Supplemental Digital Content is available in the text.
Keywords: quadrivalent influenza vaccine, children, immunogenicity, safety
Abstract
Background:
For children <3 years of age, a half dose of inactivated influenza vaccine (7.5 μg hemagglutinin per strain) has been used for more than 30 years, but several studies indicate that a full dose (15 μg hemagglutinin per strain) can be used in this population without increasing the rate of fever or other reactions. Here, we compare the safety and immunogenicity of full and half doses of quadrivalent, split-virion, inactivated influenza vaccine (IIV4) in children 6–35 months of age.
Methods:
In this phase IV, randomized, observer-blinded, multi-center study, healthy children 6–35 months of age were randomized 1:1 to be vaccinated with a half or full dose of IIV4 (NCT02915302). The primary objective was to demonstrate that the rate of any fever (≥38.0°C) up to 7 days after a full dose of IIV4 was noninferior to the rate of fever after a half dose.
Results:
The study included 1950 children. Noninferiority in the rate of fever was demonstrated for the full dose versus the half dose of IIV4 (difference in rate = 0.84%; 95% confidence interval, −2.13% to 3.80%). Solicited reactions and unsolicited adverse events were similar between the dose groups. No vaccine-related serious adverse events were reported. Noninferiority of both hemagglutination inhibition geometric mean titers and seroconversion rates was demonstrated for all 4 vaccine strains for the full dose versus the half dose.
Conclusions:
In children 6–35 months of age, a full dose of IIV4 was immunogenic and had a safety profile comparable to that of a half dose, with no new safety concerns observed.
Young children are at increased risk for influenza virus infection, as well as for severe influenza illness and influenza-related hospitalization.1,2 Although influenza A has been the focus of most study and prevention efforts, influenza B is now appreciated to be a frequent cause of illness, hospitalization and death.3 In young children, influenza B is responsible for a disproportionate amount of severe illness and hospitalization.3,4 Two distinct lineages of influenza B, Victoria and Yamagata, now cocirculate, although their distribution can vary substantially between years and regions.5
Because trivalent influenza vaccines contain only a single B-lineage strain, quadrivalent vaccines containing B strains from both lineages have been developed to reduce the risk of influenza illness and its associated morbidity and mortality as immunity to 1 B lineage does not provide adequate protection against the other.6 A quadrivalent, split-virion, inactivated influenza vaccine (IIV4; Fluzone Quadrivalent; Sanofi Pasteur, Swiftwater, PA) has been available in the United States since 2013 for individuals ≥6 months of age. Clinical trial data indicated that in children 6 months to 8 years of age, IIV4 was as immunogenic as the comparator trivalent inactivated influenza vaccine for each of the 3 shared influenza strains and, despite the additional antigen, the 2 vaccines had a similar safety profile.7
For more than 30 years, influenza vaccines for children <3 years to age have contained a half dose of antigen (7.5 μg hemagglutinin per strain)8 to reduce the risk of fever and febrile convulsions associated with earlier whole-virus influenza vaccines.9 More recent findings suggest that a full dose (15 μg hemagglutinin per strain) can be used in children <3 years without increased fever or other reactions,10–13 although this has not yet been established for IIV4, which is currently licensed for use as a half dose in young children. In the current study, we therefore evaluated the safety and immunogenicity of full versus half doses of this IIV4 in healthy children 6–35 months of age. The primary objective was to compare the rates of fever following administration of full and half doses of IIV4.
MATERIALS AND METHODS
Study Design
This was a phase IV, randomized, observer-blinded, 2-arm, multi-center study to evaluate the safety and immunogenicity of 2 different doses of IIV4 in healthy children 6–35 months of age. The study was conducted between September 2016 and March 2017 at 38 sites in the United States (ClinicalTrials.gov no. NCT02915302).
Ethics
The study was approved by the institutional review boards for all sites and conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. Before participants were included in the study, written informed consent was provided by their parents or guardians.
