Abstract
Keystone microbial species have driven eco-evolutionary processes since the origin of life. However, due to our inability to detect the majority of microbiota, members of diverse microbial communities of fungi, bacteria and viruses have largely been ignored as keystone species in past literature. Here we tested whether heritable Epichloë species of pooidae grasses modulate microbiota of their shared host plant.
Introduction
Microbe-plant interactions have existed since the origin of plants, and microbes have been demonstrated to contribute to all major aspects of plant life, including regulation of metabolism and growth, nutrient acquisition and resistance to biotic and abiotic stressors1,2. They can thus potentially modulate all ecosystems because food chains rely on primary producers converting energy from the sun to the consumers.
Traditionally, research on plant-microbe interactions has focused on one or very limited number of microbes or microbial groups. However, recent developments in molecular methodology have dramatically extended our understanding of plant microbiota, and demonstrated that the interactions between the plant and its associated microbiota, as well as microbe-microbe interactions within microbiome, impact strongly plant phenotypes and that plant microbiota are at least partially heritable3. Thus, the plant and its associated microbiome should be regarded as a co-evolving ecosystem, holobiont1. Here we propose that the most topical steps forward in biology are to untangle how selection treats plants and their symbiotic microbes individually or in concert as a holobiont, and to unravel how individual keystone microbial species drive the rest of the microbiota associated with plants, and thus potentially determine the plant performance and ecosystem functions relying on the plant4,5.
Here we test the hypothesis that individual keystone microbial species can modulate plant microbiomes using fungal endophytes of the genus Epichloë as a model. They commonly colonize cool-climate grasses6. Epichloë-grass symbiosis has been intensively studied since the 1970s when alkaloids produced by them were discovered to cause animal disorders7. Since then, Epichloë species have been shown to be able to modulate virtually all plant-plant, plant-herbivore and plant-pathogen interactions8,9 and thereby shape community structures of grassland ecosystems. Thus, they can be regarded as keystone species. Although the ecological importance of Epichloë species is demonstrated and widely recognized, their interactions with plant-associated microbes other than plant pathogens have largely been ignored (but see10,11). Here we propose that the Epichloë species, while growing systemically throughout the above ground parts of the host grass including the developing seeds, are likely to interact with a broad spectrum of taxonomically diverse microbial species occupying the host endosphere. They can e.g. compete, interact chemically and modulate plant quality to other microbes12. This calls for comprehensive community-level studies to test whether Epichloë species affect the rest of the grass microbiota.
To empirically approach the hypothesis that Epichloë species act as keystone microbial species modulating plant microbiomes we analyzed Epichloë coenophiala mediated changes in the bacterial and fungal endophytic communities of tall fescue grasses. As systemic Epichloë colonization has been reported to strongly modulate plant chemistry and physiology6,13–15, we expected that the bacterial and fungal community structures would be shaped by the Epichloë colonization status of the shared plant host.
Materials and Methods
Plant material and experimental setup
In 2003, a stock population of Epichloë colonized (E+) and endophyte-free (E−) tall fescue (Schedonorus phoenix (Scop. Holub.) [=Lolium arundinaceum (Schreb.) Darbysh. = Schedonorus arundinaceus (Schreb.) Dumort]) seeds collected from eight naturally occurring populations from Åland Island (Finland) was established in the Turku University Botanical Garden (60°26 N, 22°10.4E). A mixture of mother plant seeds, either E+ or E− tall fescue, were used in the experiment. Because the fungus is maternally inherited, the endophyte status of grasses (E+ or E−) used in the experiments was confirmed by microscopic examination of 1–3 seeds of each mother plant. The hyphae of the endophyte are visible by light microscopy among the embryonic cells of the plant16 after the seeds have been soaked overnight in solution containing water, ethanol and NaOH. Ten 1 m × 1.2 m tall fescue plots were established, 2.5 m apart from each other, in the Botanical Garden on the 25th of May 2016. Approximately 300 g of E− or 380 g of E+ seed mixture were sown to the plots. The higher amount of seed material was sown in E+ plots because this mixture had more non-seed material (seed envelopes etc.) compared to E− mixture. Every other plot was sown with E+ seeds, and every other with E− seeds. The seeded plots were covered with white mesh, which was removed after two weeks of germination. The plots were watered when needed, but not fertilized, as the soil in the plots (decomposed slurry) was expected to contain high amount of nutrients.
