Abstract
Background:
Teriflunomide is a once-daily oral immunomodulatory agent approved for the treatment of relapsing–remitting multiple sclerosis (MS). We aimed to obtain data on the effectiveness, tolerability, and subject satisfaction with teriflunomide (Aubagio®) under clinical practice conditions in unselected MS patients.
Methods:
This work was a non-interventional, prospective, longitudinal, observational study in 307 sites in Germany.
Results:
A total of 1128 patients were eligible for the efficacy analysis [67.5% female; mean age (± standard deviation) 44.9 ± 9.7 years, range 20–73 years]. Time since first MS symptoms was 10.6 ± 8.2 years, and time since MS diagnosis was 8.9 ± 7.6 years. Expanded Disability Status Scale (EDSS) score at inclusion was 2.3 ± 1.5 (70.4% with score < 3.5). The mean observation period was 16.3 ± 9.1 months. A total of 75.2% had received previous disease-modifying therapies (DMTs) at any time. Of these patients, 504 (44.7%) received no DMT within 6 months of study entry, 593 patients (52.6%) had DMT discontinued prior to study entry [glatiramer acetate in 10.6%, subcutaneous interferon-beta 1a (IFNβ-1a) in 9.3%, intramuscular IFNβ-1a or IFNβ-1b in 6.6% each, azathioprine oral in 0.4%, other in 7.3%, last medication not known in 12.0%]. The mean annualized relapse rate decreased from 0.87 in the 24 months prior to study entry to 0.35 in the 24 months after study entry (n = 468; p ⩽ 0.001). EDSS and Fatigue Severity Scale remained stable. In patients who received previous MS treatments, Treatment Satisfaction Questionnaire (TSQM-9) values (maximum = 100), for the observation at 24 months improved by 8.1 points for effectiveness, 17.0 points for convenience, and 15.3 points for global satisfaction (p ⩽ 0.001 each, compared with study entry). In the safety cohort (n = 1139), the proportion of patients with adverse events (AEs) of any severity was 35.8%, and with serious events 13.0%. The most frequently reported AEs were diarrhea (n = 55), followed by MS relapse (n = 48), hair thinning (n = 38), and viral upper respiratory tract infection (n = 31).
Conclusions:
Relapse rate was halved during the observation period in comparison with the same time period before study entry. Patient satisfaction with teriflunomide was high in this real-world observation of patients, the majority of whom switched from other DMTs. The safety and tolerability profile of teriflunomide was similar to that reported in previous clinical trials.
Keywords: multiple sclerosis, observational, oral, patient-related outcomes, treatment
Background
Multiple sclerosis (MS) is an immune-mediated chronic inflammatory disease of the central nervous system, which is characterized by demyelination and axonal damage.1 MS is the most common neurological disorder of young adults, often leading to permanent disability and premature retirement.
The course of the disease is variable, and outcomes cannot be predicted for individual patients. The majority of patients start with relapsing–remitting MS (RRMS) with clearly distinguishable attacks occurring at irregular intervals.2 As no curative therapy is available, treatment of MS aims at reducing the risk of relapses and disability progression.1 In recent years, the landscape of MS treatment has changed, with currently more than 12 disease-modifying therapies (DMTs) approved for relapsing forms of the disease, and a single DMT approved for primary progressive disease.3 This large treatment armamentarium helps to individualize medical therapy with careful balance of efficacy and safety, and treatment escalation as clinically appropriate.4
Teriflunomide (Aubagio®) is a once-daily oral immunomodulatory agent with anti-inflammatory properties, which was approved for RRMS in the EU in 2013.5 The agent selectively and reversibly inhibits the mitochondrial enzyme dihydro-orotate dehydrogenase (DHODH) which is required for de novo pyrimidine synthesis.6,7 Hence, teriflunomide blocks the proliferation of rapidly dividing cells such as activated lymphocytes. Its efficacy was demonstrated in two placebo-controlled trials (TEMSO8,9 and TOWER10) in patients with RRMS. Further studies include TENERE, a randomized, parallel-group, rater-blinded study comparing teriflunomide and interferon beta-1a subcutaneously (SC) in patients with RRMS,11 a phase II study with extension up to 8.5 years,12,13 and the phase III TOPIC study in patients with a first clinical episode suggestive of MS.14 Teriflunomide is commonly used for mild/moderate MS disease as a first line or first-switch therapy in Germany.15
Current data on the effectiveness, tolerability, and patient satisfaction of teriflunomide under routine clinical practice conditions in unselected patients are limited. Consequently, the team leading the non-interventional TAURUS-MS study aimed to collect such information in a large contemporary cohort of real-world patients in Germany.
Methods
Design
TAURUS-MS (Therapie mit Aubagio® unter Praxisbedingungen: Wirksamkeit, Lebensqualität und Verträglichkeit bei Patienten mit schubförmiger Multipler Sklerose) was a non-interventional, prospective, longitudinal study in Germany. The study was locally approved by the Ethic Committee at the Ruhr-University of Bochum, Faculty of Medicine, as well as registered in the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) public database under number 2075. It was conducted between 6 January 2014 (first patient in) and 4 April 2017 (last patient out).
