Summary
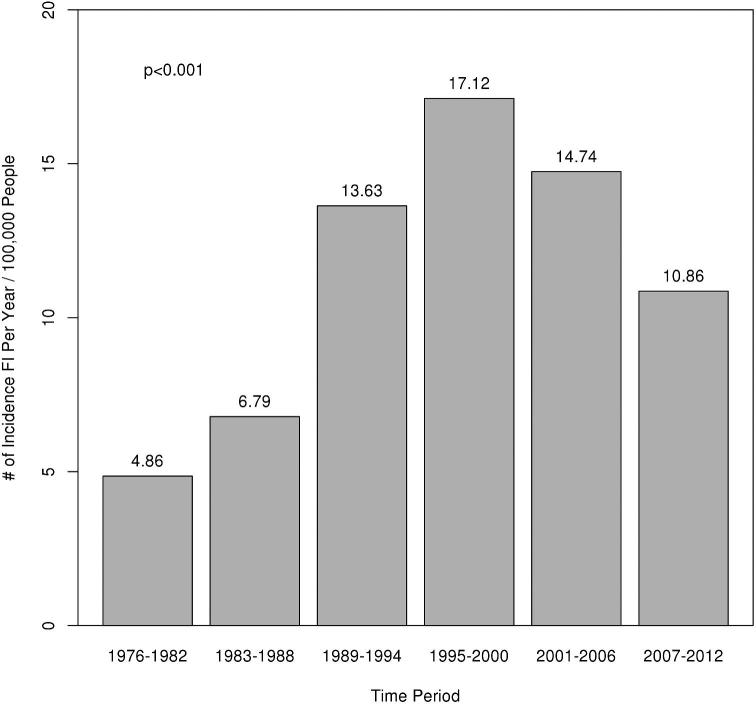
With the emergence of eosinophilic esophagitis (EoE) as a common cause of food impaction (FI) and a presumed increase in incidence of EoE in the population, the effect on the incidence of FI has not been well described. The aim of this study is to describe the incidence of FI and endoscopic findings in these patients and the association with EoE. A population-based retrospective chart review of the Rochester Epidemiology Project database was performed to identify all patients within Olmsted County that presented with FI from 1976 to 2012. A review of all endoscopic findings, biopsy results, and demographic data was performed. 497 patients were identified with FI from 1976 to 2012. The overall incidence of FI has changed from 1976 to 2012 (Fig. 1) (P < 0.001). The peak incidence of 17.12 per 100,000 people occurred in the time period 1995 to 2000. Both the incidence of comorbid gastroesophageal reflux disease (GERD) and proton pump inhibitor (PPI) use increased over the time period of the study (P < 0.001 for both). Of these patients, 188 (46.7%) had no abnormalities on their endoscopy. The most common endoscopic finding was stricture in 71 (17.6%) patients followed closely by Schatzki's ring in 68 (16.9%) patients. 139 patients had biopsies performed within 2 years of FI and 50 (36.0%) of those were diagnosed with EoE. We present for the first time the changing incidence of FI over the last 35 years in a population-based setting. We also demonstrate the rise of EoE as an important clinical consideration in patients with FI.
Keywords: dysphagia, eosinophilic esophagitis, esophagus, food impaction
ABBREVIATIONS
- EoE
eosinophilic esophagitis
- FI
food impaction
- REP
Rochester epidemiology project.
