Abstract
Post-exercise cardiac troponin (cTn) elevation is a recognised phenomenon which historically has been detected using standard sensitivity assays. More recently high-sensitivity assays have been developed and are now the gold standard for detection of cTn in the clinical setting. Although the assay's enhanced sensitivity confers benefits it has created new challenges for clinicians. By evaluating the change in cTn values over time, taking into account biological and analytical variation, the clinician is able to differentiate between a pathological and normal cTn value. As a result, serial cTn testing has become a fundamental component of the clinical assessment of chest pain patients and is included in the most recent definition for myocardial infarction and the latest guidelines for the management of acute coronary syndromes without persistent ST-segment elevation. A review of the cTn kinetics literature demonstrates a pattern of elevation and peak within the first 4 h after exercise dropping within 24 h. In contrast myocardial necrosis demonstrates a later cTn peak with a slower downslope occurring over several days. Understanding cTn kinetics facilitates clinician's decision making when presented with a chest pain patient post-exercise. Furthermore, it helps elucidate the underlying mechanism and establish the clinical significance of post-exercise cTn elevation, which in all other situations confers negative prognostic value. We recommend serial cTn testing in this scenario with a suggested algorithm included in this review.
Keywords: Troponin, Kinetics, Heart, Exercise
1. Introduction
It is well accepted that short bouts of moderate intensity exercise taken regularly are beneficial for health [1,2]. What is unclear is whether this still applies to those participating in strenuous and/or prolonged exercise. Some of this uncertainty has arisen from the growing literature demonstrating a rise in the cardiac biomarker, troponin (cTn), following endurance exercise. Understanding the significance of this is important. Firstly, to enable clinicians to give informed advice to those wishing to participate in such exercise and secondly to facilitate the interpretation of troponin levels in the context of an endurance event.
2. cTn testing in the clinical setting
Due to their ability to detect cTn much earlier, high-sensitivity cTn (HS-cTn) assays have largely replaced those of standard sensitivity in the clinical setting [[3], [4], [5]]. The enhanced sensitivity of these newer assays has led to the detection of cTn in healthy individuals [6] which in conjunction with the existence of biological and analytical variability [7,8] has made it harder to differentiate between a pathological and normal cTn value. To overcome this serial blood testing and the evaluation of troponin kinetics has become a fundamental component of the clinical assessment of chest pain patients.
The change criteria for a pathological rise between the two blood sampling points is assay specific and depends on a variety of factors including the timing of baseline sampling and the onset of symptoms. The key is that any change detected is greater than the combined biological and analytical variation. A 20% or greater change from an elevated cTn value is set as the threshold for diagnosis of myocardial necrosis [9,10] and represents a significant >3 standard deviations of variation associated with an elevated baseline concentration change in cTn on the basis of a 5–7% analytical total CV [10]. For clinical situations where the baseline sampling value is below the URL a change in the range of 50–60% is needed [11]. This is not error proof and thus it is recommended [12] that if the clinical situation is ambiguous and the pre-test likelihood of disease high, additional sampling is performed.
The fourth definition of myocardial infarction includes serial testing as a criterion for its diagnosis [13]. However, it defines this detection of a rise and/or fall of cTn with at least 1 value above the 99th percentile upper reference limit (URL) as acute myocardial injury (MIn) and explains that this may exist as a clinical entity in its own right. In addition, this latest consensus statement updates the definitions of the five subtypes of MI. Criteria required for the diagnosis of types 1, 2 and 3 includes acute MIn in conjunction with clinical evidence of myocardial ischaemia. The different subtypes are then differentiated by the aetiology of the myocardial ischaemia. MI caused by atherothrombotic coronary artery disease (CAD) and usually precipitated by atherosclerotic plaque disruption (rupture or erosion) is designated as type 1 MI. Evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute atherothrombosis meets the criteria for type 2 MI. Cardiac death in patients with symptoms suggestive of myocardial ischaemia and presumed new ischaemic ECG changes before cTn values become available or abnormal meets criteria for type 3 MI.
The 2015 ESC guidelines for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation [12] continues to recommend serial cTn testing and presents a 0 h/3 h and 0 h/1 h rule in and rule out algorithm for clinical use. Those patients presenting post-exercise typically fall within this cohort and thus utilisation of their suggested cTn testing schedule is logical.
