08095; cdg [conidia dumbbell germination]) before forming a normal germ tube (Fig. 8). Interestingly, expression of NCU08095 was significantly downregulated in both BM cultures and MSM cultures. Orthologs of cdg were found only in some genomes of Sordariomycetes, Leotiomycetes, and, Eurotiomycetes and were absent in other ascomycetes and yeast genomes—and the ortholog in Rutstroemia was annotated as a kynurenine formamidase (Fig. S6).
FIG 7.
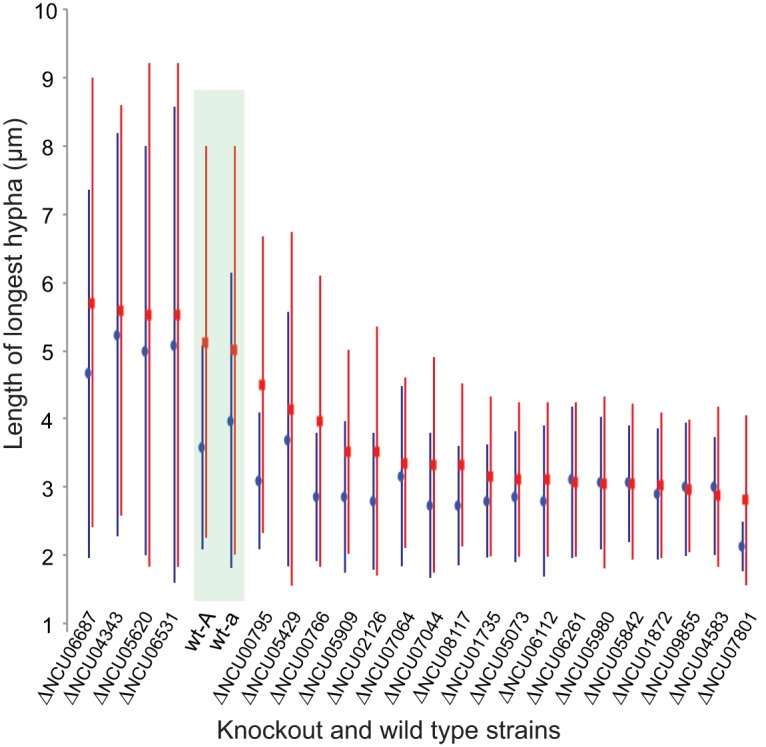
Knockout strains exhibited higher and lower average growth levels at 2 h (blue) and 3 h (red) postinoculation compared with wild-type strains of both A and a mating types (wt-A and wt-a). Phenotyping was performed on paired knockout and wild-type strains. Measurements of growth were conducted for three replicates of 20 randomly picked conidia for each strain. A high standard deviation value is evident, arising primarily as a consequence of a high variance in the starting time of germination.
TABLE 1.
Functional annotation for 13 genes that exhibited knockout phenotypes in conidial germination from previous studiesa
| Gene ID | Functional annotation at NCBI and FungiDB |
|---|---|
| NCU00795 | Cation transporter |
| NCU02126 | Isovaleryl-CoA dehydrogenase |
| NCU04343 | Ergothioneine-1 |
| NCU04583 | Acetyltransferase |
| NCU05429 | Alpha-glucan branching enzyme |
| NCU05620 | Proteasome activator |
| NCU05980 | Carboxypeptidase S1 |
| NCU06112 | Glutamate decarboxylase |
| NCU06261 | Uracil phosphoribosyltransferase |
| NCU06687 | Lycogen synthase-1 |
| NCU07044 | Metallo-beta-lactamase |
| NCU07064 | l-Galactonate dehydratase |
| NCU09855 | Nicotianamine synthase |
ID, identifier; FungiDB, The Fungal and Oomycete Genomics Resource (81); Isovaleryl-CoA, isovaleryl-coenzyme A.
FIG 8.
Phenotypes of knockouts of NCU08095 on BM at the stages of (A) polar growth and (B) doubling of the longest axis, exhibiting dumbbell-like conidial extension (arrows) before formation of the germ tube. Images of controls (representing wild-type germination on each medium) are shown in Fig. 1.
Differential expression of genes that exhibited knockout phenotypes during conidial germination. Download FIG S5, PDF file, 0.1 MB (104KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogeny of orthologs of NCU08095, illustrating their presence or absence in genomes of Leotiomycetes, Sordariomycetes, and Eurotiomycetes. Branches with significant support (MrBayes posterior probability value, >0.95) are indicated in bold characters. Download FIG S6, PDF file, 0.1 MB (153.3KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
In many cases, conidial germination is the critical first step of fungal colonization. We previously reported signatures of gene expression associated with mating type identifiable during asexual reproduction of N. crassa on BM (49). In this study, we investigated the conidial germination process and its responses to different nutritional conditions by profiling transcriptomics across four distinct morphological stages of conidial germination on two different media. Commercial maple sap provides a readily available natural nutrient source enabling investigation of diverse stages of N. crassa development, including conidial germination.
Fungal spores prepare for flexibility instead of efficiency.
