Abstract
Background
“Frailty” is associated with poor prognosis in ST-elevated myocardial infarction (STEMI). However, there is little data regarding the impact of the Canadian Study of Health and Aging Clinical Frailty Scale (CFS), a simple and semiquantitative tool for assessing frailty, on mid-term mortality in STEMI patients.
Methods
A total of 354 consecutive STEMI patients (mean age 69.8 ± 12.4 years; male 76.6%) who underwent percutaneous intervention between July 2014 and March 2017 were retrospectively reviewed. The study endpoint was mid-term mortality according to the CFS classification. Furthermore, in order to clarify the impact of CFS upon admission on mid-term mortality, the independent predictors of all-cause death were evaluated.
Results
Patients were categorized into three groups (CFS 1–3, n = 281; CFS 4–5, n = 62; and CFS 6–7, n = 11). During the study period (median 474 days), all-cause death was observed in 39 patients. After multivariate Cox regression analysis, higher CFS (adjusted hazard ratio [HR] 2.34, 95% confidence interval [CI] 1.43–3.85, p < 0.001), higher Killip score (adjusted HR 2.46, 95%CI 1.30–5.78, p = 0.002), and lower serum albumin level (adjusted HR 4.29, 95%CI 2.16–8.51, p < 0.001) were significantly associated with an increased risk of all-cause death.
Conclusion
In conclusion, severe frailty was associated with mid-term mortality in STEMI patients who underwent PCI.
Keywords: Coronary heart disease, ST-elevated myocardial infarction, Frail, Prognosis
1. Introduction
The popularity of percutaneous coronary intervention (PCI) for the treatment of ST-elevation myocardial infarction (STEMI) has increased over the past decades. Currently, the concept of “frailty” has become more common in the field of intervention in which there is an increased tendency for dependency and/or mortality when a patient is exposed to a stressor. Frailty can occur as a result of a range of diseases and medical conditions [1]. Frailty is associated with body mass index (BMI) and serum albumin level, which are reported to correlate with a poor prognosis in patients with acute coronary syndrome (ACS) [[2], [3], [4]]. There are several frailty scoring systems. The Canadian Study of Health and Aging Clinical Frailty Scale (CFS) [5] is based on the clinician's judgement derived from an interview without the need for laboratory assessments or diagnostic testing devices. The scores are based on fitness, active disease, activities of daily living, and cognition. However, the impact of CFS, which is a simple tool for assessing patient frailty, on outcomes after STEMI is unclear. The aim of this study was to evaluate the impact of CFS on mid-term mortality in STEMI patients.
2. Methods
2.1. Study design and population
The medical records of 354 consecutive patients who were admitted to the Ogaki Municipal Hospital with acute STEMI and underwent primary PCI between July 2014 and March 2017 were retrospectively reviewed. The medical ethics committee of our hospital approved this study.
STEMI was defined as follows: (a) clinical evidence of ischemia (chest pain, chest tightness, radiating pain, dyspnea, nausea, and cold sweat); (b) electrocardiogram showing new ST elevation at the J-point in two contiguous leads, with a cutoff point of ≥0.2 mV in men and ≥0.15 mV in women in leads V2–V3 or ≥0.1 mV in the other leads; and (c) at least one high myocardial biomarker level, defined as serum troponin I or creatine kinase level above the 99th percentile of the normal reference population during the first 24 h after admission [6]. Patients were excluded if they had: (a) suspected STEMI but did not undergo coronary angiography (CAG) because of the attending physician's decision or patient's request; (b) coronary spasms or Takotsubo cardiomyopathy; or (c) culprit lesions but did not undergo PCI because of the attending physician's decision.
2.2. Data collection
Baseline patient characteristics, including age, sex, BMI, systolic and diastolic blood pressure, and heart rate were recorded. Hypertension was defined as current or previous treatment with antihypertensive medication. Diabetes mellitus was defined as current or previous treatment with antidiabetic medication (insulin or oral hypoglycemic drugs) or a hemoglobin A1c level of ≥6.5% (National glycohemoglobin standardization program) [7]. Dyslipidemia was defined as current or previous treatment with anti-dyslipidemic medication. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2 [8]. Previous myocardial infarction, congestive heart failure, ischemic stroke, PCI, and coronary artery bypass grafting were recorded based on interviews with the patients and/or their relatives at admission. The time periods from the onset of myocardial infarction to reperfusion and from door to balloon were recorded. The laboratory findings recorded included the baseline white blood cell count, hemoglobin concentration, peak creatine kinase, peak creatine kinase MB isoenzyme, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (LDL-C), and albumin. The Killip classification was determined based on the physical examination findings and the systolic blood pressure. Significant cognitive disorder was defined as a score ≥ III for degree of independent living [9]. Frailty was assessed using the CFS [5,10]. Patients were categorized into three groups based on the CFS validation study [2,10,11]: CFS 1–3 (not frail), CFS 4–5 (at risk-to-mildly frail), and CFS 6–9 (frail). CFS data assessment was retrospectively performed by two investigators. CFS assessment was conducted with the primary physician using chart reviews which were prepared during very early cardiac rehabilitation by rehabilitation therapists as well as those made by nurses to assess patients' preadmission history, including activities of daily living and cognitive function, by interview to patients and their relatives.
