Abstract
Platelets regulate inflammation as well as hemostasis. Inflammatory insults often induce hemostatic function through mechanisms that are not always understood. The triggering receptor expressed in myeloid cells (TREM)-like transcript 1 (TLT-1) is an abundantly expressed platelet receptor and its deletion leads to hemorrhage and edema after lipopolysaccharide and TNF-α treatment. To define a role for TLT-1 in immune derived bleeding we used a CXCL-2 mediated local inflammatory reaction in the vessels of the cremaster muscle of treml1−/− and wild type mice. Our whole mount immunofluorescent staining of the cremaster muscle demonstrated a 50% reduction in clot size and increased extravasation of plasma molecules in treml1−/− mice compared to wild type. We demonstrate that the decreased clotting in treml1−/− mice is associated with a 2X reduction in integrin β3 phosphorylation on residue Y773 after platelet activation, which is consistent with treml1−/− mice displaying reduced outside-in signaling and smaller thrombi. We further substantiate TLT-1’s role in the regulation of immune derived bleeding using the reverse arthus reaction and demonstrate TLT-1’s role in thrombosis using the thromboplastin initiated and collagen/epinephrine models of pulmonary embolism. Thus, the data presented here demonstrate that TLT-1 regulates early clot formation though the stabilization of αIIbβ3 outside-in signaling.
Keywords: TREM-Like transcript (TLT)-1, alpha granules, αIIbβ3, outside-in signaling, platelets
Introduction:-
The relationship between inflammatory and hemostatic systems is dynamic, and although extensively characterized, the complete relationship is not understood(Herter et al., 2014). TREM-Like transcript 1 (TLT-1) is an α-granular platelet receptor that binds fibrinogen and localizes at the platelet surface upon platelet activation(Washington, 2009; Washington et al., 2002; Washington et al., 2004). TLT-1 is abundantly expressed in platelets(Coxon et al., 2017). Based on the works of Simon et. al.(Simon et al., 2014) and those of Rowley et. al(Rowley et al., 2011), TLT-1 is one of the most abundantly expressed proteins in mouse and human platelets (top 3%). These data suggest that it is more abundant than GP1β (top 7%), P-selectin, and integrin 2a (each are in the top 5%). However, TLT-1’s role in hemostasis or inflammation remains undefined.
Invocation of the Shwartzman reaction in treml1−/− mice suggests that TLT-1 controls hemorrhage, neutrophil recruitment, and possibly edema during inflammation and points to a role for TLT-1 in the translation of inflammatory response into hemostatic mechanisms(Washington, 2009).The Shwartzman reaction uses lipopolysaccharide and tumor necrosis factor- to induce an immune response(Brozna, 1990; Stetson and Good, 1951; Stetson, 1951b). In the present study, we evaluate the role of TLT-1 in inflammation and determine that TLT-1 mediates early clot formation enabling αIIbβ3 activation by facilitating downstream outside-in signaling. We further demonstrate the relevance of our finding using inflammatory and hemostatic challenges.
Methods:-
Mice
All mice were maintained under specific pathogen–free conditions at the Animal House Facility. Mice were between 8–10 weeks of age and weighed 20–23 g. Animal care was provided in accordance with the NIH Guidefor the Care and Use of Laboratory Animals (publication no. 85–23. Revised 1985).
Reverse Arthus Reaction (RAR)
RAR was completed as described in reference(Goerge et al., 2008). For quantification studies of the inflammatory area, edema and hemorrhage were subjected to quantification as described in references(Klopfleisch, 2013; Sylvestre et al., 1996).
Western blotting
Western blotting was completed as in ref(Washington et al., 2002)
Whole mount stain of cremaster muscle
Anesthetized wild type or treml1−/− mice were intra-scrotally injected with CXCL-2 (2.5 g/mL). After 3.5 hours incubation, the cremaster muscle was prepared for intravital microscopy as previously described(Zhang et al., 2001).
Pulmonary embolism
Thromboplastin induced pulmonary embolism (PE) was achieved through the I.V injection of 5 μL thromboplastin [Sigma] and mice were monitored for 30-minutes for breathing cessation after which mortality was recorded and lungs were collected for histology. Collagen/epinephrine PE was induced as in ref(Rowley et al., 2016) using 64 mg/kg of collagen and 5.4 mg/kg of epinephrine and anti-TLT-1 (100ug/100ul) 20 minutes prior to the collagen/epinephrine injection.