Nutrients represent a major factor influencing the onset of N. crassa colonization, and extended maintenance on a singular formulation of artificial medium is known to lead to degeneration of strains (50–52). Natural environments of N. crassa can be very diverse in terms of carbon level and nitrogen level, and this fungus has often been found to flourish in high-carbon environments, in particular, in the noncharred, sappy, woody remains of plants after forest fires. N. crassa has also been suggested to propagate as a plant endophyte (53); MSM resembles the nutrition accessible to the fungus within the environment of the living plant. While epigenetic regulation based on parent-of-origin expression from fungal species has been reported previously (54–56), our data exhibited no impact of parental nutrient conditions on conidial germination. Although the tested wild-type strains had been maintained on BM for multiple generations, the delayed germination of their conidiospores on BM is evidence that the conidia are equipped with germination surveillance that has evolved calibration toward a more natural environmental setting.
Genetic associations between development and the metabolism of nutrition.
Our observations are consistent with previous research on the impact of levels of fixed nitrogen on sexual development and further suggest that these effects begin in the very early stages of asexual growth, during conidial germination. Nitrogen, in the form of nitrate, is the primary source of nitrogen nutrition and is often a limiting resource in natural environments (57). Therefore, fungal growth and development on a low-nitrogen natural medium likely represent a good model for most fungal colonization of natural environments. Several standard media have been developed for studying N. crassa (58). Natural media such as carrot medium (59) have also been widely used in fungal research, especially to study pathogens whose growth conditions are challenging to mimic with artificial media (60, 61). While these media are useful for investigating specific stages in Neurospora growth and development, they all have limitations with respect to the study of growth stages that require quick changes in nutrients under laboratory conditions. BM and MSM provide different nutrition profiles (see Table S1 in the supplemental material), especially in nitrogen levels and carbon resources. MSM represents one of the likely environments for Neurospora, which has been reported to sometimes persist as a plant endophyte (53). Low nitrogen levels in MSM induced the initiation of sexual development even at very early stages of conidial germination, and key regulatory genes in sexual development were activated in MSM cultures (Fig. 3 and 4; see also Fig. S1, S2, and S4). Our results also call for further investigation of the involvement of G-proteins in nitrogen metabolism regulation in N. crassa. Upregulation of gna-1 on MSM is consistent with previous suggestions indicating an additional function of gna-1 in sexual development (62). Expression of gna-1 was downregulated in cultures on BM, where sexual development is repressed. Gpr-5 and Gpr-6 are homologs of Stem1, a transmembrane protein in yeast that signals upon nitrogen starvation (6, 38, 63). Their roles as nitrogen sensors in N. crassa growth and development have not yet been confirmed (64).
Although expression was dynamically regulated in response to available carbon and nitrogen levels in both BM and MSM, the spores appear to take longer to physiologically adapt to BM than to MSM. To illustrate the metabolic responses, consider the expression of cyn-1 (encoding cyanate lyase, a key enzyme in transforming cyanate as an alternative nitrogen resource), which was upregulated in cultures in nitrogen-poor MSM. Expression of invertase (encoded by inv)—which catalyzes degradation of sucrose to glucose—was, conversely, downregulated in glucose-rich BM (Table S2). In fact, two frequent expression patterns were identified on BM: continuous stage-to-stage upregulation and downregulated expression during germ tube formation followed by upregulated expression in subsequent stages. In contrast to these relatively homogeneous expression patterns, which are attributable to the presence of a high number of genes in BM cultures, expression patterns identified in MSM cultures were more multifarious, indicating more intricacy of function and developmental dynamism.
Response of conidial germination to various environmental factors.
We observed greater upregulation of early light-induced genes (ELIG) in cultures on MSM than on BM. ELIG are controlled by the light-activated white collar complex (WCC) and regulate activities of late light-induced genes (LLIG) in growth, conidiation, and sexual development in N. crassa (47, 65–68). The upregulation of ELIG in MSM cultures is likely associated with boosted sexual development of N. crassa on MSM. Knocking out early light-induced gene NCU01870 yields a female sterile phenotype (68). This gene is expressed in a steady stage-to-stage increase in MSM cultures but is significantly downregulated stage to stage on BM, on which sexual development is inhibited. Using MSM helped us to reveal different aspects of gene regulation during the asexual-sexual switch by light-responsive WCC and metabolic pathways, which have been obscured on nitrogen-rich artificial media such as BM.
Our analysis illustrates that with respect to the adaptively tuned decision to engage in asexual or sexual development, metabolic pathways, sensory responses to environmental stimuli, and developmental pathways are tightly associated even early during conidial germination. The asexual-to-sexual switch is an integrative process, linking the asexual and sexual modes of reproduction—modes that respond to nearly opposite environmental attributes (11, 49). In many ascomycetes, asexual reproduction is prolonged by high temperatures and by high levels of nutrition, oxygen, ROS (reactive oxygen species), and light exposure, and sexual reproduction can be induced by low temperature, low levels of nutrition and oxygen, and reduced light intensity (69).