3. cTn elevation following exercise
Earlier investigations using 2nd and 3rd generation assays reported significant elevations of cTnT and I following endurance exercise [[14], [15], [16], [17], [18]]. Deriving common findings from these studies has been challenging for several reasons. Variables, including exercise modality, intensity and duration of exercise, are not standardised between studies. Furthermore, the use of different assays, each with its own sensitivity and specificity and threshold levels for detection and limit, makes cross comparison of results difficult. Finally, the timing for sampling troponin can differ between trials. As described later, the levels of troponin rise and fall over 24 h making it imperative to standardise sampling time points.
By taking advantage of the existence of only one cTnT assay (second or third- generation) (Roche Diagnostics, Lewes, UK) and limiting the search criteria to cTnT, Shave and colleagues were able to combine data sets and overcome some of these limitations. The authors examined 1120 cases from 26 trials finding that post-exercise cTnT concentrations exceeded the assay's lower limit in approximately one-half of participants. In addition, that the detection of cTnT following cycling races was found to be approximately half of that found after running events. The incidence of a cTnT rise was found to increase the shorter the duration of the endurance event and the lower the body mass of the participant. Regwan et al. performed a systematic review of 16 studies investigating cTn elevation related to marathon running and included the detection of TnT and I by different standard sensitivity assays. In order to pool data, levels of cTnT and I were categorised according to whether they were detectable (non-negative) or above the threshold for myocardial necrosis (above the URL). 6/940 (0.6%) of subjects were reported to have a non-negative cTn prior to the race, compared to 579/936 (62%) after the race (P < 0.001). When the threshold criteria for myocardial necrosis was used, the pre-race incidence of positivity was 0/848 (0%) and post-race was 124/845 (15%).
More recently the number of studies using HS- cTn assays has increased. A systematic review performed by Vilela et al. investigates HS-cTn elevation after running [19]. Ten studies with a total number of 479 runners between 2009 and 2013 are identified. Although again not directly comparable due to the use of different assays the authors attempt to pool results by dichotomising data into those with values above and below the 99th percentile of the HS-cTn assay used. They find that all participants demonstrated a detectable HS-cTn following completion of the race and that 69.8% (296/424) had a value above the 99th percentile, the cut-off used for myocardial necrosis. More recently Richardson et al. [20] evaluate HS-cTn using a fifth generation high-sensitivity assay (Elecsys, Roche Modular E170) and find that all 52 marathon runners have an increase above the reference value, reporting mean post-race values of 74 ± 30 ng/L−. These studies do not differentiate between those elevated troponin levels of male and female participants. Although differences between the threshold set for the 99th percentile has been seen [21,22], evidence suggests that a change to differentiate between gender is not needed for diagnostic performance [23].
Enhanced sensitivity of assays has increased the number of detectable cTn values, including those above the level used to diagnose myocardial necrosis. Taking into account the central role troponin plays in the risk stratification of ACS it is imperative that the mechanism and thus significance of troponin rise is understood.
4. Mechanism of cTn release
Several theories have been proposed to explain the mechanism underlying Tn release following exercise. Currently the most well received is that of increased membrane permeability of cardiomyocytes, whereby unbound cTn found in the cytosol diffuses across a concentration gradient from the intra- to extra-cellular compartment. The initial peak, illustrated in Fig. 2, would represent this release of Tn through the sacrolemmal membrane with levels subsequently decreasing over 24 h reflecting the half-life and clearance of cTn subunits thereafter.
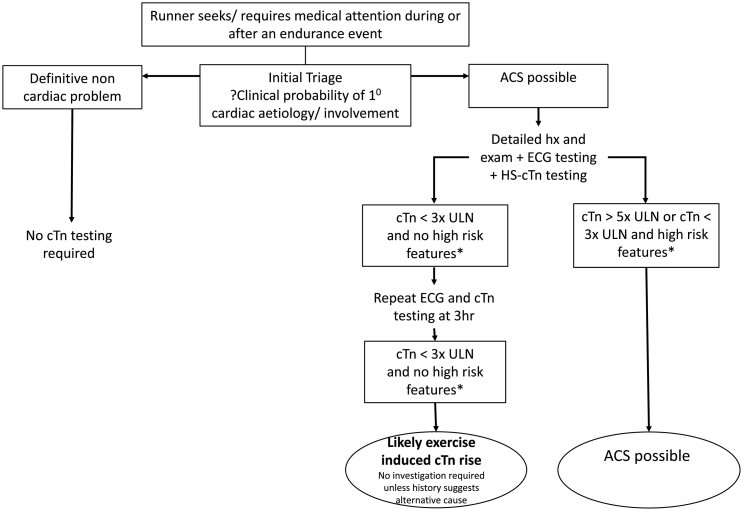
Fig. 2.