Sites
A total of 307 office-based and hospital-based neurologists in Germany documented eligible patients.
Subjects
Patients were eligible for enrolment if they met all inclusion criteria: age ⩾ 18 years, diagnosis of RRMS, written patient informed consent, capable of completing questionnaires, and with no existing contraindications. No explicit exclusion criteria were specified to avoid selection bias. According to the prescribing information, administering Aubagio® (teriflunomide) 14 mg once daily was recommended.
Documentation began about 4 weeks after treatment initiation with teriflunomide, and follow-up visits were scheduled after 3 months and in 6-month intervals thereafter, until month 24.
Documented parameters included demographics, information on MS [date of onset and diagnosis, type, number of relapses, disability, magnetic resonance imaging (MRI) results], previous DMT, fatigue as measured using the Fatigue Severity Scale (FSS), and adverse events (AEs).
The Treatment Satisfaction Questionnaire (TSQM-9) served as an instrument for the (self-) assessment of patients’ satisfaction with their current medication.16 The questionnaire comprises nine questions in three domains: effectiveness, convenience, and global satisfaction. Higher levels of satisfaction are expressed as higher TSQM scores (maximum 100 points on each scale).
Data collection and management
Data were collected on paper case-report forms (CRFs) and entered in duplicate in the data management program DMSys®, Version 5.1., Released 2005, SigmaSoft International Inc., Chicago. The data were validated according to rules previously defined in a data validation plan. specified by the clinical research organisation.
Statistical analysis
Analyses were performed in an exploratory manner using descriptive statistical methods. For continuous variables, the number of patients with nonmissing and missing data, mean, standard deviation, minimum, 25% quantile, median, 75% quantile, and maximum were calculated. For ordinal and categorical variables, frequencies were calculated. Incomplete data sets were included in the analysis. There was no imputation of missing values for any endpoint. No sensitivity analyses were done.
All effectiveness analyses were conducted on the per-protocol set (PPS) comprising all treated patients who complied with the protocol. Clinical results were analyzed by visit. For the analysis of relapse rate, the Wilcoxon matched-pair signed-ranks test was used because the number of relapses showed a positively skewed distribution (see Table 1). For the TSQM-9 and FSS (mean value of four out of five items), respectively, paired t-tests were used. Changes from baseline were analyzed by repeated-measurement analysis for time trends.
Table 1.
Demographic data at baseline.
| Characteristic | n | Value |
|---|---|---|
| Age, years, mean (SD) | 1128 | 44.9 (10.2) |
| Sex, % | ||
| Female | 761 | 67.5 |
| Male | 367 | 32.5 |
| Employment status, % | ||
| Regularly fulltime-employed (⩾ 30 h/week) | 469 | 41.6 |
| Regularly part-time-employed (⩾15–29 h/week) | 140 | 12.4 |
| Underemployed or not regularly employed (< 15 h/week) | 59 | 5.2 |
| Not employed | 451 | 40.0 |
| No data regarding employment | 9 | 0.8 |
| Marital status, % | ||
| Single/separated | 214 | 19.0 |
| Partnership | 208 | 18.4 |
| Married | 590 | 52.3 |
| Divorced | 80 | 7.1 |
| Widowed | 14 | 1.2 |
| No data | 22 | 1.95 |
| MS history | ||
| Time since first symptom of MS, mean (SD), yearsa | 1065 | 10.6 (8.2) |
| Time since diagnosis of MS, mean (SD), yearsa | 1075 | 8.9 (7.6) |
| EDSS scorea | ||
| mean (SD) | 947 | 2.3 (1.5) |
| median | 2.00 | |
| range | 0.0 – 7.0 | |
| ⩽3.5 points, % | 794 | 70.4 |
| >3.5 points, % | 153 | 13.6 |
| no data, % | 181 | 16.1 |
| MS relapses over the past 24 months, % | ||
| Mean (SD)b | 1117 | 0.95 (1.1) |
| 0 | 456 | 40.4 |
| 1 | 408 | 36.2 |
| 2 | 172 | 15.3 |
| 3 | 45 | 4.0 |
| ⩾4 | 36 | 3.2 |
| No data | 11 | 0.1 |
| Brain MRI findings, mean (SD) | ||
| MRI performed | 1078 | 0.1 |
| Time of last brain MRI before baseline visit, quarters | 968 | 3.0 (5.4) |
| Number of T2 lesions by MRI | 514 | 10.3 (8.2) |
| Number of GD+ lesions by MRI | 681 | 0.5 (1.3) |
| Symptoms, % | 1128 | |
| Fatigue | 637 | 56.5 |
| Depression (MDD) | 289 | 25.6 |
| Cognitive deficits | 294 | 26.1 |
| Spasticity | 235 | 20.8 |
| Bladder dysfunction | 261 | 23.1 |
| Other | 354 | 31.4 |
Values are means (±SD) or percentages.