Although our experiment profiled expression only during the very early stages of conidial germination and hyphal extension, genes modulating the asexual-sexual switch showed the presence of regulatory networks that diverged between the two culture conditions. Bayesian networks based on coexpression illustrated that both asexual reproduction regulation and sexual reproduction regulation were highly modular in both BM and MSM cultures. However, nitrate transporters were modular in the expression network inferred from BM cultures, in line with intensive activation of nitrogen intake and metabolism. Multiple interactions between nitrogen metabolism and a similarly modular conidiation pathway suggest coordinated responses of nitrogen metabolism and conidiation to high-nitrogen media such as BM that are associated with the promotion of asexual growth and inhibition of sexual development. The more diffuse organization of these gene interactions could be a consequence or a cause of the more labile balance of the asexual-sexual switch in MSM.
Complex genetic regulation of conidial germination implied by KO phenotypes.
Genes with critical roles in conidial germination were identified on the basis of comparative transcriptomics and focused knockout phenotyping. Of 23 genes identified for knockout phenotyping, 22 encoded knockout mutants that showed quantitative phenotypes with respect to germination ratio and growth rate. Knocking out NCU08095 (cdg) yielded a germination phenotype resembling yeast budding morphology—and this gene is not present in yeast genomes. Morphological transitions from filamentous growth to yeast-form growth have occurred many times in fungal evolution (70), and such physiological transformations are known to be associated with pathogenesis for some fungal species (71, 72).
Interestingly, no knockout strains showed distinct phenotypes on different media, despite differential expression on BM and MSM. One explanation for this homogeneity of knockout phenotypes across environments is that expression differences are largely associated with differential developmental timing on BM and MSM rather than being attributable to operatively different expression programs. In other words, most genes that we chose based on their expression differences between the cultures on different media likely represent quantitative regulators of developmental change, leading to quantitative differences in development pace, rather than qualitative differences in development, between cultures on BM and MSM.
Our results call for greater attention to isoforms of genes and their distinct functions during fungal development. For example, isoforms of the gene sub-3, which is essential for sexual reproduction, exhibited 2-fold downregulation when cultured on BM. In contrast, isoforms of sub-3 exhibited 2.5-fold upregulation when cultured on MSM, on which sexual development can be expected to occur within a week after inoculation.
Conclusion.
Synchronous metabolic and developmental processes underlying conidial germination, a rapid process that occurs in response to environmental signals, including carbon and nitrogen nutrition as well as light signals, were revealed by transcriptomics analyses performed with synthetic or natural nutrition. Results indicating modularity among elements of early sexual development, asexual growth, and nitrogen metabolism were detected in conidial germination, with a more diffuse set of network interactions in natural medium than in nitrogen-rich laboratory medium. The implication is that a more tentative balance of asexual and sexual development is typical during growth and development of N. crassa colonies in natural environments than has been previously implied by analyses relying on culture in media that suppress activation of the asexual-sexual switch. Nine genes that were previously unannotated with respect to function and that we have now identified as contributing to asexual growth after conidial germination may contribute significantly to modulating this balance and provide targets for future fungal growth control in prevention of pathogen infection, in biochemical fermentation optimization, and in bioenergy generation.
MATERIALS AND METHODS
Strains and culture conditions.
Germination studies were performed with N. crassa mat A (FGSC2489) macroconidia, harvested from 5-day cultures on solid (2% agar) Bird medium (24). Macroconidia were collected with deionized distilled water containing Tween 20 (0.1%). They were washed with autoclaved distilled water and filtered through a three-layer Mira cloth. Spores (1 × 105) were placed on top of cellophane-covered medium in petri dishes. MSM was composed of maple sap (Vertical; Feronia Forests) with agar (2%). Conidia were incubated on media at 25°C under constant white light, a protocol that avoids the dynamic expression regulation known to arise from changes of light color and intensity (47). Germination was monitored at 0, 15, 60, 120, 180, 240, 300, and 360 min. Cellophane membranes with fungal tissues were collected at 15, 120, 240, and 360 min, when the majority (51% to 92%) of active spores on BM were at one of the following stages and beyond: fresh spores, spores showing evidence of polar growth, spores having doubled their long axis, and spores having commenced their first hyphal branching. The same time points were used for sampling tissues on MSM. Tissue samples were flash frozen in liquid nitrogen and stored at −80°C. All tissues that were collected from multiple plates in one collection process were counted as one biological replicate. Three temporally segregated biological replicates were prepared for each sampled time point on both BM and MSM.
RNA isolation and preparation.
Total RNA was extracted from homogenized tissue with TRI reagent (Molecular Research Center) as described previously by Clark et al. (73). Preparation of cDNA used N6 primers following the Illumina mRNA sequencing sample preparation guide. The quality of the cDNA samples was verified with an Agilent 423 Technologies Bioanalyzer to ensure an insertion size of between 150 to 225 bp and by quantitative PCR (qPCR) (Kapa Biosystems) to ensure an RNA concentration of ≥0.5 ng/μl. Sequencing libraries were produced by the use of the Illumina TruSeq stranded-RNA protocol.
Data acquisition and analysis.
