Abstract
Background
There is no information on the prevalence and incidence of atrial fibrillation in Ethiopia. We aimed to investigate the prevalence, risk factors and anticoagulant requirements of atrial fibrillation in an elderly Jimma population.
Methods
In a community-based cross-sectional study in 634 adult (≥40 years) Jimma population, we performed cardiovascular health examinations including a 12-lead electrocardiogram to estimate AF prevalence. A standardized questionnaire was used to collect information on medical history, lifestyle and use of medications. Stroke risk stratification was done using CHA2DS2-VASc score. We used logistic regressions to determine the potential risk factors of AF.
Results
The overall prevalence of AF was 4.3%. AF was associated with sex, current smoking, hypertension and BMI. Nineteen out of twenty seven participants with AF were in need of anticoagulation to prevent risk of stroke.
Conclusion
The prevalence of AF is high and common risk factors were sex, current smoking, hypertension, and higher BMI in this cohort. More than two-third of study participants with AF were at higher need of oral anticoagulants.
Keywords: Atrial fibrillation, CHA2DS2-VASc, Jimma Town, Prevalence, Risk factors
1. Introduction
Atrial fibrillation (AF) is the commonest arrhythmia associated with a significant cardiovascular disease burden, with increased risk of a number of co-morbidities; of particular concern is the five-fold increased risk of stroke [1]. AF is often identified by the clinical observation of an irregular pulse; the suspicion must always be confirmed with a resting electrocardiogram (ECG) [2].
The principal pathophysiological mechanisms can be divided into electrophysiological and structural abnormalities [3]. Interestingly, AF is a self-perpetuating arrhythmia, inducing remodeling changes that are in themselves a cause of AF [3,4].
It has been shown that AF is probably triggered by rapidly firing focal sources. Moreover, atrial interstitial fibrosis has also been shown to increase AF vulnerability by creating a substrate for intra-atrial re-entry circuit (intracellular scar) that promotes AF. Atrial interstitial fibrosis is increased with age in humans and in congestive heart failure [4].
AF can be symptomatic with tachycardia, but is usually asymptomatic or mildly symptomatic and even up to 75% of cases are silent. AF can be permanent, but in the beginning is often paroxysmal. That explains why the detection rate of AF is low in the clinical practice and why the prevalence of atrial fibrillation varies between studies [5,6].
Data from the West reveal that the incidence and prevalence of AF are higher in men as compared to females with reported male: female rates of appx 1.1% vs 0.8%. In both Framingham Heart Study and Atherosclerosis Risk in Communities Study, men had a 1.5-fold greater risk of developing AF than women and the lifetime risk of developing AF after age of 40 in the Framingham cohort was reported to be 26% for men and 23% for women [7].
The Framingham Heart Study and other investigations have identified advancing age, male sex, hypertension, diabetes mellitus, obesity, heart failure, valve disease, myocardial infarction (MI), smoking, alcohol consumption, and khat chewing as major risk factors for AF [1,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]].
Apart from its effect on cardiac function, AF is a major risk factor for stroke and systemic embolization, and this risk is increasing significantly with the aging of the population, with up to 5% of those 70 years or older having this condition [34,35]. AF increases the risk of stroke by approximately 5-fold and doubles the rate of mortality in patients with concomitant heart disease compared with age-matched controls. It has been shown that there is a steep age-related increase in the risk of stroke in patients with AF, ranging from 1.5% at age 50 to 59 years to 23.5% at age 80 to 89 years [34,36].
The global prevalence of AF is estimated to be 0.47%, but there is significant regional variation [37]. However, most of the available data on prevalence of AF is from studies performed in North America and Western Europe. There is a significant lack of epidemiological information regarding AF in the low/middle-income nations, including Ethiopia. Prevalence studies are important in estimating the population burden of the disease and are instrumental in developing effective public health policies [38].
Stroke occurring in patients with AF is associated with increased mortality and morbidity, severe disability, greater rate of stroke recurrence, and longer hospitalization [39]. CHADS2 (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes, and prior Stroke or transient ischemic attack (TIA) [doubled]) [40], and CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75 years [doubled], Diabetes, prior Stroke or TIA [doubled] e Vascular disease, Age 65–74 years, and Sex category [female]) [41] are 2 well-validated risk-stratification scores for predicting stroke in patients with atrial fibrillation. CHA2DS2-VASc score was recommended for risk assessment of stroke by the 2010 European Society of Cardiology, and the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for the management of patients with atrial fibrillation [[42], [43], [44]].
