Abstract
Background:
We aim to evaluate the distribution in the upper urinary tract of intravesical-delivered fluids, after inducing vesicoureteral reflux (VUR) with a double J stent.
Methods:
In group 1 (n = 35) patients were maintained in a 20° Trendelenburg position and were evaluated after immediate insertion of ureteral stent, while in group 2 (n = 16) patients were evaluated after several days with ureteral stent placement. Patients in both groups were submitted to a cystogram with progressive volumes of iodine contrast and were evaluated according to VUR of contrast medium to the renal pelvis. Additionally, in group 2 visual confirmation was performed by endoscopic inspection of upper tract mucosal impregnation with methylene blue.
Results:
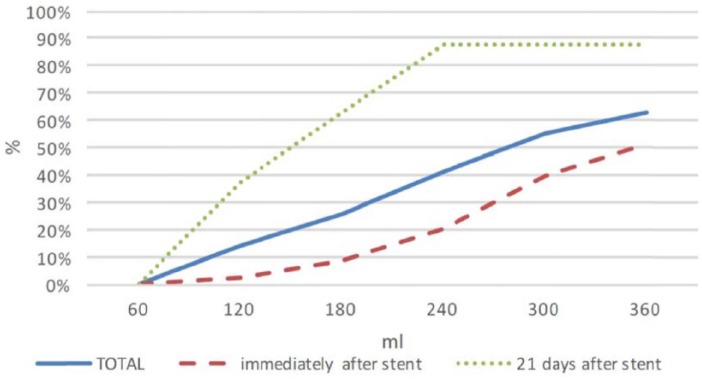
In group 1, after immediate insertion of ureteral stent reflux occurred in 51.4% (n = 18/35), and after several days with ureteral stent placement reflux was observed in 87.5% (n = 14/16) of patients. Reflux was progressively more frequent according to increasing bladder volume (p < 0.001). At 60 ml bladder volume no reflux was observed, while at 120 ml, 180 ml, 240 ml, 300 ml and 360 ml bladder volume reflux was observed in 14%, 25%, 41%, 55% and 63% of patients, respectively.
Conclusion:
Retrograde delivery of fluids such as bacillus Calmette-Guérin to the upper urinary tract through double J stents can be effective; however, it is mainly effective after several days with ureteral stent and relatively large volumes might be instilled into the bladder.
Keywords: urinary tract physiology, urothelial carcinoma, imunotherapy, BCG
Introduction
Intravesical instillation of bacillus Calmette-Guérin (BCG) for preventing recurrence of urothelial carcinoma and treating carcinoma in situ (CIS) of the bladder is currently recommended as the standard treatment.1 Upper urinary tract urothelial carcinoma (UUTUC) is a more uncommon disease, but its principles follow those applied to bladder disease.2,3 Better radiologic imaging techniques along with technical developments in flexible ureteroscopes have improved the diagnosis of flat and initial lesions. For these rare patients with low-risk UUTUCs, conservative surgery and preservation of the upper urinary renal unit might be an option. Conservative management of UUTUCs can also be considered imperative in cases such as renal insufficiency or solitary functional kidney. After endoscopic treatment, segmental resection or percutaneous access for the treatment of the main lesion, adjuvant topical agents might be required.
Intravesical perfusion of BCG for bladder CIS, first reported by Morales and colleagues in 1976, has been widely accepted as a standard treatment.2,4 For UUTUC CIS, BCG therapy was first reported by Herr in 1985.5 One of the concerns for BCG therapy in the upper urinary tract is whether the vaccine would reach and remain at the diseased site for a sufficient period to achieve satisfactory antitumor effect.
The aim of the present study was to evaluate the distribution in the upper urinary tract of intravesical delivered fluids, after inducing vesicoureteral reflux (VUR) with a double J stent.
Patients and methods
The Institutional Review Boards approved the present study and all patients have signed an informed consent form in order to participate in the present study. A total of 51patients who required double J (4.7 or 6.0 Fr) placement due to urinary stone disease were enrolled in the present study.