Statistics
Paired, two-tailed Student’s t test analysis available in Prism, version 7.01 (Graph-Pad Software) was applied to evaluate statistical differences for 2 groups. P < 0.05 was considered statistically significant.
Results and discussion:-
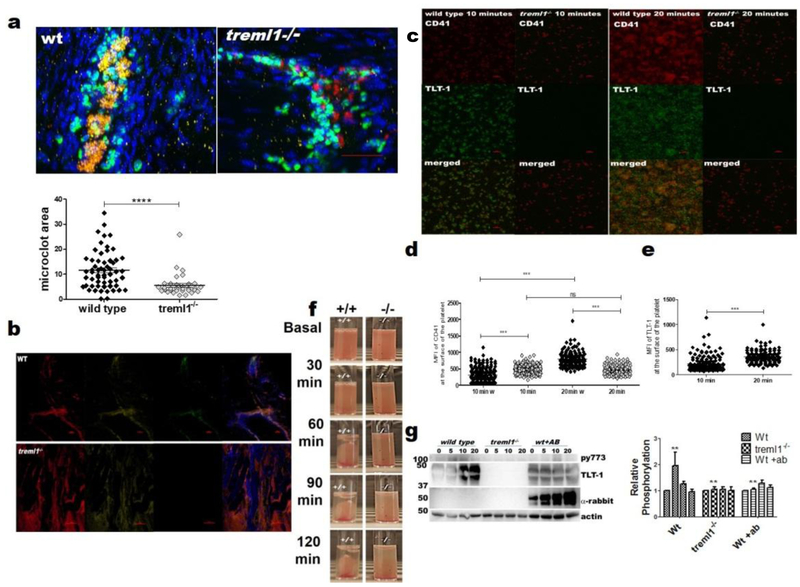
To gain insights into TLT-1 function we injected CXCL2 in to the scrotum of wild type (WT) and treml1−/− mice to visualize the inflammatory changes in the vasculature. CXCL2 attracts neutrophils and leads to clotting in the vasculature. We evaluated the cremaster muscle at 3, 4, and 5 hours after injection and found 3 – 4 hours to be the most insightful. WT mice exhibited more and larger clots with an average area that was approximately twice that of those in the treml1−/− mouse (11.68±0.99 μM2 vs. 5.59±0.80 μM2; figure1a). These results suggested that TLT-1 is responsible for the preliminary stages of clot formation after inflammatory challenge. We also stained for complement factors H (155kD) and I (88kD) to evaluate plasma leakage and found that CXCL2 caused a striking mobilization of plasma factor H and I from vasculature into the cremaster muscle of treml1−/− mice, which was not observed in WT mice (figure1b). These results support the concept that TLT-1 regulates edema as well.
Figure 1. TLT-1 controls later stages of platelet activation and function by facilitating αIIbβ3 outside-in signaling.
(a) Upper panel - Representative Z-stack images of whole mount cremaster muscle showing clot formation in wild type and treml1−/− mice (red: CD41-platelets; green: GR1-neutrophils; yellow: anti TLT-1; blue: DAPI; n=4 mice per group). The red scale bar = 50 μM. Lower panel - Quantified thrombus area in wild type and treml1−/− mice. Images were processed using a Nikon Confocal Microscope and analyzed using NIS element viewer 4.20 magnification 40X, (****p<0.001). (b) Confocal analysis of CXCL-2 treated whole mount cremaster muscle showing plasma leakage in treml1−/− mice compared to wild type mice (green: TLT-1, red: complement factor H, yellow: complement factor I, blue: DAPI). The red scale bar = 100μM (c) Time course of αIIb (CD41) and TLT-1 expression in wild type and treml1−/− platelets. These are representative results of more than 5 experiments. The red scale bar = 10μM (d) Quantification of MFI (mean fluorescence intensity) of CD41 (comparing the kinetics of surface expression of CD41 between wild type and treml1−/− mice, ***p≤0.0001). (e) Quantification of MFI of surface expression of TLT-1 in wild type platelets, ***p≤0.0001 student t-test. In d and e n>95 individual platelets measured (f) Clot retraction using 3× 108 cells/ml and 0.2 units of thrombin comparing WT and treml1−/− mice. (g) Left panel - representative western blot of protein lysates of platelets from wild type and treml1−/− mice activated with 0.025 units thrombin and probed with anti-phospho β3Y773 (Novus Biologicals) at different time points, anti TLT-1 from ref(Washington et al., 2004), anti-rabbit (Jackson Immunoresearch) and anti-β actin (Sigma) controls demonstrate differences in phosphorylation at different time points. Right panel-quantification of western blots (n=3)