INTRODUCTION
Since it was first described in 1978, eosinophilic esophagitis (EoE) has emerged as a leading cause of food impaction (FI)1 and is considered by many to be a representation of atopy in the esophagus.2,3 Epidemiologic data suggests that the incidence of atopic disorders has been increasing; the evidence suggests that the incidence of EoE has increased in the decades since its initial discovery.4–8 Two studies have shown that the percentage of esophageal biopsies with EoE has remained constant over time, and thus debate has ensued as to whether the increase in EoE over time is actually secondary to increased recognition of the disease or a true increase in incidence.9,10
A recent study demonstrated an incidence of FI of 25 per 100,000 inhabitants per year over a time period from 2008 to 201311 increased compared to a previously reported incidence of 13 per 100,000 inhabitants per year from 1993 to 1998.12 Furthermore, a study looking at all patients admitted with food bolus impaction from 1996 to 2010 demonstrated a significant increase over that time.13 It has been shown that as many as 25–100% of patients with EoE will develop an esophageal FI.4,5,14–16 It has been hypothesized that at least some of the increase in incidence of FI recently has been related to an increasing incidence of EoE associated FI.17 FI has been increasingly viewed over the last 3 to 4 decades as a surrogate marker of EoE.12,17–20
In this population-based study, we used the Rochester Epidemiology project (REP) database to retrospectively examine the charts of patients presenting to a health care provider in Olmsted County for FI from 1976 to 2012. Our aim will be to determine the incidence of FI in a stable population in Olmsted County over the last 35 years and the associated endoscopic findings and eventual EoE diagnosis among these patients. We will assess for the first time how the incidence and etiology of FI has changed over a prolonged time period. Our hypothesis is that the emergence of EoE as a clinical entity has contributed to a rising incidence of FI.
MATERIALS AND METHODS
The population of Olmsted County includes approximately 120,000 people. Over 80% of the entire population is seen at one of two major medical institutions (Mayo Clinic and Olmsted Medical Center) each year and almost the entire population is seen within any 4-year period.21 The population of Olmsted County is 89% white and sociodemographically similar to the US white population.21 The REP was created as a common medical record linkage system for care provided to patients within the county including both the Mayo Clinic and Olmsted Medical Center. Therefore, the REP medical record linkage system provides what is essentially an enumeration of the population from which samples can be drawn.
This study was a retrospective review of the EMR at the institutions included in the REP and was approved by the institutional review board at both the Mayo Clinic and Olmsted Medical Center (the two main institutions included in the REP). A text-based search was conducted through the REP database for a diagnosis of esophageal foreign body impaction, foreign body in the esophagus, or foreign body in an orifice. Patients were then excluded if impaction was revealed to be a non-food item or if the item was not impacted in the esophagus. Patients were also excluded if the symptoms resolved prior to being seen in the emergency department.
All patients living in Olmsted county who presented to an emergency department with FI from 1976 to 2012 were identified from the REP.22 FI was defined as a sensation of food being lodged in the throat with associated esophageal obstruction (inability to swallow saliva or water) for a long enough time to present to the emergency room. Dysphagia prior to impaction was defined as patient-described difficulty swallowing without FI. Endoscopic findings (when performed) and pathology results (when obtained) were recorded. Concomitant medical diagnoses were recorded if they were present prior to or within 6 months after the initial FI.
In patients in whom an EGD was performed within 2 years of the FI, endoscopic findings were recorded as identified on this EGD. If the patient had multiple EGDs, the endoscopic findings on the first were utilized. Changes suggestive of EoE (furrows and rings) were recorded but were not included as a major endoscopic finding. Inclusion of this finding alone in the study would unfairly bias the prevalence of this toward later years when this was more readily recognized by endoscopists. In those patients who had biopsies, pathologic findings were recorded. Pathology slides were reviewed by a pathologist [T.S.] for any patient that only had biopsies done before 2006 to assess for eosinophilia as EoE may not have been recognized prior to that time. Pathologic criteria utilized for diagnosis of EoE were in accordance with current diagnostic criteria for EoE and required a peak count of ≥15 eosinophils/hpf.5 Schatzki's rings were kept distinct from strictures and were classified as in the original description of the condition.23
With the emergence of EoE, the workup for a patient with FI and/or dysphagia has changed dramatically. A large number of patients with FI did not have biopsies of the esophagus performed in the earlier years of our study. In those patients that were diagnosed with EoE at any time during the time frame of this study, EoE was considered to have been present from the time of the initial FI.
To understand the change in incidence over time, our time period (1976–2012) was split up into six time periods of roughly equal duration. Age was summarized by median and interquartile range with potential differences between time periods assessed using the Kruskal-Wallis test. Categorical patient characteristics and endoscopic findings were summarized using counts and percentages. Differences across time periods for categorical variables were assessed using a Χ2 test or Fisher's exact test when frequencies were small. Incidence rates were calculated separately for each of our 6 time periods using the Olmsted County population size at the midpoint year as the denominator. The incidence rates presented are as the rate per year per 100,000 people. Poisson regression with an offset for midyear population was used to assess whether the incidence rates changed across the time periods. Statistical analyses were performed using SAS (version 9.4, Cary, NC) and R (version 3.2.3, Vienna, Austria).