Algorithm outlining proposed management of patients with suspected ACS after exercise
Algorithm for the initiation of cTnT testing in patients with suspect non ST-elevation ACS syndrome after exercise. This is proposed to supplement not replace standard clinical guidelines. High risk features include haemodynamic instability or shock, ongoing chest pain refractory to medical treatment, heart failure, life threatening arrhythmias or arrest and dynamic ST or T wave changes.
It has been suggested that mechanical stress through the transient disruption (wounding) of the sarcolemma might be responsible for this increased membrane permeability. Initially demonstrated in the skeletal muscle of rats after a period of downhill running [24], it was shown that these ‘cell wounds’ resolve within 24 h following the same time-course as the CK levels detected. These events have also been demonstrated in-vivo cardiac muscle and have been associated with the release of growth factors [25,26]. It is logical to think, therefore, that troponin elevation following exercise may be a reflection of the adaptive cellular cascades seen in exercise-induced cardiac remodelling and hypertrophy.
An alternative hypothesis is that of integrin-stimulated troponin release. Integrins are transmembrane glycoproteins that mediate the attachment of cells to the extracellular matrix [27]. They are responsive to stretch, such as that seen in haemodynamic overload, and transmit force across the plasma membrane. This has been shown to trigger intracellular signalling pathways responsible for cardiac hypertrophy [28] and it is proposed that Tn is released as a by-product of this process. Recently, Hessel et al. [29] linked the stimulation of integrin to the reversible release of Tn from cultured cardiomyocytes. The pentapeptide Gly-Arg-Gly-Asp-Ser is a known agonist for integrin stimulation. Treatment of myocytes with this peptide resulted in a two to three-fold increase in TnI release versus various controls. Reversible myocardial injury was inferred by the absence of LDH release in treated cells relative to controls.
There is uncertainty as to how the intracellular cTn moves into the bloodstream. An alternative mechanism of increased membrane permeability has been suggested. This involves the formation of blebs which bud off from the plasma membrane of the cell, these have been seen in the liver in response to cellular ischaemia [30]. If the ischaemia is limited and re‑oxygenation occurs the blebs may be released into the circulation without rupture of the plasma membrane, resulting in a one-off release of cytoplasmic contents. If the ischaemia is sustained the blebs will grow and eventually rupture leading to cell necrosis. Support for this process occurring in the heart comes from studies showing the presence of blebs on cultured cardiomyocytes subjected to hypoxia and release of cTn, without the development of cellular necrosis [31].
Whether the release of cTn from the myocardium is related to intact subunits or degradation products is also under debate. In contrast to Hessel's study [29] where intact cTn is released, Feng and colleagues [32] demonstrated the release of degraded cTn subunits in response to preload. Without having assays specific to cTn degradation products, the form of cTn released following exercise is not known.
Tn release following prolonged and/or strenuous exercise could be secondary to subclinical apoptosis or necrosis. The kinetics of exercise-induced cTn rise with a peak within the first 1–4 h and falling levels thereafter with resolution at 72 h make this suggestion unlikely. This in contrast to the peak seen at 24 h after a type 1 MI peak with continued sustained levels for several days [33]. Finally injured skeletal muscle may release proteins that are detected by TnT assays resulting in situations where elevated cTn values may originate from skeletal muscle [[34], [35], [36]]. This as yet has only been seen in those neuromuscular conditions and its applicability to healthy participants completing a bout of exercise is uncertain.
5. Kinetics of cTn release
In order to understand the underlying mechanism, it is useful to look at the kinetics of cTn release following a bout of endurance exercise. A review of the literature identifies 12 studies investigating the kinetics of cTn release following prolonged exercise [15,[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] (see Table 1). All cTn kinetic trials except that by Middleton et al. [37] demonstrate a peak within the first 24 h after exercise. To establish the timing of the cTn peak blood samples must be drawn at multiple time points within this time. The most comprehensive data set is provided by Tian et al. [45] who conduct HS-cTnT sampling at 1, 2, 3, 4, 5, 6 and 24 h. They confirm a cTn peak at 3–4 h in 13 adolescents and 13 adults after 90 min of treadmill running at 90% of ventilatory threshold. However when evaluating cTn kinetic studies conducted following a marathon using the gold standard HS-cTnT assay [41,43,44] sampling is only performed from 24 h onwards and thus a comprehensive trend of the rise and fall of troponin cannot be seen. To expand on our understanding of cTn kinetics in the field we evaluated runners at multiple time points within the first 24 h of marathon completion. We believe we are the first study to do this and thus are providing valuable new information in this field.
Table 1.