To determine the basis for the small size of TLT-1−/− thrombi and inhibition of clot retraction, we examined the kinetics of αIIbβ3 surface expression after low dose thrombin activation. Quantification of mean fluorescence intensity demonstrated that, WT platelets exhibited a time dependent increase of αIIbβ3 and TLT-1 surface expression. Although not significant, αIIb seemed to decrease on treml1−/− platelets (figure1c–e). These results suggested that TLT-1 continuously participates in agonist-induced signaling that leads into αIIbβ3 stabilization on the surface. Treml1−/− mice demonstrate reduced clot retraction compared to WT (figure 1f) and collectively our results suggest that TLT-1 affects αIIbβ3 function. We hypothesized that TLT-1 stabilizes clot formation by facilitating outside-in signaling of αIIbβ3. To test this hypothesis, we examined β3Y773 phosphorylation (pβ3Y773) over time in WT and treml1−/− platelets. pβ3Y773 is an indicator of early outside-in signaling(Unsworth et al., 2017; Xi et al., 2006). Consistent with published data(Unsworth et al., 2017) we found by 5 minutes, pβ3Y773 increased in WT mice. Treml1−/− platelets demonstrated ~2X less pβ3Y773 at 5 minutes and less over the 20-minute period (p=0.03; figure 1g). Addition of an anti-TLT-1 monoclonal antibody(Manfredi et al., 2018) yielded a significant decrease in pβ3Y773 accumulation and supports the idea that TLT-1 stabilizes αIIbβ3 on the platelet surface by mediating integrin outside in signaling.
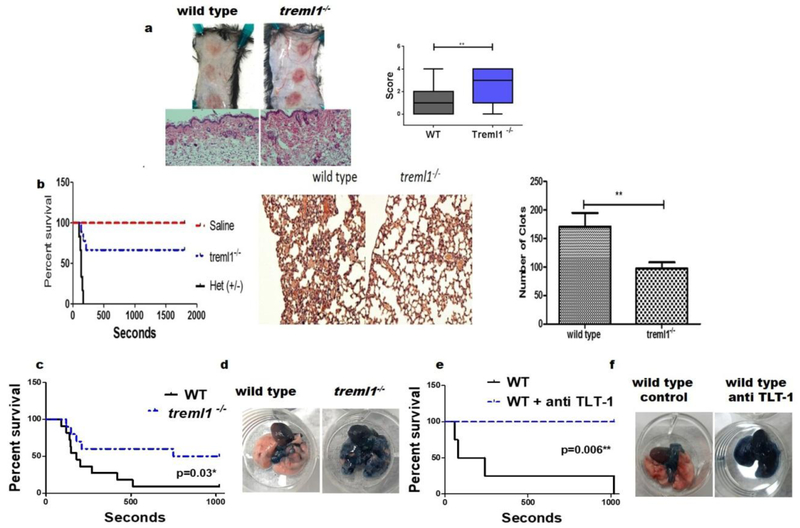
To extend the applicability of TLT-1’s mechanism beyond LPS mediated inflammation seen in the Shwarztman reaction, we used the Reverse Passive Arthus reaction which elicits a localized, dermal, immune response using immune complexes(Stetson, 1951a). Evaluations of the skin lesions show that wild type mice exhibited mild petechia surrounding the injection area (figure 2a). Blinded histological grading of the lesions (scale 1 – 4) revealed that the treml1−/− mice have a significant increase in bleeding and associated edema when compared with their wild type counterparts (score=2.4±0.23 vs 1.3±0.21). Thus TLT-1 has a broad impact in translating the immune challenge into hemostatic response.
Figure 2. TLT-1 deficiency associates with increase survival in a pulmonary embolism model but cause poor hemostasis on inflammation.
