Abstract
In this study, Rhizophora mangle L. mangrove plants and plant growth-promoting bacteria were evaluated for their ability to degrade polycyclic aromatic hydrocarbons in diesel oil-contaminated sediment. The diesel-contaminated soil was sown with plant growth-promoting bacteria in the R. mangle L. rhizosphere and monitored for 120 days in a greenhouse. The plant growth-promoting bacteria Pseudomonas aeruginosa and Bacillus sp. were analyzed for their ability to degrade eight priority polycyclic aromatic hydrocarbons, achieving a removal rate for naphthalene (80%), acenaphthene (> 60%), anthracene (> 50%), benzo(a)anthracene (> 60%), benzo(a)pyrene (> 50%) and dibenzo(a,h)anthracene (> 90%) in the treatments with and without plants. R. mangle L. demonstrated a removal rate above 50% for acenaphthene and fluoranthene. The bacterial strains promoted the development of the plant propagule in 55% of sediment contaminated with diesel. Scanning electron microscopy revealed the formation of biofilms by the strains in the roots of the plants in contact with the diesel. Thus, the interaction between Rhizophora mangle L. and the bacterial strains (Bacillus sp. and P. aeruginosa) demonstrated the potential of the strains to degrade diesel and bioremediate mangroves impacted by diesel oil.
Keywords: Rhizophora mangle L., Bacillus sp., Pseudomonas aeruginosa, Mangrove, Diesel oil
Introduction
Mangroves are typical tropical ecosystems that are located between terrestrial and marine environments, are impacted by the tidal regime, and present favorable conditions for the feeding, protection and reproduction of animal species (Schaeffer-Novelli 1995). Mangroves are important for the transformation of nutrients into organic matter, play a role in protecting against erosion and the impacts of extreme events and display economic importance for adjacent communities (Alongi 2002; Santos et al. 2011).
Rhizophora mangle L. is a typical mangrove plant that displays high salinity tolerance and morphological plasticity (Boizard and Mitchell 2011). Roots originating from the stem, such as anchors and lenticels to increase aeration, are the main feature of this plant model (Duke and Allen 2006; Colares and Melo 2013). This species has been applied in toxicity evaluations of mangroves impacted by oil activities, as it demonstrates high adaptability and resistance to chronic oil pollution. Because of this, R. mangle L. is an adequate bioindicator of the pollution of mangroves with derivatives from the petroleum production chain, since derivative contaminants triggers indicative effects on the height, presence of lateral branches, increase in leaf biomass and early aging of these plants (Orge et al. 2000; Sánchez-Arias et al. 2013).
Human activities promote changes in the physical and chemical characteristics of estuaries, reducing the water quality in these environments and, consequently, in mangrove areas (Borja et al. 2011). In fact, due to their coastal location, mangroves suffer from the pressure of industrial and anthropogenic activities, such as pollution and contamination, including oil pollution (Lang et al. 2016). Oil-derived compounds are among the most commonly found pollutants in coastal environments, many of which are persistent and highly toxic (Mucha et al. 2011). These compounds are present in commercial products, including fuel oil, which is a complex mixture of hydrocarbons that includes aliphatic and aromatic molecules. Polycyclic aromatic hydrocarbons (PAHs) are a group of compounds that display toxicity and mutagenic, teratogenic and carcinogenic properties, and due to their environmental risk, PAHs are classified as priority (16 PAHs) pollutants by the US Environmental Protection Agency (1984). They possess two or more fused aromatic rings in linear, angular and clustered arrangements, which increases their persistence in the environment (Singh et al. 2013).
Bioremediation is one of the most applicable methods for the management of environmental contaminants by biological mechanisms (including microorganisms) in soil and water (Trivedi and Ansari 2015). The rhizobacteria that colonize plant roots and promote plant growth have been associated with soil bioremediation (Huang et al. 2005; Zhuang et al. 2007). Some bacterial strains isolated from plant rhizospheres are able to metabolize petroleum-based compounds and produce biosurfactants and can be used in the decontamination of polluted environments.
This study was performed to investigate the fundamental interactions of PAHs, mangrove plants and bacteria. The objective of the present study was to evaluate the performance of the plant growth-promoting bacteria Bacillus sp. and Pseudomonas aeruginosa in addition to Rhizophora mangle L. plants in the degradation of polycyclic aromatic hydrocarbons (PAHs).
Materials and methods
Sediment and propagule sampling and processing
Sediment was collected from a typical mangrove area at the Paraguassu River (lat.12°42′11′′S; long.38°19′0.4″WO), Maragogipe, Bahia, Brazil, without the influence of petroleum hydrocarbon contamination. The sediment was air-dried at room temperature, homogenized, distributed into plastic bags (8 kg), and autoclaved at 121 °C for 30 min 3 times with 24-h intervals between each sterilization.
Rhizophora mangle L. propagules were collected along the mangrove area from the Ilha de Maré region, Bahia, Brazil. This region was chosen because propagules in the ideal development stage for planting were available during the study period. Propagules were uniform in size, lacked radicles and were collected taking into account their phytosanitary conditions, namely, the absence of chlorosis and necrosis due to the presence of pests. In the laboratory, the propagules were sanitized with 2% sodium hypochlorite for 5 min and rinsed with sterile water three times to remove possible microorganisms present on their outer surfaces.
Bacterial strains and growth conditions
Bacillus sp. (NCBI GenBank Accession Number MG461379) and Pseudomonas aeruginosa (NCBI GenBank Accession Number MG461605) bacterial strains were isolated from plant roots obtained from Chapada Diamantina—BAHIA, Brazil, and deposited in the Microorganism Culture Collection at the Health Sciences Institute, Federal University of Bahia.