Sample collection and processing
In August 15, 2016 three samples of Schedonorus phoenix fully grown, undamaged green leaves were collected from each plot, resulting in a total of 15 E+ and 15 E− samples. Samples were transported on ice to the laboratory and processed within 8 hours after harvesting. The leaves were thoroughly washed with water and surface sterilized by immersing in 70% ethanol for 1 minute, followed by 3% sodium hypochlorite for 3 min and sterile double distilled water (3 × 90 s). 300 mg of leaf blade from mid-leaf, including both vascular and leaf mesophyll tissue were excised with a sterile blade and homogenized in liquid nitrogen with a mortar and pestle. 80–100 mg of each sample was used for subsequent DNA analysis. Prior to DNA isolation, samples were homogenized with a sterilized mortar and pestle in liquid nitrogen. An Invisorb Spin Plant Mini Kit (STRATEC Biomedical AG, Germany) was used for community DNA isolation according to the manufacturer’s protocol.
16S rRNA gene was amplified following the protocol described in17 using primers 799 f/1492r18 and M13-1062f/1390r19 in a nested approach. The nested primers targeting the V6-V8 regions of 16S rRNA gene enable elimination of plant chloroplast 16S rRNA gene amplicons as well as separation of endophyte amplicons from plant mitochondrial amplicons by size fractionation (799f–1492r)18 and produce an amplicon with high phylogenetic coverage and optimal size for IonTorrent sequencing (1062f–1390r)20. Primer 1062 f was tagged with a M13 sequence to enable sample barcoding as described in19. Both reactions had 1 μl of sample DNA, 1x PCR buffer, 1 mg/ml of BSA, 0.2 mM dNTP’s, 0.3 μM of each primer and 1250 U/ml GoTaq DNA Polymerase (Promega, WI, USA) in a 30 μl reaction volume. 30 ng of DNA was used in the first PCR, and 1 μl of 1:10 diluted amplicons from the first PCR were used as a template for the second run. PCR reactions were performed as follows: 3 min denaturation at 95 °C followed by 25 cycles of denaturing, annealing, and extension at 95 °C for 45 s, 54 °C for 45 s, and 72 °C for 1 min, respectively. Final extension was carried out at 72 °C for 5 min.
Sequencing libraries were prepared by running a third PCR to attach the M-13-barcode system19. Amplicons from the second PCR were diluted 1:5 and re-amplified using barcode sequence-M13 system as forward primers and 1390r-P1 with adaptor A as a reverse primer. PCR mix and conditions were similar as described above, with an exception of using 8 cycles for amplification. Amplified libraries were purified using Agencourt AMPure XP PCR purification system (Beckman Coulter, CA, USA). Purified samples were quantified with Tape Station 2200 (Agilent, CA, USA) and were pooled based on an equivalent quantity of endophyte amplicon per sample. The pooled samples were size fractionated (size selection range of 350–550 bp) using Pippin Prep (Sage Science, MA, USA) 2% Agarose gel cassette (Marker B) following the manufacturer’s protocol. Size fractioned libraries were sequenced using an Ion 314 chip kit V2 BC on Ion Torrent PGM (Life Technologies, CA, USA) in the Biocenter Oulu, Finland.