The 24 libraries (3 replicates per condition) underwent 76-bp paired-end sequencing on an Illumina HiSeq 2500 system at the Yale Center for Genomics Analysis (YCGA). Adapter sequences, empty reads, and low-quality sequences were removed. For each read, we trimmed the first six nucleotides and the last nucleotides at the point where the Phred score of an examined base fell below 20 using in-house scripts. Any read that was less than 45 bp in length after trimming was discarded. The remaining trimmed reads were aligned to the N. crassa OR74A v12 genome (6) using Tophat v.2.0.12 with default settings (74). Only reads that mapped to a single unique location within the genome, with ≤2 mismatches in the anchor region of the spliced alignment, were tallied by alignment to exons using HTSeq v0.6.1p1. We also used HiSat2 and StringTie (54, 75, 76) to perform spliced alignments of the reads against the reference genome. Tallies were statistically analyzed with LOX v1.6 (55), yielding relative gene expression levels across the germination time points. For statements involving the statistical significance of multiple genes, P values were determined using conservative Bonferroni adjustment (56). We analyzed the differential expression of 800 genes predicted to have isoforms (http://genome.jgi.doe.gov/Neucr2/) using CuffDiff v 2.2.1 (77). We applied the fragment bias correction and strand-specific parameters, leaving other options at the default settings.
Knockout strains and phenotype identification.
Knockout strains for more than 9,600 genes (43) were acquired from the Fungal Genetic Stock Center (FGSC; 48); those acquired included deletion cassettes for genes in either or both of the two mating types, mat A and mat a, that regulate mating and sexual development in heterothallic N. crassa (40). Knockout strains of genes that showed a significant (LOX, P < 0.01) expression difference in the two stage-to-stage expression wild-type strains and a difference in the direction of expression change under the two medium conditions were examined for altered phenotypes during conidial germination. For each investigated strain, 3,000 to 5,000 conidia were plated onto 90-mm diameter plates and monitored. Strains of genotype mat A were assayed when available; otherwise, mat a strains were used. Genotype mat a strains were also assayed in parallel when mat A strains exhibited a distinct phenotype. Wild-type strains were monitored alongside each knockout strain on BM and MSM with three replicates under constant white light at 25°C. Germination and growth rates of 20 conidia picked at random were recorded. The knockout strain for NCU08095 that exhibited a significant morphological phenotype was crossed with the wild-type strain, and cosegregation of the observed phenotype with deletion of the gene in the offspring was verified to ensure that the intended deletion was responsible for the mutant phenotype (30, 61, 78).
Functional enrichment analyses.
The statistical significance of overrepresentation of gene groups in functional categories relative to the whole genome was quantified by calculating P values via the hypergeometric distribution using FungiFun (79). To evaluate each functional category, results indicating whether the genes in each functional category were differentially expressed between stages at a higher frequency than expected were assessed in comparison to the genome (FungiFun’s exact P) and were based on background gene sets (FungiFun’s adjusted P). To achieve significance, we required both an exact P value of <0.01 and an adjusted P value of <0.05. Where appropriate, further functional annotation was carried out via the biochemical pathway and annotation data in the Kyoto Encyclopedia of Genes and Genomes (KEGG; 80). Functional annotations were also obtained from FungiDB (81).
Bayesian network reconstruction.
Biological networks were modeled using the Bayesian network Web server (82) supplied with conidial germination expression data for each culture condition. Input files contained fold changes reflecting differences between adjacent sample points across the experiment [(Xt+1 − Xt)/min(Xt, Xt+1)]. Global structure learning settings were retained at default settings. The network models depicted are the 50% majority consensuses of 100 models (edge-selection threshold, 0.5; the 100 highest-scoring networks were averaged), calculated without imposition of any structural constraints.
ACKNOWLEDGMENTS
We thank the editors and the reviewers very much for the constructive comments. We thank the Broad Institute and the Munich Information Center for Protein Sequences (MIPS) for making N. crassa gene and genomic data available for oligonucleotide prediction.
This study was supported by the Agriculture and Food Research Initiative competitive grant program (no. 2015-67013-22932 from the USDA National Institute of Food and Agriculture to J.P.T. and F.T.), by funding to J.P.T. from The National Institutes of Health (P01 grant GM068067), by funding from the National Science Foundation (grants MCB 0923797 and IOS 1457044 to J.P.T.), by funding from the National Science Foundation (grants MCB 0923794 and IOS 1456482) and Michigan AgBioResearch to F.T., and by funding by the Israel Science foundation to O.Y. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have declared that no competing interests exist.
Footnotes
Citation Wang Z, Miguel-Rojas C, Lopez-Giraldez F, Yarden O, Trail F, Townsend JP. 2019. Metabolism and development during conidial germination in response to a carbon-nitrogen-rich synthetic or a natural source of nutrition in Neurospora crassa. mBio 10:e00192-19. https://doi.org/10.1128/mBio.00192-19.
