Abstract
Seminal plasma has conventionally been viewed as a transport and survival medium for mammalian sperm; however, its role now extends beyond this process to actively targeting female tissues. Studies in rodents, swine, and humans demonstrate that seminal plasma induces molecular and cellular changes within the endometrium or cervix following insemination. Seminal-plasma-induced alterations to the maternal environment have been theorized to facilitate embryo development, modulate maternal immunity toward the conceptus, and potentially improve pregnancy success. It is unknown if bovine seminal plasma modulates the uterine environment following insemination in the cow, where routine use of artificial insemination reduces maternal exposure to seminal plasma. We hypothesize that seminal plasma modulates the expression of inflammatory mediators in the endometrium, altering the maternal environment of early pregnancy. In vitro, seminal plasma altered intact endometrial explant expression of CSF2, IL1B, IL6, IL17A, TGFB1, IFNE, PTGS2, and AKR1C4. Furthermore, endometrial epithelial cell CSF2, CXCL8, TGFB1, PTGS2, and AKR1C4 expression were increased after seminal plasma exposure, while endometrial stromal cell CSF2, IL1B, IL6, CXCL8, IL17A, TGFB1, PTGS2, and AKR1C4 expression were increased following seminal plasma exposure. Endometrial expression of IL1B was increased in the cow 24 h after uterine infusion of seminal plasma, while other evaluated inflammatory mediators remained unchanged. These data indicate that seminal plasma may induce changes in the bovine endometrium in a temporal manner. Understanding the role of seminal plasma in modulating the maternal environment may aid in improving pregnancy success in cattle.
Keywords: seminal plasma, endometrium, endometrial epithelium, endometrial stroma, inflammation, insemination
Exposure of endometrial cells to seminal plasma alters gene expression of inflammatory mediators, reminiscent of the postcoital inflammatory response in other species.
Introduction
Seminal plasma (SP) is conventionally thought to have a single purpose, a survival medium for spermatozoa during carriage to the oocyte. However, the role of SP is now recognized to extend beyond this process to also optimize reproductive outcomes by targeting the female reproductive tract [1]. The periconceptional and embryo implantation phase of early pregnancy is a vulnerable period of the reproductive process [2, 3]. In livestock species, disruptions to the maternal environment and capacity to support conceptus development are implicated in constraining fertility [4, 5]. The cellular and molecular environment of the uterus during the peri-implantation period of early pregnancy is critical for implantation success and optimal fetal and placental development. In most mammalian species, it has been proven that SP is not required to achieve pregnancy, which is demonstrated by the success of artificial insemination (AI), embryo transfer, and cloning. Indeed, studies have demonstrated that in cattle AI and embryo transfer pregnancy rates are equal to or greater than natural conception [6, 7]. Despite the fact that SP is not required for successful pregnancy, several studies in multiple species indicate that pregnancy outcomes are altered if females are not exposed to SP at conception. For example, in mice, surgical removal of the seminal vesicle glands of stud males causes a reduction in subsequent pregnancy rates, due to decreased embryo implantation [1]. In dairy and beef cattle, infusion of SP at the time of AI increases pregnancy rates by 4.6% and 6.7%, respectively, albeit not significantly [8]. However, surgical removal of the seminal vesicle gland in the bull maintains a typical 69% conception rate [9]. Studies in rodents, swine, equine, and humans show that SP induces numerous changes within the endometrium or cervix [10–19]. Studies in various species have described SP-induced changes of endometrial CSF2, IL6, CXCL8, IL17A, and PTGS2 [10–19]. These changes are proposed to occur by activating a cascade of cytokine and leukocyte-mediated events induced by active molecules present in SP. Epithelial cytokines induced by SP exposure exert embryotrophic actions on the developing pre-implantation embryo and modulate maternal immunity toward the conceptus [20–23]. In the human and rodent, SP-derived transforming growth factor beta (TGFβ) has been demonstrated to be one of the active molecules within the ejaculate to facilitate these maternal tract changes [24, 25]. Herein, we hypothesize that exposure of bovine endometrial tissue to SP will modulate the expression of inflammatory mediators and alter the maternal environment of early pregnancy. We have employed in vitro and in vivo exposure of bovine endometrial tissue to semen components to better understand the impact of SP on modulating the environment of the maternal reproductive tract. We have measured targeted genes known to be regulated by SP in other species, in addition to novel factors involved in inflammation of the endometrium (IFNE) or regulation of ovarian function (OXTR). These studies are an important addition to our understanding of SP’s role in modulating the female reproductive tract environment of early pregnancy, particularly in domestic species such as cattle where SP is diluted during AI, potentially removing any paternally derived effects on the maternal tissues.
Materials and methods
All reagents were acquired from Fisher Scientific (Waltham, MA) unless otherwise stated.
Animal use and care
All animals were housed at the University of Florida, North Florida Research and Extension Center (NEFREC). All procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Semen and seminal plasma collection
Whole semen was collected from Angus bulls during routine breeding soundness evaluation. Whole semen was collected by electroejaculation from healthy bulls, evaluated and only processed further if the sample was free of blood, urine, and any other visual anomalies. Parameters pertaining to the breeding soundness evaluation were recorded for each individual sample; including volume, sperm motility, scrotal circumference, collection date, and bull ID. Collected semen volume ranged from 7.5 to 15 ml, gross motility ranged from 10% to 90%, and scrotal circumference ranged from 31 to 49 cm. Following collection, whole semen was placed on ice and transported to the laboratory for processing under aseptic conditions. A 200 μl aliquot of whole semen was placed into a sterile microcentrifuge tube and stored at –20°C. The remaining ejaculate was centrifuged at 1000 × g for 10 min at room temperature (RT) to facilitate collection of cell free SP and the ejaculate cell pellet. Cell-free SP was transferred to new labeled tubes in aliquots of 500–1000 μl and stored at –20°C until use. The remaining cell pellet was stored at –20°C.
Preparations of pooled semen, seminal plasma, and cell pellet
A whole semen pool was prepared under aseptic conditions by combining 100 μl whole semen from 11 individual bulls. Pooled whole semen was stored in 500 μl aliquots at –20°C until use. The cell pellet from individual bulls was resuspended in sterile phosphate-buffered saline (PBS) to the same volume of the original ejaculate (achieving the original ejaculates sperm concentration). The volume of cell suspension from each bull corresponding to 108 total cells was pooled and stored in 500 μl aliquots at –20°C. Ejaculates that contained a total volume of 5 ml or higher were used to generate the SP pool. The SP pool was prepared from the ejaculates of 21 individual bulls. Seminal plasma was pooled under aseptic condition by combining 1 or 2 ml of SP from each bull, and stored in 500 μl aliquots at –20°C. Each SP aliquot was used a maximum of twice before being discarded to minimize any negative effects of repeated freeze/thawing.
Preparation of female reproductive tracts for endometrial dissection
Uteri from postpubertal, nonpregnant cattle with no gross evidence of genital disease or microbial infection were collected at the local slaughterhouse (breed was not defined). Whole reproductive tracts were transported to the laboratory within approximately 3 h for further processing at RT. Reproductive tracts from a total of 36 animals were used for all experiments. The stage of the reproductive cycle (stage of estrous cycle) was determined by examination of ovarian morphology and vasculature of the corpus luteum (CL), according to Ireland et al. [26], and only reproductive tracts between days 1 and 10 of the estrous cycle were used herein. Reproductive tracts were prepared as previously described for both intact explants and semi-purified epithelial and stromal cell isolation [27]. Briefly, individual uteri were freed of surrounding fat and connective tissue and rinsed with 70% ethanol. An incision along the major curvature of the uterine horn was made to expose the endometrium ipsilateral to the CL. The exposed endometrium was washed twice with Dulbecco phosphate-buffered saline containing 50 IU/ml of penicillin, 50 μg/ml of streptomycin, and 2.5 μg/ml amphotericin B to remove mucus and potential microorganisms. Tissues were then immediately processed for isolation of intact endometrial explants or semi-purified epithelial or stromal cells (below).