RESULTS
Out of 497 patients were identified who presented with FI from 1976 to 2012. Demographic data including comorbid conditions and the number of patients that were biopsied within two years can be seen in Table 1. It is important to note that both the incidence of comorbid gastroesophageal reflux disease (GERD) and proton pump inhibitor (PPI) use increased over the time period of the study (P < 0.001 for both). The overall incidence of FI has changed from 1976 to 2012 (Fig. 1) (P < 0.001). The peak incidence of 17.12 per 100,000 people occurred in the time period 1995 to 2000.
Table 1.
Patient demographics all incident FIs
| 1976–1982 | 1983–1988 | 1989–1994 | 1995–2000 | 2001–2006 | 2007–2012 | Total | ||
|---|---|---|---|---|---|---|---|---|
| (N = 31) | N = 41) | (N = 90) | (N = 124) | (N = 117) | (N = 94) | (N = 497) | P value | |
| Total impactions | 31 | 41 | 90 | 124 | 117 | 94 | 497 | |
| Age at incident FI |
0.867* | |||||||
| Median | 61.3 | 57.8 | 57.2 | 53.9 | 50.6 | 54.6 | 54.5 | |
| Q1, Q3 | 19.1, 74.9 | 34.8, 64.8 | 35.8, 71.4 | 32.4, 70.7 | 39.1, 76.1 | 34.2, 69.5 | 35.8, 71.9 | |
| Range | (0.0-84.1) | (9.9-78.3) | (1.0-95.3) | (1.3-94.5) | (10.8-99.1) | (1.5-97.9) | (0.0-99.1) | |
| Female | 13 (41.9%) | 13 (31.7%) | 35 (38.9%) | 45 (36.3%) | 52 (44.4%) | 35 (37.2%) | 193 (38.8%) | 0.701** |
| Any allergic diathesis | 20 (64.5%) | 23 (56.1%) | 51 (56.7%) | 78 (62.9%) | 69 (59.0%) | 59 (62.8%) | 300 (60.4%) | 0.889** |
| Asthma | 6 (19.4%) | 8 (19.5%) | 18 (20.0%) | 26 (21.0%) | 16 (13.7%) | 20 (21.3%) | 94 (18.9%) | 0.722** |
| Allergic rhinitis | 8 (25.8%) | 7 (17.1%) | 20 (22.2%) | 23 (18.5%) | 22 (18.8%) | 26 (27.7%) | 106 (21.3%) | 0.533** |
| Drug allergy | 2 (6.5%) | 7 (17.1%) | 17 (18.9%) | 37 (29.8%) | 30 (25.6%) | 20 (21.3%) | 113 (22.7%) | 0.064** |
| Food allergy | 3 (9.7%) | 1 (2.4%) | 4 (4.4%) | 4 (3.2%) | 8 (6.8%) | 8 (8.5%) | 28 (5.6%) | 0.402** |
| Eosinophilia | 11 (35.5%) | 7 (17.1%) | 12 (13.3%) | 30 (24.2%) | 16 (13.7%) | 17 (18.1%) | 93 (18.7%) | 0.036** |
| Other | 7 (22.6%) | 5 (12.2%) | 17 (18.9%) | 20 (16.1%) | 24 (20.5%) | 19 (20.2%) | 92 (18.5%) | 0.793** |
| Medications | ||||||||
| H2 Blocker | 0 (0.0%) | 0 (0.0%) | 6 (6.7%) | 7 (5.6%) | 6 (5.1%) | 4 (4.3%) | 23 (4.6%) | 0.524*** |
| PPI | 0 (0.0%) | 0 (0.0%) | 2 (2.2%) | 3 (2.4%) | 14 (12.0%) | 19 (20.2%) | 38 (7.6%) | <0.001*** |
| GERD | 3 (9.7%) | 2 (4.9%) | 22 (24.4%) | 25 (20.2%) | 32 (27.4%) | 44 (46.8%) | 128 (25.8%) | <0.001** |
| Dysphagia | 14 (45.2%) | 16 (39.0%) | 42 (46.7%) | 56 (45.2%) | 74 (63.2%) | 63 (67.0%) | 265 (53.3%) | <0.001** |
| Esophageal biopsy | 3 (9.7%) | 10 (24.4%) | 16 (17.8%) | 19 (15.3%) | 49 (41.9%) | 42 (44.7%) | 139 (28%) | <0.001** |
*Kruskal Wallis; **Chi-Square; ***Fisher Exact.