Fungal internal transcribed spacer (ITS) gene libraries were amplified using fITS7 and ITS4 primer sets21. The 30 µl reaction mixture contained 1 μl (30 ng) of sample DNA, 1x PCR buffer, 1 mg/ml of BSA, 0.2 mM dNTP’s, 0.3 μM of each primer and 1250 U/ml GoTaq DNA Polymerase (Promega, WI, USA) in a 30 μl reaction volume. The amplification procedure consisted of 5 mins denaturation at 95 °C followed by 35 cycles of denaturing, annealing, and extension at 95 °C for 30 s, 55 °C for 30 secs and 72 °C for 1 min, respectively. Final extension was carried out at 72 °C for 7 mins. The sequencing libraries were prepared by attaching a M-13 barcode system to the amplicons as described above and in20. Amplicons from the first PCR were diluted 1:10 and re-amplified using a barcode attached M13 system as a forward primer and ITS4-P1 with adaptor A as a reverse primer. PCR mix and conditions were similar as described above, with the exception of only using 8 cycles for amplification. Amplified products were purified with an Agencourt AMPure XP PCR purification system (Beckman Coulter, CA, USA) and quantified using a Qubit dsDNA HS assay kit and Qubit fluorometer (Invitrogen, MA, USA). The quantified samples from each sample were pooled equimolarly and were sequenced using an Ion 314 chip kit V2 BC on Ion Torrent PGM (Life Technologies, CA, USA) in the Biocenter Oulu, Finland.
Bioinformatics and statistical analyses
The sequence reads were processed using a CLC Genomics Workbench 11.0 with a Microbial Genomics Module (Qiagen, Denmark). Raw reads imported from IonTorrent were screened for chimeras, and reads <150 bp and with Q score <25 were removed. Good quality reads were clustered into OTUs (operational taxonomic units) at 97% sequence identity, and the OTUs were assigned taxonomically using a RDP classifier22 with reference databases RDP 16S rRNA training set 16 and UNITE Fungal ITS trainset 7.123 for bacteria and fungi, respectively (https://rdp.cme.msu.edu). OTUs representing plant chloroplast or mitochondrial sequences, as well as OTUs with <10 reads were removed from the dataset prior to analyses.
Square root transformed data was used to construct Bray-Curtis distance matrixes, which were used to analyze community structures using PERMANOVA (permutational multifactorial manova)24 and visualized by PCoA ordinations at the OTU level. Taxonomic groups (phyla or OTU) with the strongest impact on differences between community structures were identified with SIMPER (Similarity Percentages - species contributions), all performed with PRIMER 6.1 software package with the PERMANOVA+ add-on (primer-e.com).
IonTorrent sequencing resulted in 270705 fungal and 322177 bacterial quality filtered sequence reads. After sequence data processing (removal of chimeric sequences, low read count samples, OTUs with less than 10 reads and plant mitochondrial sequences), fungal (ITS) and bacterial (16S rRNA) datasets consisted of 214304 and 167772 sequence reads assigned into 54 and 233 OTUs, respectively.
The ITS dataset was reanalyzed after removing the Epichloë assigned reads. After depletion, five E+ samples had less than 90 reads. Those samples were removed from the dataset, along with five randomly selected E− plant samples, and thereafter 10 samples from both E+ and E− plants were used for community analyses.
Raw sequence data are available at the EBI Sequence Read Archive under accession PRJEB29516.