(a) Top-macroscopic evaluation of wild type and treml1−/− mice subjected to the RPAR and sacrificed at 4 hours, Bottom-hematoxylin and eosin (H&E) staining of RPAR lesions, blue line equals 100μM. Right panel-blinded histological quantification of hemorrhage from wild type and treml1−/− mice (n=3 per group, **p≤ 0.001, student t-test). (b) Left panel - mortality associated with I.V. injection of either 0.9% saline or 5μl/kg thromboplastin and monitored over a 30-min observation period. Treml1−/− mice were found to be protected from thrombosis compared to the littermate heterozygous mice. Middle panel - H&E histologic analysis of the lungs after thromboplastin treatment. Right panel - quantification of the clots in the H&E analysis demonstrated a significant decrease in clot formation in the treml1−/− mice compared to treml1−/+ mice (treml1+/− n=6, treml1−/−n=9, control n=3; p=0.009). (c, e) Kaplan-Meier curves demonstrating the mortality associated with I.V. injection of 64 mg/kg of collagen and 5.4 mg/kg of epinephrine after 17 minutes. (e) mice were given anti-TLT-1 (100ug/100ul) 20 minutes prior to the collagen/epinephrine injection. Animals surviving the 17 minutes were considered survivors (wild type n=11, treml1−/−, n=10; p=0.0025). (d and f) Mice from d and f were perfused with Evans’ blue (500ul) after onset of respiratory arrest or those mice that survived the study; lungs were then excised, the treml1−/− mice showed almost complete perfusion (blue) while in the wild type the perfusion was impaired (pink) compared to wild type (wild type n=4, wild type + antibody- n=4; p=0.006).
To investigate if TLT-1 responded to hemostatic triggers, we elicited pulmonary embolism (PE) using two different experimental approaches, both of which cause widespread formation of micothrombi in the lungs(Renné et al., 2005). First, we used an I.V. injection of thromboplastin, which mimics tissue factor exposure by activating coagulation through the conversion of pro-thrombin to thrombin(Zacharski and Rosenstein, 1979). Thromboplastin administration caused a significant increase in mortality in treml1+/−heterozygous littermates compared to treml1−/− mice (65% vs 100%; figure 2b). Histological analysis revealed a reduction of clot formation in treml1−/− mice compared to treml1+/− mice. Moreover, quantification of the clots in the lungs WT mice after thromboplastin treatment demonstrated a 40% decrease in clot formation compared to treml1−/+ mice (p<0.009). Second, we injected I.V. collagen/epinephrine which causes direct platelet activation(Mustonen and Lassila, 1996),(Renné et al., 2005). Administration of collagen/epinephrine caused a significant increase in mortality in WT compared to treml1−/− mice (p=0.025; figure2c). Accordingly, administration of anti-TLT-1 antibody closely mimics the treml1−/− phenotype (p=0.006; figure 2e). The infusion of Evans’ blue after onset of respiratory arrest demonstrated almost complete blockage of perfusion on WT compared to treml1−/− mice (figure 2d, f). To our knowledge, this is the first in vivo demonstration of TLT-1 as a therapeutic target. Based on these results we conclude that TLT-1 contributes to microclot formation after hemostatic challenges as well as immune challenges.
Here, we demonstrate that TLT-1 contributes to αIIbβ3 inside-out signaling and mediates the phosphorylation of the β3 subunit, resulting in enhanced outside-in directed clot stabilization and vessel occlusion. It is possible that TLT-1 may play a receptor scaffolding role, recruiting key signaling molecules to the platelet surface. Like αIIbβ3, TLT-1 is a fibrinogen receptor(Washington, 2009). Where αIIbβ3 must convert to its active form, there is no known TLT-1 activation sequence and consequently TLT-1 should bind fibrinogen upon surface expression. Therefore, TLT-1 could sequestrate fibrinogen on the platelet surface and/or abet fibrin formation. Fibrin(ogen) sequestration would be important in forming a hierarchical thrombus core(Stalker et al., 2013), controlling fibrin deposition, and regulation of clot size(Owaynat et al., 2015).
Key points:
TLT-1 modulates αIIbβ3 fibrinogen-mediated inside-out signaling while stabilizes outside-in signaling.
Anti-TLT-1 reduced mortality in the pulmonary embolism model demonstrating therapeutic potential for TLT-1.