Participants
The study included healthy children 6–35 months of age who had not been vaccinated against influenza during the current season (2016–2017). Children 6–11 months of age had to be born at full term of pregnancy (≥37 weeks) or with a birth weight ≥2.5 kg. Children with moderate or severe acute illness or infection according to the investigator’s judgment or febrile illness (temperature ≥100.4°F [38.0°C]) who were otherwise eligible to participate were not enrolled until the illness resolved. Other exclusions are listed in Table, Supplemental Digital Content S1, http://links.lww.com/INF/D336. Enrollment was stratified by age at each site so that approximately equal numbers of children 6–23 and 24–35 months of age would be included.
Vaccine
In accordance with US Food and Drug Administration guidance for composition of Northern Hemisphere 2016–2017 influenza vaccines, IIV4 (Fluzone Quadrivalent; Sanofi Pasteur) contained the A/California/07/2009 X-179A (H1N1), A/Hong Kong/4801/2014 X-263B (H3N2), B/Brisbane/60/2008 (Victoria lineage) and B/Phuket/3073/2013 (Yamagata lineage) strains. Each half dose (0.25 mL) was formulated to contain 7.5 μg hemagglutinin per strain, and each full dose (0.5 mL) was formulated to contain 15 μg hemagglutinin per strain. Study vaccines were supplied, respectively, in 0.25- or 0.5-mL, prefilled single-dose syringes.
Study Conduct
Using a preprogrammed interactive response technology system, participants were randomized in a 1:1 ratio to be vaccinated by intramuscular injection with a half or full dose of IIV4. In accordance with US Advisory Committee on Immunization Practices guidance,8 participants received 2 doses of IIV4 28 days apart (window, 28–35 days) if they had not previously received 2 doses of influenza vaccine. The participant, study site personnel (including investigators) and sponsor’s clinical team members involved in the study were blinded to the vaccine dose administered, with the exception of unblinded qualified study staff who administered the vaccine. The unblinded qualified study staff did not collect safety data, and they were instructed to not inform parents or guardians of the dose administered.
Safety Assessment
For 7 days after each vaccination, parents or guardians took temperature readings each day, measured the size of any local reactions and recorded medications taken for any adverse reactions on a diary card. Investigators or authorized designees interviewed the parents or guardians to collect the information recorded in the diary card. Solicited local reactions consisted of injection-site tenderness, erythema and swelling; solicited systemic reactions consisted of fever, vomiting, abnormal crying, drowsiness, loss of appetite and irritability. Severity gradings for each reaction are provided in Table, Supplemental Digital Content S2, http://links.lww.com/INF/D337. Investigators also collected unsolicited adverse events (AEs) for 28 days after each vaccination and serious AEs (SAEs) up to the end of the trial according to the International Conference on Harmonisation E2A Guideline for Clinical Safety Data Management. SAEs occurring after a participant had completed the study but likely related to the product were also to be recorded.
Immunogenicity Assessment
Immunogenicity was to be assessed in a planned subset of 1600 participants randomly selected via the interactive response technology system. For the immunogenicity subset, blood samples were collected before the first vaccination and 28 days (window, 28–35 days) after the final vaccination. Hemagglutination inhibition (HAI) titers were measured as described previously.7 The lower limit of quantitation was set at the reciprocal of the lowest dilution (1:10), and the upper limit of quantitation was set as the highest dilution (1:10,240) used in the assay. Seroconversion was defined as (1) a prevaccination titer <10 and postvaccination titer ≥40; or (2) a prevaccination titer ≥10 and a ≥4-fold increase in postvaccination titer.
Statistical Analysis
The primary objective was assessed by determining if the rate of any fever (temperature ≥100.4°F [38.0°C]) within 7 days after administration of any full dose of IIV4 was noninferior to the rate of fever after administration of any half dose. All safety endpoints were assessed in all vaccinated participants according to the vaccine received. Noninferiority with respect to fever was considered demonstrated if the upper bound of the 2-sided 95% confidence interval (CI) of the fever rate difference between participants receiving full-dose vaccine and participants receiving half-dose vaccine was <5%. The 95% CI of the rate difference was computed using the Wilson Score method without continuity correction.14 Assuming a total planned enrollment sample size of approximately 2190 (1095 per group), an attrition rate of 5%, a 1-sided alpha of 2.5%, an expected 14.3% rate of fever,7 and a noninferiority margin of 5%, the study was powered at approximately 90% to demonstrate noninferiority for fever.