EDSS, Expended Disability Status Scale; GD+, gadolinium enhanced; MDD, major depressive disorder: MRI, magnetic resonance imaging; MS, multiple sclerosis; SD, standard deviation.
in relation to baseline visit.
without “missing data”.
The Safety Analysis Set (SAS) contains all patients for whom a CRF was available, and additionally patients with documented AEs or serious AEs but without an available CRF. All AEs occurring during this observational study were coded using Medical Dictionary for Regulatory Activities (MedDRA(R)), Version 13.1., Released September 2010, MedDRA(R) trademark is owned by International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of International Conference on Harmonization (ICH) The incidence of AEs and adverse drug reactions (ADRs) by MedDRA system organ class (SOC) was calculated (number, frequency) for the safety population.
Analyses were carried out with the statistical tool SPSS for Windows, Version 15.0, Released 2006, SPSS Inc., Chicago. As an exception, confidence intervals of categorical variables were calculated with the statistical software BIAS for Windows, Version 10.12, epsilon-Verlag GbR, Hochheim Darmstadt.
Results
Patient disposition
Of 1139 documented patients (100.0%, safety set), 1135 patients had available CRFs, and 1128 fulfilled the inclusion criteria and were eligible for analysis (99.4%, PPS).
Demographics and MS history
Detailed information on the patients at inclusion is provided in Table 1. At the baseline visit, 67.5% of patients were females and 32.5% were males. Mean age was 44.9 ± 10.2 years.
The mean time since first MS symptom was 10.6 ± 8.2 years before baseline visit, and the time since diagnosis was 8.9 ± 7.6 years. Mean Expanded Disability Status Scale (EDSS) was 2.3 ± 1.5, with a wide range across patients (0.0 to 7.0). The majority had an EDSS ⩽ 3.5 (70.4%). The mean number of MS relapses was 0.95 ± 1.10 over the 24-month period prior to study entry. While 40.4% of patients had no relapses within the 24 months prior to study entry, 36.2% had one, 15.3% two, and 7.2% three or more relapses (no data: 1.0%).
The most commonly reported MS-associated symptoms were fatigue (56.5%), depression (25.6%), cognitive deficits (26.1%), bladder dysfunction (23.1%) and spasticity (20.8%).
Treatment history
No previous MS treatment was documented in 24.8% of patients. In patients previously treated with other MS agents, the most commonly prescribed DMTs were glatiramer acetate (26.9%), SC interferon-beta 1a (IFNβ-1a; 23.8%), intramuscular IFNβ-1a (22.8%), and SC IFNβ-1b (19.7%).
For the 848 patients who had received a prior therapy for mild-to-moderate disease, the decision to terminate the previous treatment was taken by the patient in 37.2%, by the physician in 24.5%, and by both in 33.1% (no data: 5.2%). The main reasons for terminating the previous DMT were adverse reactions (59.0%), insufficient efficacy (24.2%), a desire to switch to an oral DMT (16.2%), and a desire for treatment break (10.4%).
An overview of the most recent DMTs before study entry is shown in Table 2.
Table 2.
Previous treatment prior to study entry.
| Characteristic | n | % |
|---|---|---|
| No pretreatment | 280 | 24.8 |
| Any previous MS medication | 848 | 75.2 |
| IFNβ-1a intramuscular | 257 | 22.8 |
| IFNβ-1a subcutaneous | 268 | 23.8 |
| IFNβ-1b subcutaneous | 222 | 19.7 |
| Glatiramer acetate subcutaneous | 303 | 26.9 |
| Azathioprine oral | 51 | 4.5 |
| Immunoglobulin intravenous | 13 | 1.2 |
| Other | 154 | 24.8 |
| Previous DMT discontinued ⩽ 6 months prior to study entry | 593 | 52.6 |
| Thereof: | ||
| IFNβ-1a intramuscular | 74 | 6.6 |
| IFNβ-1a subcutaneous | 105 | 9.3 |
| IFNβ-1b subcutaneous | 74 | 6.6 |
| Glatiramer acetate subcutaneous | 119 | 10.6 |
| Azathioprine oral | 4 | 0.4 |
| Other | 82 | 7.3 |
| Last MS medication not known | 135 | 12.0 |
| No treatment ⩽ 6 months prior to start of teriflunomide | 504 | 44.7 |
| No data regarding previous treatment | 31 | 2.8 |
| Main reason for stopping previous treatment | ||
| Lack of trust in efficacy | 75 | 14.9 |
| Fear of adverse reactions | 143 | 28.4 |
| Wish for child | 12 | 2.4 |
| Pregnancy | 10 | 2.0 |
| Assumption of patient’s noncompliance | 18 | 3.6 |
| Fear of needles | 74 | 14.7 |
| Other | 183 | 36.3 |
Values are means or percentages.
DMT, disease-modifying therapy; IFNβ, interferon beta; MS, multiple sclerosis.