Preparation of whole endometrial explants and in vitro culture
Endometrial explants were obtained from intracaruncle tissue using an 8-mm diameter biopsy punch (Stiefel Laboratories Ltd, Research Triangle Park, NC) and cut away from the underlying myometrium. Endometrial biopsies were immediately washed twice with Hank balanced salt solution (HBSS). A single biopsy was placed into a well of a 6-well culture plate (TPP, Trasadingen, Switzerland) containing 3 ml of complete culture medium (RPMI 1640 medium, 10% FBS, 2 mM of L-Glutamine, 50 IU/ml of penicillin, 50 μg/ml of streptomycin, and 2.5 μg/ml amphotericin B) with the specified treatment (below). Each experimental replicate is derived from explants of individual cows. Six independent experiments were performed for each treatment, represented by explants from six individual cows.
Isolation and culture of bovine endometrial epithelial and stromal cells
Endometrial tissue was dissected and processed as described previously by Turner et al. [28]. Briefly, thin strips of endometrium were dissected away from the underlying serosa and placed directly into HBBS containing 50 IU/ml of penicillin, 50 μg/ml of streptomycin, and 2.5 μg/ml amphotericin B. Tissue pieces were transferred into a centrifuge tube containing HBSS and placed into a water bath at 37°C. After 10 min, the sterile digestive solution (HBSS containing 100 mg BSA, 125 CDU/mg collagenase II, 250 BAEE trypsin and 4% DNase I) was added to the endometrial tissue and digested for 1 h in a shaking water bath at 37°C. The cell suspension was then filtered through a 40-μm mesh and resuspended in HBSS containing 10% FBS. The suspension was centrifuged at 700 × g for 7 min at RT and resuspended in warm complete culture medium (as above). Cells were cultured in 30 ml of equilibrated complete culture medium in 75 cm2 flasks (Greiner Bio-one, Monroe, NC) at 39°C in a humidified atmosphere of air with 5% CO2. After 18 h of culture, unattached epithelial cells in suspension were transferred to a new flask leaving semi-purified stromal cells attached. Both epithelial and stromal cells were cultured for an additional 48 h before plating at a final concentration of 1.5 × 105 cells/ml in 24-well culture plates (TPP, Trasadingen, Switzerland) at a final volume of 500 μl in complete culture medium. Cells were equilibrated for 24 h before treatment (below).
Cell culture challenge of endometrial explants or endometrial cells with semen components
Intact endometrial explants or endometrial cells were exposed to either complete culture medium alone or medium containing 1%, 2%, 5%, 10%, or 20% v/v SP; 5% v/v whole semen (SM); or 5% v/v cell pellet (CP). Treatments were added to complete culture medium according to experimental design and equilibrated at 37°C prior to exposure of explants or cells. A minimum of six replicates were performed for each treatment or time point, with each replicate representative of endometrial material from an individual cow. Following application of treatment, explants or endometrial cells were incubated at 39°C in a humidified 5% CO2 environment. Initial dose experiments using endometrial explants or cells were performed for a total of 24 h. Time course experiments of both explants and endometrial cells utilized complete culture medium alone or medium containing 5% v/v SP for 0, 15, 30, 60, 90, and 120 min. Following treatment, explants were weighed prior to bisecting and storage at –20°C in Trizol for RNA extraction. Epithelial and stromal cells cultures were stored at –20°C in Qiagen RLT lysis buffer (Qiagen, Hilden, Germany).
Synchronization and intrauterine infusion of cows
A total of 54 multiparous nonlactating Angus beef cows were randomly assigned to receive 1 ml of treatment: (1) saline, (2) conventional semen from a single sire, (3) pooled SP alone, or (4) a combination of conventional semen and SP. Each treatment was pre-loaded in 0.5 ml semen straws under aseptic conditions. Saline and SP were filter sterilized using a 0.4-μm syringe filter before loading into straws.
Estrous synchronization was performed in all cows by administering GnRH (Factrel; 100 μg gonadorelin hydrochloride; Zoetis Animal Health, Parsippany, NJ), followed by PGF2α treatment (Lutalyse; 25 mg dinoprost tromethamine; Zoetis Animal Health) 7 days later. Transrectal ultrasound (Ibex ultrasound with 5 MHz multifrequency transducer: E. I. Medical Imaging, Loveland, CO) was performed to confirm the presence and location of a dominant/ovulatory follicle at the time of PGF2α treatment and again at the time of intrauterine infusion. Treatments were administered into the uterine body 48 h after PGF2α treatment by standard AI technique. Endometrial biopsies were collected from both uterine horns, ipsilateral and contralateral to the ovulatory follicle. Briefly, animals received local anesthesia by administering 4 ml of lidocaine to the epidural space between the second and third coccygeal vertebrate. Endometrial biopsy forceps (Jorgensen Laboratories, Loveland, CO) were passed into the uterine horn, guided by rectal palpation. Once in place, the endometrium was gently pressed into the jaw of the biopsy forceps by rectal palpation and the endometrium clipped off by closing the instrument jaw. The process was repeated to facilitate the collection of endometrial tissue from both uterine horns, ipsilateral and contralateral to the dominant follicle. Endometrial biopsies were rinsed in sterile PBS, bisected into two pieces, and stored in RNAlater (Invitrogen) until RNA extraction or fixed in 10% neutral-buffered formalin. Biopsies from 43 cows with a visible dominant follicle were used for further analysis, and 11 cows were excluded from analysis as no visible dominant follicle was observed, indicating that they either ovulated prior to administration of treatments or failed to respond to synchronization of estrus.
Extraction and purification of RNA from endometrial tissues or cells
RNA was extracted from endometrial explants and biopsies using Trizol. Briefly, endometrial tissue was homogenized using a plastic pellet pestle (Kimble, Rockwood, TN). Homogenates were centrifuged at 1200 × g for 5 min at 4°C and supernatants were transferred to clean centrifuge tube. A total of 200 μl of chloroform was added to each homogenate and incubated at RT for 5 min. Samples were centrifuged at 1200 × g for 15 min at 4°C. The RNA-rich aqueous supernatant was transferred to a new tube and 500 μl of 100% isopropanol was added to the sample and incubated at RT for 10 min. Samples were centrifuged at 1200 × g for 15 min at 4°C and supernatant was removed leaving the RNA pellet. The pellet was washed with 1 ml of 75% ethanol and centrifuged at 1200 × g for 10 min at 4°C. The RNA pellet was resuspended in 50 μl of RNAase-free water. Total RNA was extracted from semi-purified endometrial cells using the RNeasy Mini kit according to the manufacturer’s instructions (Qiagen). Extracted RNA was quantified using a NanoDrop ND1000 spectrophotometer. A total of 1 μg of RNA was subjected to reverse transcription using the Verso cDNA synthesis kit according to the manufacturer’s instruction, including a genomic DNA wipe-out procedure. The recommended thermal cycling conditions for reverse transcription were one cycle of cDNA synthesis at 42°C for 30 min followed by inactivation at 95°C for 2 min.