A community-based study on the prevalence of AF has never been carried out in a multi-ethnic country such as Ethiopia. Ethiopia is the second most populous country in Africa with a population of over 100 million. Being a sub-Saharan country it is facing the double burden of diseases because of epidemiological transition. Predisposing factors for cardiovascular disease and sudden death due to cardiovascular disorders are rampant in Ethiopia. Therefore, it is important to determine the prevalence of AF and its associated risk factors in the country.
2. Materials and methods
2.1. Study population
We conducted a cross-sectional study on adults aged 40 and above living in Jimma town, Southwest Ethiopia from May to August 2017. Ethiopia is located at the horn of Africa and has a population of 106. It follows three-tier health care system which consists of tertiary level (specialized hospitals: serving 3.5–5 million people), Secondary level (General hospitals: serving 1–1.5 million people), and Primary level (primary hospital: serving 60,000–100,000 peoples; Health center: serving 15,000–25,000 people; and Health post: serving 3000–5, 000 people). In the country one medical doctor provide service for about 28, 867 people. Jimma Town is located 352 km away from the capital Addis Ababa, Southwest of Ethiopia. It has a population of over 200,000 and it has one specialized hospital which provides healthcare service for over 15 million people from nearby regions.
Sample size was determined using single population proportion formula. Since, no previous similar studies were done in Ethiopia; the p-value was taken as 0.5 and margin of error 0.05. A 10% non-response rate was used. After design effect of 1.5, extrapolated from WHO estimate for risk factor assessment studies, we get sample size of 634.
Multi-stage sampling technique was utilized. Initially six Kebeles (lowest administrative unit in Ethiopia) were selected from the seventeen Kebeles of the town by simple random sampling, then the total sample size of 634 were distributed to the six Kebeles employing proportional to size allocation to the Kebeles. A systematic sampling technique was undertaken to identify the study households from each Kebele. The sample sizes were divided proportionally to the respective Kebeles. An individual from the household was then chosen with a lottery method.
The study was approved by the Ethics Committee of Jimma University in Jimma, Ethiopia, and all procedures were performed in accordance with its ethical standards. Written consent was obtained from all participants after they had been informed of the study's objectives, and confidentiality safeguards for personal information. If the participants were illiterate, we obtained written informed consent from their proxies.
2.2. Data collection and measurement
Data were collected during a single household visit by trained General practitioners and nurses using a standard questionnaire in a face-to-face interview. All potential investigators received training on the purpose of the study, how to administer the questionnaire, the standard methods of measurement, the importance of standardization, and study procedures. Investigators were under supervision during data collection.
The questionnaire assessed data on socio-demographic characteristics, lifestyle risk factors (smoking, alcohol drinking, khat chewing), and family history of AF and related chronic diseases.
Diabetes mellitus was diagnosed if the subjects had a fasting glucose level of or higher (≥126 mg/dL) or was taking insulin or an oral hypoglycemic agent at the current examination [45]. Hypertension was defined for each examination as a systolic blood pressure of at least 140 mm Hg or a diastolic blood pressure of at least 90 mm Hg on each of two readings or the use of antihypertensive medication [46].
Weight and height were measured using stadiometer (TANITA 380, Tokyo, Japan) to the nearest 0.1 kg and 0.1 cm, respectively, with the participants in lightweight clothing and without shoes. The body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters.
Waist circumference was measured at the horizontal plane that corresponds with the mid-point between the anterior superior iliac spine and the lower costal margin at the mid-clavicular line using stretch resistant tape-meter. We used the European cut off to interpret the waist circumference measurements for the Sub-Saharan African People as per the WHO and International Diabetes Federation recommendations. According to the above guidelines the range of abnormal waist circumference of male and female are ≥94 cm and ≥80 cm, respectively [47].
Twelve-lead ECGs (ECG1200G, YSIP-155, Beijing, China) (resting, 10 s) were performed on all participants by well-trained General practitioners with a standardization of 1 mV = 10 mm and paper speed of 25 mm/s. ECG-based AF diagnoses were reviewed by at least two independent cardiologists. ECG electrode explores electrical impulse generated through extracellular fluid; we first have participants lie supine on examination bed calmly and then clean the subsequent anatomical location for each electrode with alcohol, cut if nonconductive tissue such as hair(in male participants) were there and then applied cardio-cream to enhance conductivity; electrodes were placed accordingly and participants were asked to close his/her eyes to avoid disturbances and eventually after clear waveform recruited the electrocardiogram result was printed. Our ECG machine is rechargeable and once recharged it can record for >10 h if continually used or for couple of days otherwise.