The primary endpoint was defined as the occurrence of VUR due to each technique (group 1 – cystogram in the Trendelenburg position; group 2 – cystogram and methylene blue). Secondary endpoints were defined as evaluation of volume to induce reflux to the renal pelvis and patient position for an efficient protocol for intravesical therapies after double J placement.
Group 1: cystogram in the Trendelenburg position
In 35 patients, after placing a double J stent in the completion of the main surgery, a Foley catheter was placed and a cystogram was performed. The patient was maintained in a 20° Trendelenburg position and iodine contrast volumes of 60 ml, 120 ml, 180 ml, 240 ml, 300 ml and 360 ml were inserted into the bladder. The occurrence of VUR and the volume necessary to induce reflux to the renal pelvis were monitored radiographically. The largest infusion volume was determined to be 360 ml based on average vesical capacity found in the literature, in addition to the fact that these patients had to endure for at least 2 hours the BCG vaccine inside the bladder.6,7
Group 2: cystogram and methylene blue
A total of 16 patients with prior insertion of an indwelling ureteral stent who were to undergo a flexible nephron-ureteroscopy were evaluated. These patients were submitted to a cystogram with iodine contrast added to methylene blue to the lowest concentration necessary to result in VUR to the entire calyceal system. Patients were evaluated radiographically according to VUR of contrast medium to the renal pelvis, and additionally visual confirmation was performed with endoscopic inspection of upper tract mucosal impregnation with methylene blue.
Descriptive statistical analyses were performed using IBM SPSS. Comparisons of outcomes among groups were performed using Chi-square testing for categorical variables and Student’s t testing for continuous variables. Statistical significance was considered as p < 0.05.
Results
A total of 51 patients were evaluated. Mean ± SD patient age was 46.7 ± 14.2 years, and 68.6% were males. Demographic data are shown in Table 1.
Table 1.
Demographic data.
| Parameter | n = 51 |
|---|---|
| Age (years), mean ± SD | 46.7 ± 14.2 |
| Gender | |
| Male, n (%) | 35 (68.6) |
| Female, n (%) | 16 (31.4) |
| Ureteral stent diameter | |
| 4.7 Fr, n (%) | 3 (5.9) |
| 6.0 Fr, n (%) | 48 (94.1) |
| Laterality | |
| Right, n (%) | 23 (45.1) |
| Left, n (%) | 28 (54.9) |
| Anesthesia | |
| Spinal, n (%) | 23 (45.1) |
| General, n (%) | 28 (54.9) |
| Reflux | |
| Yes, n (%) | 32 (62.7) |
| No, n (%) | 19 (37.3) |
Of the total number of patients, 35 were evaluated immediately after ureteral stent insertion and 16 after an average time of 21.6 days using the ureteral stent. Reflux was observed in 62.7% (n = 32/51) of patients using the ureteral stent. After immediate insertion of the ureteral stent, reflux occurred in 51.4% (n = 18/35) of patients, and after several days with ureteral stent placement reflux was observed in 87.5% (n = 14/16) of patients (Table 2). Even though general anesthesia (versus spinal anesthesia) was associated with higher reflux rates (p = 0.013), when corrected according to time of stent usage (immediately inserted versus previously inserted), anesthesia was no longer associated with VUR occurrence (p = 0.82).
Table 2.
Patients characteristics according to the observation of vesicoureteral reflux or no reflux after bladder instillation of contrast medium up to 360 ml (n = 51).