Figure 1.
Incidence of food impaction from 1976 to 2012.
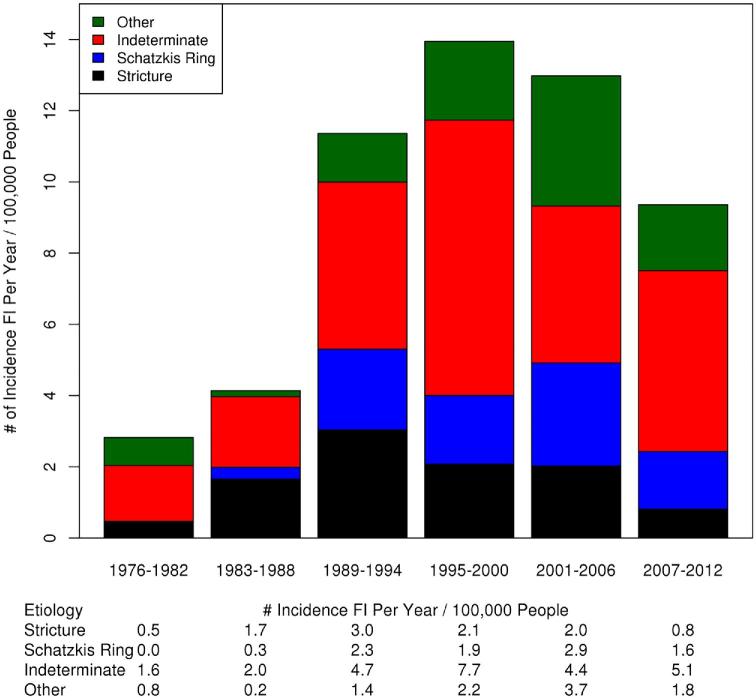
Out of 403 (81.1%) patients underwent EGD within 2 years of initial FI. Of these patients, 188 (46.7%) had no abnormalities on their endoscopy. The most common endoscopic finding was stricture in 71 (17.6%) patients followed closely by Schatzki's ring in 68 (16.9%) patients. The endoscopic findings by time period in those patients that underwent EGD within 2 years can be seen in Figure 2. Among those classified as ‘other’ findings were 55 (72% of other etiology group) with esophagitis, 8 (11%) with tortuous esophagus, 5 (7%) with congenital stenosis, 3 (4%) with masses, 2 (3%) with postoperative changes, 1 (1%) with extrinsic compression, 1 (1%) with Barrett's esophagus, and 1 (1%) with an esophageal web. Esophageal dilation was performed in 59/333 (18%) patients and 13/70 (19%) patients with eventual EoE. The frequency of dilation did not vary between those with eventual EoE and those without EoE (P = 0.87).
Figure 2.
Endoscopic findings in food impaction as determined at the time of initial endoscopy.
Among the 403 patients who had an EGD, 70 (17.4%) total patients were eventually diagnosed with EoE or had pathologic evidence for EoE on biopsies performed before 2006 when pathology slides were reviewed. Only 139 patients had biopsies performed within 2 years of incident FI and 50 (36.0%) of those were diagnosed with EoE. The final etiology of incident FI after pathologic review in those with biopsies within 2 years can be seen in Table 2. Furthermore, an additional 20 patients would be diagnosed with EoE by biopsy performed outside of the 2 year time frame. In those patients that had biopsies performed, EoE was eventually diagnosed in 17% of those with strictures on EGD, 65% with Schatzkis rings, 40% with an indeterminate EGD, and 25% with other endoscopic findings. Again, in a majority of cases of EoE, furrows and/or rings were present at the time of initial endoscopy (41/70 59%).
Table 2.