Quantitative real-time RT-PCR
Primers were designed using the NCBI database and initial specificity verified by BLAST to ensure no cross-reactivity with other loci (Supplemental Table S1). Amplification efficiency was evaluated for each primer by performing serial dilutions of cDNA. All primers met MIQE guidelines for further use (Pearson correlation coefficient r > 0.98 and efficiency between 90% and 110%). Quantitative real-time RT-PCR was performed in 20 μl reactions using iTaq Universal SYBR green chemistry (Bio-Rad, Hercules, CA) and 100 nM of each forward and reverse primer. A Bio-Rad CFX Connect light cycler was employed to perform quantitative PCR (Bio-Rad) using a two-step protocol and the recommended thermal cycling conditions outlined below: initial denaturation/enzyme activation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and annealing/extension for 30 s at 60°C. A three-step protocol was employed for IL17A and OXTR primers with an annealing temperature of 63°C. Each PCR reaction was followed by melt curve analysis to ensure single product amplification. A no template negative control was used in place of cDNA to determine nonspecific amplification. PCR products were electrophoresed on a 1% agarose gel containing Diamond Dye (Promega, Madison, WI) and visualized under UV illumination to confirm predicted PCR product size. All cell culture data were normalized independently to ACTB mRNA expression, while in vivo expression was normalized to the arithmetic mean of ACTB and GAPDH mRNA expression using the ΔΔCt method.
Histology and immunohistochemistry of endometrial tissue
Paraffin-embedded sections were cut at 7 μm and stained using hematoxylin and eosin. Immunohistochemistry was performed to detect IL-1β (#MBS2026412; MyBioSource; San Diego, CA) using Alexafluor 488 secondary detection (Invitrogen, Carlsbad, CA) and DAPI counterstaining (Vector Labs, Burlingame, CA). Tissue sections underwent antigen retrieval using Sodium Citrate buffer (pH 6.0) in a pressure cooker for 3 min. Images were taken using a Zeiss Axioplan microscope and Axiovision software (Zeiss, Gottingen, Germany).
Statistical analysis
SPSS software V24.0 (IBM Analytics, Armonk, NY) was used for statistical analysis. Relative gene expression data was log transformed and analyzed using the generalized linear mixed model with pairwise comparisons. Treatment, dose, time, stage of estrous cycle, and uterine horn were used as fixed factors and replicate was included as a random variable. Interactions were assessed as appropriate in either dose experiments or time course experiments. Pairwise comparisons were made between individual time points or concentrations with vehicle treated controls. The stage of estrous cycle had no effect on responsiveness and was removed from the analysis. Data for individual replicates according to the stage of estrous cycle are presented is Supplemental Figure S1. Data are presented as mean + standard error of the mean. A P value of ≤ 0.05 was assumed statistically significant.
Results
The effect of semen components on ex vivo endometrial explant gene expression
To assess the capacity of semen components to modulate expression of inflammatory mediators, intact endometrial explants were exposed to either vehicle control medium alone, 2%, 5%, 10%, or 20% v/v SP, 5% v/v semen cell pellet (CP), or 5% v/v whole semen (SM) for 24 h (Figure 1).
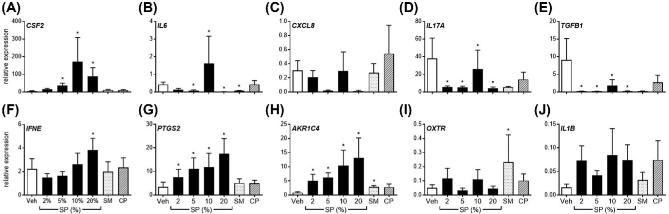
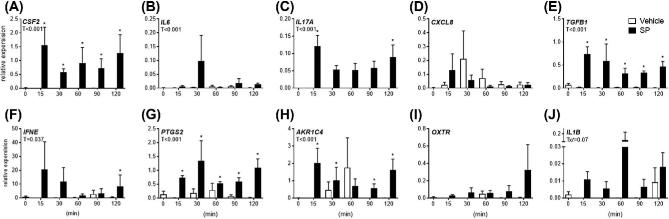
Figure 1.
Effect of semen components on endometrial explant gene expression. Intact endometrial explant expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4 OXTR, and IL1B in response to seminal plasma (SP; 2%, 5%, 10%, 20%), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (Veh) after 24 h. Data are presented as the mean relative expression + SEM. from six independent experiments using explants from six individual cows. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons only made with vehicle treated control. *, P value of ≤ 0.05.
Expression of endometrial CSF2, IFNE, PTGS2, and AKR1C4 were increased following exposure to SP compared to vehicle treated controls, whereas expression of IL6, IL17A, and TGFB1 were downregulated following exposure to SP. Interestingly, explant exposure to 5% SM only modulated IL6, AKR1C4, and OXTR expression compared to vehicle treated controls. Exposure to 5% CP had no effect on any genes measured.
Explant exposure to 10% SP maximally increased expression of CSF2 by 29.2-fold compared to vehicle treated explants (Figure 1A; P < 0.05). Exposure to 20% SP increased PTGS2 and AKR1C4 expression maximally by 5.16- and 15.0-fold compared to controls, respectively (Figure 1G and H; P < 0.05). Treatment of explants with 20% SP reduced IL6, IL17A, and TGFB1 expression by 85.7%, 89.4%, and 97.3% , respectively, compared to vehicle treated control expression, (Figure 1B, D, E; P < 0.05). On the other hand, expression of IFNE was increased by 1.7-fold following exposure to 20% SP compared to control treated explants (Figure 1F; P < 0.05). Explant expression of CXCL8 or IL1B was not modulated by any semen components (Figure 1C). The expression of IFNG was not detectable in all in vitro experiments (data not shown).
Explant viability was assessed by measuring LDH activity on supernatants after culture (Supplemental Figure S2). Minimal increases in LDH activity were observed following treatment, with the exception of a 5.6-fold increase in the 20% SP treatment group compared to uncultured medium alone. The proportion of LDH activity following treatment with 20% SP was comparable to treatment with medium containing 1% Triton X-100.
Acute effects of semen components on ex vivo endometrial gene expression
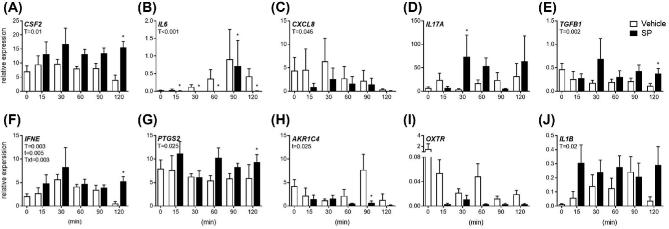
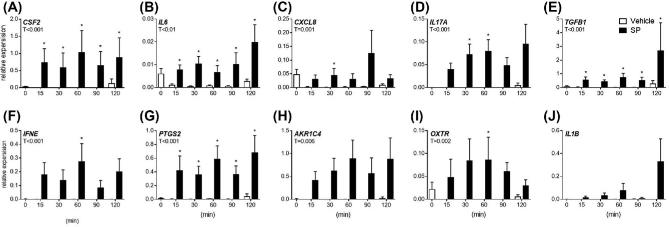
Explants were exposed to 5% SP or vehicle control medium alone for 0, 15, 30, 60, 90, and 120 min to assess acute effects of SP on gene expression (Figure 2).Treatment with SP increased CSF2, TGFB1, IFNE, PTGS2, and IL1B expression during the experimental period (Figure 2A, D, E, F, G, J; P < 0.05), while SP treatment reduced overall expression of IL6 and CXCL8 (Figure 2B and C; P < 0.05). An effect of culture time and a treatment by time interaction was observed for IFNE expression (Figure 2F; P < 0.05), while an effect of culture time was observed for PTGS2 expression (Figure 2E; P < 0.05). Specifically, treatment with SP increased CSF2, TGFB1, IFNE, and PTGS2 expression compared to vehicle treated controls at 120 min (Figure 1A, E, F, G; P < 0.05). Interestingly, expression of IL6 was reduced compared to vehicle treated controls at 15, 30, 60, 90, and 120 min (Figure 2B; P < 0.05). There was no effect of treatment on OXTR expression at any time point (Figure 2I).