2.3. Study endpoints
The study endpoint was mid-term mortality according to the CFS classification. Furthermore, in order to clarify the impact of CFS at admission on mid-term mortality, the independent predictors of mortality were evaluated.
2.4. Treatment protocol
Upon admission, the patients underwent a quick evaluation that included history taking and physical examination, electrocardiography, chest radiography, echocardiography, and blood tests. In this study, the patients underwent immediate coronary angiography and primary PCI. Written informed consent was obtained from each patient and/or the patient's relatives before PCI. None of the patients received systemic thrombolytic therapy. Other therapeutic interventions were performed at the discretion of the attending physician, including blood transfusion for anemia, mechanical ventilation for respiratory failure or shock, administration of diuretics for congestive heart failure, and administration of inotropes for hypotension or hypoperfusion.
2.5. Statistical analysis
Continuous variables are expressed as means ± standard deviations (SD) or median and interquartile range (IQR; 25%–75%). Comparisons of clinical, angiographic, or procedure-related characteristics were performed using the chi-square test for categorical covariates, the 1-way analysis of variance for continuous covariates that were listed as mean value and SD and the Kruskal-Wallis test for continuous variables that were listed as medians with interquartile range according to the CFS. Comparisons of event-free survival (Kaplan–Meier curves) were performed with the log-rank test. A Cox regression analysis, using selected covariates, was performed to determine the independent predictors of mid-term all-cause death during the follow-up period following STEMI-PCI. The selected variables were, CFS, age ≥ 75, BMI, CKD, Killip class ≥ III, serum hemoglobin level, and serum albumin level < 3.5 g/dL, based on their association with mid-term all-cause death (p < 0.1). Other variables were identified in a previously published study [12] (sex, dyslipidemia, diabetes mellitus, hypertension, and current smoking). To avoid over-fitting, the number of variables used in the final multivariable model was limited to 1 for every 8 to 10 events. The results are reported as adjusted hazard ratios (a-HR) with associated 95% confidence intervals (CIs). We used SPSS version 22 (SPSS Inc., Chicago, Illinois) for all statistical analyses. All p-values were two-tailed, and results with p < 0.05 were considered to be statistically significant for all analyses.
3. Results
During the study period, 354 consecutive patients with STEMI were treated with PCI in our institute.
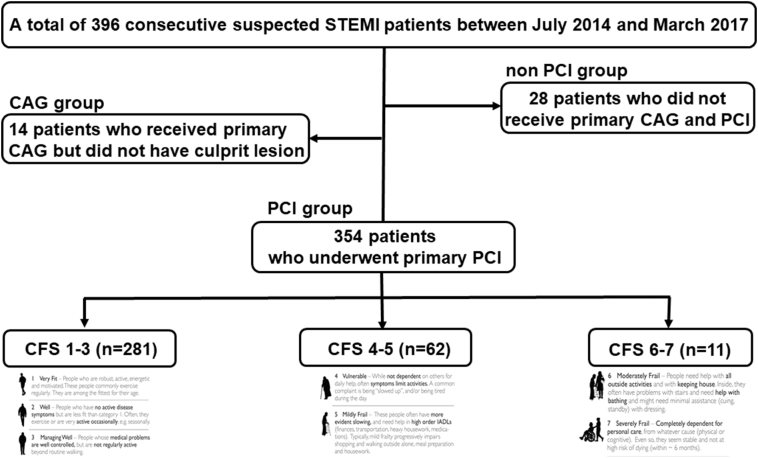
Patients (with no age limit, the youngest patient was 27 years and the oldest patient was 96 years) were further subdivided based on CFS classification: 281 patients (79.4%) in CFS 1–3, 62 patients (17.5%) in CFS 4–5, and 11 patients (3.1%) in CFS 6–7. There was no patient with CFS ≥ 8 (Fig. 1). The median clinical follow-up was 474 days (first to third quartile: 323–737 days).
Fig. 1.
Study population and classification.
Baseline clinical, laboratory, and procedural characteristics by CFS are shown in Table 1. This cohort had a mean age of 69.8 ± 12.4 years, and 76.6% were male. One hundred and ninety patients (53.7%) had hypertension, 96 (27.1%) had diabetes mellitus, and 231 (65.3%) had dyslipidemia. The median time from onset to reperfusion was 3.6 h (first to third quartile: 2.3–7.0 h), and the median time from door to balloon was 79.0 min (first to third quartile: 61.0–111.0 min). Thirty-two patients (9.0%) were classified as Killip class III or IV. Among the three study groups, significant differences were observed in mean age, gender, CKD, diastolic blood pressure, hemoglobin, total cholesterol, LDL-C, triglycerides, and serum albumin level.
Table 1.