Results
Fungal endophytic communities are strongly shaped by Epichloë symbiosis
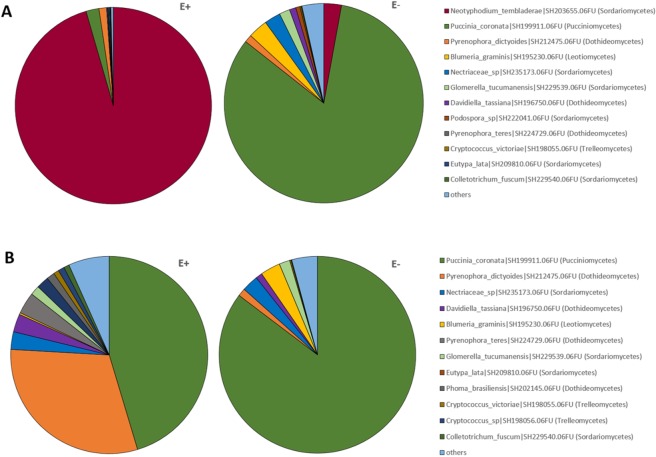
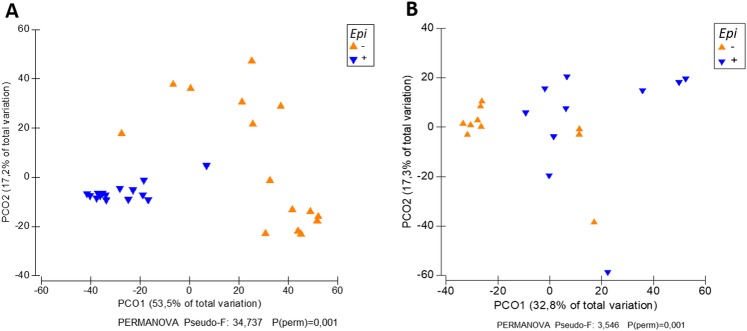
The fungal endophytic communities in tall fescue (Schedonorus phoenix) leaf samples represented 17 classes in the fungal phyla Ascomycota, Basidiomycota and Zygomycota. Communities in the plants colonized by Epichloë (E+ plants) were very strongly dominated by Epichloë (fungal class Sordariomycetes) (96% relative abundance of the communities in the E+ plants), followed by OTUs assigned as Puccinia coronata (class Pucciniomycetes) and Pyrenophora dictyoides (class Doditheomycetes) (2% and 1% relative abundance in E+ plant communities, respectively) (Fig. 1). E− plant communities were dominated by Puccinia coronata (Pucciniomycetes), with 83% relative abundance, followed by Blumeria graminis (Leotiomycetes, 3%) and Nectriaceae sp., Epichloë (both class Sordariomycetes, 6%), Glomerella tucumanensis, Pyrenophora dictyoides and Davidiella tassiana. Other OTUs were present at relative abundances of less than 1% (Fig. 1). Endophytic fungal communities in E+ and E–plants differed significantly in their community structures (PERMANOVA p = 0.001), which was also clearly visible in PCO ordination (Fig. 2A).
Figure 1.
Taxonomic composition of Schedonorus phoenix endophytic fungal communites in Epichloë colonized (E+) and uncolonized (E−) plants. (A) total fungal communities, (B) fungal communities based on Epichloë depleted dataset. Fungal communities are presented at fungal genus level. 15 biological replicates of both E+ and E− plants were used in analysis of total communities, while 10 biological replicates of E+ and E− plants were used in Epichloë depleted dataset analysis. Neotyphodium = Epichloë.
Figure 2.
Principal coordinate analysis (PCoA) of endophytic fungal communities in E+ and E− plants. (A) total communities, (B) fungal communities based on Epichloë depleted dataset. PCoAs are based on Bray-Curtis distance matrices of standardized, square root transformed community data of 15 (A) and 10 (B) biological replicates of E+ and E− plants.
To gain a better insight in the endophytic fungal communities other than the systemic Epichloë symbiont, we reanalyzed the data after removing Epichloë-assigned OTU reads from the dataset. In the Epichloë-depleted datasets, Puccinia coronata was the most abundant OTU in both E+ and E− plant communities (45% and 85% of the total relative abundance, respectively), followed by Pyrenophora dictyoides and P. teres in E+ plants (31% and 4%, respectively), and Nectriaceae sp. and Glomerella tucumanensis in E− plants (2 and 1%, respectively) (Fig. 1B). PERMANOVA analysis of the Epichloë depleted communities demonstrated, that there was small but significant difference in the fungal endophyte community structures between E+ and E− plants (Fig. 2B). SIMPER (similarity percentages species contribution) analysis identified Puccinia coronata, Pyrenophora dictyoides and Nectriaceae sp. as the major taxa driving these differences (Table 1). Several other taxa, including OTUs representing Phoma sp. and Davidiella sp. were also differentially abundant in E+ and E− plants.
Table 1.