Diagnosis of eosinophilic esophagitis (EoE) in patients with and without furrows and/or rings
| Indeterminate | Stricture | Schatzki's rings | Other | |
|---|---|---|---|---|
| With furrows and/or rings | ||||
| Total | 26 | 4 | 15 | 7 |
| EoE diagnosis | 21 (81%) | 2 (50%) | 13 (87%) | 5 (71%) |
| Without furrows and/or rings | ||||
| Total | 162 | 67 | 53 | 69 |
| EoE diagnosis | 18 (11%) | 5 (7%) | 3 (6%) | 3 (4%) |
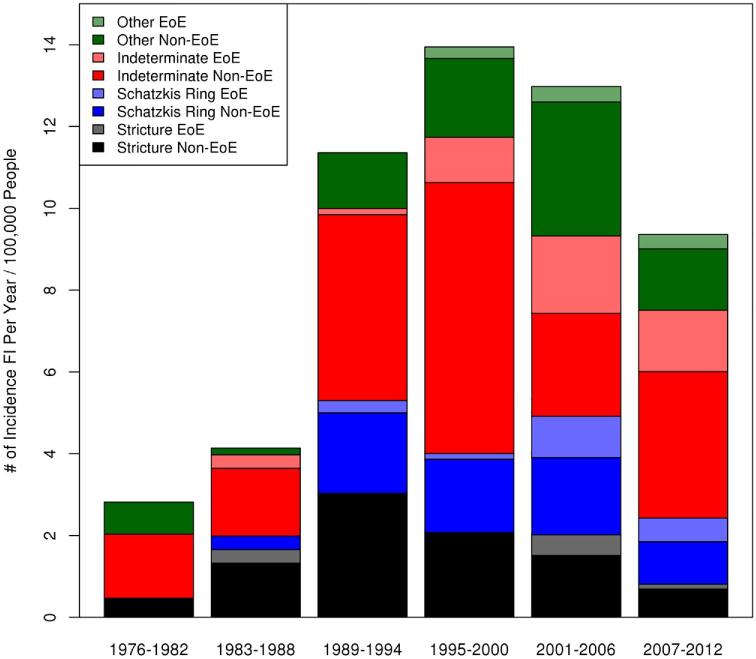
The number of patients from within each endoscopic finding category who had furrows and/or rings and association with EoE diagnosis can be seen in Table 3. There were 14 (3% of those with EGD) patients with both furrows and rings on initial EGD [11/14 (79%) eventual EoE diagnosis], 26 (6%) patients with esophageal rings but no furrows [18/26 (69%)], and 12 (3%) patients with furrows but no esophageal rings [12/12 (100%)]. Initial endoscopic findings and those diagnosed with EoE can be seen in Figure 3. There are patients that were eventually diagnosed with EoE from within each endoscopic finding group. Those patients with rings and/or furrows were more likely to be diagnosed with EoE than those that did not have rings and/or furrows (79% vs. 8%, P < 0.001).
Table 3.
Major endoscopic finding in food impaction as identified on EGD among those with biopsy done within 2 years
| 1976–1982 | 1983–1988 | 1989–1994 | 1995–2000 | 2001–2006 | 2007–2012 | Total | |
|---|---|---|---|---|---|---|---|
| (N = 3) | (N = 10) | (N = 16) | (N = 19) | (N = 49) | (N = 42) | (N = 139) | |
| FI etiology | |||||||
| Stricture [% EoE Dx] | 0 (0.0%) [0%] | 4 (40.0%) [25%] | 7 (43.8%) [0%] | 5 (26.3%) [0%] | 6 (12.2%) [33%] | 2 (4.8%) [50%] | 24 (17.3%) [17%] |
| Distal | 0 (0.0%) | 3 (75.0%) | 7 (100.0%) | 4 (80.0%) | 6 (100.0%) | 1 (50.0%) | 21 (87.5%) |
| Nondistal | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 1 (50.0%) | 3 (12.5%) |
| Schatzkis ring [% EoE Dx] | 0 (0.0%) [0%] | 1 (10.0%) [0%] | 1 (6.3%) [0%] | 0 (0.0%) [0%] | 7 (14.3%) [86%] | 8 (19.0%) [63%] | 17 (12.2%) [65%] |
| Indeterminate [% EoE Dx] | 0 (0.0%) [0%] | 4 (40.0%) [0%] | 5 (31.3%) [0%] | 11 (57.9%) [27%] | 23 (46.9%) [57%] | 27 (64.3%) [44%] | 70 (50.4%) [40%] |
| Other [% EoE Dx] | 3 (100.0%) [0%] | 1 (10.0%) [0%] | 3 (18.8%) [0%] | 3 (15.8%) [33%] | 13 (26.5%) [23%] | 5 (11.9%) [60%] | 28 (20.1%) [25%] |
| FI etiology with EoE category added | |||||||
| Stricture | 0 (0.0%) | 3 (30.0%) | 7 (43.8%) | 5 (26.3%) | 4 (8.2%) | 1 (2.4%) | 20 (14.4%) |
| Schatzkis ring | 0 (0.0%) | 1 (10.0%) | 1 (6.3%) | 0 (0.0%) | 1 (2.0%) | 3 (7.1%) | 6 (4.3%) |
| Indeterminate | 0 (0.0%) | 4 (40.0%) | 5 (31.3%) | 8 (42.1%) | 10 (20.4%) | 15 (35.7%) | 42 (30.2%) |
| Other | 3 (100.0%) | 1 (10.0%) | 3 (18.8%) | 2 (10.5%) | 10 (20.4%) | 2 (4.8%) | 21 (15.1%) |
| EoE | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) | 4 (21.1%) | 24 (49.0%) | 21 (50.0%) | 50 (36.0%) |
Figure 3.
Initial endoscopic findings and eventual diagnosis of eosinophilic esophagitis within each endoscopic finding group.