REFERENCES
- 1.Stajich JE, Berbee ML, Meredith B, Hibbett DS, James TY, Spatafora JW, Taylor JW. 2009. The fungi. Curr Biol 19:R840–R845. doi: 10.1016/j.cub.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deacon J. 2013. Fungal spores, spore dormancy, and spore dispersal, p 184–212. In Fungal biology. Blackwell Publishing Ltd, Hoboken, NJ. [Google Scholar]
- 3.Sephton-Clark PCS, Voelz K. 2018. Spore germination of pathogenic filamentous fungi. Adv Appl Microbiol 102:117–157. doi: 10.1016/bs.aambs.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz A, Chu M, Marris PI, Sagaram US, Kaur J, Shah DM, Read ND. 2014. Specific domains of plant defensins differentially disrupt colony initiation, cell fusion and calcium homeostasis in Neurospora crassa. Mol Microbiol 92:1357–1374. doi: 10.1111/mmi.12634. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Bordonaba J, Adams TH. 1994. Development of conidia and fruiting bodies in Ascomycetes, p 333–349. In Growth, differentiation and sexuality. Springer, New York, NY. [Google Scholar]
- 6.Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, Plamann M, Goodrich-Tanrikulu M, Schulte U, Mannhaupt G, Nargang FE, Radford A, Selitrennikoff C, Galagan JE, Dunlap JC, Loros JJ, Catcheside D, Inoue H, Aramayo R, Polymenis M, Selker EU, Sachs MS, Marzluf GA, Paulsen I, Davis R, Ebbole DJ, Zelter A, Kalkman ER, O'Rourke R, Bowring F, Yeadon J, Ishii C, Suzuki K, Sakai W, Pratt R. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68:1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki S, Satoshi S, Bayram ÖS, Özgür B, Braus GH. 2013. conF and conJ contribute to conidia germination and stress response in the filamentous fungus Aspergillus nidulans. Fungal Genet Biol 56:42–53. doi: 10.1016/j.fgb.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Radford A. 2004. Metabolic highways of Neurospora crassa revisited. Adv Genet 52:165–207. doi: 10.1016/S0065-2660(04)52005-9. [DOI] [PubMed] [Google Scholar]
- 9.Böhm J, Hoff B, O’Gorman CM, Wolfers S, Klix V, Binger D, Zadra I, Kürnsteiner H, Pöggeler S, Dyer PS, Kück U. 2013. Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc Natl Acad Sci U S A 110:1476–1481. doi: 10.1073/pnas.1217943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer PS, O'Gorman CM. 2012. Sexual development and cryptic sexuality in fungi: insights from Aspergillus species. FEMS Microbiol Rev 36:165–192. doi: 10.1111/j.1574-6976.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Romero J, Julio R-R, Maren H, Christian K, Sylvia M, Reinhard F. 2010. Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- 12.Fischer R, Kües U. 2006. Asexual sporulation in mycelial fungi, p 263–292. In The mycota. Springer, New York, NY. [Google Scholar]
- 13.Nelson MA, Metzenberg RL. 1992. Sexual development genes of Neurospora crassa. Genetics 132:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebbole DJ. 1998. Carbon catabolite repression of gene expression and conidiation in Neurospora crassa. Fungal Genet Biol 25:15–21. doi: 10.1006/fgbi.1998.1088. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz NH, Charlang G, Horn G, Williams NP. 1976. Isolation and identification of the conidial germination factor of Neurospora crassa. J Bacteriol 127:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawanabe Y, Yamane H, Takahashi N, Nakamura T. 1985. Identification of GA3 in Neurospora crassa and its changes during conidial germination and mycelial growth. Agric Biol Chem 49:2447–2450. doi: 10.1271/bbb1961.49.2447. [DOI] [Google Scholar]
- 17.Rao JP, Praveen Rao J, Reena G, Subramanyam C. 1997. Calmodulin-dependent protein phosphorylation during conidial germination and growth of Neurospora crassa. Mycol Res 101:1484–1488. doi: 10.1017/S0953756297004255. [DOI] [Google Scholar]
- 18.Dreyfuss JM, Zucker JD, Hood HM, Ocasio LR, Sachs MS, Galagan JE. 2013. Reconstruction and validation of a genome-scale metabolic model for the filamentous fungus Neurospora crassa using FARM. PLoS Comput Biol 9:e1003126. doi: 10.1371/journal.pcbi.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmit JC, Brody S. 1976. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev 40:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasuga T, Townsend JP, Tian C, Gilbert LB, Mannhaupt G, Taylor JW, Glass NL. 2005. Long-oligomer microarray profiling in Neurospora crassa reveals the transcriptional program underlying biochemical and physiological events of conidial germination. Nucleic Acids Res 33:6469–6485. doi: 10.1093/nar/gki953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heavner BD, Price ND. 2015. Comparative analysis of yeast metabolic network models highlights progress, opportunities for metabolic reconstruction. PLoS Comput Biol 11:e1004530. doi: 10.1371/journal.pcbi.1004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton CJ, Cabrera IE, Servin JA, Wright SJ, Cox MP, Borkovich KA. 2012. The guanine nucleotide exchange factor RIC8 regulates conidial germination through Gα proteins in Neurospora crassa. PLoS One 7:e48026. doi: 10.1371/journal.pone.0048026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel JH. 1956. A convenient growth medium for Neurospora crassa. Microb Genet Bull 13:42–43. [Google Scholar]
- 24.Metzenberg RL. 2004. Bird medium: an alternative to Vogel medium. Fungal Genet Rep 51:19–20. doi: 10.4148/1941-4765.1138. [DOI] [Google Scholar]