Baseline patient characteristics, procedural characteristics and clinical outcome.
| Overall n = 354 |
CFS 1–3 n = 281 |
CFS 4–5 n = 62 |
CFS 6–7 n = 11 |
p value | |
|---|---|---|---|---|---|
| -Patient clinical characteristics | |||||
| Age, years | 69.8 ± 12.4 | 66.2 ± 11.1 | 83.5 ± 4.6 | 84.4 ± 8.0 | <0.001 |
| Age ≥ 75 years, n (%) | 131 (37.0) | 62 (22.1) | 59 (95.2) | 10 (90.9) | <0.001 |
| male, n (%) | 271 (76.6) | 239 (85.1) | 26 (41.9) | 6 (54.5) | <0.001 |
| Body mass index, kg/m2 | 23.7 ± 4.5 | 23.9 ± 3.8 | 23.0 ± 6.8 | 23.8 ± 5.6 | 0.40 |
| Prior heart failure, n (%) | 6 (1.7) | 3 (1.1) | 2 (3.2) | 1 (9.1) | 0.076 |
| Prior MI, n (%) | 30 (8.5) | 21 (7.5) | 6 (9.7) | 3 (27.3) | 0.064 |
| Prior PCI, n (%) | 32 (9.0) | 22 (7.8) | 9 (14.5) | 1 (9.1) | 0.25 |
| Prior CABG, n (%) | 2 (0.6) | 2 (0.7) | 0 (0) | 0 (0) | 0.77 |
| Prior ischemic stroke, n (%) | 14 (4.0) | 8 (2.8) | 5 (8.1) | 1 (9.1) | 0.10 |
| Dyslipidemia, n (%) | 231 (65.3) | 191 (68) | 35 (56.5) | 5 (45.5) | 0.065 |
| Diabetes mellitus, n (%) | 96 (27.1) | 75 (26.7) | 19 (30.6) | 2 (18.2) | 0.65 |
| Hypertension, n (%) | 190 (53.7) | 150 (53.4) | 36 (58.1) | 4 (36.4) | 0.40 |
| current smoking, n (%) | 141 (39.8) | 118 (42.0) | 20 (32.3) | 3 (27.3) | 0.23 |
| CKD, n (%) | 156 (44.1) | 104 (37.0) | 45 (72.6) | 7 (63.6) | <0.001 |
| -Findings at presentation | |||||
| Systolic blood pressure, mm Hg | 132.0 ± 32.5 | 133.0 ± 33.3 | 127.6 ± 30.3 | 131.7 ± 24.5 | 0.50 |
| Diastolic blood pressure, mm Hg | 77.9 ± 21.9 | 79.8 ± 22.7 | 70.0 ± 16.8 | 74.5 ± 16.0 | 0.006 |
| Heart rate, bpm | 75.4 ± 23.4 | 75.2 ± 23.6 | 74.6 ± 20.0 | 86.2 ± 23.6 | 0.28 |
| Killip class | 1.4 ± 0.8 | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.8 ± 1.0 | 0.11 |
| Killip class ≥ 3, n (%) | 32 (9.0) | 23 (8.2) | 7 (11.3) | 2 (18.2) | 0.42 |
| Hemoglobin, g/dL | 13.6 ± 2.0 | 14.0 ± 1.8 | 11.9 ± 1.8 | 12.5 ± 2.7 | <0.001 |
| LDL-cholesterol, mg/dL | 116.5 ± 36.7 | 121.0 ± 95.6 | 99.4 ± 28.1 | 99.7 ± 33.2 | <0.001 |
| HDL-cholesterol, mg/dL | 43.5 ± 12.4 | 43.4 ± 11.4 | 43.0 ± 13.0 | 47.9 ± 26.7 | 0.47 |
| Total-cholesterol, mg/dL | 184.6 ± 43.1 | 189.4 ± 44.2 | 165.4 ± 33.0 | 170.7 ± 33.3 | <0.001 |
| Triglycerides, mg/dL | 114.5 ± 89.8 | 121.0 ± 95.6 | 95.2 ± 59.8 | 60.9 ± 27.3 | 0.016 |
| eGFR, mL/min/1.73 m2 | 63.4 ± 23.7 | 66.8 ± 22.5 | 50.2 ± 23.9 | 58.7 ± 26.4 | <0.001 |
| Albumin, g/dL | 4.1 ± 0.5 | 4.2 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.5 | <0.001 |
| Albumin < 3.5 g/dL, n (%) | 35 (9.9) | 18 (6.4) | 15 (24.2) | 2 (18.2) | <0.001 |
| White blood cell count, ×103/μL | 10.1 ± 3.6 | 10.2 ± 3.6 | 9.5 ± 3.4 | 10.8 ± 4.4 | 0.29 |
| peak CK, IU/L | 1823.5 (782.8–3216.0) | 1834.0 (798.0–3349.5) | 1457.0 (689.8–2922.3) | 1917.0 (426.0–5138.0) | 0.34 |
| peak CKMB, IU/L | 180.5 (80.8–310.8) | 181.0 (81.0–304.0) | 169.0 (72.5–313.5) | 222.0 (40.0–484.0) | 0.85 |
| -Lesion characteristics | |||||
| LMT, n (%) | 8 (2.3) | 7 (2.5) | 1 (1.6) | 0 (0) | 0.80 |
| LAD, n (%) | 179 (50.6) | 151 (53.7) | 20 (32.3) | 8 (72.7) | 0.003 |
| LCx, n (%) | 26 (7.3) | 19 (6.8) | 7 (11.3) | 0 (0) | 0.30 |
| RCA, n (%) | 140 (39.5) | 104 (37.0) | 34 (54.8) | 2 (18.2) | 0.01 |
| RCA & LCx, n (%) | 1 (0.3) | 0 (0) | 0 (0) | 1 (9.1) | <0.001 |
| -Time to reperfusion | |||||
| Onset to reperfusion time, hour | 3.6 (2.3–7.0) | 3.5 (2.2–6.4) | 4.5 (2.6–9.6) | 5.7 (3.0–15.2) | 0.052 |
| Door to balloon time, minutes | 79.0 (61.0–111.0) | 76.0 (60.0–110.8) | 85.5 (67.3–110.3) | 120.0 (82.0–163.0) | 0.078 |