Figure 2.
Acute effects of semen components on endometrial explant gene expression. Intact endometrial explant expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4 OXTR, and IL1B in response to 5% seminal plasma (SP) or control medium alone (vehicle). Gene expression was measured in terminal experiments starting at 15 min, up to 120 min. Data are presented as the mean relative expression + SEM from six independent experiments. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons made with vehicle treated control within each time point. *, P value of ≤ 0.05. T, treatment effect; t, time effect; Txt, treatment by time interaction.
The effect of semen components on semi-purified endometrial epithelial and stromal cell gene expression
Semi-purified endometrial epithelial (Figure 3) and stromal (Figure 4) cells were exposed to either vehicle control medium alone, 1%, 2%, or 5% v/v SP, 5% v/v semen cell pellet (CP), or 5% v/v whole semen (SM) for 24 h. Purity of epithelial and stromal cells was confirmed by microscopy and flow cytometry (56 ± 16% and 95 ± 3%, respectively; Supplemental Figure S3).
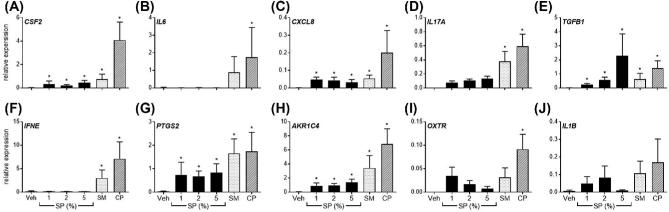
Figure 3.
Effect of semen components to alter gene expression of semi-purified endometrial epithelial cells. Semi-purified endometrial epithelial cell expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4 OXTR, and IL1B in response to seminal plasma (SP; 1%, 2%, 5%), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (Veh) after 24 h. Data are presented as the mean relative expression + SEM from six independent experiments. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons made with vehicle treated control. *, P value of ≤ 0.05.
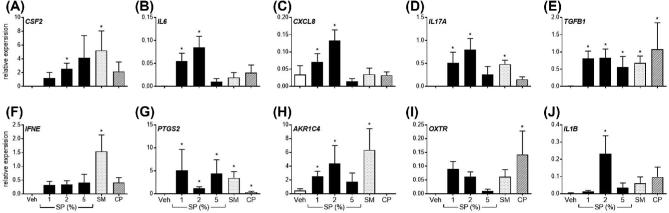
Figure 4.
Effect of semen components to alter gene expression of semi-purified endometrial stromal cells. Semi-purified endometrial stromal cell expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4 OXTR, and IL1B in response to seminal plasma (SP; 1%, 2%, 5%), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (Veh) after 24 h. Data are presented as the mean relative expression + SEM from six independent experiments. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons made with vehicle treated control. *, P value of ≤ 0.05.
Exposure to 5% SM increased epithelial expression of CSF2, CXCL8, IL17A, TGFB1, IFNE, PTGS2, and AKR1C4 compared to vehicle treated controls (Figure 3A, C–H; P < 0.05). Interestingly, exposure of epithelial cells to 5% CP maximally increased expression of all evaluated genes compared to vehicle treated controls (Figure 3; P < 0.05), while treatment of epithelial cells with SP increased expression of CSF2, CXCL8, TGFB1, PTGS2, and AKR1C4 (Figure 3A, C, E, G and H; P < 0.05). Specifically, exposure of epithelial cells to 2% SP increased CSF2, CXCL8, TGFB1, PTGS2, and AKR1C4 expression by 9.8, 28.9, 69.3, 29.9, and 27.1-fold, respectively (P < 0.05). Epithelial cells did not modulate expression of IL1B to any semen component (Figure 3J).
Exposure to 5% SM increased stromal cell expression of CSF2, IL17A, TGFB1, IFNE, PTGS2, and AKR1C4 compared to vehicle treated controls (Figure 4A, D–H; P < 0.05). Exposure of stromal cells to 5% CP increased expression of TGFB1, PTGS2, and OXTR compared to vehicle treated controls (Figure 4E, G, I; P < 0.05), while treatment with SP increased stromal cell expression of CSF2, IL6, CXCL8, IL17A, TGFB1, PTGS2, and AKR1C4 compared to vehicle treated controls (Figure 4A–E, G, H; P < 0.05). Specifically, exposure of stromal cells to 2% SP increased CSF2, IL6, CXCL8, TGFB1, PTGS2, AKR1C4, and IL1B expression by 2248.0, 134.4, 2.1, 74.1, 165.4, 9.6, and 69.0-fold, respectively, compared to vehicle treated controls, respectively (P < 0.05).
Cell viability of semi-purified epithelial and stromal cells was assessed using the MTT assay following treatment for 24 h (Supplemental Figure S3D and H). Viability of epithelial cells was reduced by 76.5% of vehicle treated controls only after exposure to 20% SP. Stromal cell viability was reduced by 26.3%, 43.1%, and 35.7% of vehicle treated controls following treatment with 5%, 10%, and 20% SP, respectively.
Acute effects of semen components on semi-purified endometrial epithelial and stromal cells
Semi-purified endometrial epithelial (Figure 5) and stromal (Figure 6) cells were exposed to 5% SP or vehicle control medium alone for 0, 15, 30, 60, 90, and 120 min to assess acute effects of SP on gene expression.
Figure 5.
Acute effects of semen components on semi-purified endometrial epithelial cell gene expression. Semi-purified endometrial epithelial cell expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4 OXTR, and IL1B in response to 5% seminal plasma (SP) or vehicle medium alone (vehicle). Gene expression was measured in terminal experiments starting at 15 min, up to 120 min. Data are presented as the mean relative expression + SEM from six independent experiments. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons made with vehicle treated control within each time point. *, P value of ≤ 0.05. T, treatment effect.
Figure 6.
Acute effects of semen components on semi-purified endometrial stromal cell gene expression. Semi-purified endometrial stromal cell expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKRIC4 OXTR, and IL1B in response to 5% seminal plasma (SP) or vehicle medium alone (vehicle). Gene expression was measured in terminal experiments starting at 15 min, up to 120 min. Data are presented as the mean relative expression + SEM from six independent experiments. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons made with vehicle treated control within each time point. *, P value of ≤ 0.05. T, treatment effect.
Treatment of epithelial cells with SP increased CSF2, IL6, IL17A, TGFB1, IFNE, PTGS2, AKR1C4, and IL1B expression during the experimental period (Figure 5A, B, D–H, J; P < 0.05). Epithelial expression of CXCL8 and OXTR were unaffected by treatment (Figure 5C and I). Specifically, epithelial expression of CSF2, TGFB1, PTGS2, and AKR1C4 were elevated above vehicle treated controls starting at 15 min for the 120 min duration of the experiment (P < 0.05). There was no effect of culture time on expression of any epithelial genes assessed.
Treatment of stromal cells with SP increased expression of CSF2, IL6, CXCL8, IL17A, TGFB1, PTGS2, AKR1C4, and OXTR during the experimental period (Figure 6; P < 0.05). Expression of stromal cell CSF2, IL6, TGFB1, and PTGS2 were all increased compared to the vehicle treated controls starting at 15 min for the 120 min duration of the experiment (Figure 6A,B, E, G; P < 0.05).
