Abstract
Background and purpose:
In acute ischaemic stroke (AIS) of the anterior circulation (AC) treated with mechanical thrombectomy (MT), data point to a decline of treatment effect with increasing time from symptom onset to treatment. However, the magnitude of the decline will depend on the clinical setting and imaging selection used. The aims of this study were (1) to evaluate the clinical effect of time to reperfusion (TTR); and (2) to assess the safety and technical efficacy of MT according to strata of TTR.
Methods:
Using the retrospective multicentre BEYOND-SWIFT registry data (ClinicalTrials.gov identifier: NCT03496064), we compared safety and efficacy of MT in 1461 patients between TTR strata of 0–180 min (n = 192), 180–360 min (n = 876) and >360 min (n = 393). Clinical effect of TTR was evaluated using multivariable logistic regression analyses adjusting for pre-specified confounders [adjusted odds ratios (aOR) and 95% confidence intervals (95% CI)]. Primary outcome was good functional outcome (modified Rankin Scale: mRS 0–2) at day 90.
Results:
Every hour delay in TTR was a significant factor related to mRS 0–2 (aOR 0.933, 95% CI 0.887–0.981) with an estimated 1.5% decreased probability of good functional outcome per hour delay of reperfusion, and mRS 0–1 (aOR 0.929, 95% CI 0.877–0.985). Patients with late TTR had lower rates of successful and excellent reperfusion, higher complication rates and number of passes.
Conclusions:
TTR is an independent factor related to long-term functional outcome. With increasing TTR, interventional procedures become technically less effective. Efforts should be made to shorten TTR through optimized prehospital and in-hospital pathways.
Keywords: endovascular, mechanical thrombectomy, stroke, symptom onset, time to reperfusion, time to treatment, thrombolysis
Introduction
A meta-analysis of randomized controlled trials (RCTs) in patients treated with mechanical thrombectomy (MT) for acute ischaemic stroke (AIS) in the anterior circulation (AC) showed that the treatment effect of recanalization decreased over time, reaching non-significance at 7.3 h.1 In those trials, patient selection was based primarily on symptom onset to groin puncture time (STG) of 6 h (MR CLEAN, EXTEND-IA, SWIFT-PRIME), 8 h (REVASCAT) and 12 h (ESCAPE). Further selection criteria were Alberta Stroke Program Early Computed Tomography Score (ASPECTS) thresholds, perfusion imaging and collateral status in four of the five trials. Recently, observational data of patients with an STG of up to 6.5 h suggested an even stronger decline of treatment effect, with an estimated 5.3% decreased probability of functional independence and a 2.2% increase in mortality at 3 months per hour increase from stroke onset to MT start.2
Contrarily, two RCTs showed that advanced imaging is able to select patients up to 24 h after symptom onset benefiting from MT,3,4 with a very strong treatment effect also in the late time window.5 Although also here a decline in efficacy of the treatment effect was noted, the slope and time of the decline were very different from the studies mentioned above.6 Therefore, the decline of treatment effect seems to be strongly influenced by patient selection, and the concept of a general time window with an uniform decline of efficacy has been challenged.7,8 Furthermore, it remains unclear whether with increasing time from symptom onset to reperfusion (TTR) there is a cut-off time with deleterious treatment effect thereafter similar to intravenous thrombolysis (IVT) or if MT at a given time point simply remains futile and not cost-effective.9,10
The aims of this study were (1) to evaluate the clinical effect of TTR; and (2) to assess the safety and technical efficacy of MT according to strata of TTR.
Methods
Details on the BEYOND-SWIFT registry are in the public domain (ClinicalTrials.gov identifier: NCT03496064) and have been published previously.11 Briefly, the registry is a retrospective, international, multicentre, non-randomized observational study to investigate the safety and efficacy of a CE-labelled market-release neurothrombectomy device (applied as initial devices) in AIS patients. An overview of included patients, rates of available follow-up data for each centre and ethical approval procedure can be found in Supplementary Table 1. Additionally, ethical approval was obtained in Bern for pooling and anonymized analyses of the registry data (KEK Bern, Bern, Switzerland ID 2018-00766).
Most patients in the registry (n = 2046) were treated for large-vessel AC strokes (n = 1832). Of the patients with known TTR (n = 1461), 1314 (89.9%) had documented 90-day follow up.
Variables and image analysis
Local investigators categorized the site of occlusion into intracranial internal carotid artery, carotid-T/L, first/second/third segment of the middle cerebral artery (M1/M2/M3), and first/second segment of the anterior cerebral artery (A1/A2). The patients with large-vessel occlusion (LVO) in the posterior circulation and seven patients with missing information on occlusion site were excluded from this analysis. The post-procedural modified thrombolysis in cerebral infarction (mTICI) scale was operator-adjudicated at each centre or rated by an independent research fellow, depending on the applicable institutional standards (see Supplementary Table 1). AC extracranial–intracranial tandem occlusion was defined as the presence of an intracranial LVO and >90% cervical stenosis or occlusion. According to the modified version of the TICI scale, we rated mTICI2b as reperfusion of at least 50% of the initially occluded target territory.12 ASPECTS was evaluated at each site (see Supplementary Table 1) based on admission non-contrast computed tomography (CT) in 954/1461 (65.3%) cases and diffusion-weighted magnetic resonance imaging (MRI) in 505/1461 (34.6%) cases, with missing information in two cases. For clinical outcome evaluation, we assessed 3-month functional outcome applying the modified Rankin Scale (mRS) either in routinely scheduled clinical visits or standardized telephone interviews.