| -Contrast and fluoroscopy | |||||
| Contrast volume, mL | 135.2 ± 54.2 | 139.8 ± 52.7 | 115.6 ± 51.6 | 127.5 ± 81.2 | 0.006 |
| Fluoroscopy time, minutes | 22.8 (15.6–31.4) | 22.9 (15.3–31.8) | 22.7 (17.7–29.8) | 27.0 (14.2–35.9) | 0.93 |
| Exposure dose, mGy | 1342.0 (915.0–1937.0) | 1375.0 (971.0–2014.0) | 1041.0 (680.8–1668.5) | 1278.0 (1050.0–1917.0) | 0.014 |
| -In hospital clinical outcome | |||||
| All death, n (%) | 20 (5.6) | 14 (5.0) | 4 (6.5) | 2 (18.2) | 0.17 |
| Cardiac death, n (%) | 18 (5.1) | 12 (4.3) | 4 (6.5) | 2 (18.2) | 0.10 |
| CABG, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Stent thrombosis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| -Clinical outcome | |||||
| All death, n (%) | 39 (11.0) | 21 (7.5) | 13 (21.0) | 5 (45.5) | <0.001 |
| Cardiac death, n (%) | 26 (7.3) | 17 (6.0) | 6 (9.7) | 3 (27.3) | 0.022 |
| Non cardiac death, n (%) | 13 (3.7) | 4 (1.4) | 7 (11.3) | 2 (18.3) | <0.001 |
| CABG, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Stent thrombosis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
Values are numbers (%) or mean ± SD. CFS, clinical frailty scale; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; eGFR, estimated glomerular filtration rate CK, creatine kinase; CKMB, creatine kinase MB; LMT, left main trunk; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
During the follow-up period, 39 patients died after PCI. The mortality of patients increased as CFS increased. All-cause death occurred in each group as follows: CFS 1–3, 21 of 281 (7.5%); CFS 4–5, 13 of 62 (21.0%); and CFS 6–7, 5 of 11 (45.5%) patients (p < 0.001). The causes of death according to the CFS classification were as follows: CFS 1–3 comprised 17 cardiac deaths, 2 malignancies, 1 due to pneumonia, and 1 due to asthenia; CFS 4–5 included 6 cardiac deaths, 2 malignancies, 1 due to pneumonia, 1 due to asthenia, 1 cerebral contusion, and 2 due to unknown causes; and CFS 6–7 included 3 cardiac deaths, and 2 due to asthenia.
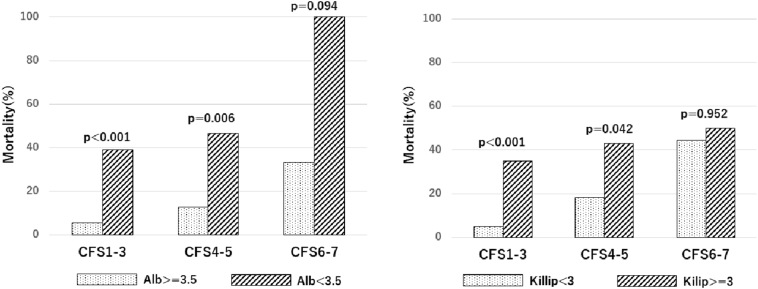
The Kaplan-Meier analysis of cumulative mortality in the three groups on the basis of CFS is presented in Fig. 2. The baseline clinical characteristics of all 354 patients and the results of the univariate regression analyses for mid-term all-cause deaths are shown in Table 2. Severe CFS, older age, higher Killip score, lower BMI, lower systolic and diastolic blood pressure, higher heart rate, lower hemoglobin, lower serum albumin, LDL-C, total cholesterol, and triglycerides levels and renal failure were significantly associated with an increased risk of mid-term all-cause mortality on univariate analysis. Furthermore, Cox multivariate regression analysis identified higher CFS (a-HR 2.46, 95% CI 1.52–3.98, p < 0.001); higher Killip score (a-HR 3.10, 95% CI 1.50–6.39, p = 0.002); and lower serum albumin level (a-HR 4.29, 95% CI 2.16–8.51, p < 0.001) as independent predictors of mid-term all-cause mortality (Table 2). CFS was a significant predictor of mid-term all-cause death. In patients with CFS 1–3 and CFS 4–5, both serum albumin level and Killip score had a significant impact on mortality but did not have an impact in patients with CFS 6–7 (Fig. 3). As shown in Fig. 4, the distribution rates of CFS differed across age groups. In younger subjects, there were few patients who were categorized as having severe CFS. Furthermore, the severity of CFS increased with age.
Fig. 2.