Immunogenicity was assessed according to the vaccine received in all participants in the immunogenicity subset who received at least 1 dose of the study vaccine, had a postvaccination serology result for at least 1 strain and completed the study according to protocol. For immunogenicity and safety variables, the 95% CIs of point estimates were calculated assuming a normal distribution. Geometric means and their 95% CIs were calculated as the anti-log of the mean and 95% of the log10 values. For point estimates of differences in proportions, 95% CIs were calculated using the Wilson Score method without continuity correction or by the exact binomial (Clopper-Pearson) method.
For each strain, noninferiority in the HAI geometric mean titer (GMT) was considered demonstrated if the lower limit of the 2-sided 95% CI of the GMT ratio (GMTfull dose/GMThalf dose) was >0.667. Noninferiority for seroconversion rates was considered demonstrated if the lower limit of the 2-sided 95% CI for the rate difference for the full dose minus the half dose was >−10%. For the difference in seroconversion rates, the 95% CI of the rate difference was computed using the Wilson Score method without continuity correction. For the 1600 participants who were to be randomly assigned to the immunogenicity subset, assuming an attrition rate of 20%, a 1-sided alpha of 2.5% for each test, the same expected GMTs for each dose group, a standard deviation of log titers against each strain of 0.7 and a noninferiority margin of 0.667, the study power was approximately 97.6% to demonstrate noninferiority for GMTs. Assuming for each strain the same expected seroconversion rates for each vaccine dosing group (90.9% for A/H1N1, 95.4% for A/H3N2, 72% for B/Victoria and 57.5% for B/Yamagata7) and a noninferiority margin of 10% for each strain, the planned study power was approximately 93.2% to demonstrate noninferiority for seroconversion rates.
Results of participants receiving exactly 1 dose of IIV4 and those receiving 2 doses were combined in all noninferiority assessments (ie, for fever rate, postvaccination HAI GMTs and seroconversion rates).
Demographic characteristics and safety were assessed in all participants who received at least 1 dose of study vaccine.
Missing data were not imputed. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics and Disposition
The study was conducted between September 23, 2016, and March 6, 2017. Due to lower than expected recruitment, 1950 participants were enrolled in the study instead of the planned 2190 (Fig. 1). The participants were randomly assigned in nearly equal proportions to receive the half dose (n = 955; 49.0%) or full dose of IIV4 (n = 995; 51.0%). Six participants randomized to the full-dose group and 3 ran domized to the half-dose group were not vaccinated. In accordance with Advisory Committee on Immunization Practices guidance, a total of 1024 (52.5%) participants received 2 doses of IIV4 (507 [53.1%] in the half-dose group and 517 [52.0%] in the full-dose group). The study was completed by 890 (93.2%) participants in the half-dose group and 917 (92.2%) in the full-dose group. The most common reasons for participant discontinuation were voluntary withdrawal not because of an AE and noncompliance with the protocol. No participants discontinued the study for an AE or SAE considered related to vaccination.
FIGURE 1.
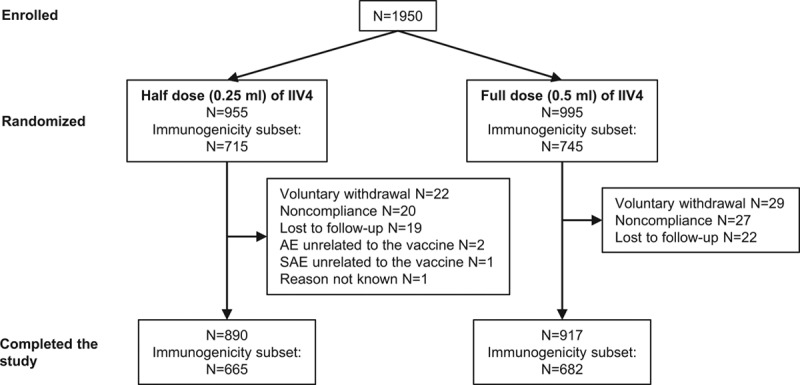
Study design and disposition of participants. Healthy children 6–35 months of age were randomly assigned 1:1 to receive the half dose or the full dose of IIV4 by intramuscular injection.