The bacterial strains previously stored at − 80 °C were inoculated in 100 mL of nutrient broth (composition in g L−1: peptic digest of animal tissue, 5.0; sodium chloride, 5.0; beef extract, 1.5; yeast extract, 1.5; agar, 15.0; pH 7.0) and incubated at 30 °C/120 rpm in an orbital shaker for 24 h to obtain a pre-inoculum. The cultures were then centrifuged at 5000 rpm for 5 min, resuspended in sodium chloride solution (0.9%) and adjusted to 109 cells ml−1 OD 600 nm.
In the greenhouse, sediment potted for each treatment was inoculated with 200 mL of each bacterial suspension. Propagules were immersed for 1 min in 1 L of the bacterial suspensions (109) and later planted in the sediment, according to the experimental design.
Biosurfactant production
The emulsification activity by biosurfactant production was measured according to Das et al. (1998), with modifications. Bacterial suspensions containing the Bacillus sp. and Pseudomonas aeruginosa strains previously grown in nutrient agar were adjusted in sodium chloride solution (0.9%) to 108 cells mL−1 OD600 nm. Suspension aliquots were inoculated in 100 mL of mineral saline medium (MSM) (composition, g L−1: K2HPO4, 4.0; Na2HPO4, 1.5; NaNO3, 1.0; MgSO4.7H2O, 0.2; CaCl2.2H2O, 0.02; FeCl3.6H2O, 0.02; agar, 15.0; pH 7.0) supplemented with 1% glycerol (v/v). The samples were then incubated (180 rpm for 5 days).
The emulsification yield was determined by the emulsification index (EI24) after 24 h, calculated according to the following formula:
A 2-mL aliquot of cell-free broth was added to 2 mL of mineral oil and then mixed to form the emulsion layer. The amount of biosurfactant produced was evaluated in triplicate.
Experimental design for the microcosm study
To set up the microcosm design, plastic boxes with taps (60 × 30 × 10 cm) and an 18-L capacity were used to retain 8 kg of sediment each. Each box received one of the following treatments (Table 1): 1 (BPD), 2 (BPDRm), 3 (DRm), 4 (Rm). Three replicates for each treatment were performed and distributed in randomized blocks.
Table 1.
Bacterial strain, diesel oil and Rhizophora mangle L. treatments in mangrove sediment
| Treatments | Composition | Initials |
|---|---|---|
| 1 | Bacillus sp. + Pseudomonas fluorescens + diesel (autoclaved) | BPD |
| 2 | Bacillus sp. + Pseudomonas fluorescens + diesel + Rhizophora mangle L. (autoclaved) | BPDRm |
| 3 | Diesel + Rhizophora mangle L. (autoclaved) | DRm |
| 4 | Rhizophora mangle L. | Rm |
To obtain contaminated sediment, 310 mL of commercial diesel oil at a final concentration of 55 μg mL−1 was incorporated 5 days before inoculation of the bacterial strains Bacillus sp. and Pseudomonas aeruginosa. The propagules were then used to set up the experiment in triplicate, with six propagules distributed by treatment. A field capacity control was carried out through seawater irrigations during the entire experimental procedure for 120 days in a greenhouse.
Polycyclic aromatic hydrocarbon analysis
The levels of eight priority PAHs (naphthalene, acenaphthene, fluorene, phenanthrene, anthracene, benzo(a)anthracene, benzo(a)pyrene, and dibenzo(a,h)anthracene) defined by the US EPA (1982) were evaluated in the diesel oil and sediment samples. The sediment hydrocarbon extractions were performed according to US EPA Method 3550C (2007), in which 2 g of the sediment was subjected to ultrasonic extraction using 10 mL of dichloromethane. The extracts were fractionated according to the US EPA 3630C (1996) method, where the aromatic fraction (PAH fraction) was diluted with dichloromethane/n-hexane (1:4, v/v). The diesel oil was diluted with dichloromethane and analyzed, along with the sediment samples, using gas chromatography/mass spectrometry (GC–MS) (GC–MS QP5050A Shimadzu model).
For the PAH determinations, the separation column was a DB-5-HP (30 m × 0.25 mm × 0.25 µm; Agilent Technologies), and helium was the carrier gas at flow rates of 2.0 mL min−1. The selected ion monitoring mode was used with a withdrawal rate of 1 μL with a microsyringe and injected into the chromatograph at a 1:10 split ratio. The chromatographic conditions were as follows: injector temperature, 300 °C, initial temperature 1 min at 45 °C, heating at 22 °C min−1 to 130 °C, heating at 10 °C min−1 to 240 °C, followed by heating at 10 °C min−1 to 310 °C, and finally an increase of 20 °C min−1 to 325 °C, which was maintained for 4 min.
The priority PAHs were quantified by the external standard method using an analytical curve with a standard mix (TCL Polynuclear Aromatic Hydrocarbons Mix, 48905-U SUPELCO) containing the eight PAHs. The sediment marine standard certificate from the National Institute of Standards and Technology (NIST) SRM 1941b was used as an external standard for the quantification of the evaluated compounds.
The percentage values of biodegradation were calculated according to equation (Isaac et al. 2015a):
where Hi is the initial concentration of hydrocarbons present in diesel oil and Hf is the residual concentration at the end of the experiment.
Evaluation of the morphology and presence of bacterial root cells by scanning electron microscopy (SEM)
Root samples from BPDRm and Rm treatments were fixed in Karnovsky’s solution (70%) at pH 7.4 (2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 mol L−1 sodium cacodylate buffer and distilled water) for 24 h. The samples were washed in 0.1 mol L−1 sodium cacodylate buffer and postfixed in osmium tetroxide 1%. After fixation, the samples were dehydrated in ethanol (30, 50, 70, 90 and 100%), dried on a critical point apparatus (Leica, model CPDO30) and metallized with a thin layer of gold (Deton Vacuum Desk IV Standard model). The roots were analyzed by a JEOL Scanning Electron Microscope JSM, model 6390LV (Chin-A-Woeng et al. 1997).
