Abstract
The prevalence of type 2 diabetes (T2D) has reached a pandemic scale. These patients are at a substantially elevated risk of developing cardiovascular disease, with heart failure (HF) being a leading cause of morbidity and mortality. Even in the absence of traditional risk factors, diabetes still confers up to a twofold increased risk of developing HF. This has led to identifying diabetes as an independent risk factor for HF and recognition of the distinct clinical entity, diabetic cardiomyopathy. Despite a wealth of research interest, the prevalence and determinants of diabetic cardiomyopathy remain uncertain. This limited understanding of the pathophysiology of diabetic heart disease has also hindered development of effective treatments. Tight blood-glucose and blood-pressure control have not convincingly been shown to reduce macrovascular outcomes in T2D. There is, however, emerging evidence that T2D is reversible and that the metabolic abnormalities can be reversed with weight loss. Increased aerobic exercise capacity is associated with significantly lower cardiovascular and overall mortality in diabetes. Whether such lifestyle modifications as weight loss and exercise may ameliorate the structural and functional derangements of the diabetic heart has yet to be established. In this review, the link between T2D and myocardial dysfunction is explored. Insights into the structural and functional perturbations that typify the diabetic heart are first described. This is followed by an examination of the pathophysiological mechanisms that contribute to the development of cardiovascular disease in T2D. Lastly, the current and emerging therapeutic strategies to prevent or ameliorate cardiac dysfunction in T2D are evaluated.
Keywords: cardiometabolic disease, diabetic cardiomyopathy, heart failure, type 2 diabetes
Introduction
The diabetes pandemic and cardiovascular disease
Diabetes mellitus (DM) is a major global health concern. Recent estimates suggest that there are currently 451 million people with diabetes worldwide and this figure is projected to increase to 693 million by 2045.1 Importantly, estimates suggest that almost half (49.7%) of people living with diabetes remain undiagnosed.1
The vast majority (90%) of people with diabetes have type 2 diabetes (T2D), which is linked to increased sedentary behaviour and obesity and is largely preventable. Whereas T2D was once rare in young people, increasingly we are seeing the condition diagnosed in children, adolescents and adults under the age of 30 years.2,3 Globally, there are now more obese than underweight people4 and this dramatic rise in obesity and sedentary lifestyles, particularly in younger age groups, has resulted in up to a 10-fold increase in the prevalence of T2D in younger adults.5
Diabetes mellitus and heart failure
The most deleterious consequence of developing T2D is a substantially elevated risk of cardiovascular disease (CVD). The risk of cardiovascular complications is two to two-and-a-half times greater in people with T2D compared with the nondiabetic population. In a meta-analysis combing data from 4,549,481 people with T2D, almost one third (32.7%) suffered from CVD and half of all deaths were attributable to CVD.6 Atherosclerotic diseases (angina, myocardial infarction and stroke) have typically been regarded as the predominant manifestations of CVD in T2D. However, recent data from the United Kingdom National Diabetes Audit 2015–2016, which includes data on over 2.7 million patients with diabetes, showed that heart failure (HF) is the commonest cardiovascular complication of T2D and a major cause of premature mortality.7 While the risk of death, myocardial infarction and stroke can be mitigated with strict risk-factor control in T2D, the excess risk of HF persists, in spite of good cardiovascular risk-factor management.8
Patients with DM have up to a 74% increased risk of developing HF, and diabetic patients with HF are four times more likely to die than those without HF.9 Importantly, unrecognized HF is highly prevalent in T2D, with over one quarter (27.7%) of over 60s having previously undiagnosed HF in one report.10 There is a high prevalence of diabetes in patients with both common forms of HF: impaired systolic left ventricular (LV) function and HF with preserved ejection fraction. Conversely, a high proportion (almost one third) of patients with DM have undiagnosed LV dysfunction.10