- 25.Crowther TW, Boddy L, Maynard DS. 2017. The use of artificial media in fungal ecology. Fungal Ecol 32:87–91. [Google Scholar]
- 26.Park G, Pan S, Borkovich KA. 2008. Mitogen-activated protein kinase cascade required for regulation of development and secondary metabolism in Neurospora crassa. Eukaryot Cell 7:2113–2122. doi: 10.1128/EC.00466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westergaard M, Mitchell HK. 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am J Bot 34:573–577. doi: 10.2307/2437339. [DOI] [Google Scholar]
- 28.Leeder AC, Jonkers W, Li J, Glass NL. 2013. Early colony establishment in Neurospora crassa requires a MAP kinase regulatory network. Genetics 195:883–898. doi: 10.1534/genetics.113.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dettmann A, Illgen J, März S, Schürg T, Fleissner A, Seiler S. 2012. The NDR kinase scaffold HYM1/MO25 is essential for MAK2 map kinase signaling in Neurospora crassa. PLoS Genet 8:e1002950. doi: 10.1371/journal.pgen.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu C, Iyer P, Herkal A, Abdullah J, Stout A, Free SJ. 2011. Identification and characterization of genes required for cell-to-cell fusion in Neurospora crassa. Eukaryot Cell 10:1100–1109. doi: 10.1128/EC.05003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104. doi: 10.1534/genetics.104.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. 2016. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Propheta O, Vierula J, Toporowski P, Gorovits R, Yarden O. 2001. The Neurospora crassa colonial temperature-sensitive 3 (cot-3) gene encodes protein elongation factor 2. Mol Gen Genet 264:894–901. [DOI] [PubMed] [Google Scholar]
- 34.Collinge AJ, Fletcher MH, Trinci APJ. 1978. Physiology and cytology of septation and branching in a temperature-sensitive colonial mutant (cot 1) of Neurospora crassa. Trans Br Mycol Soc 71:107–120. doi: 10.1016/S0007-1536(78)80012-6. [DOI] [Google Scholar]
- 35.Resheat-Eini Z, Zelter A, Gorovits R, Read ND, Yarden O. 2008. The Neurospora crassa colonial temperature sensitive 2, 4 and 5 (cot-2, cot-4 and cot-5) genes encode regulatory and structural proteins required for hyphal elongation and branching. Fungal Genet Rep 55:32–36. doi: 10.4148/1941-4765.1088. [DOI] [Google Scholar]
- 36.Yarden O, Plamann M, Ebbole DJ, Yanofsky C. 1992. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J 11:2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aharoni-Kats L, Zelinger E, Chen S, Yarden O. 2018. Altering Neurospora crassa MOB2A exposes its functions in development and affects its interaction with the NDR kinase COT1. Mol Microbiol 108:641–660. doi: 10.1111/mmi.13954. [DOI] [PubMed] [Google Scholar]
- 38.Xue C, Hsueh Y-P, Heitman J. 2008. Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol Rev 32:1010–1032. doi: 10.1111/j.1574-6976.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Won S, Michkov AV, Krystofova S, Garud AV, Borkovich KA. 2012. Genetic and physical interactions between G subunits and components of the G dimer of heterotrimeric G proteins in Neurospora crassa. Eukaryot Cell 11:1239–1248. doi: 10.1128/EC.00151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Wright SJ, Park G, Ouyang S, Krystofova S, Borkovich KA. 2012. Roles for receptors, pheromones, G proteins, and mating type genes during sexual reproduction in Neurospora crassa. Genetics 190:1389–1404. doi: 10.1534/genetics.111.136358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krystofova S, Borkovich KA. 2005. The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gbetagamma dimer required for normal female fertility, asexual development, and galpha protein levels in Neurospora crassa. Eukaryot Cell 4:365–378. doi: 10.1128/EC.4.2.365-378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Borkovich KA. 2006. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot Cell 5:1287–1300. doi: 10.1128/EC.00109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boni AC, Ambrósio DL, Cupertino FB, Montenegro-Montero A, Virgilio S, Freitas FZ, Corrocher FA, Gonçalves RD, Yang A, Weirauch MT, Hughes TR, Larrondo LF, Bertolini MC. 2018. Neurospora crassa developmental control mediated by the FLB-3 transcription factor. Fungal Biol 122:570–582. doi: 10.1016/j.funbio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Carrillo AJ, Schacht P, Cabrera IE, Blahut J, Prudhomme L, Dietrich S, Bekman T, Mei J, Carrera C, Chen V, Clark I, Fierro G, Ganzen L, Orellana J, Wise S, Yang K, Zhong H, Borkovich KA. 2017. Functional profiling of transcription factor genes in Neurospora crassa. G3 (Bethesda) 7:2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Wang J, Li N, Li J, Dunlap JC, Trail F, Townsend JP. 12 December 2017. Light sensing by opsins and fungal ecology: NOP-1 modulates entry into sexual reproduction in response to environmental cues. Mol Ecol doi: 10.1111/mec.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C-H, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. 2009. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J 28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCluskey K, Wiest A, Plamann M. 2010. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J Biosci 35:119–126. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Zheng W, Koryu K, Francesc L-G, Hanna J, Townsend JP. 2012. Sex-specific gene expression during asexual development of Neurospora crassa. Fungal Genet Biol 49:533–543. doi: 10.1016/j.fgb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin J, Xin X, Weng Y, Gui Z. 2017. Transcriptome-wide analysis reveals the progress of Cordyceps militaris subculture degeneration. PLoS One 12:e0186279. doi: 10.1371/journal.pone.0186279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Hu X, Xia Y, Xiao G, Zheng P, Wang C. 2014. Linkage of oxidative stress and mitochondrial dysfunctions to spontaneous culture degeneration in Aspergillus nidulans. Mol Cell Proteomics 13:449–461. doi: 10.1074/mcp.M113.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butt TM, Wang C, Shah FA, Hall R. 2006. Degeneration of entomogenous fungi, p 213–226. In Progress in biological control. Springer, New York, NY. [Google Scholar]