SIMPER (Similarity percentage species contribution) analysis of fungal taxa contributing to the differences in community structures between E+ and E− plants, based on Bray-Curtis dissimilarity matrix of standardized community data.
| Species | ref seq ID (Unite) | E− | E+ | AvDi | Di/SD | Co% | Cu% |
|---|---|---|---|---|---|---|---|
| AvAb | AvAb | ||||||
| A Groups E− & E+, Average dissimilarity = 86,67 | |||||||
| Neotyphodium (Epichloë) | SH203655.06 FU | 9,54 | 91,88 | 4 1,21 | 4,22 | 47,55 | 47,55 |
| Puccinia_coronata | SH199911.06FU | 52,71 | 4,40 | 25,43 | 1,27 | 29,34 | 76,89 |
| Nectriaceae_sp | SH235173.06FU | 8,66 | 0,38 | 4,34 | 0,61 | 5,01 | 81,90 |
| Davidiella_tassiana | SH196750.06FU | 6,66 | 0,58 | 3,40 | 0,51 | 3,92 | 85,82 |
| Blumeria_graminis | SH195230.06FU | 3,64 | 0,01 | 1,83 | 0,27 | 2,11 | 87,93 |
| Glomerella_tucumanensis | SH229539.06FU | 3,09 | 0,09 | 1,54 | 0,56 | 1,78 | 89,71 |
| Pyrenophora_dictyoides | SH212475.06FU | 1,58 | 1,62 | 1,37 | 0,57 | 1,58 | 91,29 |
|
B Groups E− & E+
Average dissimilarity = 70,13 | |||||||
| Puccinia_coronata | SH199911.06FU | 73,88 | 31,55 | 26,60 | 1,88 | 37,93 | 37,93 |
| Pyrenophora_dictyoides | SH212475.06FU | 0,72 | 20,99 | 10,45 | 0,72 | 14,91 | 52,84 |
| Nectriaceae_sp | SH235173.06FU | 6,04 | 14,30 | 7,82 | 1,18 | 11,15 | 63,99 |
| Blumeria_graminis | SH195230.06FU | 5,47 | 0,73 | 3,03 | 0,37 | 4,32 | 68,31 |
| Phoma_brasiliensis | SH202145.06FU | 0,00 | 5,67 | 2,83 | 0,48 | 4,04 | 72,35 |
| Cryptococcus_sp | SH198056.06FU | 0,01 | 4,40 | 2,20 | 0,44 | 3,14 | 75,49 |
| Glomerella_tucumanensis | SH229539.06F | 3,20 | 2,23 | 2,04 | 0,80 | 2,91 | 78,40 |
| Chionosphaeraceae | 0,00 | 3,88 | 1,94 | 0,33 | 2,76 | 81,16 | |
| Rhizoscyphus_ericae | SH207166.06FU | 0,00 | 2,86 | 1,43 | 0,33 | 2,04 | 83,20 |
| Podospora_sp | SH222041.06FU | 2,66 | 0,00 | 1,33 | 0,33 | 1,89 | 85,09 |
| Davidiella_tassiana | SH196750.06FU | 2,05 | 0,76 | 1,24 | 0,52 | 1,76 | 86,86 |
| Fungi_unidentified_6 | 0,00 | 2,47 | 1,24 | 0,33 | 1,76 | 88,62 | |
| Pyronemataceae_sp | SH227977.06FU | 0,00 | 1,63 | 0,82 | 0,33 | 1,16 | 89,78 |
| Candida_smithsonii | SH216776.06FU | 0,00 | 1,56 | 0,78 | 0,42 | 1,11 | 90,90 |
A: total fungal endophytic communities, B: communities with Epichloë assigned reads removed. AvAb: Average abundance, AvDi: Average dissimilarity, Di: Dissimilarity, SD: standard deviation, Co%: contribution to the observed dissimilarity, % of total, Cu%: cumulative contribution, %. Only OTUs up to 90% cumulative contribution are listed.