2.3. Statistical analysis
Data entered into EPI data manager software version 4.0.2 (Odense, Denmark) and were exported to a computer software package SPSS (V.21; SPSS, IBM, Chicago, Illinois, USA) for statistical analysis. Categorical variables were described with frequency and percentage. Binary and multivariable logistic analyses were used to compare AF and associated factors. P value ≤0.05 was considered to indicate statistical significance. Bivariate analyses were done and all covariate variables which had association with the outcome variables at P-value of 0.25 were selected for multivariate analyses. Multivariable logistic regression models were used to isolate independent predictors of AF. Normality of continuous variables was checked using graphic methods (Histograms with normality curves and QQ-plots) and models were selected depending on the type of dependent variable (Logistic regression for binary variable) and multicollinearity was checked and Hosmer Lemeshaw test was done for assessing goodness of model fitness.
3. Result
3.1. Socio-demographic characteristics of the participants
A total of 634 participants took part in this study out of whom 360 (56.8%) were females while 274 (43.2%) were males.
The mean age of the participants was 63.3 ± 11.9. Majority of study participants were in age group of 60–69 years. Regarding the ethnicity of the participants, majority of them were Oromo 295 (46.5%) followed by Amhara 108 (17.0%). Three hundred twenty seven (51.6%) participants were Orthodox Christians, 272 (42.9%) were Muslims and the remaining 5.5% were others.
Concerning the marital status, 351 (55.4%) of the participants were married, and 205 (32.3%) were widowed/widower and majority of the participants (40.5%) were unable to read and write whereas 231 (36.4%) had got primary education. About 208 (32.8%) of the study participants were house wives followed by pensioner, 84 (13.2%) (Table 1).
Table 1.
Socio-demographic characteristics of the participants, Jimma town, May to July 2017.
| Characteristics | Frequency | Percent |
|---|---|---|
| Sex | ||
| Male | 274 | 43.2 |
| Female | 360 | 56.8 |
| Age | ||
| 40–49 | 78 | 12.3 |
| 50–59 | 133 | 21.0 |
| 60–69 | 199 | 31.4 |
| 70–79 | 166 | 26.2 |
| 80+ | 58 | 9.1 |
| Ethnicity | ||
| Oromo | 295 | 46.5 |
| Amhara | 108 | 17.0 |
| *Others | 231 | 36.4 |
| Religion | ||
| Orthodox | 327 | 51.6 |
| Protestant | 28 | 4.4 |
| Muslim | 272 | 42.9 |
| °Others | 7 | 11 |
| Marital status | ||
| Married | 351 | 55.4 |
| Widowed/widower | 205 | 32.3 |
| **Others | 78 | 12.3 |
| Educational status | ||
| Illiterate | 257 | 40.5 |
| Primary (1–8) | 231 | 36.4 |
| Secondary (9–12) | 98 | 15.5 |
| Diploma and above | 48 | 7.6 |
| Occupation | ||
| Employee | 212 | 33.4 |
| Housewife | 208 | 32.8 |
| ^Others | 214 | 33.8 |
| Income | ||
| Low income | 518 | 81.7 |
| High income | 51 | 8.0 |
*Woliata, Kafa, Dawuro, Gurage, Silte, Yem etc. Catholic, J. witness, Waqefata;**single/unmarried, separated, Divorced; ^None, Pensioners.
3.2. Prevalence of atrial fibrillation
The prevalence of atrial fibrillation among the study participants was found to be 4.3% (27/634). The prevalence rose steeply with advancing age in the study participants. It was 5.1% (4/78) in people aged 40–49 year where as 6.9% (4/58) in those whose age was ≥80 yr. The mean age of individuals with AF was approximately 62.6 yr. There was no significant difference in prevalence of AF between men and women (p = 0.145) (Table 2).
Table 2.
Prevalence of atrial fibrillation according to age group and sex.
| Age groups (yr) | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| 40–49 | 2 (8.3%) | 2 (3.7%) | 4 (0.6%) |
| 50–59 | 1 (1.8%) | 5 (6.6%) | 6 (0.9%) |
| 60–69 | 2 (2.1%) | 6 (5.9%) | 8 (0.1%) |
| 70–79 | 3 (4.0%) | 2 (2.2%) | 5 (0.7%) |
| ≥80 | 0 (0.0%) | 4 (10.8%) | 4 (0.6%) |
| Total | 8 (2.9%) | 19 (5.3%) | 27 (4.3%) |
% = number of atrial fibrillation/number of participants.
3.3. Factors associated with AF
Bivariate analysis indicated that hypertension (COR = 2.42; 95% C.I = 1.103–5.301; P = 0.028) and study participants who had high risk for OSA (COR = 2.86; 95% C.I = 1.195–6.879; P = 0.018) were independently associated with AF; whereas sex, age, occupation, marital status, current smoking, current drinking, khat chewing, diabetes, prior MI, history of stroke and higher body mass index were not associated with AF (Table 3).
Table 3.