Diabetic cardiomyopathy
Large, population-based studies have shown that the occurrence of HF in diabetes cannot be accounted for solely by the increased atherosclerotic risk11–13 or the prevalence of other traditional risk factors, such as age, sex, hypertension, coronary artery disease (CAD) and dyslipidaemia that are inherent in diabetic subjects. Even after adjustment for these factors, diabetes still confers a two- to five-fold added risk for HF development.11–13 This has led to the identification of diabetes as an independent risk factor for HF and the recognition of the distinct clinical entity of ‘diabetic cardiomyopathy’,14 a term originally coined by Lundbaek in 1954.15
Diabetic cardiomyopathy is defined as myocardial disease in patients with diabetes, not attributable to hypertension, CAD or other cardiac disease.14 Four stages of diabetic cardiomyopathy are described, and there is overlap with the HF classifications of both the American College of Cardiology/American Heart Association Stage and New York Heart Association Class (Table 1).14 Patients in stage 2 have annual mortality rates up to 20%16 and are twice as likely to be hospitalized for HF than nondiabetics with HF with preserved ejection fraction.17 Over the past 2 decades, the rapid evolution of advanced non-invasive cardiac imaging techniques has enabled detailed evaluation of heart structure and function in vivo. Application of these techniques to the study of diabetic cardiomyopathy have provided key insights to the relationship between T2D and HF.
Table 1.
Classification of diabetic cardiomyopathy.
| Diabetic cardiomyopathy stage | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
| Diastolic HF with normal ejection fraction | Symptomatic HF with combined systolic and diastolic dysfunction | Symptomatic HF to which hypertension, microvascular disease or viral disease have contributed No coronary artery disease |
Symptomatic HF, with contribution from multiple confounders including coronary artery disease | |
| NYHA functional class | Class 1 | Class 2 | Class 3 | Class 4 |
| Asymptomatic, no limitation of physical activity | Slight limitation during ordinary physical activity, with fatigue, palpitation, dyspnoea or angina | Marked limitation, with symptoms occurring during minimal physical activity | Symptoms present at rest Unable to carry out any physical activity without discomfort |
|
| ACC/AHA HF stage | Stage A | Stage B | Stage C | Stage D |
| At risk of HF, but no structural heart disease or symptoms | Asymptomatic structural heart disease | Symptomatic HF with structural heart disease | Refractory HF requiring specialist interventions |
Classification of diabetic cardiomyopathy, using the New York Heart Association (NYHA) Functional Class and American College of Cardiology/American Heart Association (ACC/AHA) HF stages. There is considerable overlap across the three classification schemes.
HF, heart failure.
Clinical factors associated with HF development in T2D
Specific to diabetes populations, increased age, increasing glycosylated haemoglobin (HbA1c), increased body mass index, hypertension, CAD, longer duration of diabetes and the presence of microvascular complications are associated with HF development.18 The UK National Diabetes Audit 2015–2016 showed that the association of HF with HbA1c level was relatively weak but considerably stronger for hypertension.19 Although females with T2D have a higher risk for cardiovascular complications than males,20 there are scarce data in relation to HF.18
Morphological changes in the diabetic heart
The occurrence of structural changes of the diabetic myocardium were first observed by Rubler et al. in 1972. In four postmortem specimens from diabetic patients free from hypertension, CAD or valvular heart disease, Rubler and colleagues described findings of left ventricular (LV) hypertrophy and diffuse myocardial fibrosis.21 There is now an abundance of data to support Rubler and coworkers’ initial findings and demonstrate that diabetes is associated with several alterations in LV geometry.