Statistical analysis
The primary endpoint of this analysis was good functional outcome (mRS 0–2) at 90 days. Secondary and safety outcomes at 90 days consisted of excellent outcome (mRS 0–1), favourable outcome (mRS 0–3), all-cause mortality and symptomatic intracerebral haemorrhage (sICH), which was assessed at each centre, applying the European Co-operative Acute Stroke Study-II (ECASS II) criteria. Non-haemorrhagic neurological worsening was defined as a drop in the NIH Stroke Scale (NIHSS) ⩾4 between admission NIHSS and 24-h NIHSS without occurrence of sICH.13
Univariate comparisons between three TTR strata (0–180 min, 180–360 min, >360 min) were made using standard statistical measures [Chi-squared test for categorical variables, Kruskal–Wallis H-test for non-normally continuous or ordinally scaled variables and analysis of variance (ANOVA) for independent normally distributed data].
Association of TTR with all outcome parameters was assessed using multivariable logistic regression, adjusting for the following prespecified confounders: age (continuous), sex (categorical), NIHSS on admission (ordinal, adjusted odds ratio (aOR) per point increase), direct presentation versus transfer from another hospital (categorical), in-hospital stroke (categorical), wake-up stroke (categorical), tandem versus non-tandem (categorical, tandem defined as >90% cervical stenosis or cervical occlusion), centre (categorical, contrast type: indicator, comparator: largest centre), ASPECTS (ordinal, aOR per point increase), IVT (categorical), risk factor hypertension (categorical), risk factor dyslipidaemia (categorical), risk factor smoking (categorical), risk factor previous stroke (categorical), risk factor diabetes (categorical), type of admission imaging (CT versus MRI, categorical) and successful reperfusion ⩾TICI2b. For mRS shift analysis, multivariable ordinal regression was used to assess the association of time to reperfusion with mRS at day 90 adjusting for the abovementioned categorical factors and continuous or ordinal covariates using a logit link function. A parallel lines test was used to check for proportional odds assumption. Patients with missing data were excluded from multivariate analysis.
Results
Baseline characteristics
Of 1832 patients in the registry, TTR was available in 1461 patients (1253 documented symptom onset, 203 last seen well) and all had documented final mTICI score. Overall, 404 patients had a TTR of more than 6 h and 371 of more than 7 h. Distribution of TTR and STG is depicted in Supplementary Figures 1a and 1b. Baseline characteristics and comorbidities are presented in Table 1. Patients with late TTR had a higher rate of undocumented symptom onset, higher rate of wake-up stroke, lower rate of in-hospital stroke, were more likely to be transferred from another hospital, more often received MRI as the initial imaging modality, had a lower ASPECTS score and received IVT less often.
Table 1.
Baseline characteristics of all patients and according to time from symptom onset to reperfusion.
| Symptom onset to reperfusion 0–180 min (n=192) | Symptom onset to reperfusion 180–360 min (n=876) | Symptom onset to reperfusion >360 min (n=393) | p | All patients with available TTR (n = 1461) | |
|---|---|---|---|---|---|
| Clinical items | |||||
| Age (years) | 73 (IQR 63–82) | 74 (IQR 60–81) | 74 (IQR 62–82) | 0.871 | 73 (IQR 61–82) |
| Transfer from another hospital | 17 (8.9%) | 283 (32.3%) | 175 (44.5%) | <0.001 | 475 (32.5%) |
| Sex (female) | 101 (52.6%) | 444 (50.7%) | 192 (48.9%) | 0.679 | 737 (50.4%) |
| NIHSS on admission | 17 (IQR 12–20) | 16 (IQR 11–20) | 16 (IQR 10–20) | 0.135 | 16 (IQR 11–20) |
| Prestroke independence | 177 (92.2%) | 812 (93.2%) | 361 (92.3%) | 0.789 | 1350 (92.8%) |
| Blood pressure systolic (mmHg) | 148 (SD 29) | 151 (SD 29) | 152 (SD 28) | 0.309 | 150 (SD 28) |
| Blood pressure diastolic (mmHg) | 81 (SD 21) | 82 (SD 18) | 82 (SD 24) | 0.664 | 80 (SD 20) |
| Admission glucose (mmol/L) | 6.4 (IQR 5.7–7.7) | 6.9 (IQR 5.8–8.1) | 6.8 (IQR 5.8–8.3) | 0.156 | 6.6 (IQR 5.8–8.1) |
| Quality of symptom onset | <0.001 | ||||
| − Noticed | 186 (96.9%) | 801 (91.4%) | 266 (67.7%) | 1253 (85.8%) | |
| − Last seen well | 6 (3.1%) | 73 (8.3%) | 124 (31.6%) | 203 (13.9%) | |