Notably, 44.7% of the patients (n = 504) received no DMT within 6 months (⩽6 months) of study entry. For 52.6% of the patients (n = 593), the previous DMT was discontinued prior to study entry. These patients were most commonly treated with glatiramer acetate (10.6%), SC IFNβ-1a (9.3%), intramuscular IFNβ-1a (6.6%), or IFNβ-1b (6.6%). The most frequent reasons for discontinuations were ‘fear of adverse reactions’ (28.4%), ‘lack of trust in efficacy’ (14.9%), and ‘fear of needles’ (14.7%).
Treatment with teriflunomide
The mean observation period was 16.3 ± 9.1 months (494.6 days ± 277.2 days). Patient disposition on teriflunomide and reasons for stopping are shown in Table 3.
Table 3.
Teriflunomide treatment duration and reasons for stopping.
| Characteristic | n | Value |
|---|---|---|
| Exposure, % | ||
| Duration of observation, days, mean (SD) | 1072 | 495 (277) |
| Follow-up period, mean (days ± SD) | ||
| approximately 3 months (93.9 ± 26.8) | 989 | 87.7 |
| approximately 6 months (195.7 ± 49.5) | 882 | 78.2 |
| approximately 12 months (365.1 ± 65.7) | 758 | 67.2 |
| approximately 18 months (543.3 ± 67.4) | 630 | 55.9 |
| approximately 24 months (725.4 ± 76.9) | 512 | 45.4 |
| Main reason for discontinuation (total n = 242; multiple responses possible), % | ||
| Insufficient efficacy | 55 | 22.7 |
| Adverse events | 97 | 40.1 |
| Desire to have children | 5 | 2.1 |
| Pregnancy | 1 | 0.4 |
| Wish for treatment break | 22 | 9.1 |
| Assumed lack of compliance | 22 | 9.1 |
| Other | 56 | 23.1 |
Values are means (±standard deviation) or percentages.
SD, standard deviation.
At the 12-month visit, continuous treatment was confirmed in 67.2% (n = 758) patients, the status was unknown (lost to follow up) in 17.8% (n = 201), and 15.0% (n = 169) had stopped treatment. After 24 months, 45.4% (n = 512) patients were confirmed to be on continuous treatment, 33.2% (n = 374) patients were lost to follow up, and 21.5% (n = 242) had discontinued treatment. The most common reason for discontinuation of treatment were AEs (40.1%, n = 97). Of those, 29.9% (n = 29) patients discontinued due to diarrhea, 16.5% (n = 16) due to hair thinning, and 10.3% (n = 10) due to nausea. Further reasons for discontinuation were insufficient efficacy (22.7%, n = 55), wish for treatment break (9.1%, n = 22), and assumed lack of compliance (9.1%, n = 22). Temporary interruption of treatment at some point during the observation period was reported in 7.6% (no interruptions: 81.3%; no data: 11.1%).
Effectiveness
MS relapses
After 12 months’ treatment with teriflunomide, the mean annualized relapse rate (ARR) was 0.24 ± 0.53 [95% confidence interval (CI): 0.20–0.28], whereas the 12-month ARR before study entry was 0.59 ± 0.76 (95% CI: 0.54–0.65). After 24 months’ treatment with teriflunomide, the mean ARR was 0.35 ± 0.68 (95% CI: 0.29–0.41), while the ARR for the corresponding period before study entry was 0.87 ± 1.10 (data from 468 patients, 95% CI: 0.77–0.97, p ⩽ 0.001; Figure 1).
Figure 1.
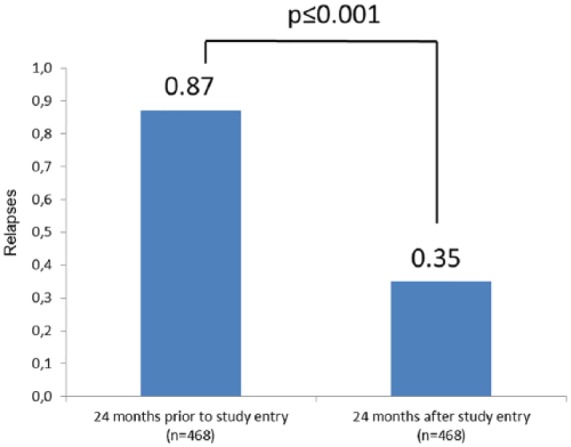
Mean annualized relapse rate (ARR).
EDSS
Mean EDSS at study entry was 2.32 and 2.37 at month 12 (in n = 555 patients with a follow-up value at this time point), or 2.28 at study entry and 2.40 at month 24 (n = 346).
Patient-reported outcomes
TSQM-9
Following treatment with teriflunomide, all three domains of the TSQM-9 improved.
For the effectiveness scale, the mean values at study entry (n = 829), 3 months (n = 879), 6 months (n = 777), 12 months (n = 664), 18 months (n = 542), 24 months (n = 444), and at the last follow-up visit (irrespective of treatment duration, n = 942) were 60.8, 67.7, 67.9, 69.9, 70.4, 71.0 and 67.5, respectively. Comparing the study entry data with that of the last visit (n = 710 patients), the mean effectiveness score increased by 5.8 ± 29.9 points.