Bivariate analysis of selected risk factors for atrial fibrillation.
| Variables | AF |
COR (95% C.I) | P value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Sex | Female | 19 | 341 | 1 | |
| Male | 8 | 266 | 1.853 [0.799, 4.298] | 0.151 | |
| Age | 40–49 | 4 | 74 | 1 | |
| 50–59 | 6 | 127 | 0.874 [0.239, 3.198] | 0.839 | |
| 60–69 | 8 | 191 | 0.775 [0.227, 2.651] | 0.684 | |
| 70–79 | 5 | 161 | 0.575 [0.150, 2.201] | 0.419 | |
| ≥80 | 4 | 54 | 1.370 [0.328, 5.724] | 0.666 | |
| Occupation | Employee | 9 | 203 | 0.786 [0.287, 2.150] | 0.638 |
| Housewife | 7 | 201 | 1.222 [0.496, 3.013] | 0.663 | |
| Others | 11 | 203 | 1 | ||
| Marital status | Married | 10 | 341 | 0.428 [0.142, 1.29] | 0.132 |
| Widowed/wer | 12 | 193 | 0.908 [0.309, 2.67] | 0.860 | |
| Others | 5 | 73 | 1 | ||
| Current smoker | Yes | 4 | 44 | 2.225 [0.737, 6.720] | 0.156 |
| No | 23 | 563 | 1 | ||
| Current drinker | Yes | 7 | 207 | 0.676 [0.281, 1.626] | 0.382 |
| No | 20 | 400 | 1 | ||
| Khat chewing | Yes | 7 | 170 | 0.900 [0.374, 2.166] | 0.814 |
| No | 20 | 437 | 1 | ||
| HTN | Yes | 16 | 228 | 2.42 [1.103, 5.301] | 0.028⁎ |
| No | 11 | 379 | 1 | ||
| DM | Yes | 5 | 74 | 1.64 [0.602, 4.454] | 0.33 |
| No | 22 | 533 | 1 | ||
| OSA | Low risk for OSA | 7 | 304 | 1 | 0.018⁎ |
| High risk OSA | 20 | 303 | 2.86 [1.195, 6.879] | ||
| Previous MI | Yes | 3 | 82 | 0.8 [0.236, 2.718] | 0.72 |
| No | 24 | 525 | 1 | ||
| History of stroke | Yes | 1 | 25 | 0.895 [0.117, 6.86] | 0.915 |
| No | 26 | 582 | 1 | ||
| BMI | <18.5 | 4 | 81 | 1 | |
| 18.5–24.9 | 10 | 346 | 0.441 [0.134, 1.45] | 0.178 | |
| 25–29.9 | 8 | 134 | 0.910 [0.264, 3.14] | 0.882 | |
| ≥30 | 5 | 66 | 1.155 [0.296, 4.50] | 0.835 | |
Candidate variables for multivariate logistic regression.
A multivariate logistic regression analysis revealed that sex, current smoking, hypertension and BMI were independent risk factors for AF (P < 0.05) (Table 4). The strongest risk factor for AF was being male with an odds ratio of 2.71; 95% C.I = 1.025–7.173 and P = 0.04.
Table 4.
Multivariable logistic regression model predicting AF among study participants in Jimma Town, 2017.
| Variables | AOR | 95% C.I | P value |
|---|---|---|---|
| Intercept | 0.008 | ||
| Sex | 2.71 | 1.025, 7.173 | 0.04 |
| Current smoker | 0.25 | 0.72, 0.84 | 0.02 |
| Hypertension | 0.41 | 0.17, 0.93 | 0.03 |
| BMI | 1.04 | 1.000, 1.071 | 0.04 |
3.4. Stroke risk stratification scores of AF cases
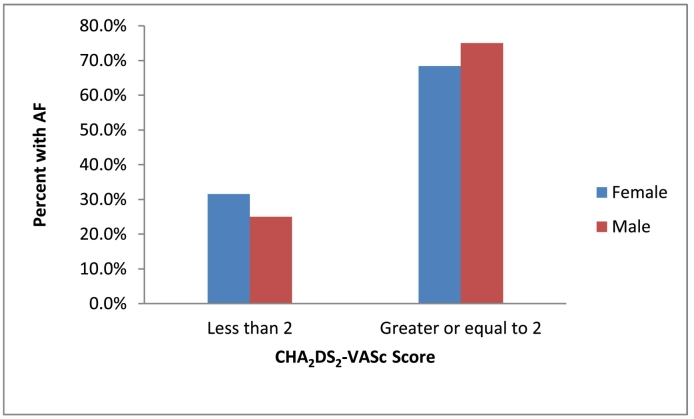
Stroke risk stratification in participants with AF was done using validated CHA2DS2-VASc score which includes: congestive heart failure, Age ≥75, DM, Stroke, Vascular diseases, Age from 65 to 74 years and female sex category. All components have score of 1 each except age ≥75 and stroke which have score of 2 each. Accordingly, CHA2DS2-VASc score of ≥2 in male and CHA2DS2-VASc score ≥3 in female mandates for start of anti-coagulations. So in current study nineteen out of twenty seven participants with AF have to take anticoagulation to prevent risk of ischemic attack (Fig. 1).