| Wake-up stroke | 4 (2.1%) | 27 (3.1%) | 82 (20.9%) | <0.001 | 113 (7.7%) |
| In-hospital stroke | 15 (7.8%) | 14 (1.6%) | 7 (1.8%) | <0.001 | 36 (2.5%) |
| Medication | |||||
| Antiplatelet | 0.558 | ||||
| − Monotherapy | 51 (27.1%) | 242 (29.8%) | 97 (27.1%) | 390 (28.7%) | |
| − Dual Therapy | 5 (2.7%) | 15 (1.8%) | 4 (1.1%) | 24 (1.8%) | |
| Statin | 60 (33.5%) | 237 (30.7%) | 90 (25.9%) | 0.137 | 387 (29.8%) |
| Anticoagulation | 0.247 | ||||
| − None | 157 (83.5%) | 670 (82.4%) | 308 (86.0%) | 1135 (83.5%) | |
| − VKA | 20 (10.6%) | 102 (12.5%) | 41 (11.5%) | 163 (12.0%) | |
| − NOAC | 11 (5.9%) | 41 (5.0%) | 9 (2.5%) | 61 (4.5%) | |
| Risk factors | |||||
| Diabetes | 33 (17.2%) | 151 (17.3%) | 69 (17.6%) | 0.990 | 253 (17.4%) |
| Arterial hypertension | 139 (72.4%) | 573 (65.9%) | 258 (65.8%) | 0.200 | 970 (66.7%) |
| Dyslipidaemia | 102 (53.1%) | 450 (21.8%) | 199 (51.0%) | 0.892 | 751 (51.8%) |
| Smoking | 51 (27.7%) | 255 (30.3%) | 99 (25.9%) | 0.278 | 405 (28.8%) |
| Previous stroke | 33 (17.2%) | 120 (13.7%) | 47 (12.1%) | 0.253 | 200 (13.8%) |
| Coronary artery disease | 31 (25.2%) | 140 (22.3%) | 61 (20.5%) | 0.572 | 232 (22.1%) |
| TOAST aetiology | 0.067 | ||||
| − Large artery | 19 (10.0%) | 110 (12.8%) | 59 (15.3%) | 188 (13.1%) | |
| − Cardiac embolism | 100 (52.6%) | 419 (48.6%) | 168 (43.6%) | 687 (47.8%) | |
| − Other specific | 19 (10.0%) | 50 (5.8%) | 31 (8.1%) | 100 (7.0%) | |
| − Unknown | 52 (27.4%) | 283 (32.8%) | 127 (33.0%) | 462 (32.2%) | |
| Imaging | |||||
| Imaging type | 0.016 | ||||
| − MRI | 58 (30.2%) | 289 (33.0%) | 158 (40.3%) | 505 (34.6%) | |
| − CT | 134 (69.8%) | 586 (67.0%) | 234 (59.7%) | 954 (65.4%) | |
| Hemisphere | 0.451 | ||||
| − Left | 100 (54.3%) | 418 (49.6%) | 177 (46.3%) | 695 (49.4%) | |
| − Right | 82 (44.6%) | 410 (48.7%) | 197 (51.6%) | 689 (48.9%) | |
| − Midline/bilateral | 2 (1.1%) | 14 (1.7%) | 8 (2.1%) | 24 (1.7%) | |
| Occlusion site | 0.259 | ||||
| − iICA | 3 (1.6%) | 31 (3.5%) | 18 (4.6%) | 52 (3.6%) | |
| − Carotid-T/L | 49 (25.5%) | 195 (22.3%) | 95 (24.2%) | 339 (23.2%) | |
| − M1 | 109 (56.8%) | 535 (61.1%) | 229 (58.3%) | 873 (59.8%) | |
| − M2 | 28 (14.6%) | 110 (12.6%) | 51 (13.0%) | 189 (12.9%) | |
| Collaterals | 0.432 | ||||
| − 0 | 9 (20.5%) | 49 (17.8%) | 25 (20.2%) | 83 (18.7%) | |
| − 1 | 17 (38.6%) | 109 (39.6%) | 37 (39.8%) | 163 (36.8%) | |
| − 2 | 18 (40.9%) | 117 (42.5%) | 62 (50.0%) | 197 (44.5%) | |
| Tandem occlusion | 22 (11.5%) | 123 (14.1%) | 79 (20.1%) | 0.006 | 224 (15.3%) |
| Dissection | 3 (1.6%) | 35 (4.0%) | 21 (5.4%) | 0.091 | 59 (4.0%) |
| ASPECTS | 9 (IQR 7–10) | 8 (IQR 7–10) | 8 (IQR 7–9) | <0.001 | 8 (IQR 7–9) |
| Treatment | |||||
| IVT use | 92 (47.9%) | 522 (59.6%) | 130 (33.1%) | <0.001 | 744 (50.9%) |
| Time from onset of symptoms to IVT needle (min) | 85 (IQR 57–103) | 130 (IQR 90–170) | 180 (IQR 126–225) | <0.001 | 120 (IQR 83–170) |
| Time from onset of symptoms to admission (min) | 53 (IQR 39–69) | 110 (IQR 70–173) | 315 (IQR 240–477) | <0.001 | 133 (IQR 69–230) |
TTR: time from symptom onset to reperfusion ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CT, computed tomography; iICA, intracranial internal carotid artery; IVT, intravenous thrombolysis; M1, first segment of middle cerebral artery; M2, second segment of middle cerebral artery; MRI, magnetic resonance imaging; NIHSS, National Institute of Health Stroke Scale; NOAC, non-vitamin-K antagonist oral anticoagulant; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; VKA, vitamin K antagonist.
Outcome
On unadjusted analysis, better long-term outcomes were observed in patients with short TTR as indicated by significantly higher rates of mRS 0–2 (55.0% versus 46.8% versus 38.1%, p = 0.001, Table 2) and mRS 0-1 (39.2% versus 28.4% versus 24.9%, p = 0.003). Also, NIHSS at 24 h was lower and median NIHSS improvement at 24 h greater in shorter TTR (p < 0.001; Supplementary Figures 2 and 3). However, mortality (19.3% versus 22.3% versus 23.7%, p = 0.520) did not differ significantly. The overall incidence of non-haemorrhagic neurological worsening was 7.8% (95% CI 6.4–9.2%), with no significant differences among TTR strata (p = 0.138). Rate of sICH was highest in the medium TTR stratum (3.1% versus 7.7% versus 5.4%, p = 0.04; see Table 4).
Table 2.