Alterations in left-ventricular mass and volumes
Increased LV mass is independently associated with diabetes in many (but not all) echocardiographic22–24 and cardiovascular magnetic resonance imaging (CMR) studies.25–27 A 1% rise in HbA1c level was associated with a 3.0 g [95% confidence interval (CI) 1.5–4.6 g] increase in LV mass in one report.23 While these changes in LV mass are modest, increased LV mass is a recognized predictor of cardiovascular morbidity and mortality,28,29 and is likely to be a key contributor to HF development in T2D. When the increased LV mass is indexed for body surface area, however, the differences between diabetics and controls become inconsistent. These inconsistencies arise because adjustment of LV mass for body surface area inherently ‘permits’ obese individuals to have higher LV mass;30 this is why indexing of LV mass/height is advocated.31,32
Other markers of LV remodelling are also apparent in diabetes; LV mass/volume,26,33 relative wall thickness,23,34–36 and septal thickness36,37 are all increased in diabetes. LV mass may be increased as a consequence of increased ventricular wall thickness or from chamber dilatation, that is, the spectrum of LV hypertrophy ranges from concentric to eccentric hypertrophy. While there is variation in both the degree and pattern of hypertrophy observed in patients with diabetes, concentric LV hypertrophy represents the main structural characteristic of diabetic cardiomyopathy. Concentric LV remodelling is associated with an increased risk of developing HF and other adverse cardiac events and appears to be the predominant remodelling pattern in diabetes.38,39 However, LV geometry is also altered by sex,40 ethnicity,41 obesity42 and hypertension,43 common confounders in diabetes, and controlling for these confounders may in fact prevent the development of LV remodelling.44 The lack of standardization in reported markers of LV remodelling makes comparisons between studies difficult45 and limits knowledge of LV remodelling patterns in diabetes.
Functional impairments in the diabetic heart
Cardiac dysfunction in diabetes is thought to lie on a continuum (Table 1), ranging from asymptomatic diastolic dysfunction through subclinical systolic dysfunction and then overt HF with reduced ejection fraction.14
Diastolic dysfunction
It is often stated that diastolic dysfunction is the earliest functional change occurring in diabetic cardiomyopathy. Observational studies have found an increased frequency of diastolic dysfunction in T2D by echo and CMR. The prevalence and severity of diastolic dysfunction was shown to be directly proportional to HbA1c level in one study of 1810 people with T2D.46 Despite controlling metabolic risk factors in T2D [e.g. elevated HbA1c, hypertension, raised body mass index (BMI), dyslipidaemia, albuminuria], diastolic dysfunction persists in the absence of LV remodelling or systolic impairment.44 There are, however, inconsistencies in the prevalence of diastolic dysfunction found in asymptomatic subjects with T2D. Reported prevalence rates vary from 15% to 78%47–50 and differ according to the technique used for diagnosis.47
Systolic dysfunction
Despite the association of T2D with HF, few studies have shown that T2D causes a reduction in global LV ejection fraction, which remains the most utilized measure of LV systolic performance. Using myocardial strain and strain-rate measurement, subclinical impairments in systolic function with normal ejection fraction are now frequently reported in T2D. Tissue Doppler imaging,35,51,52 speckle tracking echocardiography23,53 and CMR33 data confirm systolic LV global longitudinal strain is lower in T2D than in nondiabetics. These impairments in global longitudinal strain worsen with time54 and vary across the glycaemic spectrum (e.g. one study found global longitudinal strain in controls was −18.5 ± 2.3%; in subjects with prediabetes it was −18.1 ± 2.5% versus −17.8 ± 2.4% in those with T2D).23 These subclinical abnormalities in contractility are widely considered a precursor to the onset of clinical HF in diabetes. Indeed, longitudinal studies have found global longitudinal strain to be an independent predictor of cardiovascular events and may provide incremental prognostic value in asymptomatic people with T2D.55,56 However, in the first of these studies, the sample size was modest and there was significant risk of overfitting of the multivariable regression model,55 and in the second study, only echocardiographic parameters as predictors of outcomes were explored with no mention of clinical predictors.56 Importantly, the majority of cardiovascular events in these studies were atherosclerotic (myocardial infarction and stroke) and not HF-related events and it remains unclear why reductions in global longitudinal strain would predict atherothrombotic events independent of other clinical risk factors.