Acknowledgements:-
Source of funding: Research reported in this publication was supported by the NIH grants (R01HL090933, HL126547, F31HL136183 and HL112311) from the National Heart Lung and Blood Institute and a NIGMS INBRE grant (P20GM103475). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:-
Nothing to disclose
References:-
- Brozna JP (1990). Shwartzman reaction. Semin Thromb Hemost 16, 326–332. [DOI] [PubMed] [Google Scholar]
- Coxon CH, Geer MJ, and Senis YA (2017). ITIM receptors: more than just inhibitors of platelet activation. Blood 129, 3407–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O’Donnell E, Zhao BQ, Cifuni SM, and Wagner DD (2008). Inflammation induces hemorrhage in thrombocytopenia. Blood 111, 4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter JM, Rossaint J, and Zarbock A (2014). Platelets in inflammation and immunity. J Thromb Haemost 12, 1764–1775. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R (2013). Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology--a systematic review. BMC Vet Res 9, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi B, Morales-Ortiz J, Díaz-Díaz L, Hernandez-Matias, Barreto-Vázquez D, Menéndez-Pérez J, Rodríguez-Cordero J, Villalobos-Santos J, Santiago-Rivera E, Rivera-Dompenciel A, et al. (2018). Characterization of monoclonal antibodies to mouse TLT-1 suggests that TLT-1 plays a role in wound healing. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen P, and Lassila R (1996). Epinephrine augments platelet recruitment to immobilized collagen in flowing blood--evidence for a von Willebrand factor-mediated mechanism. Thromb Haemost 75, 175–181. [PubMed] [Google Scholar]
- Owaynat H, Yermolenko IS, Turaga R, Lishko VK, Sheller MR, and Ugarova TP (2015). Deposition of fibrinogen on the surface of in vitro thrombi prevents platelet adhesion. Thromb Res 136, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, and Nieswandt B (2005). Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med 202, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Chappaz S, Corduan A, Chong MM, Campbell R, Khoury A, Manne BK, Wurtzel JG, Michael JV, Goldfinger LE, et al. (2016). Dicer1-mediated miRNA processing shapes the mRNA profile and function of murine platelets. Blood 127, 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, and Weyrich AS (2011). Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118, e101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, Ma L, Fortina P, Kunapuli S, Holinstat M, et al. (2014). Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood 123, e37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, and Brass LF (2013). Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 121, 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson CA, and Good RA (1951). Studies on the mechanism of the Shwartzman-phenomenon; evidence for the participation of polymorphonuclear leucocytes in the phenomenon. J Exp Med 93, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson CA Jr. (1951a). Similarities in the mechanisms determining the Arthus and Shwartzman phenomena. J Exp Med 94, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson CA Jr. (1951b). Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med 93, 489–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre D, Clynes R, Ma M, Warren H, Carroll MC, and Ravetch JV (1996). Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med 184, 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth AJ, Kriek N, Bye AP, Naran K, Sage T, Flora GD, and Gibbins JM (2017). PPARγ agonists negatively regulate αIIbβ3 integrin outside-in signaling and platelet function through up-regulation of protein kinase A activity. J Thromb Haemost 15, 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington AV (2009). TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. The Journal of Clinical Investigation 119, 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington AV, Quigley L, and McVicar DW (2002). Initial characterization of TREM-like transcript (TLT)-1: a putative inhibitory receptor within the TREM cluster. Blood 100, 3822–3824. [DOI] [PubMed] [Google Scholar]
- Washington AV, Schubert RL, Quigley L, Disipio T, Feltz R, Cho EH, and McVicar DW (2004). A TREM family member, TLT-1, is found exclusively in the alpha-granules of megakaryocytes and platelets. Blood 104, 1042–1047. [DOI] [PubMed] [Google Scholar]
- Xi X, Flevaris P, Stojanovic A, Chishti A, Phillips DR, Lam SC, and Du X (2006). Tyrosine phosphorylation of the integrin beta 3 subunit regulates beta 3 cleavage by calpain. J Biol Chem 281, 29426–29430. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, and Rosenstein R (1979). Reduction of salivary tissue factor (thromboplastin) activity by warfarin therapy. Blood 53, 366–374. [PubMed] [Google Scholar]
- Zhang XW, Liu Q, Wang Y, and Thorlacius H (2001). CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br J Pharmacol 133, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]