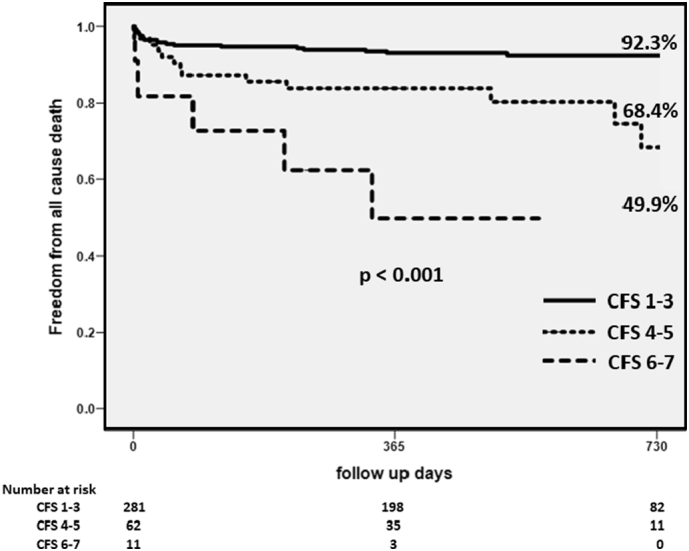
Kaplan-Meier analysis of cumulative mortality in the three groups with respect to CFS.
CFS – clinical frailty scale.
Table 2.
Univariate and Multivariate regression analysis for the association between mid-term all-cause mortality and clinical findings.
| Factors for predicting | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Mid-term all-cause mortality | ||||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| -Patient clinical characteristics | ||||||
| CFS (1–3, 4–5, 6–7) | 2.98 | 1.91–4.65 | <0.001 | 2.46 | 1.52–3.98 | <0.001 |
| Age (per 1 year increase) | 1.07 | 0.81–0.98 | <0.001 | |||
| Age ≧ 75 | 3.42 | 1.77–6.59 | <0.001 | |||
| Gender (male) | 0.68 | 0.34–1.34 | 0.26 | |||
| Body mass index, kg/m2 | 0.89 | 0.81–0.98 | 0.012 | |||
| Prior heart failure | 1.49 | 0.20–10.83 | 0.70 | |||
| Prior MI | 1.66 | 0.65–4.23 | 0.29 | |||
| Prior PCI | 1.17 | 0.42–3.30 | 0.76 | |||
| Prior CABG | 0.05 | 0.001–1.11 × 107 | 0.76 | |||
| Prior ischemic stroke | 0.64 | 0.09–4.65 | 0.66 | |||
| Dyslipidemia | 0.63 | 0.33–1.20 | 0.16 | |||
| Diabetes mellitus | 0.68 | 0.31–1.47 | 0.33 | |||
| Hypertension | 0.74 | 0.40–1.39 | 0.35 | |||
| Current smoking | 0.88 | 0.46–1.68 | 0.70 | |||
| CKD | 4.67 | 2.21–9.88 | <0.001 | |||
| -Findings at presentation | ||||||
| Systolic blood pressure, mm Hg | 0.98 | 0.97–0.99 | <0.001 | |||
| Diastolic blood pressure, mm Hg | 0.97 | 0.96–0.99 | <0.001 | |||
| Heart rate, bpm | 1.02 | 1.01–1.03 | 0.002 | |||
| Killip class | 2.23 | 1.76–2.84 | <0.001 | |||
| Killip class ≥ 3 | 5.66 | 2.87–11.2 | <0.001 | 3.10 | 1.50–6.39 | 0.002 |
| Hemoglobin, g/dL | 0.74 | 0.64–0.84 | <0.001 | |||
| LDL-cholesterol, mg/dL | 0.99 | 0.98–1.00 | 0.004 | |||
| HDL-cholesterol, mg/dL | 0.97 | 0.94–1.00 | 0.059 | |||
| Total-cholesterol, mg/dL | 0.98 | 0.98–0.99 | 0.001 | |||
| Triglycerides, mg/dL | 0.99 | 0.99–1.00 | 0.013 | |||
| eGFR, mL/min/1.73 m2 | 0.97 | 0.96–0.98 | <0.001 | |||
| Albumin, g/dL | 0.14 | 0.09–0.22 | <0.001 | |||
| Albumin <3.5 g/dL, n(%) | 7.46 | 3.93–14.13 | <0.001 | 4.29 | 2.16–8.51 | <0.001 |
| White blood cell count, ×103/μL | 1.00 | 1.00–1.00 | 0.060 | |||
HR, hazard ratio; CI, confidence interval; other abbreviations as in Table 1.
Fig. 3.
The distribution of mortality according to the CFS classification and serum albumin level and CFS and Killip classifications.
CFS – clinical frailty scale.
Fig. 4.
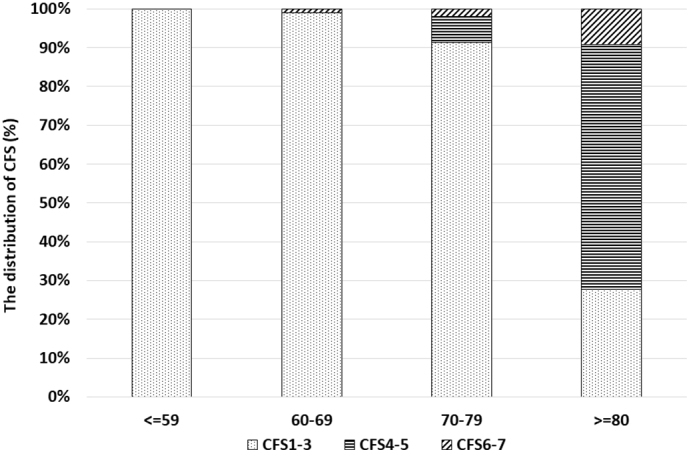
Distribution of CFS scores by age.