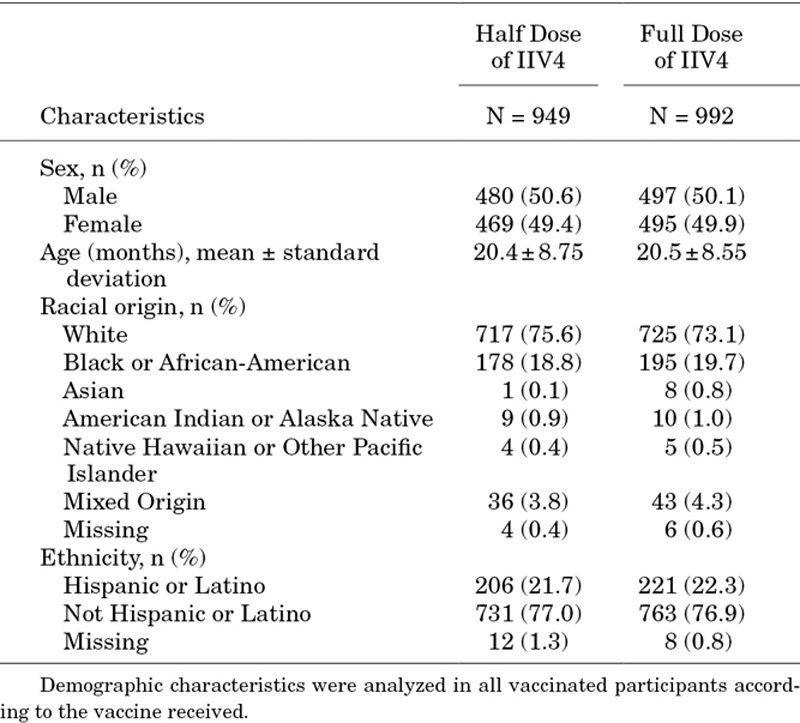
At enrollment, the full- and half-dose groups included nearly equal proportions of males and females (50.6% male in the half-dose group and 50.1% male in the full-dose group). Mean ages and distributions of racial and ethnic origins were also similar between the 2 groups (Table 1).
TABLE 1.
Demographic Characteristics
Immunogenicity was assessed in a randomly selected subset of 715 participants in the half-dose group and 745 in the full-dose group (Fig. 1). Of these, 665 (93.0%) participants in the half-dose group and 682 (91.5%) in the full-dose group completed the study.
Fever
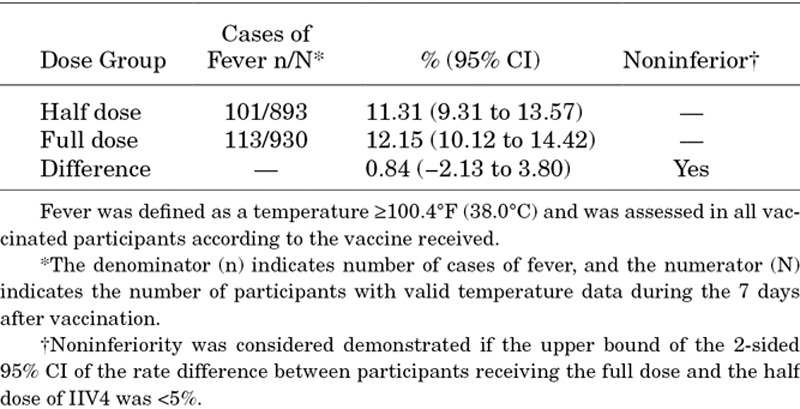
The difference in rate of fever for the full dose minus the half dose of IIV4 was 0.84% (95% CI, −2.13% to 3.80%) (Table 2). Thus, noninferiority in the rate of fever was demonstrated for the full dose compared with the half dose of IIV4.