Evaluation of plant propagule germination and biomass
Propagule germination was evaluated at the end of the experiment by the presence and development of the root system. Fresh and dry weights were determined, with dry weight evaluated after stabilization of dry mass after oven drying at 80 °C.
Statistical analyses
The analysis of variance (ANOVA) and Tukey’s test were applied to assess the weights of the fresh and dry plants. Assumptions of normality and homogeneity of variances were met in the Shapiro–Wilk and F tests, respectively. Statistical tests were performed using the Assistat software version 7.7 (2015).
Results and discussion
Biosurfactant production
The emulsification index found for Pseudomonas aeruginosa was 59.2% after 24 h. The produced biosurfactant was able to emulsify substrates containing n-alkanes and aromatic compounds (Barathi and Vasudevan 2001). Bacteria producing biosurfactants improve the bioremediation process by reducing the surface tension between hydrocarbons and water and by aiding carbon source degradation by bacterial cells (Ayed et al. 2015; Zhang and Miller 1994), which increases the transfer of hydrocarbons to the aqueous phase and optimizes the biodegradation process.
PAH removal analysis
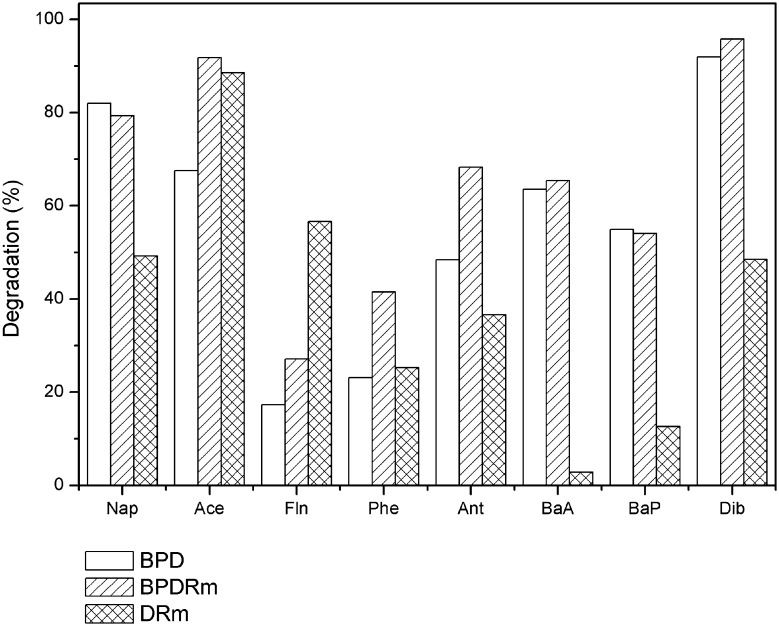
Of the eight PAHs evaluated in diesel oil (Fig. 1), the compounds that showed the greatest decrease after 120 days in all treatments were naphthalene (Nap), acenaphthene (Ace), benzo(a)anthracene (BaA), benzo(a)pyrene (BaP) and dibenzo(a,h)anthracene (Dib). The treatments containing the bacterial strains reached higher rates of degradation for these compounds compared to the treatment containing only the plant. According to Cerniglia (1993), gram-negative and gram-positive bacteria isolated from soil may have the metabolic capacity to use HPAs, such as naphthalene, phenanthrene and anthracene, as energy sources. HPA-degrading bacteria use cometabolism, mainly for molecules with three fused benzene rings or more, as in the case of benzo(a)anthracene and benzo(a)pyrene observed in this study.
Fig. 1.
PAH degradation in diesel oil after BPD, BPDRm and DRm treatment for 120 days
The bacterial strains from the rhizosphere environment interacted with Rhizophora mangle L. in the BPD and BPDRm treatments and provided a better rate removal of naphthalene from the sediment than the DRm treatment. The effectiveness in removing naphthalene can be attributed to the low molecular weight and simple structure of the hydrocarbon and the low complexity catabolic pathways, which can be used as a carbon source by microorganisms (Nwinyi et al. 2016).
In our study, fluorene (Fln) 165.34 µg g−1, phenanthrene (Phe) 745.96 µg g−1 and anthracene (Ant) 105.52 µg g−1 concentrations in the DRm treatment were lower when compared to the diesel oil 381.48 µg g−1 (Fln), 997.56 µg g−1 (Phe), 166.62 µg g−1 (Ant) and 111.12 µg g−1 (Pyr). The observed decrease in acenaphthene (Ace) (89%) and fluorene (Fln) (57%) in the DRm treatment demonstrates that Rhizophora mangle L. was efficient in the degradation of these compounds. In accordance with these results, Moreira et al. (2011) performed a comparative study between phytoremediation techniques using R. mangle L. and intrinsic bioremediation, with the aim of evaluating the effectiveness of these techniques in the removal of total petroleum hydrocarbons (TPHs) in mangrove sediment contaminated with residual oil. The authors obtained a degradation rate of 87% of total petroleum hydrocarbons when using R. mangle L., while the intrinsic bioremediation was able to remove 70% of TPHs after 90 days.
The mechanisms used by plants to remove compounds from the sediment may occur through stimulation of the microbiota present in their rhizosphere or through the absorption of organic compounds and their subsequent degradation by phytoremediation processes, such as phytovolatilization, where the compound is transformed into a less toxic substance and released into the atmosphere through the leaves, or phytodegradation, where the organic compounds are degraded by enzymatic activity (Arslan et al. 2017). However, a limiting factor in HPA degradation in mangrove sediment is salinity. The degradation rate of PAHs can be reduced in the presence of high salinity, especially when bacteria are not applied in a consortium (Tam et al. 2002).