Fig. 1.
Sex based distributions of atrial fibrillation by CHA2DS2-VASc Scoring system in study participants, Jimma Town, May to July 2017.
4. Discussions
This community-based study confirmed a 4.3% prevalence of AF in an adult urban Jimma population. This result is in line with a study conducted in an urban South African community which reported a 4.6% AF prevalence [48].
In contrary to the current study, the prevalence of AF in Ivory Coast, and Senegal were 5.5% and 5.4%, respectively [49]. The difference in the findings might be due to study setting. The studies in Ivory Coast and Senegal were hospital based where as our study was community based. Similarly, a study done in India found a 5.1% prevalence of AF; which is higher than our finding. This discrepancy might be attributed to the difference in the screening tool used to detect AF and their sample size is by half lower than our study [50].
A strikingly lower prevalence rate of AF was reported in a Kenyan and a Tanzanian study in which the prevalence of AF among the participants of both studies was 0.7% [51,52]. This low prevalence might be due to the patients have been taken medication for underlying cause. Yet, a recent nationwide, retrospective, observational Italian study involving 233 general practitioners and screening almost 300,000 patients representative of the population, the prevalence of AF was 2.0% [53].
In this study, sex, current smoking, hypertension and BMI were independent risk factors for AF. However, khat chewing, diabetes, prior MI, history of stroke, and occupation were not associated with an increasing risk of AF. The results of our study are consistent with previous reports [15,54,55].
In this study, men were found to be two times at higher risk for AF than women. Other studies have also reported that men are more prone to the development of atrial fibrillation [56,57]. In the Framingham Heart Study, men were found to have a 1.5-fold higher risk of developing atrial fibrillation compared with women [58]. Our result indicated that current cigarette smokers were 25% more likely to develop AF than non-smokers. Similar finding was reported by Alanna M. et al. that smoking was associated with the incidence of AF, with more than a 2-fold increased risk of AF attributed to current smoking [15,59].
In the present study hypertensives were 0.41 times more likely to have AF. Similar study done in Malaysia reported significant association between AF and hypertension [60] and in a study done to determine prevalence of AF in patient with HTN it found to be 3.46%, indicating hypertension as independent predictor for commonest sustained arrhythmia [61]. Hypertension has two major consequences on the heart: left ventricular hypertrophy, and morphological and functional alterations of the coronary macro- and micro-vessels. These two cardiac modifications are responsible for 3 types of complications: myocardial ischemia, left ventricular dysfunction and electrical instability, which are involved in the pathogenesis of atrial and ventricular arrhythmias in hypertensive patients [62].
The finding of the current study has indicated that study participants whose body mass index were greater than or equal to thirty were at higher risk for AF than those who were normal weight. Obesity is one of the very few identified modifiable risk factors for the occurrence and progression of atrial fibrillation, and the mechanisms linking atrial fibrillation and obesity include: structural and electrophysiological atrial remodeling, metabolic factors, sympatho-vagal imbalance, clinical links (obstructive sleep apnea, cardiovascular comorbidities) and inflammation [63].
In current study we used CHA2DS2-VASc scoring to evaluate the anticoagulant requirement of study participants with atrial fibrillation. According to ‘Ten Commandments’ of 2016 European Society of Cardiology guideline for management of atrial fibrillation, stroke risk evaluation is based on the CHADS-VASc score. With a score ≥2 in male and ≥3 in female patients, anticoagulation for stroke prevention is clearly recommended, while in a score of 1 in males and 2 in females, anticoagulation should be considered. No antithrombotic therapy of any kind should be prescribed in patients with a CHADS-VASc score of 0 (males) or 1 (females) [64]. Accordingly nineteen out of twenty seven study participants with diagnosis of atrial fibrillation have CHA2DS2-VASc ≥2 so that they deserved anticoagulation for prevention of stroke.
This study had some limitations. First, it was cross-sectional and could only reflect the associations between risk factors and AF. Second, we didn't use Holter-monitor ECG so we may have missed paroxysmal AF and also echocardiography was not employed. Thus a large scale community based study using Holter-monitor and echocardiography is highly recommended.
5. Conclusion
In conclusion, there is a high prevalence of AF (4.3%) in adults living in Jimma Town. Male sex, current smoking, hypertension, and higher BMI were identified as independent risk factors for AF in this population. Early screening and management of modifiable risk factors are an important way out to mitigate the burden of the disease.