Table 2.
Rates of Fever and Noninferiority Comparison
HAI Titers
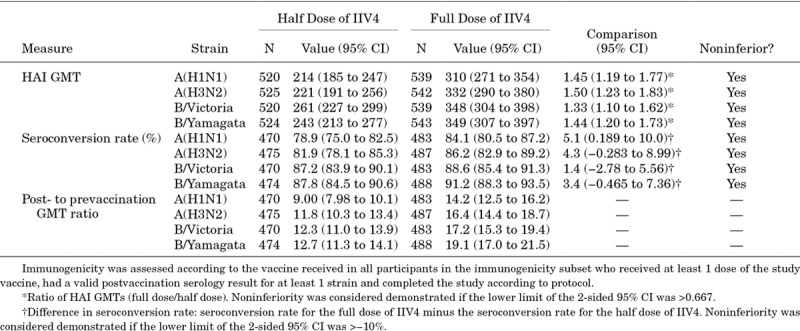
Of the 1950 participants in the study, 1460 were randomized to the immunogenicity subset. Noninferiority of both HAI GMTs and seroconversion rates was demonstrated for all 4 vaccine strains for the full dose versus the half dose (Table 3). Overall, HAI GMTs and post-/prevaccination GMT ratios were higher for the full dose than for the half dose, as indicated by nonoverlapping 95% CIs. For seroconversion rates, point estimates were higher for the full dose than for the half dose, but 95% CIs overlapped. In analyses stratified by age (6–23 months vs. 24–35 months) and by number of doses received (1 vs. 2 doses)—analyses for which this study was not powered—postvaccination HAI GMTs were generally higher among participants 24–35 months of age and among participants receiving 1 dose of IIV4, with the full dose generally inducing higher HAI GMTs compared with the half dose, regardless of age subgroup or number of doses received (Tables, Supplemental Digital Content S3–S6, http://links.lww.com/INF/D338; http://links.lww.com/INF/D339; http://links.lww.com/INF/D340; http://links.lww.com/INF/D341).
TABLE 3.
Postvaccination Immunogenicity and Noninferiority Comparisons
Solicited Reactions
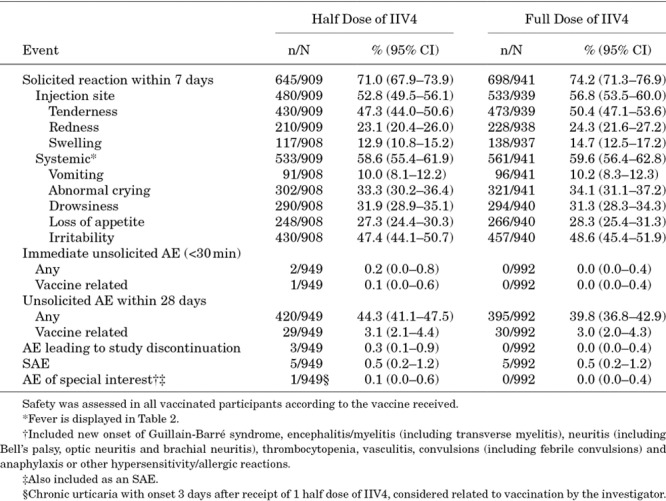
Proportions of participants reporting any solicited reactions, solicited injection-site reactions and solicited systemic reactions were similar for the full- and half-dose groups (Table 4). In most participants, solicited reactions resolved within 3 days (data not shown). Rates of grade 3 solicited reactions were generally similar for the full and half dose of IIV4 (Table, Supplemental Digital Content S7, http://links.lww.com/INF/D342). The most common grade 3 solicited injection-site reaction was tenderness (1.2% in the full-dose group and 1.7% in the half-dose group), and the most common grade 3 solicited systemic reactions included irritability (4.0% in the full-dose group and 3.6% in the half-dose group) and abnormal crying (2.6% in the full-dose group and 3.1% in the half-dose group). Grade 3 fever was reported for 0.6% (95% CI, 0.2–1.3) of participants who received the half dose and 1.2% (95% CI, 0.6–2.1) who received the full dose. In analyses stratified by age, rates of solicited systemic reactions were generally higher among participants 6–23 months of age who received the full dose compared with participants 24–35 months of age who received the full dose. Within each age subgroup, reactogenicity between the full and half doses was similar (Table, Supplemental Digital Content S8, http://links.lww.com/INF/D343). Among participants receiving 2 doses of IIV4, reactogenicity was generally similar between the first and second doses, irrespective of dosing volume received (Table, Supplemental Digital Content S9, http://links.lww.com/INF/D344).