CFS – clinical frailty scale.
Furthermore, we examined the patients in whom the CFS classification altered during admission. There were 11 patients who had an altered CFS classification. Of these 11 patients, 4 patients had died during the follow-up period (Table 3).
Table 3.
Patients characteristics who changed CFS class during admission.
| Patients | Age | Sex | Pre CFS | Post CFS | Killip class | Intensive care | Mid-term all-cause death | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | 1 | 7 | 1 | Yes | Yes | Asthenia |
| 2 | 72 | M | 1 | 7 | 1 | Yes | Yes | Cardiac |
| 3 | 83 | F | 4 | 7 | 1 | – | Yes | Malignancy |
| 4 | 74 | M | 5 | 6 | 1 | – | Yes | Malignancy |
| 5 | 58 | M | 1 | 7 | 4 | Yes | – | – |
| 6 | 68 | M | 1 | 6 | 2 | Yes | – | – |
| 7 | 80 | M | 4 | 6 | 1 | Yes | – | – |
| 8 | 87 | M | 4 | 6 | 1 | – | – | – |
| 9 | 94 | F | 4 | 7 | 2 | – | – | – |
| 10 | 88 | M | 5 | 6 | 1 | – | – | – |
| 11 | 88 | F | 5 | 6 | 1 | – | – | – |
PCI, percutaneous coronary intervention; CFS, clinical frailty scale; M, male; F, female; Intensive care; any use of intra aortic balloon pumping, percutaneous cardio-pulmonary support, and tracheal intubation.
4. Discussion
CFS is a simple and effective semiquantitative marker for a baseline objective and non-invasive assessment of patients' characteristics. In this study, we evaluated consecutive patients with STEMI regardless of age with a mid-term follow-up period. To the best of our knowledge, this is the first study to show that CFS was independently associated with a poor prognosis in patients of all ages who underwent PCI for STEMI. Geriatric patients have been investigated in most frailty studies, and frailty has been associated with mortality in these patients [10,13]. For ACS, Matsuzawa et al. reported that slow gait speed (as a marker of frailty) was significantly associated with an increased risk of cardiovascular events in 472 patients with STEMI [14]. Furthermore, White et al. showed the correlation between frailty and worse outcomes in 9326 patients with ACS [15] and Alegre et al. reported that another frailty assessment tool, the FRAIL scale, was a significant predictor of mortality in ACS patients [16]. Corresponding with previous reports, our study indicated that CFS is independently associated with poor mid-term outcomes in patients with STEMI.
CFS was developed using data from the Canadian of Health and Aging on elderly Canadians aged 65 years or older. Our study population was relatively younger than those of previous studies and included Japanese patients <65 years old. This study also showed that CFS can be applicable for non-elderly and Asian patients.
Notably, this study demonstrated that a frailty-associated parameter, serum albumin level (<3.5 g/dL), was also associated with an increased risk of mid-term all-cause mortality according to multivariate analysis, thereby emphasizing the impact of frailty. Generally, albuminemia, which is an indicator of malnutrition, has been considered an important parameter of frailty and is associated with a poor prognosis in cardiac disease patients [17]. In addition, BMI is also associated with an increased risk of mid-term mortality in univariate analysis and is reported to be related to poor clinical outcomes in patients with STEMI [18]. Although obesity is associated with cardiovascular risk factors, such as hypertension, diabetes mellitus, and dyslipidemia [19], this study showed that a markedly lower BMI was related to a poor outcome following PCI in patients with STEMI due to cardiac cachexia, malnutrition, or depression [20]. A low serum albumin level and low BMI are identified as markers of frailty [21], and these findings underpin the important role of “frailty” in mid-term mortality. Considering the poor outcome in patients with severe frailty who were treated with PCI for STEMI, the indication for intervention in those patients should be carefully determined. Indeed, Ekerstad et al. demonstrated that CFS was strongly and independently associated with in-hospital all-cause mortality and 1-month mortality in 307 patients with non-STEMI, and angiography was not performed in most of the frail patients (CFS ≥ 6) [22]. In STEMI patients with severe frailty, it may be possible to consider a rework of therapeutic strategy, including whether to perform PCI.
Generally, a higher Killip score, lower systolic blood pressure, higher heart rate, older age, and higher serum creatinine level are associated with high mortality in ACS patients [23]. Indeed, our data showed that a higher Killip score, lower systolic and diastolic blood pressure, and higher heart rate were significantly associated with mid-term all-cause death on univariate analysis. Of these, only the Killip score retained a significant correlation with both mortality indexes on multivariate analysis. However, it is important that the CFS and low albuminemia, but not comorbidities, such as diabetes mellitus and CKD, and older age, could predict mid-term mortality in the consecutive patients with STEMI, as CFS is useful for assessing prognosis in other clinical conditions [11,24].
CFS is a useful and simple predictor of mid-term mortality in patients who undergo PCI for STEMI. Frailty can be conceptualized as a phenotype of weight loss, fatigue, and weakness or a multidimensional state of vulnerability arising from a complex interplay of biological, cognitive, and social factors. Our report showed that severe frailty was a significant predictor of both cardiac and non-cardiac death. Our findings suggest that frailty is strongly relevant to mortality in STEMI patients due to multifactorial variables. In other words, it is possible to improve the outcome in frail patients with STEMI by interventions targeted at these factors. Therefore, early intervention in synergy with social work, home care, and cardiac rehabilitation, in addition to functional independence, social support, medication compliance, and nutrition should be considered to improve mortality in these patients [[25], [26], [27]]. Moreover, considering that a relatively high mortality was recorded in patients whose CFS classification were worse following primary PCI, preventing the worsening of CFS could also be important during STEMI treatment in daily practice.