List of abbreviations
Acknowledgments
Acknowledgements
This study was funded by Jimma University.
Conflicts of interest
The authors declare no conflict of interest in this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.02.003.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mcaloon C.J. Atrial Fibrillation: the nuts and bolts for primary care. 2016;10(3):142–157. [Google Scholar]
- 2.Camm J., Kirchoff P., Lip G. Guidelines for the management of atrial fibrillation. Eur. Soc. Cardiol. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S., Harada M. Atrial remodeling and atrial fibrillation. Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014;63(22):2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 4.Yap Y.G., Camm J. Essentials of Atrial Fibrillation. Springer Healthcare; Tarporley: 2014. Pathogenesis of atrial fibrillation; pp. 7–10. [Google Scholar]
- 5.Li L.H., Sheng C.S., Hu B.C., Huang Q.F., Zeng W.F., Li G.L., Liu M., Wei F.F., Zhang L., Kang Y.Y., Song J. The prevalence, incidence, management and risks of atrial fibrillation in an elderly Chinese population: a prospective study. BMC Cardiovasc. Disord. 2015 Dec;15(1):31. doi: 10.1186/s12872-015-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell A., Haynes J., Smith R. Evaluation of asymptomatic atrial fibrillation. Am. Fam. Physician. 2012;86(6) 15. [PubMed] [Google Scholar]
- 7.Chandh Raja D., Kapoor A. 2016. Epidemiology of Atrial Fibrillation -An Indian Perspective; pp. 7–10. [PubMed] [Google Scholar]
- 8.Sun G.Z., Guo L., Wang X.Z., Song H.J., Li Z., Wang J., Sun Y.X. Prevalence of atrial fibrillation and its risk factors in rural China: a cross-sectional study. Int. J. Cardiol. 2015 Mar 1;182:13–17. doi: 10.1016/j.ijcard.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 9.Jayed D., Al-huthi M.A. 2016. Khat Chewing Induces Cardiac Arrhythmia; pp. 1–7. [Google Scholar]
- 10.Al-Motarreb A., Al-Kebsi M., Al-Adhi B., Broadley K.J. Khat chewing and acute myocardial infarction. Heart. 2002 Mar 1;87(3):279–280. doi: 10.1136/heart.87.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan N.A.G.M., Gunaid A.A., Murray-Lyon I.M. 2007. Khat(Catha edulis): Health Aspects of Chewing. [PubMed] [Google Scholar]
- 12.Vanwalleghem I.E., Vanwalleghem P.W., De Bleecker J.L. Khat chewing can cause stroke. Cerebrovasc. Dis. 2006;22(2–3):198–200. doi: 10.1159/000093807. [DOI] [PubMed] [Google Scholar]
- 13.Shan H., Zhang Y., Lu Y., Zhang Y., Pan Z., Cai B. 2009. Downregulation of miR-133 and miR-590 Contributes to Nicotine-Induced Atrial Remodelling in Canines; pp. 465–472. [DOI] [PubMed] [Google Scholar]
- 14.Alessandro A.D., Boeckelmann I., Goette A., Hammwho M. 2011. Nicotine, Cigarette Smoking and Cardiac Arrhythmia: An Overview. [DOI] [PubMed] [Google Scholar]
- 15.Heeringa J., Kors J.A., Hofman A., Van Rooij F.J.A., Witteman J.C.M. Cigarette smoking and risk of atrial fibrillation: the Rotterdam study. Am. Heart J. 2008;156(6):1163–1169. doi: 10.1016/j.ahj.2008.08.003. [Internet] [DOI] [PubMed] [Google Scholar]
- 16.Nakou E.S., Mavrakis H., Vardas P. Editorial are diabetic patients at increased risk of arrhythmias? Hell. J. Cardiol. 2012;53:335–339. [PubMed] [Google Scholar]
- 17.Sarapultsev P., Yushkov B., Sarapultsev A. Prevalence of arrhythmias in patients with type 2 diabetes and the role of structural changes in myocardium in their development, DiabetesMetab. Syndr. Clin. Res. Rev. 2017:S567–S576. doi: 10.1016/j.dsx.2017.04.006. [Internet]. https://www.scopus.com/record/display.uri?eid=2-s2.0-85017452337&origin=inward. [DOI] [PubMed] [Google Scholar]
- 18.Shenasa M., Shenasa H., Rouhani S. Atrial fibrillation in different clinical subsets. Manag. Atrial Fibrillation. 2014:25–74. [Google Scholar]
- 19.Mozos I. Arrhythmia risk and obesity. J. Mol. Genet. Med. 2014;s1(1):1–10. [Google Scholar]
- 20.Mahajan R., Lau D.H., Brooks A.G., Shipp N.J., Manavis J., Wood J.P.M. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J. Am. Coll. Cardiol. 2015;66(1):1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 21.Markides V., Schilling R.J. Atrial fibrillation: classification. Pathophysiol. Mech. Drug Treat. 2003:939–943. doi: 10.1136/heart.89.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrisan S. The state of the art. Korea Times. 2007;91:1778–1810. [Google Scholar]