| Parameter | Reflux (n = 32) |
No reflux (n = 19) |
p |
|---|---|---|---|
| Age (years), mean ± SD | 51.1 ± 13.6 | 39.3 ± 11.6 | 0.003 |
| Gender | |||
| Male, n (%) | 25 (71.4) | 10 (28.6) | 0.057 |
| Female, n (%) | 7 (43.8) | 9 (53.6) | |
| Laterality | |||
| Right, n (%) | 17 (73.9) | 6 (26.1) | 0.228 |
| Left, n (%) | 15 (53.6) | 13 (46.4) | |
| Ureteral stent diameter | |||
| 4.7 Fr, n (%) | 1 (33.3) | 2 (66.7) | 0.639 |
| 6.0 Fr, n (%) | 31 (64.6) | 17 (35.4) | |
| Time with stent | |||
| Immediately inserted, n (%) | 18 (51.4) | 17 (48.6) | 0.031 |
| Previously implanted, n (%) | 14 (87.5) | 2 (12.5) | |
| Anesthesia | |||
| Spinal, n (%) | 10 (43.5) | 13 (56.5) | 0.013 |
| General, n (%) | 22 (81.5) | 5 (18.5) | |
| Infused volume (ml), mean ± SD | 242.1 ± 76.0 | 281.0 ± 82.6 | 0.049 |
Bold values represent p <0.05.
Reflux was progressively more frequent according to increasing bladder volume (p < 0.001). At 60 ml bladder volume no reflux was observed, while at 120 ml, 180 ml, 240 ml, 300 ml and 360 ml of bladder volume reflux was observed in 14%, 25%, 41%, 55% and 63% of patients, respectively (Figure 1).
Figure 1.
Vesicoureteral reflux occurrence in each of the groups of patients according to bladder volume.
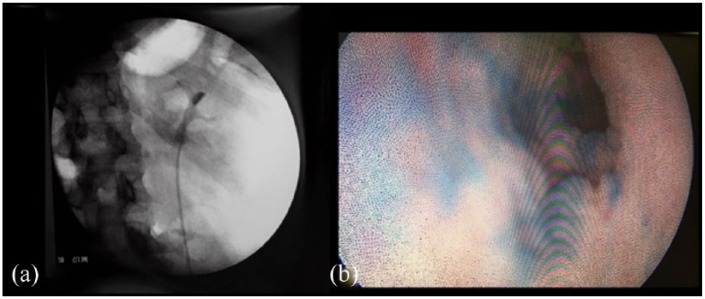
In all cases of patients with previous ureteral stent, methylene blue was used along with iodine contrast for cystography. During nephron-ureteroscopy in all patients, upper tract urothelial impregnation with methylene blue was also observed (Figure 2).
Figure 2.
(a) Radioscopic view of a patient with reflux after 300 ml of contrast injected into the bladder. (b) Endoscopic view of the ureter with methylene blue impregnation on the urothelium after bladder instillation and occurrence of vesicoureteral reflux.
Discussion
Radical nephroureterectomy is the standard treatment for UUTUC.8 However, minimally invasive endoscopic techniques, including percutaneous surgery and ureteroscopy, have emerged as alternative therapies with acceptable oncologic outcomes when appropriately applied in well-selected patients.3 When CIS of the upper urinary tract is diagnosed, renal preservation is desired mainly in patients with bilateral disease, a solitary kidney or renal insufficiency. In such a context, adjuvant measures such as topical chemotherapy or BCG might be of value.9,10
However, even though bladder instillation of BCG is simple and requires only a bladder catheter placement, it becomes a more complex issue when addressing the upper urinary tract. Various routes have been reported for the administration of BCG to the upper urinary tract, including percutaneous nephrostomy, retrograde catheterization and those using VUR. Studer and colleagues reported antegrade BCG perfusion therapy using percutaneous nephrostomy in 1989 and Sharpe and colleagues reported retrograde BCG perfusion in 1993.11–13 These reports represented the basis for treatment of UUTUC CIS with BCG perfusion therapy. Subsequently, only a few groups reported their experience with this form of therapy, probably because BCG perfusion therapy for UUTUC was not promoted other than as an experimental therapy. The short-term efficacy of BCG perfusion therapy for UUTUC CIS has been reported to be 62.5–100%.12–18 This rate is similar to that for bladder CIS.19 Therefore, the initial response to short-term BCG therapy for UUTUC CIS appears to be reasonably good.