TABLE 4.
Solicited Reactions and Adverse Events
Adverse Events
Rates of vaccine-related unsolicited AEs were similar (3.1% for the half-dose group and 3.0% for the full-dose group) (Table 4). Three AEs leading to study discontinuation were reported, all in the half-dose group. None of the AEs leading to study discontinuation were considered related to vaccination.
SAEs were experienced by 5 (0.5%) participants in the half-dose group and 5 (0.5%) in the full-dose group, none of which were considered related to the vaccine. A single AE of special interest (chronic urticaria first appearing 3 days post-vaccination and continuing for >6 weeks) was considered by the investigator to be related to vaccination.
DISCUSSION
In this study, we showed that in children 6–35 months of age, comparable rates of fever were reported among those receiving a full dose and a half dose of IIV4. Other solicited reactions and unsolicited AEs also occurred at similar rates between for the full and half dose of IIV4, and few cases of grade 3 fever and no cases of febrile convulsion were reported. Thus, no new safety concerns were observed following administration of a full dose of IIV4 in this population. The study also showed that antibody responses induced by the full dose of IIV4 were at least as high as those induced by the half dose, suggesting that a full dose of IIV4 can protect this age group at least as well as a half dose. Of note, 1 dose of IIV4 induced HAI GMTs that were generally higher than those induced by 2 doses, which likely reflects 1-dose participants having been immunologically primed, whereas 2-dose recipients were immunologically naive or relatively naive.
Similar results with respect to the reactogenicity and immunogenicity between full and half doses have been found in several studies, although most have studied trivalent influenza vaccines. Skowronski et al13 examined the immunogenicity and reactogenicity of 2 full versus 2 half doses of a trivalent inactivated influenza vaccine in previously unimmunized infants (6–11 months of age) and toddlers (12–23 months). They found that the full dose induced higher HAI titers for all 3 vaccine components in infants but not toddlers and that the rate of fever did not increase in either age group. Similarly, Pavia-Ruz et al12 and Langley et al11 reported that in children 6–35 months of age, a full dose of trivalent inactivated influenza vaccine induced higher HAI titers than the half dose for all strains without affecting rates of fever or other reactogenicity or safety endpoints. Finally, Jain et al10 compared the US-licensed standard half-dose IIV4 with an investigational full-dose IIV4 in children 6–35 months of age. Noninferior HAI GMTs and seroconversion rates were demonstrated against all 4 vaccine strains. Also, superior HAI GMTs were demonstrated against both vaccine B strains in children 6–17 months of age and unprimed children 6–35 months of age. As in the other studies, the safety profiles, including the rate of fever, were similar for the 2 vaccines. Thus, all studies to date, including the current one, have indicated that full-dose inactivated influenza vaccines can generally increase HAI antibody titers and can be safely used in young children. Moreover, several studies have demonstrated the effectiveness and safety of full-dose inactivated influenza vaccine in preventing influenza disease in children younger than 3 years of age.15–17
This study had some limitations. The study did not assess clinical protection against influenza but rather HAI titers, which are not always predictive of protection18; nonetheless, the results suggest that full dose should be at least as effective as the half dose in young children. Another potential limitation is that the planned study size due was not reached to unexpectedly low recruitment. Regardless, the study size was sufficient (ie, power >80%) to demonstrate noninferiority for the primary (rates of fever) and secondary (HAI GMTs and rates of seroconversion) outcomes.