Over the last two time periods of our study from 2001 to 2012, 52 patients were diagnosed with EoE. The average diagnostic latency in that time period was 7 months. From 2001 to 2003, 12 patients were eventually diagnosed with EoE with a diagnostic latency of 24 months on average. Only 5/12 (41%) were diagnosed within a year of their initial FI. Among the 40 patients diagnosed with EoE from 2004 to 2012, the average time to diagnosis was 1.7 months. 37/40 patients were diagnosed within a year. It is important to note that from 2001 to 2012, we did not perform an EGD in 28/211 (13%) patients and we did not biopsy 120/211 (57%). Earlier time periods were excluded from this analysis as many of the patients were diagnosed based upon review of pathologic specimens and diagnostic latency was very long for patients that were actually diagnosed.
DISCUSSION
We present the only population-based retrospective cohort of FI over the last 35 years currently in the literature. It has been previously demonstrated that the number of patients being admitted with FI is increasing in an Australian cohort, but this is the first population-based study to demonstrate a changing incidence in FI.13 The Rochester epidemiology project provides a unique opportunity to examine the true incidence of a disease in a population over a long period of time.22 The entire population of Olmsted County has health care delivered at only two institutions and the Rochester epidemiology project captures the health history of the county with a high degree of accuracy. We clearly demonstrate over this time period that the incidence of FI has changed dramatically. We are also able to demonstrate the rise of EoE as a major diagnostic consideration in patients with FI.
Gretarsdottir et al. recently reported an incidence of FI of 25 per 100,000 people in an Icelandic population-based study in the time period 2008 to 2013.11 In comparison to their findings, we demonstrate a lower incidence of FI from 2007 to 2012 at 10.86 per 100,000 population. The changing incidence that we report is supportive of recent publications suggesting that the number of FI is increasing.13,17 However, these previous publications were not population-based and unable to report an incidence of FI. We report for the first time the changing incidence of FI over the last 35 years and demonstrate a peak in the incidence of FI from 1995 to 2000 of 17.12 per 100,000 people (Fig. 1).
Recent publications have suggested that the rise of EoE may be the reason behind the change in the incidence of FI.13,17 The rise of the incidence of EoE in our cohort seems to support this claim. Interestingly, we failed to show an increase in atopic disorders, which has been hypothesized to be the cause of the emergence of EoE.24 It is possible that increased recognition of EoE recently is the cause of the rise in diagnosis and that underdiagnosis in the earlier time-periods could be contributing to the apparent rise in the incidence of EoE. We attempted to account for this by reviewing pathology from all cases that were biopsied, but this cannot account for the large number of patients that were not biopsied.