Bacterial community structures are unaffected by Epichloë symbiosis
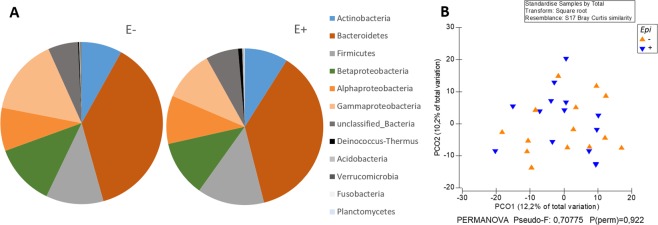
Bacterial communities in S. phoenix plants were dominated by OTUs assigned to the phyla Bacteroidetes (five most abundant OTUs in the family Chitinophagaceae) and Proteobacteria (two OTUs in the family Pseudomonadaceae), which collectively comprised 37% and 34% of the total dataset reads, respectively. Community structures of the bacterial endophytic communities were not affected by Epichloë symbiosis (PERMANOVA p = 0.925, Fig. 3). Analysis was repeated at several levels of taxonomy (OTU at 99% identity, bacterial families, orders and classes), but no significant differences or trends were detected (data not shown).
Figure 3.
Taxomonic composition (A) and principal coordinate analysis (PCoA) of community structures (B) of endophytic bacterial communities in E+ and E− plants. PCoA is based on Bray-Curtis distance matrix of standardized, square root transformed community data.
Discussion
In this study, we tested the hypothesis that systemic Epichloë endophytes as a keystone species would simultaneously shape plant fungal and bacterial communities in host plant endospheres. Our data partially supported the hypothesis. Epichloë symbiosis strongly shaped the endophytic fungal community of its host plant, Schedonorus phoenix. Epichloë dominated the fungal communities of the E+ plants and furthermore, relative abundances of other fungal taxa markedly differed from E− plants. When comparing fungal communities, excluding Epichloë, 45%, 31% and 4% of the total relative abundance were Puccinia coronata, Pyrenophora dictyoides and P. teres in E+ plants, respectively. In E− plants, latent infections of common pathogen P. coronata strongly dominated the fungal communities (85% of total relative abundance), followed by Blumeria graminis (3%), Nectriaceae sp. (2%) and Glomerella tucumanensis (1%). Several other taxa were also differentially abundant in E+ and E− plants. In general, these results suggest that fungal endophyte communities are dominated by one keystone species that shapes the taxonomic composition of rest of the fungal community. In the case of Epichloë species noteworthy is that the dominance is heritable in mother plant lineages. In contrast to our hypothesis, however, bacterial community structures in S. phoenix plants appeared to be unaffected by Epichloë presence.
Negligible effects of Epichloë on the bacterial community in S. phoenix plants suggests that Epichloë modulates the fungal community either via direct fungus-fungus interactions, such as competition (space occupation, nutrient limitation) or antagonism, or fungal-specific molecular or physiological mechanisms. Systemic Epichloë species have been shown to modulate plant physiology and metabolism on several fronts, including increased production of alkaloids and phenolic root exudates, and upregulation of systemic hormone and defense responses12,25–27. Plant associated bacterial communities have been reported to respond to changes in many or all of these aspects28,29. Thus, we expected to see an impact of Epichloë colonization status on bacterial community structures as well.
Contrasting findings have been reported on impact of Epichloë colonization on plants in past literature. While several studies report significant changes in plant gene regulation and systemic changes in synthesis and processing of the plant hormones salicylic acid, jasmonate, gibberellin, ethylene, abscisic acid, cytokinin and auxin26,27,30, others report only subtle differences in gene expression and a lack of detectable ultrastructural changes31,32. These contrasting plant responses to fungal endophytes are most likely explained by different combinations of symbiont and plant species with different symbiotic histories of host plants as well as by experimental conditions6,8,13. Studies reporting significant shifts in plant gene expression in response to Epichloë are mainly conducted with artificial fungus-plant combinations and using fungal removal and/or inoculation manipulations26,27,30, while studies reporting subtle plant responses have been conducted with E+ plant lines with long symbiotic histories and naturally endophyte-free E− plants31. Plants in our experiment were naturally colonized by compatible, vertically transmitted endophyte. Lack of plant transcriptional responses in naturally compatible Epichloë-plant symbiosis33 combined with our observations of highly similar bacterial communities in E+ and E− plants suggests that one of the major triggers of plant defense, recognition of non-self 34, is not induced in compatible Epichloë symbiosis of long symbiotic history.