The antegrade instillation of BCG in the upper urinary tract by percutaneous nephrostomy has the advantage of the easiest prediction of the distribution of the medication and of the reduction of symptoms related to bladder irritation and to ureteral stents. However, this technique is more invasive, requiring the placement and maintenance of a nephrostomy tube for long periods of time, which may reduce the patient quality of life during treatment. In addition, it may disseminate cancer cells along the nephrostomy path.20
Retrograde instillation through a single J ureteric stent plus double J stent placement to maintain the reflux or through surgical resection of the vesicoureteral junction have also been used.13,17–19,21 The single J stent and ureteral catheter require transurethral replacement of the catheter for each instillation, with potential complications and higher costs. The use of the double J stent might be limited by the presence or absence of VUR and is associated with difficulty in collecting selective urine samples to assess treatment outcomes. In addition, double J ureteral stents can cause severe symptoms in a subset of patients, especially if BCG is administered.19 However, the quality of life of the patients remains good without hospitalization and the pressure in the renal pelvis is low. These seem good arguments for using the retrograde approach.
Additionally, it must be assured that there is adequate VUR and an optimal timing of BCG contact with the urothelium.22 Most authors describe the technique as initiating the procedure with a cystogram to confirm reflux and optimize the volume necessary to obtain VUR.19 However, to the best of our knowledge, the volume and factors associated with VUR have not been studied in a larger number of patients. In addition, very few studies have been able to evaluate the efficacy and reproducibility of delivering fluids to the upper urinary tract through these approaches.17,19,21,23
Our study has some important findings. First, we have observed that with lower bladder volumes VUR did not occur. For bladder BCG instillation, a volume of 60 ml is normally infused. In our study, VUR was not observed in any patient with 60 ml bladder volume. VUR occurred progressively starting from 120 ml, and at 360 ml only 63% presented with VUR. This is a very low rate of VUR, and with a bladder volume that might be intolerable for a period of 2 h for most patients. Even though the costs might seem lower with this method of BCG delivery, if treatment is ineffective then bladder cancer recurrence might bring much greater costs.
Second, patients with a double J stent for a longer period of time had a significantly higher rate of VUR (p = 0.031), with 87.5% achieving this after a mean time of 21.6 days. In this group of patients, all patients who demonstrated VUR had reflux at a bladder volume of 240 ml. Our finding is similar to that of a previously reported series of patients, in whom VUR was obtained at up to 250 ml and a mean volume of 120 ml.19 The mean volume to obtain VUR in our group of patients was 184 ml.
Third, reflux was more common in older patients. The population in our study was young since we evaluated patients with urinary stone disease. But even in this subset of patients, VUR was more common in older patients (p = 0.003; Table 2). Patients with UUTUC tend to be much older, which could be associated with even higher rates of VUR.24 Aging is related to various changes in the lower urinary tract, such as higher collagen-to-elastin ratio, which leads to a reduction in bladder capacity and compliance, and consequently greater occurrence of VUR.25–28
Our study has demonstrated that retrograde delivery of fluids such as BCG to the upper urinary tract through double J ureteral stents can be effective in a significant number of patients. However, several considerations must be noted. It is most effective after several days with a ureteral stent, and mainly in older people. Relatively large volumes might need to be instilled into the bladder, and some patients may not tolerate these large volumes for the time needed to achieve the desired effect. Since it is not completely predictable, imaging with retrograde cystogram must be performed if this method is used.
Our study has some limitations. First, as the cystograms were performed with patients under anesthesia, the conditions obtained were not exactly the same as for patients undergoing ambulatory intravesical BCG instillations with ureteral stents for UUTUC. However, in accordance with our study, it has previously been demonstrated that VUR occurred in about 80% of awake patients with a double J ureteral stent.29 Our study has also demonstrated that VUR increased with voiding. Since VUR during voiding occurs briefly, the contact with the upper tract urothelium would probably occur for a much shorter period of time than is desirable for UUTUC CIS treatment. Additionally, our patients did not have UUTUC. Nonetheless, since it is a relatively rare disease and there are only small case series reported in the current literature, this experimental study might bring additional insights to optimize current treatment approaches.