Plant exudates are released by roots and modulate rhizosphere communities, which improves the stress response of the microorganisms (Kuiper et al. 2004). The ability of rhizobacteria to metabolize aromatic compounds, such as PAHs, is attributed to the presence of aromatic compounds in root exudates, such as phenolic compounds, whose structure stimulates bacterial metabolic capacity (Ely and Smets 2017). In addition, the efficacy of the degradation of PAHs by rhizosphere bacteria is related to soil properties, which influence the bioavailability of these compounds (Haritash and Kaushik 2009).
In the BPD and BPDRm treatments containing the evaluated bacterial strains, 82% and 79% naphthalene degradation, respectively, was observed, while the naphthalene degradation rate was 49% in the DRM treatment. The study by Zhang et al. (2013) obtained 76–80% degradation of diesel in the rhizosphere of indigenous estuarine plants associated with bacteria degrading petroleum. According to the authors, rhizosphere bacteria use organic compounds produced by plants to increase biomass and metabolic activity. Lower rates for naphthalene removal have been reported by Kong et al. (2018) in mesocosm with bacterial bioassay and bioaugmentation in association with phytoremediation after 175 days of experimentation, with removal rates of 35% and 52%, respectively.
The degradation of naphthalene using bacteria has been widely investigated, as in the study conducted by Pathak et al. (2009) using Pseudomonas spp., where a degradation of up to 2000 ppm of naphthalene was observed. Lin et al. (2010) achieved a 99.1% removal rate for naphthalene with Bacillus fusiformis. Cerqueira et al. (2011) evaluated the degradation of saturated and aromatic hydrocarbons present in mineral saline containing a sludge oil bacterial consortium by applying Bacillus, Pseudomonas and Stenotrophomonas alone and in a consortium. In the latter study, the strains, when tested in isolation, showed removal rates of the aromatic fraction ranging from 33.2 to 64.3%. When tested in a consortium, the strains reached a 51.8% removal rate of the aromatic hydrocarbons. As observed in this study, naphthalene was also the fraction that had the highest degradation rates. Similar to that observed for naphthalene and phenanthrene degradation in the present study, better degradation rates of naphthalene, phenanthrene, fluoranthene and pyrene in soil inoculated with the bacterial species Micrococcus luteus and Kocuria rosea were reported by Haritash and Kaushik (2016).
The decreases in aromatic compound concentrations in the bacterial treatments are correlated with the bioavailability of the biosurfactants produced by the bacteria (Sharma et al. 2015; Xia et al. 2014). In situ biosurfactant production is economically important in the bioremediation process, emulsification and the use of the contaminant as a carbon source. Bezza and Chirwa (2016) observed increased rates of PAH biodegradation in soil supplemented with nutrients and inoculated with Pseudomonas aeruginosa and Bacillus subtilis biosurfactant-producing species.
Physiological biosurfactant functions have been reported, such as emulsification and solubilization of oil or water insoluble compounds, hydrocarbon transport, regulation of adhesion-release of cell surfaces and antibiotic activity, conferring a greater chance of survival and competitiveness to the microorganisms (DesaI and Banat 1997; Fiechter 1992).
Pseudomonas species have genes related to the metabolism of naphthalene designated the nah genes (Isaac et al. 2015b). The nah genes encode the dioxygenases and dehydrogenases responsible for the cleavage of the aromatic ring of naphthalene. Some genes, such as nah7, are organized into two operons, one operon is responsible for the conversion of naphthalene to salicylic acid and another operon converts salicylic acid to pyruvate and then to acetyl-CoA via the β-oxidative pathway (Izmalkova et al. 2013).
Enzymes that participate in the naphthalene metabolism pathway in bacteria are classified as naphthalene oxygenases and mediate phenanthrene oxidation. The enzymatic complex naphthalene oxygenase comprises NADH oxidoreductase, ferredoxin and oxygenases (Bamforth and Singleton 2005; Tomás-Gallardo et al. 2014). Some bacteria, such as those belonging to the genus Pseudomonas, oxidize phenanthrene by generating naphthalene-1-dihydrodiol molecules, resulting in the intermediate phthalic acid, which is oxidized to acetyl-CoA (Chebbi et al. 2017).
Germination and plant growth
The results of the Rhizophora mangle L. propagule germination are displayed in Table 2.
Table 2.
Rooting (%) of Rhizophora mangle L. propagules after 120 days
| Treatment | Number of propagules | Germination percentage (%) |
|---|---|---|
| Rm | 18 | 100.00 |
| BPDRm | 18 | 55.50 |
| DRm | 18 | 22.20 |
During the Rm treatment, all propagules germinated, with the plants displaying developed leaves and roots (Fig. 2a), which demonstrated Rhizophora mangle L. adaptability to ex situ cultivation. Although leaves and a root system with a higher level of complexity were not observed in the BPDRm treatment (Fig. 2b) as they were in the Rm treatment, the BPDRm treatment resulted in 10 germinated propagules, corresponding to 55.5% of the total. This did not occur for the DRm treatment, in which 22.2% of germinated propagules were observed. Maila and Cloete (2002) obtained a reduction in the germination of Lepidium sativum seeds in soil contaminated with diesel oil. The increase in PAH concentration reduced seed germination potential, reaching 16% germination in 1000 ppm of diesel oil and 75% germination in 50 ppm.
Fig. 2.
Rooting and germination of Rhizophora mangle L. propagules. Rm (a), BPDRm (b) and DRm (c)
Hydrocarbon compounds may cause phytotoxicity due to the volatile components present in the mixture, which can move through the cell membrane and produce toxic effects, and their hydrophobicity, which may hinder the infiltration of water and aeration necessary for plant growth (Fernández et al. 2011).