In recent decades, the in-hospital mortality of patients with STEMI has been low (2.6%) as reported by the CVIT 2018 annual report in 41,774 patients [28]. However, a higher age (68.5 years vs. 69.8 years) and more comorbidities (renal insufficiency; 12.8% vs. 37.0%) might increase the in-hospital mortality in our institute. On the other hand, in the MIYAGI-AMI Registry, which is located in the rural areas, Hao et al. reported that the in-hospital mortality of 8640 patients with acute myocardial infarction (AMI) between 2002 and 2010 was 6.4% [29]. Therefore, the covered area might be associated with an increased risk of in-hospital mortality due to prolonged onset to balloon time and insufficient information of coronary artery disease.
4.1. Limitations
This study had several limitations. First, it had a single-center retrospective design. Second, no patient was categorized as CFS 8 or 9, and a relatively small number of patients were categorized as CFS 4–5 and CFS 6–7. Therefore, care should be taken while interpreting the indication for these patients with STEMI. Third, the CFS classification was not evaluated at admission because of the retrospective design, and in cases in which the primary physician was not present, we estimated the CFS score based on chart review. Fourth, the synergistic effects of social work, home care, and cardiac rehabilitation were not evaluated in this study. Fifth, the possibility of CFS in improving the currently available risk prediction models (such as GRACE score, TIMI risk score) is unclear because of the limited number of patients in this study. Finally, as the patients with severe frailty usually had more comorbidities, it is difficult to interpret whether frailty itself had a direct impact on all-cause death. Further studies are necessary to provide new risk scores, such as CFS for in-hospital and mid-term mortality. Further investigations are needed to clarify the indications for PCI for frail patients with STEMI.
5. Conclusions
The simple parameters, CFS, hypoalbuminemia, and a higher Killip score were strongly associated with mid-term mortality in patients treated with PCI for STEMI.
Conflict of interest
The authors have no financial relationships to disclose and there are no conflicts of interest regarding the content of the manuscript.
Acknowledgments
Acknowledgements
None.
Sources of funding
None.
Disclosures
None.
References
- 1.Morley J.E., Vellas B., van Kan G.A., Anker S.D., Bauer J.M., Bernabei R., Cesari M., Chumlea W.C., Doehner W., Evans J., Fried L.P., Guralnik J.M., Katz P.R., Malmstrom T.K., McCarter R.J., Gutierrez Robledo L.M., Rockwood K., von Haehling S., Vandewoude M.F., Walston J. Frailty consensus: a call to action. J. Am. Med. Dir. Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sujino Y., Tanno J., Nakano S., Funada S., Hosoi Y., Senbonmatsu T., Nishimura S. Impact of hypoalbuminemia, frailty, and body mass index on early prognosis in older patients (>/=85 years) with ST-elevation myocardial infarction. J. Cardiol. 2015;66:263–268. doi: 10.1016/j.jjcc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Alonso Salinas G.L., Sanmartin M., Pascual Izco M., Rincon L.M., Pastor Pueyo P., Marco Del Castillo A., Garcia Guerrero A., Caravaca Perez P., Recio-Mayoral A., Camino A., Jimenez-Mena M., Zamorano J.L. Frailty is an independent prognostic marker in elderly patients with myocardial infarction. Clin. Cardiol. 2017;40:925–931. doi: 10.1002/clc.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M., Rihal C.S., Lennon R.J., Spertus J.A., Nair K.S., Roger V.L. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circulation Cardiovascular Quality and Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical Frailty Scale-Geriatric Medicine Research-Dalhousie University. Faculty of Medicine GMR, research/projects, clinical frailty scale. Dalhousie University, Halifax, Canada http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm.