Overview of trials showing cTn release kinetics.
| Study (year) | N | Male/ female | Mean age (years) | Type of race | Troponin | Timing of sample collection (bolded timepoint giving highest prevalence) | Highest prevalence of post-exercise cTn elevation |
|---|---|---|---|---|---|---|---|
| Neumayr et al (2001) [15] | 38 | 38/ 0 | 35 | 230km mountain cycle at 5500km altitude | TnI | Baseline, immediately and 1 day after the race |
13/38 (34%) above URL of 0.05ug/L |
| Hermann et al (2003) [46] | 46 | 40/ 6 | 40 | Marathon | TnT TnI |
Baseline, <15 mins, 3 and 24 hours (9/46) after the race | TnT – not documented TnI – 33/46 (72%) above URL of 0.04ug/L and 27/46 (58%) above AMI cut off of 0.06ug/L |
| Frassl et al (2008) [42] | 15 | 0/ 15 | 37 | Marathon | TnT TnI |
Baseline, immediately and 24 and 72 hours after the race | TnT – 8/15 (53%) above URL/AMI cut off of 0.01ng/mL TnI – 5/15 (33%) above URL of 0.03ug/mL |
| La Gerche et al (2008) [38] | 27 | 20/ 7 | 32 | Ultra endurance triathlon (3.8km swim, 180km cycle, 42.2km run) | TnI | Baseline, immediately and 1 week after the race | 15/26 (58%) above URL of 0.16ug/L |
| Middleton et al (2008) [37] | 9 | 9/ 0 | - | Treadmill marathon | TnT | Baseline and then every 30 mins within the race and 1, 3, 6, 12 and 24 hours after race completion | 9/9 (100%) within race and 8/9 (89%) after race completion, URL not documented |
| Serrano-Osteriz et al (2011) [47] | 21 | 19/ 2 | 38 | Treadmill running for 45, 90 & 180 mins at 85 and 90% IAT | TnI | Baseline, within 30 mins and 3 hours after the race | 0/19 (0%) above URL of 0.04ug/L |
| Scherr et al (2011) [41] | 102 | 102/ 0 | 42 | Marathon | HS-TnT | Baseline, within 1 hour, 24 and 72 hours after the race | 91/102 (89%) above URL of 14ng/L |
| Nie et al (2011) [39] | 12 | 12/ 0 adolescents | 16 | Track running | TnT TnI |
Baseline, 2, 4 and 24 hours after the race | TnT – 2/3 (67%) above threshold for MIn 0.03ng/mL and for MI 0.05ng/ml TnI – 11/12 () above threshold for MIn 0.06ng/mL and ¼ (25%) for MI 0.5ng/mL |
| Tian et al (2012) [45] | 26 | 13/ 0 (adults) and 13/ 0 (adolescents) | 24 and 14 respectively | 90 minutes treadmill running at 90% VT | HS-TnT | Baseline, immediately after and 1, 2, 3, 4, 5, 6, and 24 hours after the race | Adults – 11/13 (85%) above URL of 14ng/L Adolescents - 12/13 (92%) above URL of 14ng/L |
| Wilhelm et al (2012) [44] | 10 | 10/ 0 | 34.9 | Marathon | HS-TnT | Baseline, immediately after and 1, 5 and 8 days after the race |
9/10 (90%) above URL of 14ng/L |
| Roca (2017) [43] | 79 | 57/ 22 | 39 | Marathon | HS-TnT | Baseline, within 2 hours and 48 hours after the race |
79/79 (100%) above URL of 14ng/L |
| Legaz-Arrese (2017) [40] | 66 | 7/9 (adults) and 25/25 (adolescents) | 31 and 15 respectively | Swimming | HS-TnT | Baseline, immediately after and 1, 3, 6, 12 and 24 hours after the race | 41/66 (62%) above URL of 14ng/L |
| Baker et al (2019) unpublished | 26 | 18/ 8 | 40 | Marathon | HS-TnT | Baseline, immediately after and 3, 6 and 24 hours after race | 26/26 (100%) above URL of 14ng/L |
BNP = brain natriuretic protein; TnI = cardiac troponin I; TnT = cardiac troponin T; CK = creatine kinase; h-FABP = heart-type fatty acid binding protein; HS-CRP = high-sensitivity C reactive protein; HS-TnT high-sensitivity troponin T; HBD = hydroxybutyrate dehydrogenase; IL-6 = interleukin 6; NT-proBNP = N terminal pro-hormone of brain natriuretic peptide; N = number of runners.
This table illustrates all studies demonstrating cTn kinetics after exercise. It describes the basic demographics of the participants (age, sex), exercise performed and sampling points used.