Outcome data comparing patients with large-vessel occlusion according to time from symptom onset to reperfusion.
| Symptom onset to reperfusion 0–180 min (n = 192) |
Symptom onset to reperfusion 180–360 min (n = 876) |
Symptom onset to reperfusion >360 min (n = 393) |
p | All patients with available TTR (n = 1461) | |
|---|---|---|---|---|---|
| Outcome | |||||
| mRS 0–1 at 3 months | 67 (39.2%) | 224 (28.4%) | 88 (24.9%) | 0.003 | 379 (28.8%) |
| mRS 0–2 at 3 months | 94 (55.0%) | 369 (46.8%) | 135 (38.1%) | 0.001 | 598 (45.5%) |
| mRS 0–3 at 3 months | 117 (68.4%) | 484 (61.3%) | 189 (53.4%) | 0.002 | 790 (60.1%) |
| Mortality at 3 months | 33 (19.3%) | 176 (22.3%) | 84 (23.7%) | 0.520 | 293 (22.3%) |
| NIHSS at 24 h | 5 (IQR 2–13) | 8 (IQR 3–15) | 10 (IQR 4–17) | <0.001 | 8 (IQR 3–15) |
| Delta NIHSS admission – 24 h | 9 (IQR 5–15) | 6 (IQR 1–11) | 3 (IQR 0–7) | <0.001 | 5 (IQR 1–11) |
| Non-haemorrhagic worsening | 7 (4.2%) | 54 (8.0%) | 27 (9.4%) | 0.138 | 88 (7.8%) |
TTR: time from symptom onset to reperfusion; Delta NIHSS, difference in NIHSS between NIHSS at admission compared to 24 h; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale.
Table 4.
Tolerability and efficacy data comparing patients with large-vessel occlusion according to symptom onset to reperfusion time.
| Symptom onset to reperfusion 0–180 min (n = 192) |
Symptom onset to reperfusion 180–360 min (n = 876) |
Symptom onset to reperfusion >360 min (n = 393) |
p | All patients with available TTR (n = 1461) | |
|---|---|---|---|---|---|
| Efficacy | |||||
| mTICI 3 | 111 (57.8%) | 423 (48.3%) | 152 (38.7%) | <0.001 | 686 (47.0%) |
| mTICI 2b, 2c or 3 | 187 (97.4%) | 774 (88.4%) | 316 (80.4%) | <0.001 | 1277 (87.4%) |
| Time from onset of symptoms to groin puncture (min) | 117 (IQR 94–135) | 205 (IQR 170–245) | 382 (IQR 332–561) | <0.001 | 225 (IQR 162–309) |
| Time from groin puncture to reperfusion (min) | 30 (IQR 21–42) | 47 (IQR 30–75) | 54 (IQR 33–88) | <0.001 | 45 (IQR 30–72) |
| General anaesthesia | 70 (36.5%) | 484 (55.6%) | 225 (57.5%) | <0.001 | 779 (53.6%) |
| Additional intra-arterial thrombolytics | 7 (3.7%) | 90 (10.3%) | 18 (4.6%) | <0.001 | 115 (7.9%) |
| Balloon guiding catheter | 89 (46.4%) | 469 (53.6%) | 223 (56.7%) | 0.061 | 781 (53.5%) |
| Intermediate aspiration catheter | 97 (54.8%) | 405 (50.9%) | 187 (51.7%) | 0.640 | 689 (51.6%) |
| Manoeuvre count | 1 (IQR 1–2) | 1 (IQR 1–2) | 2 (IQR 1–3) | <0.001 | |
| Intracranial stenting | 2 (1.0%) | 19 (2.2%) | 5 (1.3%) | 0.382 | 26 (1.8%) |
| Extracranial stenting | 14 (7.3%) | 92 (10.5%) | 57 (14.5%) | 0.022 | 163 (11.2%) |
| Safety | |||||
| sICH ECASS II definition | 6 (3.1%) | 67 (7.7%) | 21 (5.4%) | 0.040 | 94 (6.5%) |
| Systemic bleeding | 1 (2.1%) | 11 (3.4%) | 0 (0.0%) | 0.098 | 12 (2.4%) |
| Craniectomy | 3 (1.6%) | 29 (3.3%) | 14 (3.6%) | 0.389 | 46 (3.2%) |
| Non-haemorrhagic worsening | 7 (4.2%) | 54 (8.0%) | 27 (9.4%) | 0.138 | 88 (7.8%) |
| Any interventional complication of: | 14 (7.3%) | 118 (13.5%) | 59 (15.0%) | 0.029 | 191 (13.1%) |
| − Vasospasms | 4 (28.6%) | 29 (24.4%) | 17 (27.9%) | 49 (25.7%) | |
| − Cervical dissection | 3 (21.4%) | 28 (23.5%) | 8 (13.1%) | 38 (19.9%) | |
| − Perforation | 2 (14.3%) | 9 (7.6%) | 5 (8.2%) | 16 (8.4%) | |
| − Other | 2 (14.3%) | 16 (13.4%) | 9 (14.8%) | 26 (13.6 %) | |
| − Emboli to new territory | 3 (21.4%) | 37 (31.1%) | 22 (36.1%) | 62 (32.5%) |
TTR: time to reperfusion; LVO, large-vessel occlusion; mTICI, modified treatment in cerebral ischaemia; sICH, symptomatic intracranial haemorrhage according to European Co-operative Acute Stroke Study-II definition.
TTR per hour increase was a significant factor related to mRS 0–2 (aOR 0.933, 95% CI 0.887 –0.981; Table 3), mRS 0–1 (aOR 0.929, 95% CI 0.877–0.985) and mRS 0–3 (aOR 0.955, 95% CI 0.911–1.000) in multivariable binary logistic regression analysis adjusting for prespecified confounders outlined in the methods section (Figure 1). Associations for non-haemorrhagic neurological worsening approached significance (aOR 1.068, 95% CI 0.996–1.144) and were non-significant for all-cause mortality at 3 months (aOR 0.988, 95% CI 0.933–1.046) and sICH (aOR 1.020, 95% CI 0.927–1.123). Per hour delay of reperfusion there was a 1.5% decreased probability of good functional outcome (Figure 2). The point estimates were similar and remained significant for mRS 0–2 and mRS 0–1 in the subgroup of patients with TTR of more than 6 h (Supplementary Table 2).