The inoculated rhizobacteria induced propagule germination and root colonization and increased plant survival by decreasing water stress caused by plant contact with diesel oil in the Rm treatment (Fig. 2b). Plants in the DRm treatment had a highly branched root system (Fig. 2c), increasing the efficiency of oxygen and water transport in hypoxic conditions (Visser et al. 1997). Similar results were reported by Kechavarzi et al. (2007) when evaluating the behavior of Lolium perenne roots before different degrees of contamination by diesel oil. These authors indicated that plant roots did not grow in depth and tended to spread horizontally in the presence of increased contamination and that, by decreasing the concentration of the contaminant, the effects on the plant were minimized.
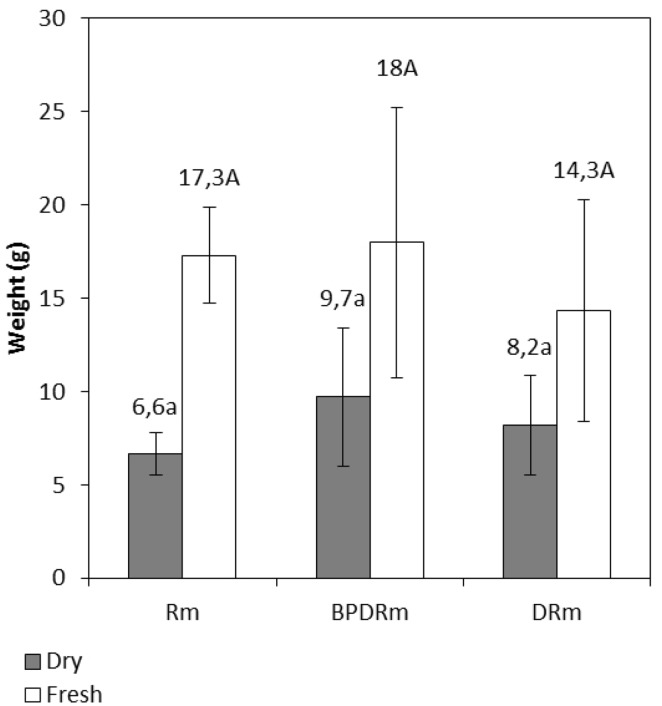
Although Rm plants displayed better development, no significant difference in fresh and dry plant biomass was observed between the Rm treatment and treatments containing diesel oil (Fig. 3).
Fig. 3.
The dry and fresh weights of Rhizophora mangle L. plants after 120 days. Data are expressed as the mean ± standard error. When the means are followed by at least one letter in the column, they do not differ from each other by the Tukey test (P > 0.05)
The plants in the Rm treatment were better developed when than the plants in the treatments in which the sediment was contaminated with diesel oil. This can be attributed to a better efficiency of water and nutrient collection due to their higher availability, since the intrinsic characteristics of the sediment are maintained when there is no contamination.
The fresh and dry weight values of the plants in the BPDRm treatment were the highest among all treatments, and the rhizobacteria Bacillus sp. and Pseudomonas aeruginosa are the probable contributors to this increased biomass production. This is in accordance with the results obtained by Hou et al. (2015), in which an increase in the biomass of the plant Festuca arundinacea L. was observed, as well as PAH degradation during phytoremediation treatments using bacteria with plant growth-promoting activity and biosurfactant production in oil-contaminated soil. The authors attribute the increase in the biomass of the roots to the activity of the rhizobacteria. Root biomass is associated with the availability of nutrients, providing favorable conditions for the growth and maintenance of hydrocarbon-degradation activity by bacteria due to plant–bacteria ratios.
Root scanning electron microscopy
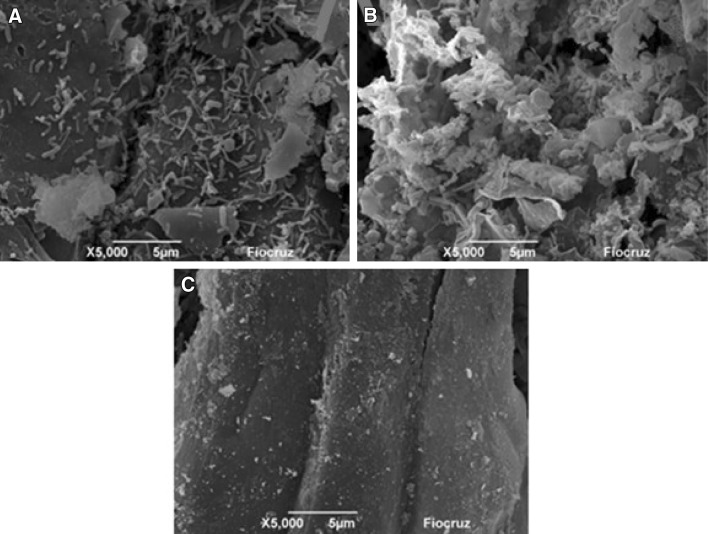
Root analysis by SEM demonstrated bacterial colonization of the plants in the BPDRm treatment (Fig. 4), including the presence of sporulating bacilli, which can characteristically become resistance to certain environmental pressures (Danhorn and Fuqua 2007). The occurrence of biofilms in the roots in the BPDRm treatment is thus a form of resistance to the adverse conditions of the environment caused by PAH contamination.
Fig. 4.