- 23.Conen D., Tedrow U.B., Koplan B.A., Glynn R.J., Buring J.E., Albert C.M. 2009. Influence of Systolic and Diastolic Blood Pressure on the Risk of Incident Atrial Fibrillation in Women; pp. 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau Y., Yiu K., Siu C., Tse H. Hypertension and atrial fibrillation: epidemiology, pathophysiology and therapeutic implications. 2011;26(10):563–569. doi: 10.1038/jhh.2011.105. [DOI] [PubMed] [Google Scholar]
- 25.Grundvold I., Skretteberg P.T., Liestøl K., Erikssen G., Kjeldsen S.E., Arnesen H. 2012. Epidemiology/Population Science Upper Normal Blood Pressures Predict Incident Atrial Fibrillation in Healthy Middle-Aged Men a 35-Year Follow-Up Study. [DOI] [PubMed] [Google Scholar]
- 26.Cintra F.D., Leite R.P., Storti L.J., Bittencourt L.A., Poyares D., De L. 2014. Sleep Apnea and Nocturnal Cardiac Arrhythmia: A Populational Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gami A.S., Pressman G., Caples S.M., Kanagala R., Gard J.J., Davison D.E. Arrhythmia/Electrophysiology. 2004. et al. [Google Scholar]
- 28.Apnea S. 2003. Part II: Central Sleep Apnea; pp. 1822–1826. [Google Scholar]
- 29.Thihalolipavan S., Morin D.P. ScienceDirect atrial fibrillation and heart failure: update 2015. Prog. Cardiovasc. Dis. 2015;58(2):126–135. doi: 10.1016/j.pcad.2015.07.004. [Internet] [DOI] [PubMed] [Google Scholar]
- 30.Gamra H., Alam S., Ponikowski P., Lewalter T. 2012. Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice; pp. 632–640. [DOI] [PubMed] [Google Scholar]
- 31.Dar B. 2016. Magnitude of Stroke and Associated Factors Among Patients Who Attended the Medical Ward of Felege Hiwot Referral. [Google Scholar]
- 32.Teodorescu C., Narayanan K., Chugh H., Gunson K., Jui J., Chugh S.S. The association between atrial fibrillation and sudden cardiac death the relevance of heart failure. 2014;2(3) doi: 10.1016/j.jchf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Johnson B., Francis J. Stress and cardiac arrhythmias. Indian Pacing Electrophysiol. J. 2014;14(5):230–232. doi: 10.1016/s0972-6292(16)30793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannon N., Sheehan O., Kelly L., Marnane M., Merwick A., Moore A., Kyne L., Duggan J., Moroney J., McCormack P.M., Daly L. Stroke associated with atrial fibrillation–incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc. Dis. 2010;29(1):43–49. doi: 10.1159/000255973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menezes A.R., Lavie C.J., DiNicolantonio J.J., O'keefe J., Morin D.P., Khatib S., Milani R.V. Mayo Clinic Proceedings. vol. 88. Elsevier; 2013 Apr 1. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies; pp. 394–409. No. 4. [DOI] [PubMed] [Google Scholar]
- 36.Miller P.S., Andersson F.L., Kalra L. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke. 2005;36:360–366. doi: 10.1161/01.STR.0000153002.56324.8c. [DOI] [PubMed] [Google Scholar]
- 37.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saggu D.K., Sundar G., Nair S.G., Bhargava V.C., Lalukota K., Chennapragada S., Narasimhan C., Chugh S.S. Prevalence of atrial fibrillation in an urban population in India: the Nagpur pilot study. Heart Asia. 2016 Apr 1;8(1):56–59. doi: 10.1136/heartasia-2015-010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saposnik G., Gladstone D., Raptis R., Zhou L., Hart R.G., Investigators of the Registry of the Canadian Stroke Network, Stroke Outcomes Research Canada (SORCan) Working Group Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013 Jan 1;44(1):99–104. doi: 10.1161/STROKEAHA.112.676551. [DOI] [PubMed] [Google Scholar]
- 40.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 41.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 42.Camm A.J., Lip G.Y., De Caterina R. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guide- lines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 43.January C.T., Wann L.S., Alpert J.S., American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Saliba W., Gronich N., Barnett-Griness O., Rennert G. Usefulness of CHADS2 and CHA2DS2-VASc scores in the prediction of new-onset atrial fibrillation: a population-based study. Am. J. Med. 2016 Aug 1;129(8):843–849. doi: 10.1016/j.amjmed.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 45.WHO Follow-up report on the diagnosis of diabetes mellitus. 2003;26(11) doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 46.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018 May 7;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Federation I.D. 2006. The IDF Consensus Worldwide Definition of the Metabolic Syndrome Part 1: Worldwide Definition for Use in Clinical Practice. [Google Scholar]