- 6.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., Hindricks G., Kastrati A., Lenzen M.J., Prescott E., Roffi M., Valgimigli M., Varenhorst C., Vranckx P., Widimsky P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 7.Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: diabetes care 2009; 32(7): 1327–1334. The Clinical Biochemist Reviews. 2009;30:197–200. [PMC free article] [PubMed]
- 8.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health LaW http://www.mhlw.go.jp/file/06-Seisakujouhou-12300000-Roukenkyoku/0000077382.pdf
- 10.Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I., Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregorevic K.J., Hubbard R.E., Lim W.K., Katz B. The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: a prospective cohort study. BMC Geriatr. 2016;16:117. doi: 10.1186/s12877-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y., Konishi M., Akiyama E., Suzuki H., Nakayama N., Kiyokuni M., Sumita S., Ebina T., Kosuge M., Hibi K., Tsukahara K., Iwahashi N., Endo M., Maejima N., Saka K., Hashiba K., Okada K., Taguri M., Morita S., Sugiyama S., Ogawa H., Sashika H., Umemura S., Kimura K. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J. Am. Coll. Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 15.White H.D., Westerhout C.M., Alexander K.P., Roe M.T., Winters K.J., Cyr D.D., Fox K.A., Prabhakaran D., Hochman J.S., Armstrong P.W., Ohman E.M. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur. Heart J. Acute Cardiovasc. Care. 2016;5:231–242. doi: 10.1177/2048872615581502. [DOI] [PubMed] [Google Scholar]
- 16.Alegre O., Formiga F., Lopez-Palop R., Marin F., Vidan M.T., Martinez-Selles M., Carol A., Sionis A., Diez-Villanueva P., Aboal J., Palau-Vendrel A., Bueno H., Rivera A.P., Sanchis J., Abu-Assi E., Corbi M., Castillo J.C., Baneras J., Gonzalez-Salvado V., Cequier A., Ariza-Sole A. An easy assessment of frailty at baseline independently predicts prognosis in very elderly patients with acute coronary syndromes. J. Am. Med. Dir. Assoc. 2018;19:296–303. doi: 10.1016/j.jamda.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Horwich T.B., Kalantar-Zadeh K., MacLellan R.W., Fonarow G.C. Albumin levels predict survival in patients with systolic heart failure. Am. Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Moscarella E., Spitaleri G., Brugaletta S., Senti Farrarons S., Pernigotti A., Ortega-Paz L., Cequier A., Iniguez A., Serra A., Jimenez-Quevedo P., Mainar V., Campo G., Tespili M., den Heijer P., Bethencourt A., Vazquez N., Valgimigli M., Serruys P.W., Sabate M. Impact of body mass index on 5-year clinical outcomes in patients with ST-segment elevation myocardial infarction after everolimus-eluting or bare-metal stent implantation. Am. J. Cardiol. 2017;120:1460–1466. doi: 10.1016/j.amjcard.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Nigam A., Wright R.S., Allison T.G., Williams B.A., Kopecky S.L., Reeder G.S., Murphy J.G., Jaffe A.S. Excess weight at time of presentation of myocardial infarction is associated with lower initial mortality risks but higher long-term risks including recurrent re-infarction and cardiac death. Int. J. Cardiol. 2006;110:153–159. doi: 10.1016/j.ijcard.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Knudtson M.D., Klein B.E., Klein R., Shankar A. Associations with weight loss and subsequent mortality risk. Ann. Epidemiol. 2005;15:483–491. doi: 10.1016/j.annepidem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kappetein A.P., Head S.J., Genereux P., Piazza N., van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.A., Hahn R.T., Kirtane A.J., Krucoff M.W., Kodali S., Mack M.J., Mehran R., Rodes-Cabau J., Vranckx P., Webb J.G., Windecker S., Serruys P.W., Leon M.B. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ekerstad N., Swahn E., Janzon M., Alfredsson J., Lofmark R., Lindenberger M., Carlsson P. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 23.Granger C.B., Goldberg R.J., Dabbous O., Pieper K.S., Eagle K.A., Cannon C.P., Van De Werf F., Avezum A., Goodman S.G., Flather M.D., Fox K.A. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 24.Shimura T., Yamamoto M., Kano S., Kagase A., Kodama A., Koyama Y., Tsuchikane E., Suzuki T., Otsuka T., Kohsaka S., Tada N., Yamanaka F., Naganuma T., Araki M., Shirai S., Watanabe Y., Hayashida K. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135:2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 25.Graham M.M., Galbraith P.D., O'Neill D., Rolfson D.B., Dando C., Norris C.M. Frailty and outcome in elderly patients with acute coronary syndrome. The Canadian Journal of Cardiology. 2013;29:1610–1615. doi: 10.1016/j.cjca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 26.George M., Azhar G., Pangle A., Peeler E., Dawson A., Coker R., Coleman K.S., Schrader A., Wei J. Feasibility of conducting a 6-month long home-based exercise program with protein supplementation in elderly community-dwelling individuals with heart failure. Journal of Physiotherapy & Physical Rehabilitation. 2017;2 doi: 10.4172/2573-0312.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIsaac D.I., Jen T., Mookerji N., Patel A., Lalu M.M. Interventions to improve the outcomes of frail people having surgery: a systematic review. PLoS One. 2017;12 doi: 10.1371/journal.pone.0190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozaki Y., Katagiri Y., Onuma Y., Amano T., Muramatsu T., Kozuma K., Otsuji S., Ueno T., Shiode N., Kawai K., Tanaka N., Ueda K., Akasaka T., Hanaoka K.I., Uemura S., Oda H., Katahira Y., Kadota K., Kyo E., Sato K., Sato T., Shite J., Nakao K., Nishino M., Hikichi Y., Honye J., Matsubara T., Mizuno S., Muramatsu T., Inohara T., Kohsaka S., Michishita I., Yokoi H., Serruys P.W., Ikari Y., Nakamura M. CVIT expert consensus document on primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) in 2018. Cardiovasc. Interv. Ther. 2018;33:178–203. doi: 10.1007/s12928-018-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao K., Takahashi J., Ito K., Miyata S., Nihei T., Nishimiya K., Tsuburaya R., Matsumoto Y., Sakata Y., Yasuda S., Shimokawa H. Clinical characteristics of patients with acute myocardial infarction who did not undergo primary percutaneous coronary intervention- report from the MIYAGI-AMI Registry study. Circ J. 2015;79:2009–2016. doi: 10.1253/circj.CJ-15-0440. [DOI] [PubMed] [Google Scholar]