6. cTn kinetics data following 2017 Brighton Marathon
As part of a subgroup analysis looking at cTnT rise following completion of the 2017 Brighton Marathon, 28 runners consented to further blood draws. Sampling timing included baseline, 0, 3, 6 and 24 h after the marathon. The HS-cTnT assay used (Roche Modular E170 (fifth generation); Basel, Switzerland) had a lowest limit of detection (LLD) of 3 ng/L and diagnostic levels for myocardial necrosis, based on the 99th percentile of the individual cTnT assay with a coefficient of variation ≤10%, were 14 ng/L−. Baseline characteristics are given in Table 1.
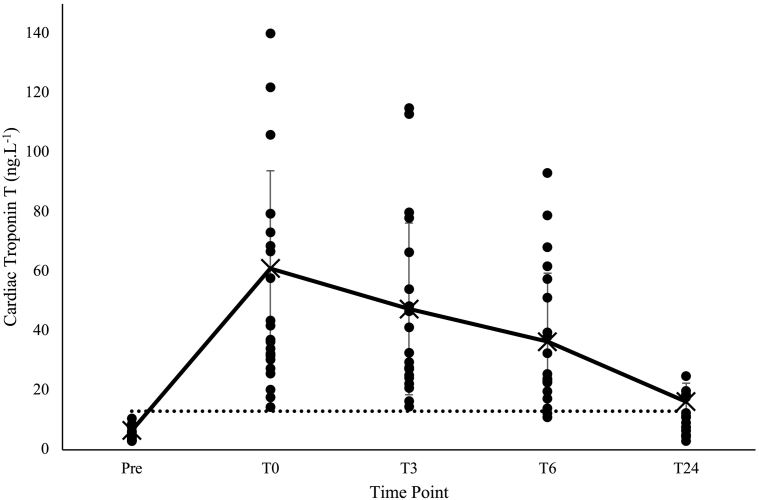
All samples, including those taken before the race, were detectable for troponin with the HS-cTn assay. Immediately following and 3 h after the marathon troponin levels for all participants exceeded the threshold for diagnosis of myocardial infarction. At 6 h this fell to 90% and at 24 h to 22% of participants (Fig. 1).
Fig. 1.
CTnT kinetics pre-marathon and 0, 3, 6 and 24 h post marathon.
Mean (X) and standard deviation of high-sensitivity cTnT values at baseline and 0, 3, 6 and 24 h. The dotted line indicates reference value (99th percentile) of 14 ng/L−.
When comparing our data to that seen in the study by Tian et al. several differences are seen. Firstly, the timing of the cTn peak is earlier in our study with a maximal cTn value occurring at 1 or 3 h, with the majority occurring within the first hour after the marathon (17/26). Furthermore, data within Tian's study demonstrates reduced variance which may be related to the matching of training status in participants and the exposure to an exercise regime with a shorter fixed duration and intensity. This is in contrast to our study where runners would set their own running pace and be exposed to additional environmental factors such as temperature. The disparity between the timing of peak cTnT is more likely to be related duration rather exercise intensity. Richardson et al. [20] showed that marathon runners exercised at 101 ± 5% of ventilatory threshold, the findings also demonstrated that working at a greater relative exercise intensity was associated with greater cTn. This intensity is much greater than that used by Tian et al. [45] or by Serrano-Ostariz et al. [47] who also demonstrated peak values to occur earlier after lower intensity work. Data showed working at 85% of the individual anaerobic threshold (IAT) induced peak values 30 min post exercise in comparison to 3 h for 95% of IAT. Therefore, the duration of exercise (265 ± 52 min in this study) seems to be the most important comparative factor, allowing Tn levels longer to rise before post-race measurement can occur.
Our data, and that of the studies found above, is at odds with the study by Middleton et al. [37]. They investigated nine trained males over the duration of a treadmill marathon. Although the data showed a cTn rise during marathon running, cTn then returned to baseline before the end of activity. Additionally, participants demonstrated a biphasic response with peak values returning 12–24 h post-race. This response is difficult to explain but could be as a result of participants recovering and attempting to restart daily activity 12–24 h post marathon. The lack of in-exercise blood sampling in the present study and the literature base is a limitation and requires further investigation.