In patients who discontinued a previous DMT within 6 months of study entry, the mean change from study entry to 12 months for the effectiveness scale was 9.2 ± 28.0 (n = 318; p < 0.001), and 8.1 ± 27.7 between study entry and 24 months (n = 229; p ⩽ 0.001; Figure 2).
Figure 2.
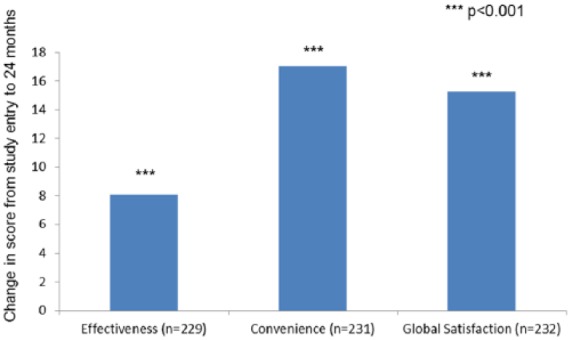
TSQM-9: Changes in effectiveness, convenience, and global satisfaction scores from study entry to 24 months for patients receiving teriflunomide who discontinued previous DMT within 6 months of study entry.
***p < 0.001.
DMT, disease-modifying therapy; TSQM-9, Treatment Satisfaction Questionnaire.
For the convenience scale, the mean values at study entry (n = 854), 3 months (n = 896), 6 months (n = 793), 12 months (n = 667), 18 months (n = 546), 24 months (n = 446), and at the last follow-up visit (n = 950) were 74.8, 89.8, 90.5, 90.8, 91.2, 90.9, and 90.2, respectively. Comparing the study entry data with that of the last visit (n = 734 patients), the mean convenience score increased by 15.60 ± 27.41 points.
In patients who discontinued a previous DMT within 6 months of study entry, the mean change from study entry to 12 months for the convenience scale was 16.8 ± 26.4 (n = 325; p ⩽0.001), and 17.0 ± 26.6 between study entry and 24 months (n = 231; p < 0.001; Figure 2).
For the global satisfaction scale, the mean values at study entry (n = 854), 3 months (n = 894), 6 months (n = 791), 12 months (n = 663), 18 months (n = 544), 24 months (n = 444), and at the last follow-up visit (n = 947) were 62.5, 72.4, 73.3, 74.8, 76.9, 77.5, and 72.3, respectively. Comparing the study entry data with that of the last visit (n = 731 patients), the mean global satisfaction score increased by 9.82 ± 29.1 points.
In patients who discontinued a previous DMT within 6 months of study entry, the mean change from study entry to 12 months for the global satisfaction scale was 12.6 ± 28.0 (n = 324; p ⩽ 0.001), and 15.3 ± 27.4 between study entry and 24 months (n = 232; p < 0.001; Figure 2).
Fatigue Severity Scale (FSS)
Only slight changes on the FSS were observed during treatment with teriflunomide. The mean values at study entry (n = 965), 6 months (n = 790), 12 months (n = 674), 18 months (n = 552), 24 months (n = 448), and at the last follow-up visit (n = 836) were 4.48, 4.27, 4.23, 4.10, 4.10, and 4.28, respectively. Comparing the study entry data with that of the last visit [n = 724 patients, not significant (n.s.)], the mean FSS score changed by −0.11 ± 1.60 points, between study entry and 12 months by −0.13 ± 1.44 points (n = 589, p < 0.05), and between study entry and 24 months by −0.16 ± 1.69 points (n = 397, n.s.).
Patient satisfaction from the perspective of the treating physician
The question on patient satisfaction could be rated from 1 ‘very dissatisfied’ to 5 ‘very satisfied’ at each visit. The mean scores increased during the treatment with teriflunomide. After 12 and 24 months of teriflunomide treatment, the scores increased by 1.35 ± 1.36 points (n = 355) and 1.42 ± 1.30 points (n = 248), respectively, compared with study entry.
Safety
Adverse events
In the safety analysis set comprising 1139 patients, 408 patients (35.8%) reported a total of 893 AEs.
The most frequently reported AEs related to the MedDRA primary SOC were ‘infections and infestations’ (13.3% of patients), ‘nervous-system disorders’ (9.7%), ‘gastrointestinal disorders’ (8.1%), ‘general disorders and administration-site conditions’ (6.8%), and ‘skin and subcutaneous-tissue disorders’ (5.4%). The most frequently reported nervous-system disorders were MS relapses or other disease-related symptoms. The detailed breakdown of AEs by seriousness and SOC is shown in Table 4.
Table 4.