Table 3.
Analysis was done using time to reperfusion information in minutes, but association of TTR per hour increase with outcome data comparing patients with large-vessel occlusion in the anterior circulation is reported. Analysis was done using multivariable binary or ordinal logistic regression analysis adjusting for prespecified confounders outlined in the methods section for TTR per hour increase except aOR for successful reperfusion, which was analysed without successful reperfusion as variable.
| Outcome | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| mRS 0–1 at 3 months | 0.928 (0.886–0.971) | 0.929 (0.877–0.985) |
| mRS 0–2 at 3 months | 0.928 (0.892–0.965) | 0.933 (0.887–0.981) |
| mRS 0–3 at 3 months | 0.951 (0.918–0.986) | 0.955 (0.911–1.000) |
| Reperfusion at intervention (mTICI ⩾2B) | 0.945 (0.906–0.985) | 0.934 (0.885–0.984) |
| mRS at 3 months (shift) | 1.046 (1.014–1.078) | 1.038 (1.001–1.077) |
| Non-haemorrhagic worsening at 24 h | 1.053 (0.998–1.110) | 1.068 (0.996–1.144) |
| Mortality at 3 months | 1.018 (0.979–1.058) | 0.988 (0.933 – 1.046) |
| Symptomatic intracranial haemorrhage ECASS II definition | 1.008 (0.944–1.076) | 1.020 (0.927–1.123) |
ECASS II, European Co-operative Acute Stroke Study-II; mRS, modified Rankin Scale; mTICI, modified treatment in cerebral ischaemia.
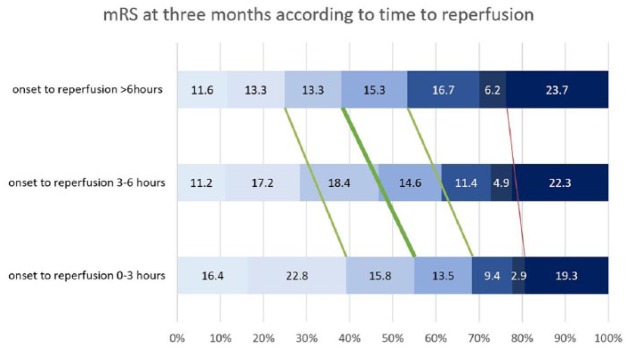
Figure 1.
Day 90 functional outcome at 3 months according to time to reperfusion.
Distribution of mRS scores at 3 months, according to time to reperfusion strata. Analysis was done using multivariable binary logistic regression analysis adjusting for prespecified confounders outlined in the methods section for TTR per hour increase. The thick green line proposed by Goyal and colleagues indicates a highly significant beneficial treatment effect with higher rate of the primary outcome mRS 0–2 (aOR 0.933, 95% CI 0.887–0.981, p = 0.007). The thin green line indicates a significant beneficial treatment effect with higher rate of mRS 0–1 (aOR 0.929, 95% CI 0.877–0.985, p = 0.013) and mRS 0–3 (aOR 0.955, 95% CI 0.911–1.000, p = 0.049). The thin red line indicates a beneficial, but non-significant, treatment effect for mortality (aOR 0.988, 95% CI 0.933–1.046, p = 0.680).
mRS, modified Rankin scale.
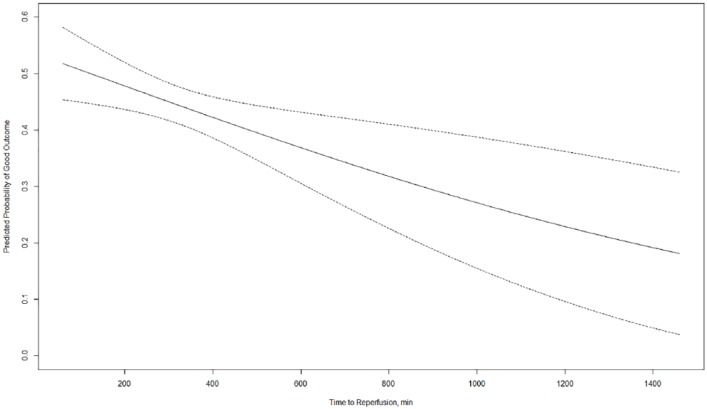
Figure 2.
Probability of good functional outcome (mRS 0–2) with margins plot, including 95% confidence intervals.
For this analysis, time to reperfusion was used as a continuous variable and multivariable binary logistic regression analysis was performed, adjusting for prespecified confounders outlined in the methods section. Per hour delay of reperfusion there was a 1.5% decreased probability of good functional outcome.
mRS, modified Rankin scale.
On ordinal regression analysis adjusting for prespecified confounders outlined in the methods section, TTR per hour increase significantly worsened long-term functional outcome (aOR 1.038, 95% CI 1.001–1.077). However, the assumption of proportional odds was violated, as indicated by the parallel lines test (p < 0.001). Nevertheless, the aOR lies in the same range as indicated by dichotomized multivariable binary logistic regression analysis and the parallel lines test may be overly sensitive when continuous explanatory variables are present or output variable number or sample size are large.14
STG per hour increase was neither related to long-term functional outcome nor to mortality (Supplementary Table 3) in multivariate analysis.
Tolerability and efficacy
Treatment metrics with efficacy and tolerability outcomes are presented in Table 4. Reperfusion by IVT and/or MT defined as ⩾TICI2b was successful in 1277 patients (87.4%). On univariate analysis, patients with late TTR had lower rates of successful and excellent reperfusion, more extracranial stenting and higher rate of general anaesthesia. Additionally, complication rate, number of passes and intervention duration were higher in late TTR. In multivariable logistic regression analysis adjusting for prespecified confounders outlined in the methods section, TTR per hour increase was a significant factor related to successful reperfusion (TICI⩾2b, aOR 0.934, 95% CI 0.885–0.984), excellent reperfusion (TICI 3, aOR 0.924, 95% CI 0.883-0.967) and number of passes (aOR 1.092, 95% CI 1.048-1.138), but not a significant factor for overall complication occurrence (aOR 1.008, 95% CI 0.950-1.067).