Scanning electron microscopy images from the root epidermis of plants from the BPDRm and Rm treatments showing a bacterial colonization in the BPDRm treatment; b biofilm formation in the BPDRm treatment; and c the Rm-treatment surface without bacterial colonization
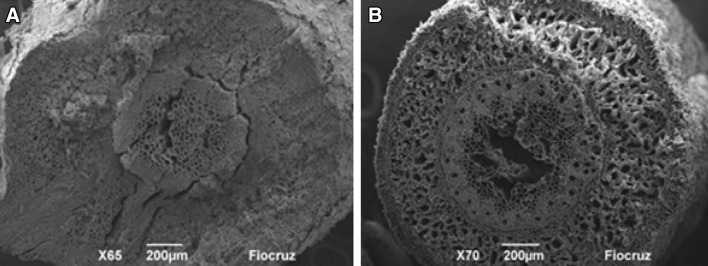
The Rm-treatment plants showed no obstruction in the cortex and central cylinder region of the roots. However, BPDRm plants showed obstructions of the pericycle, parenchymal and cortex cells caused by the deposition of the diesel oil compounds (Fig. 5). Al-Baldawi et al. (2015) also observed cellular deformation and diesel oil deposition in different regions of Scirpus grossus plants, demonstrating the toxic effects of this contaminant.
Fig. 5.
Scanning electron microscopy image showing a the obstruction of the cortex region and central cylinder of the root of a plant in the BPDRm treatment; and b the cortex and central cylinder of the root of a plant in the Rm treatment without obstruction
Low molecular weight PAHs are more easily absorbed by plant roots, while high molecular weight aromatics tend to be trapped in the sediment because of their low solubility in water (Whang et al. 2012). The presence of aerenchyma tissue in mangrove plants facilitates oil penetration (Naidoo 2016) and the deposition of hydrocarbons into plant tissues. In addition to reducing the gas exchange capacity of the mangrove plants by reducing the porosity of the sediment, the presence of hydrocarbons also blocks tissues and prevents root tissues from excluding salt, which increases the sodium concentrations in the plant tissues (Chindah et al. 2008).
Similar results were reported by Farias et al. (2009), who observed an increase in intercellular spaces and compacted root cortex cells of cultivated plants in oil-contaminated soil. Despite the occurrence of oil deposition in the root tissues, the germination and PAH degradation results indicate that rhizobacteria played an important role in preventing the adverse toxicological effects caused by diesel oil in Rhizophora mangle L. and in hydrocarbon degradation. Thus, interactions between plants and bacteria show potential for use in bioremediation processes, depending on the efficient colonization of the roots and internal tissues of the plant by the bacteria, as described by Khan et al. (2013).
Conclusions
Rhizophora mangle L. demonstrated good adaptability to ex situ cultivation conditions, allowing further studies with this plant as a phytoremediation model. The bacterial strains applied in this study, Bacillus sp. and Pseudomonas aeruginosa, demonstrated efficient root colonization of Rhizophora mangle L., conferred protection and stimulated propagule germination and degraded PAH in sediment. Thus, these bacterial strains demonstrate potential for use in bioaugmentation and phytoremediation studies and for further studies aiming at elucidating the mechanisms involved in plant–rhizobacteria interactions concerning diesel-contaminated sediment.
Acknowledgements
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel-CAPES for the financial support in this study.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Al-Baldawi IA, Abdullah SRS, Anuar N, Suja F, Mushrifah I. Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecol Eng. 2015;74:463–473. [Google Scholar]
- Alongi DM. Present state and future of the world’s mangrove forests. Environ Conserv. 2002;29:331–349. [Google Scholar]
- Arslan M, Imran A, Khan QM, Afzal M. Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ Sci and Pollut Res. 2017;24:4322–4336. doi: 10.1007/s11356-015-4935-3. [DOI] [PubMed] [Google Scholar]
- Ayed HB, Jemil N, Maalej H, Bayoudh A, Hmidet N, Nasri M. Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int Biodeterior Biodegrad. 2015;99:8–14. [Google Scholar]
- Bamforth SM, Singleton I. Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol. 2005;80:723–736. [Google Scholar]
- Barathi S, Vasudevan N. Utilization of petroleum hydrocarbons by Pseudomonas aeruginosa isolated from a petroleum-contaminated soil. Environ Int. 2001;26:413–416. doi: 10.1016/s0160-4120(01)00021-6. [DOI] [PubMed] [Google Scholar]
- Bezza FA, Chirwa EMN. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAH’s) in creosote contaminated soil. Chemosphere. 2016;144:635–644. doi: 10.1016/j.chemosphere.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Boizard SD, Mitchell SJ. Resistance of red mangrove (Rhizophora mangle L.) seedlings to deflection and extraction. Trees. 2011;25:371–381. [Google Scholar]
- Borja A, Basset A, Bricker S, Dauvin J, Elliot M, Harrison T, Marques JC, Weisberg S, West R. Classifying ecological quality and integrity of estuaries. Treat Est Coast Sci. 2011;1:125–162. [Google Scholar]
- Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4:331–338. [Google Scholar]
- Cerqueira VS, Hollenbach EB, Maboni F, Vainstein MH, Camargo FAO, Peralba MCR, Bento FM. Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol. 2011;102:11003–11010. doi: 10.1016/j.biortech.2011.09.074. [DOI] [PubMed] [Google Scholar]
- Chebbi A, Hentati D, Zaghden H, Baccar N, Rezgui F, Chalbi M, Sayadi S, Chamkha M. Polycyclic aromatic hydrocarbon degradation and biosurfactant production by a newly isolated Pseudomonas sp. strain from used motor oil-contaminated soil. Int Biodeterior Biodegrad. 2017;122:128–140. [Google Scholar]