In conclusion, there is a paucity of studies evaluating the best method for delivery of medications to the upper urinary tract. Our study brings additional knowledge to this field and might be useful when choosing the best approach for each patient. Retrograde delivery of fluids such as BCG to the upper urinary tract through double J ureteral stents can be effective in a significant number of patients. However, it is mainly effective after several days with a ureteral stent and relatively large volumes might be instilled into the bladder. Since it is not completely predictable, imaging with retrograde cystogram should be performed if this method is chosen. Other techniques must be considered if a more reliable method is desired, such as direct retrograde infusion through externalized ureteral stents, use of “tandem” ureteral stents or antegrade infusion through a nephrostomy tube.
Footnotes
Authors’ contribution: FK: project development, data collection, data analysis, manuscript writing. WB: data collection, data analysis, manuscript writing. MAS: project development, data collection.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical statement: Our study was approved by The Hospital Israelita Albert Einstein Research Ethics Committee (approval no. 2837-16). All patients provided written informed consent prior to enrollment in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The Institutional Review Boards approved the present study and all patients have signed an informed consent form in order to participate in the present study.
ORCID iD: Willy Baccaglini  https://orcid.org/0000-0001-8653-3913
https://orcid.org/0000-0001-8653-3913
Contributor Information
Fernando Korkes, Discipline of Urology, Faculdade de Medicina do ABC, Santo André, SP, Brazil Division of Urology, Hospital Israelita Albert Einstein, São Paulo, SP, Brazil.
Willy Baccaglini, Discipline of Urology – Faculdade de Medicina do ABC, Av. Lauro Gomes, 2000 – Anexo II, Vila Sacadura Cabral – Santo André, SP, CEP: 09060-870, Brazil.
Marcel Aranha Silveira, Discipline of Urology, Faculdade de Medicina do ABC, Santo André, SP, Brazil.
References
- 1. Lamm D, Colombel M, Persad R, et al. Clinical practice recommendations for the management of non-muscle invasive bladder cancer. Eur Urol 2008; 7: 651–666. [Google Scholar]
- 2. Rouprêt M, Babjuk M, Compérat E, et al. European guidelines on upper tract urothelial carcinomas. 2013 update. Eur Urol 2013; 63: 1059–71. [DOI] [PubMed] [Google Scholar]
- 3. Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012; 110: 614–628. [DOI] [PubMed] [Google Scholar]
- 4. Morales A, Eidinger D, Bruce A. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976; 116: 180–183. [DOI] [PubMed] [Google Scholar]
- 5. Herr HW. Durable response of a carcinoma in situ of the renal pelvis to topical bacillus Calmette-Guerin. J Urol 1985; 134: 531–532. [DOI] [PubMed] [Google Scholar]
- 6. Nager CW, Albo ME, Fitzgerald MP, et al. Reference urodynamic values for stress incontinent women. Neurourol Urodyn 2007; 26: 333–340. [DOI] [PubMed] [Google Scholar]
- 7. Lamm DL, Dehaven JI, Riggs DR. Keyhole limpet hemocyanin immunotherapy of bladder cancer: laboratory and clinical studies. Eur Urol 2000; 37(Suppl. 3): 41–44. [DOI] [PubMed] [Google Scholar]
- 8. Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009; 115: 1224. [DOI] [PubMed] [Google Scholar]
- 9. Fang D, Li X, Xiong G, et al. Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas: a systematic review and meta-analysis. Urol Int 2013; 91: 291. [DOI] [PubMed] [Google Scholar]
- 10. Hayashida Y, Nomata K, Noguchi M, et al. Long-term effects of bacille Calmette-Guérin perfusion therapy for treatment of transitional cell carcinoma in situ of upper urinary tract. Urology 2004; 68: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 11. Studer UE, Casanova G, Kraft R, et al. Percutaneous bacillus Calmette-Guerin perfusion of the upper urinary tract for carcinoma in situ. J Urol 1989; 142: 975–977. [DOI] [PubMed] [Google Scholar]