Effect of intrauterine infusion of seminal plasma on endometrial gene expression
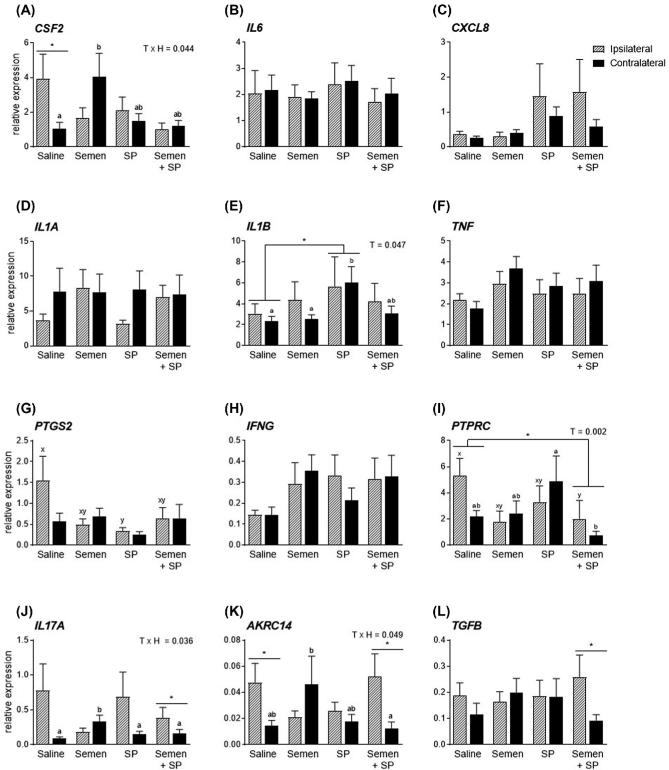
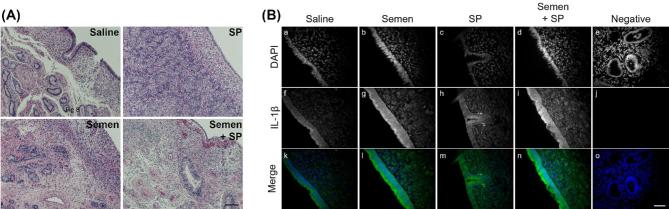
Endometrial biopsies were collected 24 h after intrauterine infusion of semen, SP, or saline in synchronized cows to determine the endometrial expression of inflammatory mediators (Figure 7). Histological evaluation of endometrial biopsies demonstrated the presence of heterogeneous cell types, including luminal and glandular epithelium, stroma, vasculature, and immune cells (Figure 8A). Endometrial expression of IL1B was increased by 2.2-fold in the SP treatment group compared to the saline infused controls (Figure 7E; P < 0.05). The effect of SP-induced IL1B expression was due mainly to increased expression in the contralateral horn where expression was increased by 2.6-fold compared to the contralateral horn of the saline infused group. Immunohistochemistry of endometrial biopsies confirmed the presence of IL-1β in endometrial tissue in all treatment groups (Figure 8B). Expression of IL-1β was concentrated in the endometrial luminal epithelium, with some immunoreactivity present in glandular epithelium and less so in the stromal tissue. A treatment effect was also observed in the semen + SP group where PTPRC (formerly CD45) expression was reduced by 63.1% (Figure 7I; P < 0.05). Reduced PTPRC expression was due primarily to a 62.0% reduction in the ipsilateral horn of the semen + SP infused group compared to the ipsilateral horn of the saline infused controls. Interestingly, PTPRC expression in the contralateral horn was elevated in the SP infused group compared to the semen + SP group (Figure 7I; P < 0.05), while expression of PTGS2 in the ipsilateral horn of saline treated cows was 4.6-fold higher than expression of the ipsilateral horn in SP treated cows (Figure 7G; P < 0.05). Expression of endometrial CSF2 in the contralateral horn was increased by 3.9-fold following semen infusion compared to the contralateral horn of saline infused cows (Figure 7A; P < 0.05), while saline infused cows had a 3.8-fold increase in CSF2 expression of the ipsilateral horn compared to the contralateral horn (Figure 7A; P < 0.05). Expression of IL17A, AKRC14, and TGFB were increased in the ipsilateral horn of the semen + SP group (Figure 7J–L; P < 0.05), while AKRC14 expression was increased in the ipsilateral horn compared to the contralateral horn of saline infused cows (Figure 7K; P < 0.05).
Figure 7.
Effect of intrauterine infusion of semen on endometrial gene expression. Endometrial biopsies were collected 24 h following intrauterine infusion of conventional semen (n = 12), pooled seminal plasma (SP; n = 11), a combination of conventional semen and SP (n = 9), or saline (n = 11) as a control. Biopsies were collected from uterine horns ipsilateral (hashed bars) and contralateral (solid bars) of the dominant follicle, identified by ultrasound the day before intrauterine infusion. Expression of CSF2, IL6, CXCL8, IL1A, IL1B, TNF, PTGS2, IFNG, and PTPRC were evaluated by real-time RT-PCR. Data are presented as the mean relative expression + SEM. Data were analyzed using repeat measures in the generalized linear mixed model with pairwise comparisons. The dam was used as the subject, treatment, horn, and treatment x horn were used as fixed effect. *, P value of ≤ 0.05. Superscripts a and b refer to differences observed between treatments within the contralateral horn; superscripts x and y refer to differences observed between treatments within the ipsilateral horn. T, treatment effect; TxH, treatment by horn interaction.
Figure 8.
Effect of intrauterine infusion of semen components on endometrial expression of IL-1β. Representative micrographs of endometrial biopsies collected 24 h following intrauterine infusion of saline, conventional semen, pooled seminal plasma (SP), or a combination of conventional semen and SP. (A) Biopsies were fixed in neural-buffered formalin, paraffin-embedded, sectioned, and H&E stained. Scale bar represents 100 μm. (B) Biopsies were fixed in neural-buffered formalin, paraffin embedded, sectioned, and stained for immunoreactive IL-1β. Separate black and white images are shown for DAPI (a–e) and IL-1β staining (f–j), in conjunction with a merged image showing DAPI in blue and IL-1β in green (k–o). A negative control (e, i, o) is shown that was not incubated with primary antibody. Scale bar represents 50 μm.
Seven days following uterine infusion, serum progesterone was evaluated and no differences were observed between the treatment groups (Supplemental Figure S4B).
Discussion
The cell-free component of semen, SP, has been demonstrated to modulate the molecular and cellular environment of the maternal reproductive tract of rodents, swine, horse, and humans [18–20, 29, 30]. The data herein describe the impact of semen, and SP specifically, in modulating the endometrial environment of the bovine. Our data suggest that while SP alters the expression of endometrial inflammatory genes in isolated culture systems, the degree of alteration in the cow endometrium 24 h after uterine infusion is significantly less.
Pregnancy in many species, including cattle can be readily achieved in the absence of SP, using IVF or AI using washed sperm. Over 10 million bovine AIs are performed each year in the USA, where SP is considerably diluted during semen extension to maximize the efficiency of a single ejaculate [31]. Studies have demonstrated that pregnancy rates from cows undergoing AI or embryo transfer in the absence of SP are at least equal to natural conception [6, 7] However, data in the rodent suggest that SP has the capacity to modulate the maternal environment and improve pregnancy outcomes, including offspring phenotype [1]. Pregnancies of mice achieved in the absence of SP result in increased embryo mortality and metabolic perturbations in resultant offspring due to a compromised uterine/oviductal environment [1]. In the bovine the addition of SP at the time of AI increases conception rates, albeit not significantly, suggesting a potential role of SP in cattle reproduction that requires further investigation [8]. Indeed, similar evidence exists in the swine and human, where SP exposure at the time of AI or embryo transfer increases conceptions rates [30, 32].