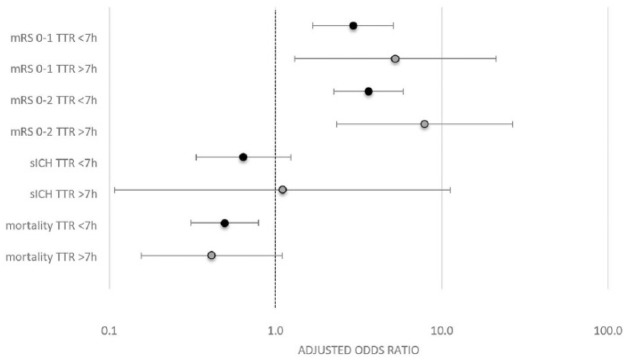
Among patients with TTR of more than 7 h, successful reperfusion significantly increased the odds of mRS 0–2 (aOR 7.879, 95% CI 2.325–26.699) and mRS 0–1 (aOR 5.249, 95% CI 1.301–21.183). Non-significant associations were found for all-cause mortality at day 90 (aOR 0.414, 95% CI 0.156–1.099) and sICH (aOR 1.102, 95% CI 0.108–11.232) in multivariable binary logistic regression analysis adjusting for prespecified confounders outlined in the methods section (Figure 3).
Figure 3.
Adjusted odds ratio of successful reperfusion (mTICI⩾2b/3) for outcome parameters.
Adjusted odds ratio of successful reperfusion (mTICI⩾2b/3) for outcome parameters stratified for patients with time to reperfusion from symptom onset (TTR) of less or more than 7 h.
mRS, modified Rankin scale; sICH, symptomatic intracranial haemorrhage; TTR, time to reperfusion from symptom onset.
Discussion
In this retrospective multicentre registry dataset we analysed the impact of TTR on 3-month functional outcome as well as tolerability and efficacy of MT with the following main findings: (1) TTR is an independent factor related to long-term functional outcome with an estimated 1.5% decreased probability of good functional outcome per hour delay. Additionally, fast TTR is associated with early neurologic recovery. (2) With increasing TTR, interventional procedures become technically less effective; however, increasing TTR was not significantly related to mortality or sICH.
In this analysis we chose to analyse TTR rather than STG, because when analysing STG many patients who are subjected to MT but never actually achieve reperfusion are part of the analysis, which weakens the true TTR effect because those patients are subjected to the potential downsides of MT without having the chance to benefit from it. However, it is rather successful reperfusion than intention to treat that is a major predictor of outcome, with every 10% increase in the rates of successful reperfusion accounting for an estimated 17% increase in the probability of achieving excellent outcomes.15 Our finding that STG was not a significant factor related to long-term functional outcome when adjusting for confounders confirms this view. Second, STG in patients who do not achieve reperfusion reflects other evidence-based treatments like earlier IVT, medical treatment or stroke unit care which might have biased the effect ascribed to STG. Contrarily to Al Sultan and Hill,16 arguing for an imprecise measurement of TTR, we hence favour TTR to be a more precise and meaningful parameter compared to STG. This view is in line with the ‘time-reset effect’, arguing for the quickest possible reperfusion once imaging identifies relevant salvageable tissue,7 rendering time from imaging to reperfusion the main time dependence because of the used imaging selection.
Our analysis has several complementary features to published data.1,2,8 First, whereas STG was limited in two of the studies, we had no such restriction including patients more than 40 h after symptom onset. Second, due to a larger sample size and good data quality, we were able to correct for several confounders that might have influenced the strong effect seen with TTR. Third, because of a markedly higher recanalization rate, our estimates probably more reliably reflect the true effect of TTR as compared to STG analysis for the reasons mentioned above.
In comparison to the association of TTR times with functional outcome in the HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) meta-analysis of previous RCTs, our results suggest that the association for TTR is less pronounced in real-world patients. We explain those findings by advances in imaging-based patient selection better identifying patients benefiting from MT. For example, a patient with complete fluid-attenuated inversion recovery demarcation of M2-occlusion might have been denied MT, although presenting 4.5 h after symptom onset.
Since almost all RCTs used some kind of imaging selection or other inclusion criteria, the true decline of TTR treatment effect when subjecting all AIS patients with a target LVO in the AC has and will probably never been elucidated. However, it is very important to keep in mind that the slope of decline in efficacy is strongly influenced by patient selection,7 and treatment effect may be distinct also in late TTR as shown by recent DAWN and DEFUSE-3 RCTs.3,4 In this registry, we observed heterogeneous aOR of TTR on good functional outcome for each centre (Supplementary Table 5), probably due to different approaches to patient selection.