- 48.Sliwa K., Carrington M.J., Klug E. Predisposing factors and incidence of newly diagnosed atrial fibrillation in an urban African community: insights from the Heart of Soweto Study. Heart. 2010;96(23):1878–1882. doi: 10.1136/hrt.2010.206938. [DOI] [PubMed] [Google Scholar]
- 49.Stambler B.S., Ngunga L.M. 2015. Atrial Fibrillation in Sub-Saharan Africa: Epidemiology, Unmet Needs, and Treatment Options; pp. 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soni A., Earon A., Handorf A., Fahey N., Talati K., Chon K. 2016. High Burden of Unrecognized Atrial Fibrillation in Rural India: An Innovative Community-Based Cross-Sectional Screening Program Corresponding Author. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shavadia J., Yonga G., Mwanzi S., Jinah A., Moriasi A., Otieno H. Clinical characteristics and outcomes of atrial fibrillation and flutter at the Aga Khan University Hospital, Nairobi. Cardiovasc. J Afr. 2013;24(2):6–9. doi: 10.5830/CVJA-2012-064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.20. Dewhurst M.J., Adams P.C., Gray W.K. Strikingly low prevalence of atrial fibrillation in elderly Tanzanians. J. Am. Geriatr. Soc. 2012;60(6):1135–1140. doi: 10.1111/j.1532-5415.2012.03963.x. [DOI] [PubMed] [Google Scholar]
- 53.Zoni-Berisso M., Filippi A., Landolina M. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study) Am. J. Cardiol. 2013;111:705–711. doi: 10.1016/j.amjcard.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Jeong J.H. 2005. Prevalence of and Risk Factors for Atrial Fibrillation in Korean Adults Older than 40 Years; pp. 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatem S.N. 2015. Atrial Fibrillation and Obesity: Not Just a Coincidence. [DOI] [PubMed] [Google Scholar]
- 56.Pothineni N.V., Vallurupalli S. Gender Differences in the Pathogenesis and Management of Heart Disease. Springer; Cham: 2018. Gender differences in atrial fibrillation: incidence, mechanistic basis of the differences and treatment options; pp. 271–285. [Google Scholar]
- 57.Roten L., Rimoldi S.F., Schwick N., Sakata T., Heimgartner C., Fuhrer J., Delacrétaz E., Tanner H. Gender differences in patients referred for atrial fibrillation management to a tertiary center. Pacing Clin. Electrophysiol. 2009 May;32(5) doi: 10.1111/j.1540-8159.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 58.Misier R., Klinieken I., Paul J., Isala O., Elvan A., Klinieken I. 2014. Sex-Based Differences in Cardiac Arrhythmias, ICD Utilisation and Cardiac Resynchronisation Therapy Sex-Based Differences in Cardiac Arrhythmias, ICD Utilisation and Cardiac Resynchronisation Therapy. [Google Scholar]
- 59.Chamberlain A.M., Agarwal S.K., Folsom A.R., Duval S., Soliman E.Z., Ambrose M. NIH Public Access. 2012;8(8):1160–1166. [Google Scholar]
- 60.Lim C.W., Kasim S., Ismail J.R., Yul N., Chua C., Khir R.N. 2016. Prevalence of Atrial Fibrillation in the Malaysian Communities; pp. 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krittayaphong R., Rangsin R., Thinkhamrop B., Hurst C., Rattanamongkolgul S., Sripaiboonkij N. Prevalence and associating factors of atrial fibrillation in patients with hypertension: a nation-wide study. 2016;16(1):57. doi: 10.1186/s12872-016-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishaq M., Samad A. 2010. Cardiac Arrhythmias and Left Ventricular Hypertrophy in Systemic Hypertension Riffat Sultana, Nuzhat Sultana, Abdul Rashid, Syed Zahid Rasheed, Mansoor Ahmed; pp. 2008–2011. [PubMed] [Google Scholar]
- 63.Mozos I. Arrhythmia risk and obesity. J. Mol. Genet. Med. 2014;s1(1):1–10. [Google Scholar]
- 64.“Ten Commandments” of 2016 ESC Guidelines for the Management of Atrial Fibrillation. 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material