- Chin-A-Woeng TFC, de Priester W, van der Bij AJ, Lugtenberg BJJ. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365, using scanning electron microscopy. Mol Plant Microbe Interact. 1997;10:79–86. [Google Scholar]
- Chindah AC, Braide SA, Amakiri JO, Onokurhefe J. Effect of crude oil on the development of white mangrove seedlings (Avicennia germinans) in the Niger delta, Nigeria. Biologia. 2008;30:77–90. [Google Scholar]
- Colares GB, Melo VMM. Relating microbial community structure and environmental variables in mangrove sediments inside Rhizophora mangle L habitats. Appl Soil Ecol. 2013;64(171):177. [Google Scholar]
- Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annu Rev Microbiol. 2007;61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- Das M, Das SK, Mukherjee RK. Surface active properties of the culture filtrates of a Micrococcus species grown on n-alkanes and sugars. Bioresour Technol. 1998;63:231–235. [Google Scholar]
- Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke NC, Allen JA. Rhizophora mangle, R. samoensis, R. racemosa, R. x harrisonii (Atlantic–East Pacific red mangroves) Species Profiles for Pac Isl Agrofor. 2006;1:1–18. [Google Scholar]
- Ely CS, Smets BF. Bacteria from wheat and cucurbit plant roots metabolize PAHs and aromatic root exudates: implications for rhizodegradation. Int J Phytoremed. 2017;19:877–883. doi: 10.1080/15226514.2017.1303805. [DOI] [PubMed] [Google Scholar]
- Farias V, Maranho LT, Vasconcelos EC, Filho MAS, Lacerda LG, Azevedo JAM, Pandey A, Soccol CR. Phytodegradation potential of Erythrina crista-galli L., Fabaceae, in petroleum-contaminated soil. Appl Biochem Biotechnol. 2009;157:10–22. doi: 10.1007/s12010-009-8531-1. [DOI] [PubMed] [Google Scholar]
- Fernández MD, Pro J, Alonso C, Aragonese P, Tarazona JV. Terrestrial microcosms in a feasibility study on the remediation of diesel-contaminated soils. Ecotoxicol Environ Saf. 2011;74:2133–2140. doi: 10.1016/j.ecoenv.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Fiechter A. Biosurfactants, moving toward industrial application. Trends Biotechnol. 1992;10:208–217. doi: 10.1016/0167-7799(92)90215-h. [DOI] [PubMed] [Google Scholar]
- Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater. 2009;169:1–15. doi: 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Haritash AK, Kaushik CP. Degradation of low molecular weight polycyclic aromatic hydrocarbons by microorganisms isolated from contaminated soil. Int J Environ Sci. 2016;6:472–482. [Google Scholar]
- Hou J, Liu W, Wang B, Wang Q, Luo Y, Franks AE. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere. 2015;138:592–598. doi: 10.1016/j.chemosphere.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Huang XD, El-Alawi Y, Gurska J, Glick BR, Greenberg BM. A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem J. 2005;81:139–147. [Google Scholar]
- Isaac P, Martínez FL, Bourguignon N, Sánchez LA, Ferrero MA. Improved PAHs removal performance by a defined bacterial consortium of indigenous Pseudomonas and actinobacteria from Patagonia, Argentina. Int Biodeterior Biodegrad. 2015;101:23–31. [Google Scholar]
- Isaac P, Lozada M, Diosini HM, Estévez MC, Ferrero MA. Differential expression of the catabolic nahAc gene and its effect on PAH degradation in Pseudomonas strains isolated from contaminated Patagonian coasts. Int Biodeterior Biodegrad. 2015;105:1–6. [Google Scholar]
- Izmalkova TY, Sazonova OI, Nagornih MO, Sokolov SL, Kosheleva IA, Boronin AM. The organization of naphthalene degradation genes in Pseudomonas putida strain AK5. Res Microbiol. 2013;164:244–253. doi: 10.1016/j.resmic.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Kechavarzi C, Pettersson K, Leeds-Harrison P, Richie L, Ledin S. Root establishment of perennial ryegrass (L. perenne) in diesel contaminated subsurface soil layers. Environ Pollut. 2007;145:68–74. doi: 10.1016/j.envpol.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Khan S, Afzal M, Iqbal S, Khan QM. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere. 2013;90:1317–1332. doi: 10.1016/j.chemosphere.2012.09.045. [DOI] [PubMed] [Google Scholar]
- Kong F, Sun G, Liu Z. Degradation of polycyclic aromatic hydrocarbons in soil mesocosms by microbial/plant bioaugmentation: performance and mechanism. Chemosphere. 2018;198:83–91. doi: 10.1016/j.chemosphere.2018.01.097. [DOI] [PubMed] [Google Scholar]
- Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg JJ. Rhizoremediation: a beneficial plant-microbe interaction. Mol Plant Microbe Interact. 2004;17:6–15. doi: 10.1094/MPMI.2004.17.1.6. [DOI] [PubMed] [Google Scholar]
- Lang FS, Destain J, Delvigne F, Druart P, Ongena M, Thonart P. Characterization and evaluation of the potential of a diesel-degrading bacterial consortium isolated from fresh mangrove sediment. Water Air Soil Pollut. 2016;227:58–77. [Google Scholar]
- Lin C, Gan L, Chen ZL. Biodegradation of naphthalene by strain Bacillus fusiformis (BFN) J Hazard Mater. 2010;182:771–777. doi: 10.1016/j.jhazmat.2010.06.101. [DOI] [PubMed] [Google Scholar]
- Maila MP, Cloete TE. Germination of Lepidium sativum as a method to evaluate polycyclic aromatic hydrocarbons (PAHs) removal from contaminated soil. Int Biodeterior Biodegrad. 2002;50:107–113. [Google Scholar]