The current study indicates that whole endometrial explants increase expression of CSF2, PTGS2, and AKR1C4 following exposure to SP, but not semen cells. This is consistent with SP-induced modulation reported in humans, rodents, and swine. However, whole endometrial explants are complex tissues comprised of epithelial, stromal, endothelial, and immune cells which may mediate various responses to semen components. Thus, we assessed the capacity of semi-purified endometrial epithelial or stromal cells to respond to SP. Purification of epithelial and stromal cells in our model system was consistent with the purity achieved by others, with epithelial purity difficult to achieve above 70% purity [28]. In semi-purified cultures, the responses to SP were more pronounced than in the complex explant cultures. Both epithelial and stromal cells responded to whole semen, which is consistent with cells responding to SP alone. Interestingly, there was a greater capacity of epithelial cells to respond to semen cell pellet, compared to stromal cells. This may be a reflection of the anatomical separation of the stromal cell compartment from the luminal space where semen (and SP) may be present following insemination. Indeed, the higher degree of responsiveness of semi-purified epithelial cells compared to endometrial explants may due to the lower proportional density of epithelial cells present in explants compared to stromal and other cell types which make up the majority of explant biopsies (as described in the histological evaluation).
Previous work in rodents has demonstrated that SP exposure at insemination improves pregnancy outcomes and reduces the incidence of metabolic syndrome in offspring [1]. It is hypothesized that the mechanism of SP improving pregnancy outcomes is multifaceted, improving expression of maternal embryokines to support preimplantation development, facilitating tissue remodeling for implantation and placental development, and finally modulation of the maternal immune response to the semi-allogeneic conceptus [23]. Data here from in vitro experiments support the hypothesis that SP may aid in the expression the embryokine CSF2, which is known to be beneficial to preimplantation embryo development in the cow, rodent, and human [33–36]; however, it is important to note that SP did not alter the expression of endometrial CSF2 24 h following intrauterine infusion.
The postcoital inflammatory response of rodents reported by Yanagamich in 1963 [19] was later demonstrated to be the result of SP-derived TGFβ modulating expression of endometrial epithelial cytokine expression [15, 24]. Here, we have yet to define the active moieties in bovine semen responsible for alterations in endometrial gene expression, or subsequent cellular inflammation in the bovine; however, SP exposure increase expression of TGFB1 by epithelial and stromal cells which may suggest either amplification of a SP TGFβ signal or a unique mechanism to induce endometrial inflammation in the cow. More recently, SP-induced IL-17A has been implicated as an intermediary cytokine responsible for endometrial inflammation following insemination [37]. Our data suggest that SP and whole semen increase IL17A expression in epithelial and stromal cells, which may aid in cellular inflammation and the influx of γδ T cells into the endometrium, known to be important in early ruminant pregnancy [38]. However, our data show that no SP-mediated increases of IL17A expression were observed in explants or endometrial biopsies following intrauterine infusion; it may be that complex cell–cell interactions regulate SP-mediated IL17A expression in tissues where multiple cell types are exposed to SP, or that expression is very acute, as observed by stromal cell increased expression at 30 min post treatment. Expression of IFNϵ is increased in human cervical cells following exposure to SP [39], while here bovine epithelial or stromal cells only increased IFNE expression following exposure to whole semen. It has been hypothesized that elevated IFNϵ in the reproductive tract confers protection against sexually transmitted pathogens by increasing the number of resident effector immune cells within reproductive tissues of sex workers frequently exposed to semen [40]. Due to the limited exposure of the bovine reproductive tract to semen, it may be that IFNϵ expression is most related to chronic exposure to semen. Endometrial synthesis of PGF2α and PGE2 are implicated in the modulation of the CL, in addition to their role as classical inflammatory mediators. Here, we demonstrate an increase in both PTGS2 and AKR1C4 in epithelial and stromal cells in response to whole semen and SP, which may act as localized inflammatory mediators by increasing prostaglandin synthesis and subsequent vascular dilation and immune cell infiltrate. Indeed, SP itself is rich in prostaglandins (derived from the seminal vesicles) which may be a direct mechanism to increase localized inflammation within female reproductive tissues [41, 42]. In swine, exposure to SP increased expression of prostaglandin synthase enzymes in the endometrium and elevated circulating serum progesterone on day 4 of pregnancy [30, 43]. While it is not clear if SP endometrial prostaglandin modulation is responsible for increased progesterone by altering CL function, we did not find any difference in circulating progesterone following exposure to SP in the cow. While bioactive molecules in SP continue to be described in other species, the method of semen collection used in the current studies should also be considered as it may alter the composition of ejaculated semen. A number of studies in rams, men, and bulls have reported various differences in semen quality, volume, or sperm concentration determined by the method of collection (artificial vagina, electroejaculation, or spontaneous ejaculation), while others report no discernable differences in overall semen quality [44–47]. A proteomic profile of bull SP collected by artificial vagina or electroejaculation reported differential expression of 47 proteins in semen collected by electroejaculation compared to artificial vagina; none of which have been previously identified as molecules involved in the SP induction of endometrial inflammation [48]. Conversely, a similar study in the ram reported no differences in SP proteins [48, 49]. It may be here that the responses observed in our various experimental systems could be altered by using semen collected with an artificial vagina. Similarly, it is not clear if the concentrations of SP used in the current experiments reflect the volume of the ejaculate that would be present in the upper reproductive tract following natural service using bulls. A standard ejaculate volume may be between 2 and 7 ml in volume, only a fraction of which is likely to arrive cranial of the cervix and be in contact with the endometrium. Similarly, it is not clear if doses of semen utilized for intrauterine infusion are reminiscent of the doses used in in vitro experimentation. The distribution of 1 ml of semen throughout the entire uterus may be a significantly lower concentration than those applied to in vitro cell cultures. Estimates suggest that the uterine volume of a dairy cow uterus is between 125 and 154 cm3 [50], resulting in a dose of approximately 0.6 to 0.8% v/v. Future experimentation should also include doses of semen components that are significantly less than those used here.
Seminal plasma is known to increase cellular inflammation in the human cervix, and endometrium of the horse, swine, goats, and rodents (reviewed by Bromfield [51]). While we have not evaluated cellular inflammation in the endometrium of cows exposed to semen or SP, we evaluated the expression of the immune cell marker PTPRC (formerly CD45). Interestingly, we did not find an expected increase in the expression of endometrial PTPRC following exposure to SP, which would be indicative of increased immune cell infiltrate. Conversely, we found a decrease in PTPRC expression when cows were exposed to SP in combination with conventional AI semen, compared to the saline treated controls. While it is unclear whether this downregulation of PTPRC is truly representative of decreased cellular inflammation, it is worthy of note that expression in the SP + semen group was not different from treatment with either SP or semen alone. Immunohistochemical evaluation is warranted to confirm an absence of SP induced cellular inflammation in the cow model. The limited SP induction of endometrial inflammation was of surprise in the cow, considering the role of SP in other species and evidence presented here in culture systems. It is possible that the timing of sample collection after treatment here was protracted, or evaluation by other means (such as uterine flushing or cytobrush) is required to capture specific effects of SP-mediated inflammation in the cow. Our in vitro data suggest that many observable changes induced by SP exposure occur within 15 min of treatment, and thus SP-induced changes measured may be resolved by 24 h after treatment.