7. Clinical approach to patients with post-exercise troponin elevation
CTn testing continues to play a central role in the risk stratification and subsequent management of those with an acute coronary syndrome. Updated guidance on the diagnosis and management of MI and Non ST-elevation ACS recommends the use of the 5th generation HS- cTn assays [9,12]. Serial testing, of at least two sampling points, is recommended to overcome the analytical and biological variation that is associated with HS-cTn assays. Using our data, and that from other kinetics studies, we support the use of repeated samples to establish cTn kinetics when attempting to differentiate between cTn elevation related to exercise and that of other causes. Using this approach in combination with the latest ESC guidelines for the management of acute coronary syndrome without persistent ST-elevation [12] allows the formulation of the below algorithm (Fig. 2) which updates that proposed by Shave et al. [48]. It is important to note that the flow chart is no replacement for clinical judgement and is meant to facilitate the clinical in their decision making process. We would urge, in the situation of clinical uncertainty, that repeated cTn testing and further investigations including evaluation by an experienced cardiologist occur in the hospital setting.
8. Further studies
Although a variety of plausible mechanisms for post-exercise cTn elevation have been proposed no one has been confirmed. Without understanding the mechanism, elucidating the clinical significance is difficult. With a large body of evidence indicating that cTn elevation in other clinical situations has a quantitative negative prognosis the need to establish this information has never been greater. Currently cTn elevation following exercise is considered benign and affected individuals are not counselled nor treated. Further investigations studying the peak cTn value and/or the kinetics and its relation to future medical events are warranted to ensure this line of action is appropriate.
9. Conclusion
CTn testing continues to be central to the assessment of patients with a suspected ACS. The current use of HS-cTn assays reveals cTn elevation in all those participating in exercise. In the short term these individuals show no increased risk of cardiac events however long term data is lacking. Our findings and that from other studies suggest an early cTn peak and that time frame therefore should be considered when individuals present with elevated values following exercise. Taking into account the kinetics of post-exercise cTn elevation and the characteristics of HS-cTn assays we propose a new updated clinical algorithm for the management of those presenting with clinical symptoms compatible with a cardiac event after exercise.
Acknowledgments
Acknowledgements
Thanks must go to the Brighton marathon organisers, medical team and of course the willing runners. We would also like to thank the laboratory support team of undergraduate and postgraduate students from the University of Brighton. The project was funded by the British Association of Sport and Exercise Medicine (BASEM) and the Centre of Sport and Exercise Medicine (SESAME), University of Brighton. The study was undertaken as part of the Brighton Marathon Research Group.
Funding
Funding for the project came from the 2016/2017 British Association of Sport and Exercise Medicine (BASEM) research bursary, an award applied for and won by the corresponding author, Dr. Polly Baker. BASEM is a charitable organisation that provides support for those working in Sport and Exercise Medicine.
Disclosures
There are no relationships with industry nor any conflicts of interests.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Contributor Information
Polly Baker, Email: pollybaker@cantab.net, @drpollybaker.
Todd Leckie, Email: t.d.leckie@gmail.com, @Todd_Leckie.
Derek Harrington, Email: derek.harrington@nhs.net.
Alan Richardson, Email: A.J.Richardson@brighton.ac.uk, @AlanRichardson.
References
- 1.Warburton D.E.R., Nicol C.W., Bredin S.S.D. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers J. Exercise and Cardiovascular Health. Circulation. 2003;107(1):2e–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 3.Cullen L., Mueller C., Parsonage W.A., Wildi K., Greenslade J.H., Twerenbold R. 62(14) 2013. Validation of High-Sensitivity Troponin I in a 2-Hour Diagnostic Strategy to Assess 30-Day Outcomes in Emergency Department Patients With Possible Acute Coronary Syndrome; pp. 1242–1249. [DOI] [PubMed] [Google Scholar]
- 4.Than M., Cullen L., Aldous S., Parsonage W.A., Reid C.M., Greenslade J. 2-hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J. Am. Coll. Cardiol. 2012;59(23):2091–2098. doi: 10.1016/j.jacc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Paiva L., Providência R., Barra S., Dinis P., Faustino A.C., Gonçalves L. Universal definition of myocardial infarction: clinical insights. Cardiol. 2015;131(1):13–21. doi: 10.1159/000371739. [DOI] [PubMed] [Google Scholar]