- Moreira ITA, Oliveira OMC, Triguis JA, Santos AMP, Queiroz AFS, Martins CMS, Silva CS, Jesus RS. Phytoremediation using Rhizophora mangle L. in mangrove sediments contaminated by persistent total petroleum hydrocarbons (TPH’s) Microchem J. 2011;99:376–382. [Google Scholar]
- Mucha AP, Almeida CMR, Magalhães CM, Vasconcelos MTSD, Bordalo AA. Salt marsh plant–microorganism interaction in the presence of mixed contamination. Int Biodeterior Biodegrad. 2011;65:326–333. [Google Scholar]
- Naidoo G. Mangrove propagule size and oil contamination effects: does size matter? Mar Poll Bull. 2016;110:362–370. doi: 10.1016/j.marpolbul.2016.06.040. [DOI] [PubMed] [Google Scholar]
- Nwinyi OC, Ajayi OO, Amund OO. Degradation of polynuclear aromatic hydrocarbons by two strains of Pseudomonas. Braz J Microbiol. 2016;47:551–562. doi: 10.1016/j.bjm.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orge MDR, Porsché IJ, Costa MC, Lima JS, Soares SED, Justino R. Assessment of oil refinery waste on Rhizophora mangle L. seedling growth in mangroves of Todos os Santos Bay, Bahia, Brazil. Aquat Ecosyst Health Manag. 2000;3:471–477. [Google Scholar]
- Pathak H, Kantharia D, Malpani A, Madamwar D. Naphthalene degradation by Pseudomonas spp. HOB1: in vitro studies and assessment of naphthalene degradation efficiency in simulated microcosms. J Hazard Mater. 2009;166:1466–1473. doi: 10.1016/j.jhazmat.2008.12.074. [DOI] [PubMed] [Google Scholar]
- Sánchez-Arias LE, Remolina DA, Alvarez-León R. Evaluation of a recovery technique for mangrove soils affected by oil spills, using as indicator plantules of Rhizophora mangle L. (Rhizophoraceae) Panam J Aquat Sci. 2013;8:79–88. [Google Scholar]
- Santos HF, Carmo FL, Paes JES, Rosado AS, Peixoto RS. Bioremediation of mangroves impacted by petroleum. Water Air Soil Pollut. 2011;216:329–350. [Google Scholar]
- Schaeffer-Novelli Y. Manguezal: ecossistema entre a terra e o mar. São Paulo: Caribbean Ecological Research; 1995. [Google Scholar]
- Sharma D, Ansari MJ, Al-Ghamdi A, Adgaba N, Khan KA, Pruthi V, Al-Waili N. Biosurfactant production by Pseudomonas aeruginosa DSVP20 isolated from petroleum hydrocarbon-contaminated soil and its physicochemical characterization. Environ Sci Pollut Res. 2015;22:17636–17643. doi: 10.1007/s11356-015-4937-1. [DOI] [PubMed] [Google Scholar]
- Singh SN, Kumari B, Upadhyay SK, Mishra S, Kumar D. Bacterial degradation of pyrene in minimal salt medium mediated by catechol dioxygenases: enzyme purification and molecular size determination. Bioresour Technol. 2013;133:293–300. doi: 10.1016/j.biortech.2013.01.068. [DOI] [PubMed] [Google Scholar]
- Tam NFY, Guo CL, Yau WY, Wong YS. Preliminary study on biodegradation of phenanthrene by bacteria isolated from mangrove sediments in Hong Kong. Mar Pollut Bull. 2002;45:1–12. doi: 10.1016/s0025-326x(02)00108-x. [DOI] [PubMed] [Google Scholar]
- Tomás-Gallardo L, Gómez-Álvarez H, Santero E, Floriano B. Combination of degradation pathways for naphthalene utilization in Rhodococcus sp. strain TFB. Microb Biotechnol. 2014;7:100–113. doi: 10.1111/1751-7915.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S, Ansari AA. Molecular mechanisms in the phytoremediation of heavy metals from coastal waters. Phytoremed Manag Environ Contam. 2015;2:219–231. [Google Scholar]
- US Environmental Protection Agency (1984) Health Effects Assessment for Polycyclic Aromatic Hydrocarbons (PAH). Environmental Criteria and Assessment Office. EPA 540/1-86-013, Washington DC
- US Environmental Protection Agency (US EPA) (1982) Appendix A to PART 423-126 priority pollutants
- US Environmental Protection Agency (US EPA) EPA 3630C Silica gel cleanup. Revision 3. Washington, DC: US EPA; 1996. [Google Scholar]
- US Environmental Protection Agency (US EPA) EPA 3550C Ultrasonic extraction. Revision 2. Washington, DC: US EPA; 2007. [Google Scholar]
- Visser EJW, Nabben RHM, Blom CWPM, Voesenek LACJ. Elongation by primary lateral roots and adventitious roots during conditions of hypoxia and high ethylene concentration. Plant Cell Environ. 1997;20:647–653. [Google Scholar]
- Whang Z, Liu Z, Yang Y, Li T, Liu M. Distribution of PAHs in tissues of wetland plants and the surrounding sediments in the Chongming wetland, Shanghai, China. Chemosphere. 2012;89:221–227. doi: 10.1016/j.chemosphere.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Xia W, Du Z, Cui Q, Dong H, Wang F, He P, Tang Y. Biosurfactant produced by novel Pseudomonas spp. WJ6 with biodegradation of n-alkanes and polycyclic aromatic hydrocarbons. J Hazard Mater. 2014;276:489–498. doi: 10.1016/j.jhazmat.2014.05.062. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Miller RM. Effect of a Pseudomonas rhamnolipids biosurfactant on cell hydrophobicity and biodegradation of octadecane. Appl Environ Microbiol. 1994;60:2101–2106. doi: 10.1128/aem.60.6.2101-2106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Whang Z, Liu X, Hu X, Liang X, Hu Y. Degradation of diesel pollutants in Huangpu-Yangtze River estuary wetland using plant-microbe systems. Int Biodeterior Biodegrad. 2013;76:71–75. [Google Scholar]
- Zhuang X, Chen J, Shim H, Bai Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int. 2007;33:406–413. doi: 10.1016/j.envint.2006.12.005. [DOI] [PubMed] [Google Scholar]