It is unclear whether the developmental environment of the oviduct and uterus of the cow is similar following natural live cover or AI. We propose that SP contributes to alteration in the developmental environment of the bovine endometrium which may subsequently influence early pregnancy. However, there appears to be some discrepancy in our data between in vitro culture exposure of endometrial cells to SP and exposure within the cow. Further studies are required to evaluate temporal changes within the cow and to identify the active components of SP responsible for endometrial alterations during early pregnancy. The execution of large field trials is required to ascertain if SP exposure at the time of AI ultimately increases pregnancy success; however, based on the success of AI in the dairy industry it is unlikely to see dramatic increases in conception rates with the addition of SP. However, it maybe that SP has a more subtle effect on the phenotype of offspring. Recently, the method of conception was shown to supersede the heritable genetic merit of offspring to produce milk in the first lactation; specifically high genetic merit cows that were derived from IVF had reduced milk production compared to lower genetic merit cows produced by AI [52]. It is likely that these effects are the consequence of having preimplantation embryos develop in a suboptimal in vitro environment. It could be hypothesized that SP exposure at conception could further optimize the developmental environment of the preimplantation in vivo. Investigations are required to determine the metabolic phenotype and production value of calves conceived in the presence of SP compared to traditional AI. These studies may extend the economic value of SP supplementation at AI. Ultimately, we hope to supplement AI semen with these SP molecules to develop new AI protocols in which the developmental environment of the preimplantation embryo is optimized for reproductive and phenotypic outcomes in commercial cattle production.
Supplementary data
Supplemental Figure S1. Effect of semen components on endometrial explant gene expression according to stage of estrous cycle. Intact endometrial explant expression of CSF2, IL6, CXCL8, IL17A, TGFB1, IFNE, PTGS2, AKR1C4, and OXTR in response to seminal plasma (SP; 2%, 5%, 10%, 20%), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (Veh) after 24 h. Data are presented for individual replicates representing explants isolated from individual cows. Explants derived from cows at stage 1 of the estrous cycle are depicted by open circles, while explants derived from cows at stage 2 of the estrous cycle are depicted as filled circles. Data were log transformed and analyzed using the generalized linear mixed model with pairwise comparisons only made with vehicle treated control. *, P value of ≤ 0.05.
Supplemental Figure S2. Effect of semen components on viability of explants. Intact endometrial explant viability was assessed by measuring LDH activity in supernatants following treatment with seminal plasma (SP; 2%, 5%, 10%, 20%), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (0) for 24 h. Uncultured medium (Veh) was used as a negative control as serum is known to contain elevated LDH. Supernatants collected from explants cultured in the presence of medium containing 1% Triton X-100 were used as a positive control. Data were normalized to the uncultured complete medium (Veh) and are presented as the mean fold change mU/ml LDH activity + S.E.M. from 3–10 independent experiments.
Supplemental Figure S3. Purity of endometrial epithelial and stromal cell cultures. Epithelial cell cultures (A–D) were confirmed as small round cells with epithelial-like morphology, while stromal cell cultures (E–H) had a typical fibroblast-like morphology. To quantify cell purity, flow cytometry using cell specific markers for epithelial (cytokeratin) and stromal (vimentin) cells was performed, in addition to identification of contaminating leukocytes (CD45). Three independent cell isolations were assessed for purity. Endometrial cell cultures contained minimal leukocytes in either epithelial (C; 5 ± 3% CD45+ total cells) or stromal cell cultures (G; 4 ± 1% CD45+ total cells). Stromal cell cultures were 95 ± 3% vimentin positive (F), while purity for epithelial cell cultures was lower at 56 ± 16% cytokeratin positive (B). Additionally, viability of epithelial (D) and stromal (H) cells was evaluated using the MTT assay following treatment with seminal plasma (SP), 5% whole semen (SM), 5% semen cell pellet (CP), or vehicle medium alone (Veh). Data from three independent experiments were normalized to vehicle controls and displayed as mean fold change + S.E.M.
Supplemental Figure S4. Effect of intrauterine infusion of semen on day 7 serum progesterone. Serum progesterone was measured in cows 7 days after intrauterine infusion of saline (n = 11), conventional semen (12), pooled seminal plasma (SP; n = 11), or a combination of conventional semen and SP (n = 9). Data are presented as mean ng/ml of progesterone + S.E.M. Data were analyzed using the generalized linear mixed model with pairwise comparisons. *, P value of ≤ 0.05. T, treatment effect.
Supplemental Table S1. List of qPCR primer sequences for target gene analysis.
Supplementary Material
Acknowledgments
The authors would like to thank Matt Utt and Bo Harstine of Select Sires for their support. The authors would also like to thank the staff and students of the University of Florida, North Florida Research and Education Center for their support, assistance and use of facilities to handle cattle. Semen was generously provided by Darren Henry, Luara Canal, Joel Yelich and Deborah Price at the time of bull breeding soundness exams.
Notes
Edited by Dr. Romana Nowak, PhD, University of Illinois Urbana-Champaign
Footnotes
Grant Support: This study was supported by Select Sires, The Southeast Milk checkoff and Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA 2014; 111:2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med 2001; 345:1400–1408. [DOI] [PubMed] [Google Scholar]
- 3. Cockburn K, Rossant J. Making the blastocyst: Lessons from the mouse. J Clin Invest 2010; 120:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kridli RT, Khalaj K, Bidarimath M, Tayade C. Placentation, maternal-fetal interface, and conceptus loss in swine. Theriogenology 2016; 85:135–144. [DOI] [PubMed] [Google Scholar]
- 5. Wiltbank MC, Baez GM, Garcia-Guerra A, Toledo MZ, Monteiro PL, Melo LF, Ochoa JC, Santos JE, Sartori R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016; 86:239–253. [DOI] [PubMed] [Google Scholar]
- 6. Lima FS, Risco CA, Thatcher MJ, Benzaquen ME, Archbald LF, Santos JE, Thatcher WW. Comparison of reproductive performance in lactating dairy cows bred by natural service or timed artificial insemination. J Dairy Sci 2009; 92:5456–5466. [DOI] [PubMed] [Google Scholar]
- 7. Sartori R, Gumen A, Guenther JN, Souza AH, Caraviello DZ, Wiltbank MC. Comparison of artificial insemination versus embryo transfer in lactating dairy cows. Theriogenology 2006; 65:1311–1321. [DOI] [PubMed] [Google Scholar]
- 8. Odhiambo JF, Poole DH, Hughes L, Dejarnette JM, Inskeep EK, Dailey RA. Pregnancy outcome in dairy and beef cattle after artificial insemination and treatment with seminal plasma or transforming growth factor beta-1. Theriogenology 2009; 72:566–571. [DOI] [PubMed] [Google Scholar]
- 9. Faulkner LC, Hopwood ML, Wiltbank JN. Seminal vesiculectomy in bulls. II. Seminal characteristics and breeding trials. Reproduction 1968; 16:179–182. [DOI] [PubMed] [Google Scholar]
- 10. Lovell JW, Getty R. Fate of semen in the uterus of the sow: Histologic study of endometrium during the 27 hours after natural service. Am J Vet Res 1968; 29:609–625. [PubMed] [Google Scholar]