The relative merits of achieving successful reperfusion for dichotomizations of the mRS scale, mortality and sICH were comparable between patients reperfused before and after 7 h (Figure 3), with significant effect of reperfusion on mRS 0–2 and mRS 0–1 also in TTR of more than 7 h. However, due to large confidence intervals, findings only approached significance for mortality in patients reperfused after 7 h. In patients with STG of more than 6 h, the rates of 3-month mRS categories and mortality were roughly comparable to DAWN and DEFUSE-3 MT patients (Supplementary Figure 4).3,4 This finding confirms that tertiary stroke centres apply the change from a merely time-based approach to MT towards a tissue-based protocol in late-presenting patients correctly, with similar benefits of reperfusion also in late TTR. Even the participating tertiary stroke centres performed CT in 80/154 (52%) of patients with TTR of more than 8 h, underlining the importance of a CT-based algorithm to select patients for MT in the late time window as an alternative when MRI is not available.17
The recanalization rate of 82.8% of the whole AC registry is similar to recent observational studies with smaller patient numbers,18–20 but higher compared to a meta-analysis that included older MT devices20 and recent registries.2,8 The mortality rate of about 22% is almost identical to the published data; however, contrarily to a recent meta-analysis we found no increase in mortality when early and late recanalization were compared.20 The rate of complications in patients reperfused after 6 h was 15.0%, similar to rates previously reported,21 and matched the frequency of complications observed in patients reperfused between 3 and 6 h.21 In regards to serious complications in patients reperfused after 7 h, we observed three perforations with no associated mortality. Patients with an unsuccessful recanalization had higher complication rates (22.5% versus 10.6%, p < 0.001).
Although the median time interval for when a patient was last known to be well and the occurrence of treatment was about 13 h in DAWN and 12 h in DEFUSE-3, the actual number of patients with warranted late MT treatment was quite low due to the high percentage of wake-up stroke.3,4 Our registry did have a higher percentage of truly late-presenting patients with documented symptom onset. However, patients treated very late after symptom onset were scarce (see Supplementary Table 4), but we still found a beneficial effect of MT also in late TTR. Further evidence needs to definitely confirm tolerability and efficacy of MT in this scenario and establish reliable imaging criteria for patient selection.
Limitations
Being a single-arm multicentre retrospective registry, this study has associated limitations. Most importantly, patient selection for MT was not specified or centre-specific. No control group of medical management only was available. No core lab adjudication was performed for neuroradiological variables like mTICI score and ASPECTS. Subgroup analyses were generally confined to small cohorts, introducing a large uncertainty of the presented with wide confidence intervals. Another limitation, but also a strength, of this study is heterogeneous patient selection by each centre based on either CT or MRI and clinical criteria, which allows generalizability at least to tertiary stroke centres.
Conclusion
TTR is an independent factor related to long-term functional outcome, with an estimated 1.5% decreased probability of good functional outcome per hour delay in the selected patients of this registry. However, the timing and slope of decline in efficacy are strongly influenced by (imaging) patient selection, hence a general assumption regarding this decline should be questioned. STG was not a significant factor related to long-term functional outcome. With increasing TTR, interventional procedures become technically less effective. Efforts should be made to shorten TTR through optimized prehospital and in-hospital pathways.
Supplemental Material
Supplemental material, ONLINE_SUPPLEMENT for Outcome, efficacy and safety of endovascular thrombectomy in ischaemic stroke according to time to reperfusion: data from a multicentre registry by Thomas Raphael Meinel, Johannes Kaesmacher, Pasquale Mordasini, Pascal J. Mosimann, Simon Jung, Marcel Arnold, Mirjam Rachel Heldner, Patrik Michel, Steven D. Hajdu, Marc Ribo, Manuel Requena, Christian Maegerlein, Benjamin Friedrich, Vincent Costalat, Amel Benali, Laurent Pierot, Matthias Gawlitza, Joanna Schaafsma, Vitor Mendes Pereira, Jan Gralla and Urs Fischer in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: The study was supported by Medtronic (Dublin, Ireland). Medtronic did not take part in the conception, design or manuscript drafting of this study. The work of JK was supported by the SAMW/Bangerter foundation and the Swiss Stroke Society.
Conflict of interest statement: U. Fischer is a consultant for Medtronic and Stryker and Co-PI of the SWIFT DIRECT trial (Medtronic). J. Gralla is a global principal investigator of STAR (Solitaire FR Thrombectomy for Acute Revascularisation), clinical event committee member of the PROMISE study (European Registry on the ACE Reperfusion Catheters and the Penumbra System in the Treatment of Acute Ischemic Stroke; Penumbra) and a principal investigator and consultant for the SWIFT DIRECT study (Medtronic), and receives Swiss National Science Foundation (SNSF) grants for magnetic resonance imaging in stroke. L. Pierot serves as a consultant for Balt, Microvention, and Penumbra. J. Kaesmacher has received travel grants from Pfizer and Stryker. M. Ribo serves as a consultant for Medtronic, Stryker, Anaconda, Apta Targets and Perflow Medical, and as a speaker for Neuravi. P. Michel has received funding for speaker honoraria from Boehringer. He has served on scientific advisory boards also for Boehringer. He has received research grants from Bristol-Myers Squibb, Boehringer and the Swiss Heart Foundation. V.M. Pereira is a consultant for Stryker (SC for DAWN trial), Penumbra (SC for PROMISE study), BALT (proctorship of products unrelated to ischaemic stroke), Phenox, Rapid Medical and Neurovasc, and receives a research grant from Philips. All other authors have nothing to disclose.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Thomas Raphael Meinel  https://orcid.org/0000-0002-0647-9273
https://orcid.org/0000-0002-0647-9273
Contributor Information
Thomas Raphael Meinel, Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Johannes Kaesmacher, Institute of Diagnostic and Interventional Neuroradiology, Institute of Diagnostic, Interventional and Pediatric Radiology and Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Pasquale Mordasini, University Institute of Diagnostic and Interventional Neuroradiology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Pascal J. Mosimann, University Institute of Diagnostic and Interventional Neuroradiology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland
Simon Jung, Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Marcel Arnold, Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Mirjam Rachel Heldner, Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Patrik Michel, Department of Neurology, CHUV Lausanne, Lausanne, Switzerland.
Steven D. Hajdu, Department of Radiology, CHUV Lausanne, Lausanne, Switzerland
Marc Ribo, Department of Neurology, Vall d’Hebron University Hospital, Barcelona, Spain.