Nonserious versus serious adverse events by MedDRA primary system organ class.
| Nonserious |
Serious |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Blood and lymphatic-system disorders | 9 | 1.3 | 4 | 1.8 |
| Cardiac disorders | 1 | 0.2 | 8 | 3.7 |
| Ear and labyrinth disorders | 5 | 0.7 | 0 | 0.0 |
| Endocrine disorders | 1 | 0.2 | 1 | 0.5 |
| Eye disorders | 9 | 1.3 | 0 | 0.0 |
| Gastrointestinal disorders | 100 | 14.8 | 18 | 8.3 |
| General disorders and administration-site conditions | 83 | 12.3 | 5 | 2.3 |
| Hepatobiliary disorders | 1 | 0.2 | 3 | 1.4 |
| Immune-system disorders | 2 | 0.3 | 0 | 0.0 |
| Infections and infestations | 151 | 22.4 | 59 | 27.1 |
| Injury, poisoning and procedural complications | 19 | 2.8 | 8 | 3.7 |
| Investigations | 49 | 7.3 | 3 | 1.4 |
| Metabolism and nutrition disorders | 6 | 0. 9 | 1 | 0.5 |
| Musculoskeletal and connective tissue disorders | 28 | 4.2 | 10 | 4.6 |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 0.2 | 4 | 1.8 |
| Nervous-system disorders | 71 | 10.5 | 63 | 28.9 |
| Psychiatric disorders | 17 | 2.5 | 5 | 2.3 |
| Renal and urinary disorders | 9 | 1.3 | 4 | 1.8 |
| Reproductive system and breast disorders | 4 | 0.6 | 2 | 0.9 |
| Respiratory, thoracic, and mediastinal disorders | 17 | 2.5 | 5 | 2.3 |
| Skin and subcutaneous-tissue disorders | 73 | 10.8 | 2 | 0.9 |
| Social circumstances | 3 | 0.4 | 0 | 0.0 |
| Surgical and medical procedures | 0 | 0.0 | 6 | 2.8 |
| Vascular disorders | 16 | 2.4 | 7 | 3.2 |
| Total | 675 | 100.0 | 218 | 100.0 |
MedDRA = Medical Dictionary for Regulatory Activities.
The incidence rates of AEs by MedDRA preferred term, sorted according to frequencies, is presented in Table 5. The most frequently reported AEs were diarrhea (4.8%, n = 55), followed by MS relapse (4.2%, n = 48), hair thinning (3.3%, n = 38), and viral upper respiratory tract infection (2.7%, n = 31).
Table 5.
Adverse events by preferred term.
| n | % | |
|---|---|---|
| Total number of patients | 1139 | 100 |
| Total patients with AEs | 408 | 35.8 |
| Of these, patients with serious AEs | 148 | 13.0 |
| AEs (multiple entries possible) | ||
| Diarrhea | 55 | 4.8 |
| MS relapse | 48 | 4.2 |
| Hair thinning* | 38 | 3.3 |
| Viral upper respiratory tract infection | 31 | 2.7 |
| Influenza | 22 | 1.9 |
| Drug ineffective | 19 | 1.5 |
| Urinary tract infection | 18 | 1.6 |
| Bronchitis | 17 | 1.5 |
| Hypertension | 16 | 1.4 |
| Influenza-like illness | 16 | 1.4 |
| Nausea | 15 | 1.3 |
Sorted by frequencies. Adverse events with incidence ⩾ 1%.
Medical Dictionary for Regulatory Activities (MedDRA) preferred term is alopecia.
AEs, adverse events.
Mean alanine transaminase (ALT) values increased from 25.3 ± 16.3 U/l (n = 989) at first report to 34.7 ± 88.7 U/l at 3 months (n = 899), and were 27.8 ± 22.0 U/l at 12 months (n = 661) and 24.9 ± 15.0 U/l at 24 months (n = 447). Six patients had elevated hepatic enzymes which led to therapy discontinuation in four patients. Additionally, liver disorders occurred in three patients (hepatic lesion, hepatocellular injury and liver tenderness, with the latter case being associated with teriflunomide treatment according to the sponsor’s safety assessment).
In 148 patients (13.0% of patients), 218 serious AEs occurred. One patient died due to an opportunistic infection (bronchopulmonary aspergillosis). The reporting investigator assessed the event as related to teriflunomide. However, the patient’s age (61 years) and concomitant medication (phenprocoumon, tiotropium bromide, metoprolol tartrate, torasemide, omeprazole, metamizole sodium, fluticasone propionate/salmeterol xinafoate and prednisolone) could be possible confounding factors.
Five neoplasms occurred during the study: breast cancer (n = 2), cervix carcinoma (n = 1), rectal neoplasm (n = 1), and non-Hodgkin’s lymphoma (n = 1). The two reported pregnancies led to one induced abortion in one case (for personal reasons, it was unknown whether anomalies were detected with prenatal diagnostics) and delivery of a healthy baby in the second case.
Discussion
The TAURUS-MS study was a prospective, non-interventional study to document the treatment with teriflunomide (Aubagio®) in patients with RRMS over a 24-month observation period. The study is the first to report experience with teriflunomide under real-life-practice conditions, and provides data on treatment satisfaction and fatigue in a real-world MS population, in which about 25% of the MS patients were treatment naïve.