- 11. Phillips DM, Mahler S. Leukocyte emigration and migration in the vagina following mating in the rabbit. Anat Rec 1977; 189:45–59. [DOI] [PubMed] [Google Scholar]
- 12. Pandya IJ, Cohen J. The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril 1985; 43:417–421. [DOI] [PubMed] [Google Scholar]
- 13. De M, Choudhuri R, Wood GW. Determination of the number and distribution of macrophages, lymphocytes and granulocytes in the mouse uterus from mating through implantation. J Leukoc Biol 1991; 50:252–262. [DOI] [PubMed] [Google Scholar]
- 14. McMaster MT, Newton RC, Dey SK, Andrews GK. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol 1992; 148:1699–1705. [PubMed] [Google Scholar]
- 15. Robertson SA, Mau VJ, Tremellen KP, Seamark RF. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. Reproduction 1996; 107:265–277. [DOI] [PubMed] [Google Scholar]
- 16. Rozeboom KJ, Troedsson MH, Hodson HH, Shurson GC, Crabo BG. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine. J Anim Sci 2000; 78:443–448. [DOI] [PubMed] [Google Scholar]
- 17. Alghamdi AS, Foster DN, Troedsson MH. Equine seminal plasma reduces sperm binding to polymorphonuclear neutrophils (PMNs) and improves the fertility of fresh semen inseminated into inflamed uteri. Reproduction 2004; 127:593–600. [DOI] [PubMed] [Google Scholar]
- 18. Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012; 188:2445–2454. [DOI] [PubMed] [Google Scholar]
- 19. Yanagimachi R, Chang MC. Infiltration of leucocytes into the uterine lumen of the golden hamster during the oestrous cycle and following mating. J Reprod Fertil 1963; 5:389–396. [DOI] [PubMed] [Google Scholar]
- 20. Robertson SA, Mau VJ, Hudson SN, Tremellen KP. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol 1997; 37:438–442. [DOI] [PubMed] [Google Scholar]
- 21. Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod 2011; 85:397–408. [DOI] [PubMed] [Google Scholar]
- 22. Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 2009; 80:1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bromfield JJ. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction 2016; 152:R223–R232. [DOI] [PubMed] [Google Scholar]
- 24. Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod 1998; 58:1217–1225. [DOI] [PubMed] [Google Scholar]
- 25. Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol 2012; 189:1024–1035. [DOI] [PubMed] [Google Scholar]
- 26. Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci 1980; 63:155–160. [DOI] [PubMed] [Google Scholar]
- 27. Borges AM, Healey GD, Sheldon IM. Explants of intact endometrium to model bovine innate immunity and inflammation ex vivo. Am J Reprod Immunol 2012; 67:526–539. [DOI] [PubMed] [Google Scholar]
- 28. Turner ML, Cronin JG, Healey GD, Sheldon IM. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014; 155:1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Troedsson MH, Loset K, Alghamdi AM, Dahms B, Crabo BG. Interaction between equine semen and the endometrium: The inflammatory response to semen. Anim Reprod Sci 2001; 68:273–278. [DOI] [PubMed] [Google Scholar]
- 30. O’Leary S, Jasper MJ, Warnes GM, Armstrong DT, Robertson SA. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction 2004; 128:237–247. [DOI] [PubMed] [Google Scholar]
- 31. Vishwanath R. Artificial insemination: The state of the art. Theriogenology 2003; 59:571–584. [DOI] [PubMed] [Google Scholar]
- 32. Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, Norman RJ, Robertson SA, Simon C. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod 2000; 15:2653–2658. [DOI] [PubMed] [Google Scholar]
- 33. Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod 2002; 67:1817–1823. [DOI] [PubMed] [Google Scholar]
- 34. Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, Gabrielsen A, Hnida C, Zobel DP, Munding B, Bendz SH, Robertson SA. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril 2013; 99:1600–1609.e2. [DOI] [PubMed] [Google Scholar]
- 35. Block J, Hansen PJ, Loureiro B, Bonilla L. Improving post-transfer survival of bovine embryos produced in vitro: Actions of insulin-like growth factor-1, colony stimulating factor-2 and hyaluronan. Theriogenology 2011; 76:1602–1609. [DOI] [PubMed] [Google Scholar]
- 36. Robertson SA, Seamark RF. Granulocyte-macrophage colony stimulating factor (GM-CSF): One of a family of epithelial cell-derived cytokines in the preimplantation uterus. Reprod Fertil Dev 1992; 4:435–448. [DOI] [PubMed] [Google Scholar]
- 37. Song ZH, Li ZY, Li DD, Fang WN, Liu HY, Yang DD, Meng CY, Yang Y, Peng JP. Seminal plasma induces inflammation in the uterus through the gammadelta T/IL-17 pathway. Sci Rep 2016; 6:25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Majewski AC, Tekin S, Hansen PJ. Local versus systemic control of numbers of endometrial T cells during pregnancy in sheep. Immunology 2001; 102:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13:491–501. [DOI] [PubMed] [Google Scholar]
- 40. Abdulhaqq SA, Zorrilla C, Kang G, Yin X, Tamayo V, Seaton KE, Joseph J, Garced S, Tomaras GD, Linn KA, Foulkes AS, Azzoni L et al. HIV-1-negative female sex workers sustain high cervical IFNvarepsilon, low immune activation, and low expression of HIV-1-required host genes. Mucosal Immunol 2016; 9:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bielanski W, Rzasa J, Okolski A. Prostaglandins in stallion semen. Theriogenology 1982; 17:167–173. [DOI] [PubMed] [Google Scholar]
- 42. Taylor PL, Kelly RW. 19-HydroxyIated E prostaglandins as the major prostaglandins of human semen. Nature 1974; 250:665–667. [DOI] [PubMed] [Google Scholar]
- 43. O’Leary S, Jasper MJ, Robertson SA, Armstrong DT. Seminal plasma regulates ovarian progesterone production, leukocyte recruitment and follicular cell responses in the pig. Reproduction 2006; 132:147–158. [DOI] [PubMed] [Google Scholar]
- 44. Jimenez-Rabadan P, Ramon M, Garcia-Alvarez O, Maroto-Morales A, del Olmo E, Perez-Guzman MD, Bisbal A, Fernandez-Santos MR, Garde JJ, Soler AJ. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim Reprod Sci 2012; 132:88–95. [DOI] [PubMed] [Google Scholar]
- 45. Moore RW. A comparison of electro-ejaculation with the artifical vagina for ram semen collection. N Z Vet J 1985; 33:22–23. [DOI] [PubMed] [Google Scholar]
- 46. Hovav Y, Almagor M, Yaffe H. Comparison of semen quality obtained by electroejaculation and spontaneous ejaculation in men suffering from ejaculation disorder. Hum Reprod 2002; 17:3170–3172. [DOI] [PubMed] [Google Scholar]
- 47. Austin JW, Hupp EW, Murphree RL. Comparison of quality of bull semen collected in the artificial vagina and by electroejaculation. J Dairy Sci 1961; 44:2292–2297. [Google Scholar]
- 48. Rego JP, Moura AA, Nouwens AS, McGowan MR, Boe-Hansen GB. Seminal plasma protein profiles of ejaculates obtained by internal artificial vagina and electroejaculation in Brahman bulls. Anim Reprod Sci 2015; 160:126–137. [DOI] [PubMed] [Google Scholar]
- 49. Marco-Jimenez F, Vicente JS, Viudes-de-Castro MP. Seminal plasma composition from ejaculates collected by artificial vagina and electroejaculation in Guirra ram. Reprod Domest Anim 2008; 43:403–408. [DOI] [PubMed] [Google Scholar]
- 50. Baez GM, Barletta RV, Guenther JN, Gaska JM, Wiltbank MC. Effect of uterine size on fertility of lactating dairy cows. Theriogenology 2016; 85:1357–1366. [DOI] [PubMed] [Google Scholar]
- 51. Bromfield JJ. Review: The potential of seminal fluid mediated paternal-maternal communication to optimise pregnancy success. Animal 2018; 12:s104–s109. [DOI] [PubMed] [Google Scholar]
- 52. Siqueira LGB, Dikmen S, Ortega MS, Hansen PJ. Postnatal phenotype of dairy cows is altered by in vitro embryo production using reverse X-sorted semen. J Dairy Sci 2017; 100:5899–5908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.