In short, our results highlight the importance of comprehensive phytobiome-level studies on bacterial and fungal interactions. We demonstrated that Epichloë species can be regarded as keystone species in shaping fungal communities. However, the observed divergent impact of Epichloë on bacterial and fungal communities in the leaf endosphere of S. phoenix supports the idea of the context dependency of microbial interactions. Although the modulation of fungal communities by Epichloë species appears to be directed via fungal-fungal-interactions rather than via modulation of counteracting SA and JA regulated plant response pathways, we cannot rule out the pivotal role of signaling and chemical cross-talk among fungal and bacterial members of plant associated microbiomes. We may expect that horizontally transmitted micro-fungi with short evolutionary history with their hosts may affect host quality of the bacterial community as well. Future studies will reveal whether the remarkable subtlety in Epichloë-plant symbiosis has evolved to block/prevent some conserved induced molecular or physiological plant responses to other microbes.
Author Contributions
R.N. led writing and microbial analysis, M.K. contributed to microbial analyses, M.H. and K.S. created the research idea and designed the field experiment, and all authors contributed to writing and draft revision.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zilber‐Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol. Rev. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 2.Saikkonen K, Young CA, Helander M, Schardl CL. Endophytic Epichloë species and their grass hosts: from evolution to applications. Plant Mol. Biol. 2016;90:665–675. doi: 10.1007/s11103-015-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-Jaramillo JE, Carrión VJ, de Hollander M, Raaijmakers JM. The wild side of plant microbiomes. Microbiome. 2018;6:146. doi: 10.1186/s40168-018-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Heijden, M. G. A. & Hartmann, M. Networking in the plant microbiome. PLoS Biol. 14, 10.1371/journal.pbio.1002378 (2016). [DOI] [PMC free article] [PubMed]
- 5.Agler, M. T et al. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 14, 10.1371/journal.pbio.1002352 (2016). [DOI] [PMC free article] [PubMed]
- 6.Cheplick, G. & Faeth, S. H. Ecology and Evolution of the Grass-Endophyte Symbiosis. New York, NY: Oxford University Press (2009).
- 7.Bacon CWJ, Porter JK, Robbins JD, Luttrell ES. Epichloë typhina from tall fescue grasses. Appl. Environ. Microbiol. 1977;34:76–81. doi: 10.1128/aem.34.5.576-581.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saikkonen K, Lehtonen P, Helander M, Koricherva J, Faeth SH. Model systems in ecology: dissecting the endophyte-grass literature. Trends Plant Sci. 2006;11:428–433. doi: 10.1016/j.tplants.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Kauppinen M, Saikkonen K, Helander M, Pirttilä AM, Wäli PR. Epichloë grass endophytes in sustainable agriculture. Nature Plants. 2016;2:15224. doi: 10.1038/nplants.2015.224. [DOI] [PubMed] [Google Scholar]
- 10.König. J., Guerreiro, M. A., Peršoh, D., Begerow, D. & Krauss, J. Knowing your neighbourhood—the effects of Epichloë endophytes on foliar fungal assemblages in perennial ryegrass in dependence of season and land-use intensity. PeerJ6, 10.7717/peerj.4660 (2018). [DOI] [PMC free article] [PubMed]
- 11.Zhong R, et al. Effects of Epichloë gansuensis on root-associated fungal communities of Achnatherum inebrians under different growth conditions. Fungal Ecol. 2018;31:29–36. doi: 10.1016/j.funeco.2017.10.005. [DOI] [Google Scholar]