- 6.Frankenstein L., Wu A.H.B., Hallermayer K., Wians F.H., Giannitsis E., Katus H.A. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin. Chem. 2011;57(7):1068–1071. doi: 10.1373/clinchem.2010.158964. [DOI] [PubMed] [Google Scholar]
- 7.Vasile V.C., Saenger A.K., Kroning J.M., Jaffe A.S. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin. Chem. 2010;56(7):1086–1090. doi: 10.1373/clinchem.2009.140616. [DOI] [PubMed] [Google Scholar]
- 8.Wu A.H.B., Quynh A.L., Todd J., Moecks J., Wians F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin. Chem. 2009;55(1):52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Third universal definition of myocardial infarction. Eur. Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 10.Morrow D.A., Cannon C.P., Jesse R.L., Newby L.K., Ravkilde J., Storrow A.B. National Academy of Clinical Biochemistry Laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin. Chem. 2007;53(4):552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 11.Wu A.H.B., Christenson R.H., Greene D.N., Jaffe A.S., Kavsak P.A., Ordonez-Llanos J. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the academy of the American Association for Clinical Chemistry and the task force on clinical applications of cardiac bio-markers. Clin. Chem. 2018 Apr 1;64(4):645–655. doi: 10.1373/clinchem.2017.277186. [DOI] [PubMed] [Google Scholar]
- 12.Roffi M., Patrono C., Collet J.-P., Mueller C., Valgimigli M., Andreotti F. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv408. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. Circulation ESC/ACC/AHA/WHF expert consensus document fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 14.Neilan T.G., Januzzi J.L., Lee-Lewandrowski E., Ton-Nu T.T., Yoerger D.M., Jassal D.S. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston Marathon. Circulation. 2006;114(22):2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- 15.Neumayr G., Gaenzer H., Pfister R., Sturm W., Schwarzacher S.P., Eibl G. Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise. Am. J. Cardiol. 2001;87(3):369–371. doi: 10.1016/s0002-9149(00)01382-5. [DOI] [PubMed] [Google Scholar]
- 16.Fortescue E.B., Shin A.Y., Greenes D.S., Mannix R.C., Agarwal S., Feldman B.J. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007;49(2) doi: 10.1016/j.annemergmed.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Scharhag J., George K., Shave R., Urhausen A., Kindermann W. Exercise-associated increases in cardiac biomarkers. Med. Sci. Sports Exerc. 2008;40(8):1408–1415. doi: 10.1249/MSS.0b013e318172cf22. [DOI] [PubMed] [Google Scholar]
- 18.Shave R., George K.P., Atkinson G., Hart E., Middleton N., Whyte G. Exercise-induced cardiac troponin T release: a meta-analysis. Med. Sci. Sports Exerc. 2007;39(12):2099–2106. doi: 10.1249/mss.0b013e318153ff78. [DOI] [PubMed] [Google Scholar]
- 19.Vilela E.M., Bastos J.C.C., Rodrigues R.P., Nunes J.P.L. High-sensitivity troponin after running – a systematic review. Neth. J. Med. 2014;72(1):5–9. [PubMed] [Google Scholar]
- 20.Richardson A.J., Leckie T., Watkins E.R., Fitzpatrick D., Galloway R., Grimaldi R. Post marathon cardiac troponin T is associated with relative exercise intensity. J. Sci. Med. Sport. 2018 Mar;21(9):880–884. doi: 10.1016/j.jsams.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Eggers K.M., Johnston N., James S., Lindahl B., Venge P. Cardiac troponin I levels in patients with non–ST-elevation acute coronary syndrome—the importance of gender. Am Heart J. Sep 1 2014;168(3):317–324.e1. doi: 10.1016/j.ahj.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Shah A.S.V., Griffiths M., Lee K.K., McAllister D.A., Hunter A.L., Ferry A.V. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015 Jan 21;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balmelli C., Meune C., Twerenbold R., Reichlin T., Rieder S., Drexler B. Comparison of the performances of cardiac troponins, including sensitive assays, and copeptin in the diagnostic of acute myocardial infarction and long-term prognosis between women and men. Am. Heart J. 2013 Jul 1;166(1):30–37. doi: 10.1016/j.ahj.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 24.McNeil P.L., Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 1992;140(5):1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke M.S., Caldwell R.W., Chiao H., Miyake K., McNeil P.L. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 1995;76(6):927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 26.George K., Whyte G.P., Green D.J., Oxborough D., Shave R.E., Gaze D. The endurance athletes heart: acute stress and chronic adaptation. Br. J. Sports Med. 2012;46(Suppl. 1):i29–i36. doi: 10.1136/bjsports-2012-091141. [DOI] [PubMed] [Google Scholar]