Manuel Requena, Department of Neurology, Vall d’Hebron University Hospital, Barcelona, Spain.
Christian Maegerlein, Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Benjamin Friedrich, Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Vincent Costalat, Department of Neuroradiology, CHU Montpellier, Montpellier, France.
Amel Benali, Department of Neuroradiology, CHU Montpellier, Montpellier, France.
Laurent Pierot, Department of Neurology, Toronto Western Hospital, Toronto, Canada.
Matthias Gawlitza, Department of Neuroradiology, CHU Reims, Reims, France.
Joanna Schaafsma, Department of Neurology, Toronto Western Hospital, Toronto, Canada.
Vitor Mendes Pereira, Joint Department of Medical Imaging, Toronto Western Hospital, Toronto, Canada.
Jan Gralla, University Institute of Diagnostic and Interventional Neuroradiology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland.
Urs Fischer, Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Freiburgstrasse 8, CH-3010, Switzerland.
References
- 1. Saver JL, Goyal M, Van Der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 2. Mulder MJHL, Jansen IGH, Goldhoorn RJB, et al. Time to endovascular treatment and outcome in acute ischemic stroke. Circulation 2018; 138: 232–240. [DOI] [PubMed] [Google Scholar]
- 3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. Epub ahead of print 11 November 2017. DOI: NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 4. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jadhav AP, Desai SM, Kenmuir CL, et al. Eligibility for endovascular trial enrollment in the 6- to 24-hour time window. Stroke. Epub ahead of print 16 March 2018. DOI: STROKEAHA.117.020273. [DOI] [PubMed] [Google Scholar]
- 6. Nogueira RG, Haussen DC, Jadhav A, et al. Time to endovascular treatment and outcomes in the DAWN trial. Stroke 2018; 49(Suppl. 1) Available at: https://www.ahajournals.org/doi/10.1161/str.49.suppl_1.78. [Google Scholar]
- 7. Fiehler J. The time–reset effect. Clin Neuroradiol 2017; 27: 3–5. [DOI] [PubMed] [Google Scholar]
- 8. Mokin M, Abou-Chebl A, Castonguay AC, et al. Real-world stent retriever thrombectomy for acute ischemic stroke beyond 6 hours of onset: analysis of the NASA and TRACK registries. J Neurointerv Surg. Epub ahead of print 2018. DOI: 10.1136/neurintsurg-2018-014272. [DOI] [PubMed] [Google Scholar]
- 9. Berkhemer OA. Do we still need selection criteria for stroke patients with acute large vessel occlusion? ESOC: Göteborg, 2018. [Google Scholar]
- 10. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 11. Neurol J . 2019. Mar;266(3):598-608. Kaesmacher J, Chaloulos-Iakovidis P, Panos L, et al. Clinical effect of successful reperfusion in patients presenting with NIHSS < 8: data from the BEYOND-SWIFT registry. J Neurol. 2019. ;266(3):598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003; 14: E1–E31. [DOI] [PubMed] [Google Scholar]
- 13. Mazya MV, Cooray C, Lees KR, et al. Minor stroke due to large artery occlusion: when is intravenous thrombolysis not enough? Results from the SITS international stroke thrombolysis register. Eur Stroke J 2017; 3(1): 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Connell A. Logistic regression models for ordinal response variables. Thousand Oaks, CA: SAGE Publications, Inc, 2006. [Google Scholar]
- 15. Manning NW, Warne CD, Meyers PM. Reperfusion and clinical outcomes in acute ischemic stroke: systematic review and meta-analysis of the stent-retriever-based, early window endovascular stroke trials. Front Neurol 2018; 9: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al Sultan AS, Hill MD. Acute ischemic stroke biology demands fast treatment. Circulation 2018; 138: 241–243. [DOI] [PubMed] [Google Scholar]
- 17. Santos T, Carvalho A, Almeida Cunha A, et al. NCCT and CTA-based imaging protocol for endovascular treatment selection in late presenting or wake-up strokes. J Neuro Intervent Surg 2018; 1–5. [DOI] [PubMed] [Google Scholar]
- 18. Motyer R, Thornton J, Power S, et al. Endovascular thrombectomy beyond 12 hours of stroke onset: a stroke network’s experience of late intervention. J Neurointerv Surg 2019; 11: 200–203. [DOI] [PubMed] [Google Scholar]
- 19. Desai SM, Rocha M, Molyneaux BJ, et al. Thrombectomy 6–24 hours after stroke in trial ineligible patients. J Neurointerv Surg 2018; 10: 1–6. [DOI] [PubMed] [Google Scholar]
- 20. Wareham J, Phan K, Renowden S, et al. A meta-analysis of observational evidence for the use of endovascular thrombectomy in proximal occlusive stroke beyond 6 hours in patients with limited core infarct. Neurointervention 2017; 12: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Behme D, Gondecki L, Fiethen S, et al. Complications of mechanical thrombectomy for acute ischemic stroke: a retrospective single-center study of 176 consecutive cases. Neuroradiology 2014; 56: 467–476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ONLINE_SUPPLEMENT for Outcome, efficacy and safety of endovascular thrombectomy in ischaemic stroke according to time to reperfusion: data from a multicentre registry by Thomas Raphael Meinel, Johannes Kaesmacher, Pasquale Mordasini, Pascal J. Mosimann, Simon Jung, Marcel Arnold, Mirjam Rachel Heldner, Patrik Michel, Steven D. Hajdu, Marc Ribo, Manuel Requena, Christian Maegerlein, Benjamin Friedrich, Vincent Costalat, Amel Benali, Laurent Pierot, Matthias Gawlitza, Joanna Schaafsma, Vitor Mendes Pereira, Jan Gralla and Urs Fischer in Therapeutic Advances in Neurological Disorders