Compared with the placebo-controlled registration studies that also included mostly RRMS patients (91.5% in TEMSO and 97.5% in TOWER), patients in TAURUS-MS differed substantially. They were older (44.9 years in TAURUS-MS versus 37.9 years in both TEMSO and TOWER), had a longer disease duration (10.6 years versus 8.7 and 8.0 years), a lower mean EDSS score (median 2.0 versus 2.5 in both TEMSO and TOWER), a lower proportion of patients with EDSS > 3.5 (13.6% versus 22.8% and 25.5%), and a lower mean ARR (0.95 versus 1.4 in both TEMSO and TOWER). Also, the percentage of patients with previous DMT use was higher in TAURUS-MS (75.2%) than in TEMSO and TOWER (73% and 67.2% in the 2 years prior to study entry).
In terms of effectiveness, in the teriflunomide 14 mg cohorts of the registration studies TEMSO and TOWER, an ARR of 0.37 and 0.32, respectively, was reported. In TAURUS-MS, the mean ARR of 0.55 was numerically higher, but compared with before study entry, the number of MS relapses during teriflunomide treatment decreased by more than half (0.59 relapses in the 12 months prior to study entry versus 0.24 in the 12 months following study entry). In line with this finding, in TAURUS-MS, disability did not progress as shown by a stable EDSS (+0.1 points at 24 months). Also, fatigue remained unchanged as measured using the FSS (−0.13 between study entry and the 12-month visit) which provides supporting evidence for the long-lasting effects of teriflunomide therapy in terms of MS disease stabilization.
The importance of the patient perspective on treatment in various indications including MS has been highlighted in recent years.17–19 The TSQM, which is not limited to specific medications, has been used in many MS studies, including the recent THEPA-MS cross-sectional study in Germany with over 3000 patients on injectable DMTs.20 For SC IFNβ-1a or -1b therapy, for example, values across domains ranged between 68 and 74 points. For patients who had been on a previous medication and switched to teriflunomide in TAURUS-MS, effectiveness or global satisfaction levels were also in this range, while the convenience score for teriflunomide was higher (up to 91 points). This is in line with recent research showing that oral medications are perceived to be more convenient than the DMTs administered by injection or infusion.21,11
Results were confirmed by the analysis of the physicians’ assessment of patient satisfaction, which also showed improvement. According to the results, more than 90% of the patients perceived the administration of teriflunomide as easy, and could easily integrate it in their daily routines, especially in comparison with the previous DMT.
Despite these results, 21.5% of patients discontinued teriflunomide therapy prematurely, and for 33.2%, it was unknown whether the treatment was continued or not (lost to follow up). The main reasons for premature discontinuation of teriflunomide appear to be AEs (40.1%) and insufficient efficacy (22.7%).
High discontinuation rates are a typical finding in clinical studies, as well as in observational research in MS. In a meta-analysis by Giovannoni and colleagues on 50 randomized studies and 19 observational studies in MS, mean discontinuation rates of 17–36% for such therapies were noted.22 One of the underlying reasons could be, according to a recent systematic review, that patients do not satisfactorily understand the benefits and risks of DMTs.23 Further, there is the possibility that patients decide to stop participation in the study, but continue the drug.
Teriflunomide was generally well tolerated. In total, AEs occurred in 35.8% of patients, with 97 patients (8.6%) discontinuing treatment due to AEs.
In the safety database of teriflunomide with nearly 4400 cumulative patient-years, the ‘very frequent’ treatment-emergent adverse reactions (defined as occurring in ⩾10%) were headache, diarrhea/nausea, elevated ALT, and hair thinning. In TAURUS-MS, no single AE was reported more frequently than 5%, with the leading events being diarrhea/nausea (4.8%/1.3%), MS relapses (4.2%), and hair thinning (3.3%).
The reported AEs were in line with the registration studies, and no new safety signals were identified. The observed initial slight increase of the mean ALT level in patients normalized during treatment. Elevated liver transaminases led to discontinuation in only four cases.
When interpreting the results of this study, methodological considerations need to be taken into account. The study used an observational design, which may lead to unquantifiable bias in the selection of MS patients (e.g. under-representation of critically ill individuals).24 Furthermore, neurologists willing to participate in the observational study are likely to represent a selection of physicians with a particular interest and knowledge in the field of MS management. It is known that adherent patients are more likely to provide their informed consent for study participation.25 The lost-to-follow-up rate over time was substantial, as in other observational MS studies;26 yet at 1 year, within the 20% range is usually regarded as acceptable within the confines of evidence-based medicine.27 Among the strengths of the study is its large number of patients, with complete coverage of all regions in Germany, and strong focus on the ambulatory setting rather than on university or specialist centers.
Conclusion
The results from this non-interventional study demonstrate the sustained effectiveness of teriflunomide in the treatment of patients with RRMS over a 24-month period. The number of relapses decreased after treatment initiation, and patient satisfaction improved in the different areas covered by the TSQM-9.
The benefit–risk profile of teriflunomide remains favorable, and is consistent with that reported for other studies of teriflunomide.
Acknowledgments
The authors and Sanofi would like to thank the patients for their participation in the trial, as well as the TAURUS-MS study team. Input to the interpretation of results and to the first draft of the manuscript was provided by David Pittrow, MD, PhD from 3P Consulting, Seefeld, Germany. Statistical analyses were done by factum GmbH, Offenbach, Germany. This manuscript was reviewed by Sigbert Jahn, PhD, Darren P Baker, PhD, Kathleen Somera-Molina, PhD.