- 53.Kuo H-C, Hui S, Choi J, Asiegbu FO, Valkonen JPT, Lee Y-H. 2014. Secret lifestyles of Neurospora crassa. Sci Rep 4:5135. doi: 10.1038/srep05135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. 2016. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, López-Giráldez F, Townsend JP. 2010. LOX: inferring Level Of eXpression from diverse methods of census sequencing. Bioinformatics 26:1918–1919. doi: 10.1093/bioinformatics/btq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan JF. 2007. P value fetishism and use of the Bonferroni adjustment. Evid Based Ment Health 10:34–35. [DOI] [PubMed] [Google Scholar]
- 57.Follett RF. 1995. Fate and transport of nutrients: nitrogen. Natural Resources Conservation Service, Annapolis, MD. [Google Scholar]
- 58.Vogel HJ. 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am Nat 98:435–446. doi: 10.1086/282338. [DOI] [Google Scholar]
- 59.Klittich C, Leslie JF. 1988. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Zheng W, Nina L, Frances T, Townsend JP. 2012. Differential impact of nutrition on developmental and metabolic gene expression during fruiting body development in Neurospora crassa. Fungal Genet Biol 49:405–413. doi: 10.1016/j.fgb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Lopez-Giraldez F, Lehr N, Farré M, Common R, Trail F, Townsend JP. 2014. Global gene expression and focused knockout analysis reveals genes associated with fungal fruiting body development in Neurospora crassa. Eukaryot Cell 13:154–169. doi: 10.1128/EC.00248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright SJ, Inchausti R, Eaton CJ, Krystofova S, Borkovich KA. 2011. RIC8 is a guanine-nucleotide exchange factor for G subunits that regulates growth and development in Neurospora crassa. Genetics 189:165–176. doi: 10.1534/genetics.111.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung KS, Won M, Lee SB, Jang YJ, Hoe KL, Kim DU, Lee JW, Kim KW, Yoo HS. 2001. Isolation of a novel gene from Schizosaccharomyces pombe: stm1+ encoding a seven-transmembrane loop protein that may couple with the heterotrimeric Galpha 2 protein, Gpa2. J Biol Chem 276:40190–40201. doi: 10.1074/jbc.M100341200. [DOI] [PubMed] [Google Scholar]
- 64.Cabrera IE, Pacentine IV, Lim A, Guerrero N, Krystofova S, Li L, Michkov AV, Servin JA, Ahrendt SR, Carrillo AJ, Davidson LM, Barsoum AH, Cao J, Castillo R, Chen W-C, Dinkchian A, Kim S, Kitada SM, Lai TH, Mach A, Malekyan C, Moua TR, Torres CR, Yamamoto A, Borkovich KA. 2015. Global analysis of predicted G protein-coupled receptor genes in the filamentous fungus, Neurospora crassa. G3 (Bethesda) 5:2729–2743. doi: 10.1534/g3.115.020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith KM, Sancar G, Dekhang R, Sullivan CM, Li S, Tag AG, Sancar C, Bredeweg EL, Priest HD, McCormick RF, Thomas TL, Carrington JC, Stajich JE, Bell-Pedersen D, Brunner M, Freitag M. 2010. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot Cell 9:1549–1556. doi: 10.1128/EC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olmedo M, Ruger-Herreros C, Corrochano LM. 2010. Regulation by blue light of the fluffy gene encoding a major regulator of conidiation in Neurospora crassa. Genetics 184:651–658. doi: 10.1534/genetics.109.109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olmedo M, Ruger-Herreros C, Luque EM, Corrochano LM. 2010. A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet Biol 47:352–363. doi: 10.1016/j.fgb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Wu C, Yang F, Smith KM, Peterson M, Dekhang R, Zhang Y, Zucker J, Bredeweg EL, Mallappa C, Zhou X, Lyubetskaya A, Townsend JP, Galagan JE, Freitag M, Dunlap JC, Bell-Pedersen D, Sachs MS. 2014. Genome-wide characterization of light-regulated genes in Neurospora crassa. G3 (Bethesda) 4:1731–1745. doi: 10.1534/g3.114.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Li N, Li J, Dunlap JC, Trail F, Townsend JP. 2016. The fast-evolving phy-2 gene modulates sexual development in response to light in the model fungus Neurospora crassa. mBio 7:e02148. doi: 10.1128/mBio.02148-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagy LG, Ohm RA, Kovács GM, Floudas D, Riley R, Gácser A, Sipiczki M, Davis JM, Doty SL, de Hoog GS, Lang BF, Spatafora JW, Martin FM, Grigoriev IV, Hibbett DS. 2014. Latent homology and convergent regulatory evolution underlies the repeated emergence of yeasts. Nat Commun 5:4471. doi: 10.1038/ncomms5471. [DOI] [PubMed] [Google Scholar]