The fall of stricture-related FI after a peak in 1989 to 1994 further supports the notion that another pathologic process is likely contributing to the increasing incidence of FI. The decrease in stricture-related FI may in part be related to the significant rise in use of proton pump inhibitors and/or histamine blockers over the course of our study. We had a significantly lower proportion of strictures as compared to previously reported cohorts, 17.6% compared to 45%.11 Finally, we hypothesize that the overall fall in FI in the most recent time period of our study may be explained at least partially by more effective identification and treatment of EoE following the publication of treatment guidelines though the increased use of proton pump inhibitors is also likely contributing.5
Our data shows that EoE should be considered in all patients that present with FI regardless of endoscopic findings, but we found that EoE was particularly prevalent among those that presented with furrows and/or rings on endoscopy. The association of furrows and/or rings on endoscopy with EoE has been clearly documented in the literature previously.5 Our patients eventually diagnosed with EoE presented with a wide range of endoscopic findings with or without furrows and/or rings including Schatzki's rings, strictures, and normal-appearing esophagi. Therefore, a high index of suspicion for EoE must be maintained in all patients presenting with FI.
We demonstrate that we are diagnosing patients with EoE relatively quickly after their initial presentation with FI. It is clear that from 2004 to 2012, our diagnostic latency is improved even when compared with 2001 to 2003. We also demonstrate that even in these later time periods a large proportion of patients are not being fully evaluated for EoE after presentation with FI. A substantial number of these did not even have an EGD performed (13%) and even fewer had a biopsy (57%). It is certainly possible that we are still missing cases of EoE.
The major limitations of this study are inherent to the retrospective nature. The quality of the data for all of the patients of this study is limited to the quality of the medical record. Additionally, given the relatively recent discovery of EoE,5 it is quite possible that findings such as rings and furrows on EGD or pathologic specimens showing eosinophils may have been ignored and not mentioned in the record in earlier time periods. Pathology slides from pre-2006 were reviewed by a pathologist to ensure that no cases of EoE in which a biopsy was performed were missed. However, as can be seen in Table 1, the percentage of patients being biopsied has increased dramatically over the course of the study. Patients that were not ever biopsied could not be diagnosed with EoE and this has very likely led to an increase in diagnosis over the course of the study.
Distinguishing EoE from a GERD/EoE overlap syndrome is very difficult in a retrospective review. We did not require that patients have a trial of proton pump inhibitor prior to diagnosis of EoE. Only 18/70 (26%) patients in the EoE group clearly had a trial of PPI prior to diagnosis. This is felt largely related to inadequate documentation in the EMR. Additionally, patients that had pathologic findings in the earlier period of this study would not have had this guideline available to guide their treatment. We are unable to report if the eosinophils were in the proximal, mid, or distal esophagus. We would suggest that a finding of eosinophilia on biopsy is suggestive of a similar pathologic process regardless of PPI-responsiveness and location within the esophagus and that the distinction of GERD-related EoE is not an important one. This is a population-based study and thus the tertiary nature of the academic institution is unlikely to have affected the results. Nevertheless, this study is representative of populations similar to that of Olmsted County a predominantly white population in a rural area with a temperate climate. This is a population which may be at increased risk for EoE compared to others.
In summary, we present the largest population-based study of FI in the literature. We demonstrate for the first time in a population-based study that the etiology and incidence of FI has changed significantly over the last 35 years. With the rise in FI over 35 years, the incidence of stricture-related FI has declined and there is strong suggestion of a true rise in the incidence of EoE-related food impaction.
Notes
Specific author contributions: Jeffrey A. Alexander will serve as the guarantor. Study concept and design: Charles Lenz, Jeffrey Alexander; Acquisition of data: Charles Lenz, Jeffrey Alexander; Analysis and interpretation of data: Charles Lenz, Jeffrey Alexander; Drafting manuscript: Charles Lenz, Jeffrey Alexander; Interpretation of data: Cadman Leggett, David A. Katzka, Joseph B. Larson, Felicity T. Enders; Statistical analysis: Joseph B. Larson, Felicity T. Enders; All authors participated in critical review and approve of the final draft submitted.
Funding information: Supported in part by the Rochester Epidemiology Project, R01 AG034676 from the National Institute on Aging of the National Institutes of Health.