All authors contributed to the design of the study and interpretation of the results. AC and JK wrote the first version of the manuscript, and the other authors provided input into the concept and the interpretation of results. All authors reviewed and approved the final version.
Footnotes
Funding: This study was sponsored by Sanofi-Aventis Deutschland GmbH, Neu-Isenburg, Germany.
Conflict of interest statement: BAK has received compensation for activities with Bayer, Biogen, Sanofi Genzyme, Merck, Novartis, Roche, and Teva.
KTW has received honoraria for lectures, studies, and consultancy from Almirall, Bayer, Biogen, Genzyme, Ipsen, Merck Serono, Merz Pharma, Novartis, Roche, Sanofi, and Teva.
AC has received compensation for activities with Actelion, Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and Teva for use of university research funds. He receives research support from UCB, and from Sanofi for basic research on drug transport mechanisms relevant to teriflunomide.
UE and JK are full-time employees of Sanofi-Aventis Deutschland GmbH.
Statement on data sharing: Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case-report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.
Contributor Information
Boris A. Kallmann, Multiple-Sclerosis-Center Bamberg, Bamberg, Germany
Klaus Tiel-Wilck, Neurologisches Facharztzentrum Berlin, Berlin, Germany, for the NeuroTransData Study Group.
Jennifer S. Kullmann, Medical Management MS, Medical Affairs, Sanofi-Aventis Deutschland GmbH, Siemensstraße 5b, 63263 Neu-Isenburg, Germany.
Ulrich Engelmann, Medical Affairs, Sanofi-Aventis Deutschland GmbH, Neu-Isenburg, Germany.
Andrew Chan, Department of Neurology, Bern University Hospital, University of Bern, Switzerland.
References
- 1. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 2018; 25: 215–237. [DOI] [PubMed] [Google Scholar]
- 2. Antel J, Antel S, Caramanos Z, et al. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol 2012; 123: 627–638. [DOI] [PubMed] [Google Scholar]
- 3. Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 789–800. [DOI] [PubMed] [Google Scholar]
- 4. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 5. European Medicines Agency. Aubagio (teriflunomide). European public assessment report. EMEA/H/C/002514 -N/0015, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002514/WC500148682.pdf. (2017, accessed 26 April 2018).
- 6. Miller AE. Oral teriflunomide in the treatment of relapsing forms of multiple sclerosis: clinical evidence and long-term experience. Ther Adv Neurol Disord 2017; 10: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan A, De Seze J, Comabella M. Teriflunomide in patients with relapsing-remitting forms of multiple sclerosis. CNS Drugs 2016; 30: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 9. Miller AE, O’Connor P, Wolinsky JS, et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler 2012; 18: 1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- 11. Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014; 20: 705–716. [DOI] [PubMed] [Google Scholar]
- 12. O’Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006; 66: 894–900. [DOI] [PubMed] [Google Scholar]
- 13. Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler 2012; 18: 1278–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 977–986. [DOI] [PubMed] [Google Scholar]
- 15. Gold R, Hemmer B, Wiendl H (eds.), DGN / KKNMS. Leitlinie zur Diagnose und Therapie der MS, http://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2016/02/dgn-kknms_ms-ll_20140813.pdf (2014, accessed 26 February 2019).
- 16. Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coons SJ, Gwaltney CJ, Hays RD, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health 2009; 12: 419–429. [DOI] [PubMed] [Google Scholar]
- 19. Rieckmann P, Boyko A, Centonze D, et al. Achieving patient engagement in multiple sclerosis: a perspective from the multiple sclerosis in the 21st Century Steering Group. Mult Scler Relat Disord 2015; 4: 202–218. [DOI] [PubMed] [Google Scholar]
- 20. Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing-remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord 2016; 9: 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eagle T, Stuart F, Chua AS, et al. Treatment satisfaction across injectable, infusion, and oral disease-modifying therapies for multiple sclerosis. Mult Scler Relat Disord 2017; 18: 196–201. [DOI] [PubMed] [Google Scholar]
- 22. Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler 2012; 18: 932–946. [DOI] [PubMed] [Google Scholar]
- 23. Reen GK, Silber E, Langdon DW. Multiple sclerosis patients’ understanding and preferences for risks and benefits of disease-modifying drugs: a systematic review. J Neurol Sci 2017; 375: 107–122. [DOI] [PubMed] [Google Scholar]
- 24. Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health 2004; 58: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Onzenoort HA, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension 2011; 58: 573–578. [DOI] [PubMed] [Google Scholar]
- 26. Sormani MP, Bruzzi P. Can we measure long-term treatment effects in multiple sclerosis? Nature Rev Neurol 2015; 11: 176–182. [DOI] [PubMed] [Google Scholar]
- 27. Sackett D, Strauss S, Wea Richardson. Evidence-based medicine: how to practice and teach EBM. Edinburgh: Churchill Livingstone, 2000. [Google Scholar]