- 12.van Overbeek LS, Saikkonen K. Impact of bacterial-fungal interactions on the colonization of the endosphere. Trends Plant Sci. 2016;21:230–242. doi: 10.1016/j.tplants.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Saikkonen K, Gundel P, Helander M. Chemical ecology mediated by fungal endophytes in grasses. J. Chem. Ecol. 2013;39:962–968. doi: 10.1007/s10886-013-0310-3. [DOI] [PubMed] [Google Scholar]
- 14.Li, T., Blande, J. D., Gundel, P. E., Helander, M. & Saikkonen, K. Epichloë endophytes alter inducible indirect defences in host grasses. PLOS ONE9, 10.1371/journal.pone.0101331 (2014). [DOI] [PMC free article] [PubMed]
- 15.Helander M, et al. Alkaloid quantities in endophyte infected tall fescue are affected by the plant-fungus combination and environment. J. Chem. Ecol. 2016;42:118–126. doi: 10.1007/s10886-016-0667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, M., van Elsas, J. D. & Nissinen, R. Strong regionality and dominance of anaerobic bacterial taxa characterize diazotrophic bacterial communities of the Arcto-Alpine plant species Oxyria digyna and Saxifraga oppositifolia. Front. Microbiol. 8, 10.3389/fmicb.2017.01972 (2017). [DOI] [PMC free article] [PubMed]
- 18.Chelius MK, Triplett EW. The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb. Ecol. 2001;4:252–263. doi: 10.1007/s002480000087. [DOI] [PubMed] [Google Scholar]
- 19.Mäki A, Rissanen JA, Tiirola M. A practical method for barcoding and size-trimming PCR templates for amplicon sequencing. BioTechniques. 2016;60:88–90. doi: 10.2144/000114380. [DOI] [PubMed] [Google Scholar]
- 20.Ghyselinck, J., Pfeiffer, S., Heylen, K., Sessitsch, A. & De Vos, P. The effect of primer choice and short read sequences on the outcome of 16S rRNA gene based diversity studies. PlosONE8, 10.1371/journal.pone.0071360 (2013). [DOI] [PMC free article] [PubMed]
- 21.Ihrmark K, et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol. 2012;82:666–77. doi: 10.1111/j.1574-6941.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kõljalg U, et al. Molec. Ecol. 2013;22:5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- 24.Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods, PRIMER‐E, Plymouth, UK (2008).
- 25.Compant S, Saikkonen K, Mitter B, Campisano A, Mercado-Blanco J. Editorial special issue: Soil, plants and endophytes. Plant Soil. 2016;405:1–11. doi: 10.1007/s11104-016-2927-9. [DOI] [Google Scholar]
- 26.Bastías DA, et al. The plant hormone salicylic acid interacts with the mechanism of anti‐herbivory conferred by fungal endophytes in grasses. Plant Cell Environ. 2018;41:395–405. doi: 10.1111/pce.13102. [DOI] [PubMed] [Google Scholar]
- 27.Schmid J, et al. Host tissue environment directs activities of an Epichloë endophyte, while it induces systemic hormone and defense responses in its native perennial ryegrass host. Mol. Plant Microbe Interact. 2017;30:138–149. doi: 10.1094/MPMI-10-16-0215-R. [DOI] [PubMed] [Google Scholar]
- 28.Miché L, Battistoni F, Gemmer S, Belghazi M, Reinhold-Hurek B. Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol. Plant Microbe Interact. 2006;19:502–511. doi: 10.1094/MPMI-19-0502. [DOI] [PubMed] [Google Scholar]
- 29.Lebeis SL, et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- 30.Dupont PY, et al. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 2015;208:122–1240. doi: 10.1111/nph.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinkins RD, Nagabhyru P, Graham MA, Boykin D, Schardl CL. Transcriptome response of Lolium arundinaceum to its fungal endophyte Epichloë coenophiala. New Phytol. 2017;213:324–337. doi: 10.1111/nph.14103. [DOI] [PubMed] [Google Scholar]
- 32.Christensen, M. J. et al. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 45, 84–93 (2008). [DOI] [PubMed]
- 33.Saikkonen, K., Wäli, P. & Helander, M. Genetic compatibility determines endophyte-grass combinations. PLoSONE5, 10.1371/journal.pone.0011395 (2010). [DOI] [PMC free article] [PubMed]
- 34.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]