- 71.Noble SM, Gianetti BA, Witchley JN. 2017. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 15:96–108. doi: 10.1038/nrmicro.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polvi EJ, Veri AO, Liu Z, Hossain S, Hyde S, Kim SH, Tebbji F, Sellam A, Todd RT, Xie JL, Lin Z-Y, Wong CJ, Shapiro RS, Whiteway M, Robbins N, Gingras A-C, Selmecki A, Cowen LE. 2019. Functional divergence of a global regulatory complex governing fungal filamentation. PLoS Genet 15:e1007901. doi: 10.1371/journal.pgen.1007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clark TA, Guilmette JM, Renstrom D, Townsend JP. 2008. RNA extraction, probe preparation, and competitive hybridization for transcriptional profiling using Neurospora crassa long-oligomer DNA microarrays. Fungal Genet Rep 55:18–28. doi: 10.4148/1941-4765.1086. [DOI] [Google Scholar]
- 74.Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chinnici JL, Fu C, Caccamise LM, Arnold JW, Free SJ. 2014. Neurospora crassa female development requires the PACC and other signal transduction pathways, transcription factors, chromatin remodeling, cell-to-cell fusion, and autophagy. PLoS One 9:e110603. doi: 10.1371/journal.pone.0110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Priebe S, Kreisel C, Horn F, Guthke R, Linde J. 2015. FungiFun2: a comprehensive online resource for systematic analysis of gene lists from fungal species. Bioinformatics 31:445–446. doi: 10.1093/bioinformatics/btu627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stajich JE, Harris T, Brunk BP, Brestelli J, Fischer S, Harb OS, Kissinger JC, Li W, Nayak V, Pinney DF, Stoeckert CJ Jr, Roos DS. 2012. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res 40:D675–D681. doi: 10.1093/nar/gkr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ziebarth JD, Bhattacharya A, Cui Y. 2013. Bayesian Network Webserver: a comprehensive tool for biological network modeling. Bioinformatics 29:2801–2803. doi: 10.1093/bioinformatics/btt472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphological development of N. crassa in (A to C) 3-day cultures and (D to F) 10-day cultures on (A and D) Bird medium (BM), (MSM) (B and E) maple sap medium, and (C and F) synthetic crossing medium (SCM). Abundant conidia were produced in the 3-day old cultures on (A) BM and (C) SCM. (D) No protoperithecia were detected in the 10-day cultures on BM. (E and F) Protoperithecia were produced in the 10-day cultures on MSM and SCM media (inset). Download FIG S1, PDF file, 2.3 MB (2.4MB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The analytical compositions of BM and MSM. Download Table S1, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Analyses of RNAseq data. (A) Summary of transcriptome sequencing (RNA-seq) data and mapping quality. (B) Gene expression levels during conidial germination on BM. (C) Gene expression levels during conidial germination on MSM. Download Table S2, XLSX file, 3.9 MB (3.9MB, xlsx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Functional analyses of selected genes during conidial germination in N. crassa. (A) Results of KEGG functional enrichment analysis for genes classified with stage-specific expression patterns. (B) Functional annotations of genes that were identified for the Bayesian network of asexual-sexual developmental responses to environmental factors during the conidial germination. Download Table S3, DOCX file, 0.05 MB (50.2KB, docx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Differential expression of MAPK genes between BM and MSM cultures. Download FIG S2, PDF file, 0.1 MB (126.7KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Differential expression of genes involved in hyphal growth between cultures on Bird medium and cultures on maple sap medium, including (A) genes involved in bud emergence and cell polarity, (B) genes involved in bud site selection, (C) genes involved in septins development and regulation, (D) genes involved in syntenic homolog actin-ring formation, (E) genes involved in syntenic homolog formation, (F) genes involved in transport and secretion during hyphal growth, (G) genes involved in morphological growth and hyphal polarity showing infrequent expression regulation in BM and MSM, and (H) genes involved in nitrogen metabolism showing differential expression regulation in BM and MSM. Download FIG S3, PDF file, 0.2 MB (262.5KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Significant expression changes between stages observed for isoforms in Neurospora crassa during germination of conidia on BM and MSM. (B) Isoforms whose expression results were opposite and significantly different during germination of conidia on BM and on MSM. Download Table S4, XLSX file, 0.1 MB (97.5KB, xlsx) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Expression of early light-responsive genes (ELRGs) was generally downregulated in cultures on BM and MSM during the first two stages of conidial germination. (B) Expression of late light-responsive genes (LLRGs) was generally downregulated in cultures on BM but upregulated in cultures on MSM. Download FIG S4, PDF file, 0.1 MB (143.4KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Differential expression of genes that exhibited knockout phenotypes during conidial germination. Download FIG S5, PDF file, 0.1 MB (104KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogeny of orthologs of NCU08095, illustrating their presence or absence in genomes of Leotiomycetes, Sordariomycetes, and Eurotiomycetes. Branches with significant support (MrBayes posterior probability value, >0.95) are indicated in bold characters. Download FIG S6, PDF file, 0.1 MB (153.3KB, pdf) .
Copyright © 2019 Wang et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.