- 12. Thalmann GN, Markwalder R, Walter B, et al. Long-term experience with bacillus Calmette-Guerin therapy of upper urinary tract transitional cell carcinoma in patients not eligible for surgery. J Urol 2002; 168: 1381–1385. [DOI] [PubMed] [Google Scholar]
- 13. Sharpe JR, Duffy G, Chin JL. Intrarenal bacillus Calmette-Guerin therapy for upper urinary tract carcinoma in situ. J Urol 1993; 149: 457–459. [DOI] [PubMed] [Google Scholar]
- 14. Giannarini G, Kessler TM, Birkhäuser FD, et al. Antegrade perfusion with bacillus Calmette-Guérin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: who may benefit? Eur Urol 2011; 60: 955–960. [DOI] [PubMed] [Google Scholar]
- 15. Bellman GC, Sweetser P, Smith AD. Complications of intracavitary bacillus Calmette-Guerin after percutaneous resection of upper tract transitional cell carcinoma. J Urol 1994; 151: 13–15. [DOI] [PubMed] [Google Scholar]
- 16. Palou J, Piovesan L, Huguet J, et al. Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: recurrence and long-term followup. J Urol 2004; 172: 66–69. [DOI] [PubMed] [Google Scholar]
- 17. Patel A, Fuchs GJ. New techniques for the administration of topical adjuvant therapy after endoscopic ablation of upper urinary tract transitional cell carcinoma. J Urol 1998; 159: 71–75. [DOI] [PubMed] [Google Scholar]
- 18. Mukamel E, Vilkovsky E, Hadar H, et al. The effect of intravesical bacillus Calmette-Guerin therapy on the upper urinary tract. J Urol 1991; 146: 980–981. [DOI] [PubMed] [Google Scholar]
- 19. Irie A, Iwamura M, Kadowaki K, et al. Intravesical instillation of bacille Calmette-Guérin for carcinoma in situ of the urothelium involving the upper urinary tract using vesicoureteral reflux created by a double-pigtail catheter. Urology 2002; 59: 53–57. [DOI] [PubMed] [Google Scholar]
- 20. Audenet F, Traxer O, Bensalah K, et al. Upper urinary tract instillations in the treatment of urothelial carcinomas: a review of technical constraints and outcomes. World J Urol 2013; 31: 45–52. [DOI] [PubMed] [Google Scholar]
- 21. Rastinehad AR, Smith AD. Bacillus Calmette-Guerin for upper tract urothelial cancer: is there a role? J Endourol 2009; 23: 563–568. [DOI] [PubMed] [Google Scholar]
- 22. Schneider B, Thanhauser A, Jocham D, et al. Specific binding of bacillus Calmette-Guerin to urothelial tumor cells in vitro. World J Urol 1994; 12: 337–344. [DOI] [PubMed] [Google Scholar]
- 23. Yossepowitch O, Lifshitz DA, Dekel Y, et al. Assessment of vesicoureteral reflux in patients with self-retaining ureteral stents: implications for upper urinary tract instillation. J Urol 2005; 173: 890–893. [DOI] [PubMed] [Google Scholar]
- 24. Korkes F, Silveira TS, Castro MG, et al. Carcinoma of the renal pelvis and ureter. Int Bras J Uro 2006; 32: 648–655. [DOI] [PubMed] [Google Scholar]
- 25. Diokno AC, Brock BM, Brown MB, et al. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol 1986; 136: 1022–1025. [PubMed] [Google Scholar]
- 26. Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction: II. Aging detrusor: normal versus impaired contractility. J Urol 1993; 150: 1657–1667. [DOI] [PubMed] [Google Scholar]
- 27. Homma Y, Imajo C, Takahashi S, et al. Urinary symptoms and urodynamics in a normal elderly population. Scand J Urol Nephrol Suppl 1994; 157: 27–30. [PubMed] [Google Scholar]
- 28. Madersbacher S, Pycha A, Klingler CH, et al. Interrelationships of bladder compliance with age, detrusor instability, and obstruction in elderly men with lower urinary tract symptoms. Neurourol Urodyn 1999; 18: 3–15. [DOI] [PubMed] [Google Scholar]
- 29. Hübner WA, Plas EG, Stoller ML. The double-J ureteral stent: in vivo and in vitro flow studies. J Urol 1992; 148: 278–280. [DOI] [PubMed] [Google Scholar]