In summary, the results of this study indicate that healthcare providers should be able to safely use the full dose of IIV4 for children 6–35 months of age. As suggested by Jain et al,10 clinical information should now be sufficient to support using full-dose influenza vaccines for this age group. Indeed, full-dose influenza vaccine has already been recommended for use in children as young as 6 months of age in some countries (eg, Canada, the United Kingdom, Finland and the United States) as part of their national immunization programs.8,19,20 The ability to use full-dose influenza vaccine should provide healthcare providers with additional flexibility and convenience when vaccinating young children against influenza.
Supplementary Material
Footnotes
This work is funded by Sanofi Pasteur. N.P.K. reports grants from Sanofi Pasteur during the conduct of the study and grants from GlaxoSmithKline, Protein Sciences, Pfizer, Merck, Dynavax and MedImmune outside the submitted work. C.A.R., M.M., A.S. and D.P.G. are employees of Sanofi Pasteur. R.J. declares no conflicts of interest. The authors have no other conflicts of interest or funding to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine. 2011;29:7524–7528. [DOI] [PubMed] [Google Scholar]
- 2.Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis. 2007;20:259–263. [DOI] [PubMed] [Google Scholar]
- 3.Paul Glezen W, Schmier JK, Kuehn CM, et al. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103:e43–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Review of the 2010–2011 winter influenza season, northern hemisphere. Wkly Epidemiol Rec. 2011;86:222–227. [PubMed] [Google Scholar]
- 5.Rota PA, Wallis TR, Harmon MW, et al. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. [DOI] [PubMed] [Google Scholar]
- 6.Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg DP, Robertson CA, Landolfi VA, et al. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J. 2014;33:630–636. [DOI] [PubMed] [Google Scholar]
- 8.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2017-18 Influenza Season. MMWR Recomm Rep. 2017;66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright PF, Dolin R, La Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines–II. J Infect Dis. 1976;134:633–638. [DOI] [PubMed] [Google Scholar]
- 10.Jain VK, Domachowske JB, Wang L, et al. Time to change dosing of inactivated quadrivalent influenza vaccine in young children: evidence from a phase III, randomized, controlled trial. J Pediatric Infect Dis Soc. 2017;6:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley JM, Vanderkooi OG, Garfield HA, et al. Immunogenicity and safety of 2 dose levels of a thimerosal-free trivalent seasonal influenza vaccine in children aged 6-35 months: a randomized, controlled trial. J Pediatric Infect Dis Soc. 2012;1:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavia-Ruz N, Angel Rodriguez Weber M, Lau YL, et al. A randomized controlled study to evaluate the immunogenicity of a trivalent inactivated seasonal influenza vaccine at two dosages in children 6 to 35 months of age. Hum Vaccin Immunother. 2013;9:1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skowronski DM, Hottes TS, Chong M, et al. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics. 2011;128:e276–e289. [DOI] [PubMed] [Google Scholar]
- 14.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen S, Silvennoinen H, Lehtinen P, et al. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. Lancet Infect Dis. 2011;11:23–29. [DOI] [PubMed] [Google Scholar]
- 16.Nohynek H, Baum U, Syrjanen R, Ikonen N, Sundman J, Jokinen J. Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds - a nationwide cohort study Finland, influenza season 2015/16. Euro Surveill. 2016;21:pii=30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claeys C, Zaman K, Dbaibo G, et al. ; Flu4VEC Study Group. Prevention of vaccine-matched and mismatched influenza in children aged 6-35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2:338–349. [DOI] [PubMed] [Google Scholar]
- 18.Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother. 2013;9:405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS): statement on seasonal influenza vaccine for 2014–2015. Can Commun Dis Rep. 2014;40:1–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halasa NB, Gerber MA, Berry AA, et al. Safety and immunogenicity of full-dose trivalent inactivated influenza vaccine (TIV) compared with half-dose TIV administered to children 6 through 35 months of age. J Pediatric Infect Dis Soc. 2015;4:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]