References
- 1. Landres R T, Kuster G G, Strum W B. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978; 74: 1298–301. [PubMed] [Google Scholar]
- 2. Arora A S, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 2004; 2: 523–30. [DOI] [PubMed] [Google Scholar]
- 3. Yakoot M. Eosinophilic digestive disease (EDD) and allergic bronchial asthma; two diseases or expression of one disease in two systems? Ital J Pediatr 2011; 37: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dellon E S, Gibbs W B, Fritchie K J et al.. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009; 7: 1305–13; quiz 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liacouras C A, Furuta G T, Hirano I et al.. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20.e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 6. Noel R J, Putnam P E, Rothenberg M E. Eosinophilic esophagitis. N Engl J Med 2004; 351: 940–1. [DOI] [PubMed] [Google Scholar]
- 7. Prasad G A, Alexander J A, Schleck C D et al.. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 2009; 7: 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115: 418–9. [DOI] [PubMed] [Google Scholar]
- 9. Vanderheyden A D, Petras R E, DeYoung B R, Mitros F A. Emerging eosinophilic (allergic) esophagitis: increased incidence or increased recognition? Arch Pathol Lab Med 2007; 131: 777–9. [DOI] [PubMed] [Google Scholar]
- 10. Franciosi J P, Osman J, Li C et al.. Increasing incidence or increasing detection of esophageal eosinophilia: a re-evaluation of esophageal biopsies from 1985 to 2005. J Allergy Clin Immunol 2008; 121: S71. [Google Scholar]
- 11. Gretarsdottir H M, Jonasson J G, Bjornsson E S. Etiology and management of esophageal food impaction: a population based study. Scand J Gastroenterol 2015; 50: 513–8. [DOI] [PubMed] [Google Scholar]
- 12. Longstreth G F, Longstreth K J, Yao J F. Esophageal food impaction: epidemiology and therapy. A retrospective, observational study. Gastrointest Endosc 2001; 53: 193–8. [DOI] [PubMed] [Google Scholar]
- 13. Mahesh V N, Holloway R H, Nguyen N Q. Changing epidemiology of food bolus impaction: is eosinophilic esophagitis to blame? J Gastroenterol Hepatol 2013; 28: 963–6. [DOI] [PubMed] [Google Scholar]
- 14. Mackenzie S H, Go M, Chadwick B et al.. Eosinophilic oesophagitis in patients presenting with dysphagia—a prospective analysis. Aliment Pharmacol Ther 2008; 28: 1140–6. [DOI] [PubMed] [Google Scholar]
- 15. Prasad G A, Talley N J, Romero Y et al.. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102: 2627–32. [DOI] [PubMed] [Google Scholar]
- 16. Straumann A, Bussmann C, Zuber M, Vannini S, Simon H U, Schoepfer A. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol 2008; 6: 598–600. [DOI] [PubMed] [Google Scholar]
- 17. Sperry S L, Crockett S D, Miller C B, Shaheen N J, Dellon E S. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc 2011; 74: 985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai T K, Stecevic V, Chang C H, Goldstein N S, Badizadegan K, Furuta G T. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61: 795–801. [DOI] [PubMed] [Google Scholar]
- 19. Prasad G A, Reddy J G, Boyd-Enders F T, Schmoll J A, Lewis J T, Wongkeesong L M. Predictors of recurrent esophageal food impaction. J Clin Gastroenterol 2008; 42: 771–5. [DOI] [PubMed] [Google Scholar]
- 20. Tilakaratne S, Day A, Lemberg D. Eosinophilic esophagitis and food impaction: an instructive case. Turk J Gastroenterol 2012; 23: 294–7. [DOI] [PubMed] [Google Scholar]
- 21. Melton LJ., 3rd History of the Rochester epidemiology project. Mayo Clin. Proc. 1996; 71: 266–74. [DOI] [PubMed] [Google Scholar]
- 22. St Sauver J L, Grossardt B R, Yawn B P et al.. Data resource profile: the Rochester epidemiology project (REP) medical records-linkage system. Int J Epidemiol 2012; 41: 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schatzki R. The lower esophageal ring. Long term follow-up of symptomatic and asymptomatic rings. Am J Roentgenol Radium Ther Nucl Med 1963; 90: 805–10. [PubMed] [Google Scholar]
- 24. Dellon E S. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am 2014; 43: 201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]