- 27.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brancaccio M., Hirsch E., Notte A., Selvetella G., Lembo G., Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc. Res. 2006;70(3):422–433. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Hessel M.H.M., Atsma D.E., Van Der Valk E.J.M., Bax W.H., Schalij M.J., Van Der Laarse A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch Eur J Physiol. 2008;455(6):979–986. doi: 10.1007/s00424-007-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gores G.J., Herman B., Lemasters J.J. Plasma membrane bleb formation and rupture: a common feature of hepatocellular injury. Hepatology. 1990 Apr 1;11(4):690–698. doi: 10.1002/hep.1840110425. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz P., Piper H.M., Spahr R., Spieckermann P.G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am. J. Pathol. 1984;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- 32.Feng J., Schaus B.J., Fallavollita J.A., Lee T., Canty J.M. Myocardial ischemia. Young. 2001:2035–2037. doi: 10.1161/01.cir.103.16.2035. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan V.S., Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124(21):2350–2354. doi: 10.1161/CIRCULATIONAHA.111.023697. [DOI] [PubMed] [Google Scholar]
- 34.Wens S.C.A., Schaaf G.J., Michels M., Kruijshaar M.E., Van Gestel T.J.M., In 'T Groen S. Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ. Cardiovasc. Genet. 2016;9(1):6–13. doi: 10.1161/CIRCGENETICS.115.001322. [DOI] [PubMed] [Google Scholar]
- 35.Olson K.N., Saenger A.K., Jaffe A.S., Vasile V.C., Apple F.S., Milone M. Diseased skeletal muscle. J. Am. Coll. Cardiol. 2011;58(17):1819–1824. doi: 10.1016/j.jacc.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Rittoo D., Jones A., Lecky B., Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. J. Am. Coll. Cardiol. 2014;63(22):2411–2420. doi: 10.1016/j.jacc.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Middleton N., George K., Whyte G., Gaze D., Collinson P., Shave R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J. Am. Coll. Cardiol. 2008;52(22):1813–1814. doi: 10.1016/j.jacc.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 38.La Gerche A., Connelly K.A., Mooney D.J., Macisaac A.I., Prior D.L. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart. 2008;94(7):860–866. doi: 10.1136/hrt.2006.101063. [DOI] [PubMed] [Google Scholar]
- 39.Nie J., Tong T.K., George K., Fu F.H., Lin H., Shi Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports. 2011;21(5):625–629. doi: 10.1111/j.1600-0838.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 40.Legaz-arrese A., Carranza-garcía L.E., Navarro-orocio R., Valadez-lira A., Mayolas-pi C., Munguía-izquierdo D. Cardiac biomarker release after endurance exercise in male and female adults and adolescents. J. Pediatr. 2017;191:96–102. doi: 10.1016/j.jpeds.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 41.Scherr J., Braun S., Schuster T., Hartmann C., Moehlenkamp S., Wolfarth B. 72-h kinetics of high-sensitive troponin T and inflammatory markers after Marathon. Med. Sci. Sports Exerc. 2011;43(10):1819–1827. doi: 10.1249/MSS.0b013e31821b12eb. [DOI] [PubMed] [Google Scholar]
- 42.Frassl W., Kowoll R., Katz N., Speth M., Stangl A., Brechtel L. Cardiac markers (BNP, NT-pro-BNP, Troponin I, Troponin T) in female amateur runners before and up until three days after a marathon. Clin. Lab. 2015;3(May):81–87. [PubMed] [Google Scholar]
- 43.Roca E., Nescolarde L., Lupón J., Barallat J., Januzzi J.L., Liu P. The dynamics of cardiovascular biomarkers in non-elite Marathon runners. J. Cardiovasc. Transl. Res. 2017;10(2):206–208. doi: 10.1007/s12265-017-9744-2. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm M., Zueger T., De Marchi S., Rimoldi S.F., Brugger N., Steiner R. Inflammation and atrial remodeling after a mountain marathon. Scand J Med Sci Sport. 2014;24(3):519–525. doi: 10.1111/sms.12030. [DOI] [PubMed] [Google Scholar]
- 45.Tian Y., Nie J., Huang C., George K.P. The kinetics of highly sensitive cardiac troponin T release after prolonged treadmill exercise in adolescent and adult athletes. J. Appl. Physiol. 2012;113(3):418–425. doi: 10.1152/japplphysiol.00247.2012. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann M., Scharhag J., M M., Urhausen A., Herrmann W., Kindermann W. Post-race kinetics of cardiac troponin T and I and N-terminal pro-brain natriuretic peptide in marathon runners. Clin. Chem. 2003;49(5):831–834. doi: 10.1373/49.5.831. [DOI] [PubMed] [Google Scholar]
- 47.Serrano-Ostáriz E., Terreros-Blanco J.L., Legaz-Arrese A., George K., Shave R., Bocos-Terraz P. The impact of exercise duration and intensity on the release of cardiac biomarkers. Scand J Med Sci Sport. 2011;21(2):244–249. doi: 10.1111/j.1600-0838.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 48.Shave R., Baggish A., George K., Wood M., Scharhag J., Whyte G. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J. Am. Coll. Cardiol. 2010;56(3):169–176. doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]