Combined systolic and diastolic dysfunction
The vast majority of studies that have examined both have shown that impaired systolic strain is associated with diastolic dysfunction.23,36,50,52,53,57–60 A small number have, however, reported reduced systolic strain without diastolic dysfunction.33,61,62 This could indicate that diastolic dysfunction is not the earliest functional change in the diabetic heart and is in fact preceded by impaired systolic strain. In most of these studies, diastolic function was determined by tissue Doppler velocities and not strain analyses, likely reducing the sensitivity of identifying diastolic impairments.61,62 Furthermore, it is acknowledged that different guidelines for grading diastolic dysfunction by echocardiography yield inconsistent results and may only be accurate for identifying the most severe cases.63 Assessment of diastolic strain rate may be a more sensitive measure of early diastolic impairment.26 Only one CMR study33 reported reduced LV systolic global longitudinal strain (controls −11.4 ± 2.8 versus T2D −9.6 ± 2.9, p = 0.049) and preserved diastolic strain rate (controls 0.65 ± 0.13 versus 0.62 ± 0.26 s−1, p = 0.749), but these values are much lower than those seen in the prevailing echo and CMR literature where they are typically ~20% and 1.5–2.0 s−1, respectively, in controls.
Biomarkers in diabetic cardiomyopathy
Several studies have investigated the role of natriuretic peptides in asymptomatic patients with T2D.64 Serum N-terminal pro-B-type natriuretic peptide levels are higher in asymptomatic T2D with isolated diastolic dysfunction compared with controls.65,66 At present B-type natriuretic peptide is often used in routine practice, however natriuretic peptide levels can be influenced by many other factors, several of which are more prevalent in diabetes, including obesity, increasing age, use of renin–angiotensin–aldosterone-system inhibitors and renal dysfunction.67 And diabetes itself is associated with elevated natriuretic peptide levels.67 Numerous other biomarkers are being evaluated in HF64 and specifically, diabetic HF. In one study comparing biomarker profiles between diabetic and nondiabetic patients with HF with preserved ejection fraction, endothelin-1 (a potent vasoconstrictor), galectin-3 and carboxy-terminal telopeptide of collagen type 1 (profibrotic biomarkers) were higher in patients with diabetes.17 Of more interest, is a biomarker analysis of the ADVANCE trial, which assessed the prediction of new and worsening HF in a nested case-control analysis.68 This study demonstrated that measurement of N-terminal pro-B-type natriuretic peptide gave a similar predictive accuracy as clinical risk factors and was of additive value, although the effects were attenuated when patients with levels > 400 pg/ml (in the HF diagnostic range) were excluded. Markers of inflammation [interleukin 6, high-sensitivity C-reactive protein (IL-6, hsCRP)] and myocardial damage (troponin) were not of added value.68 The identification of plasma biomarkers in HF is an area of intense research interest and may impact on clinical management in the future.64
Aetiological factors in the development of LV dysfunction
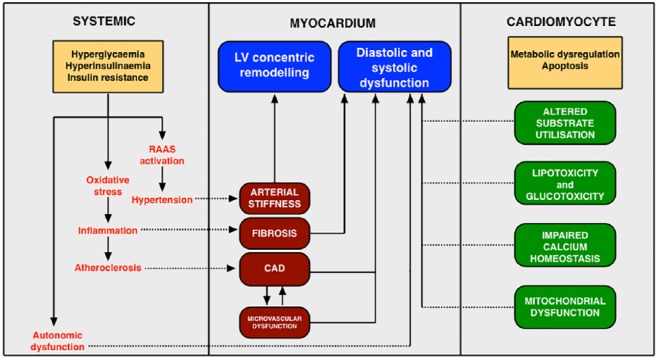
The mechanisms contributing to the development of diabetic cardiomyopathy have been extensively explored in animal models.69 These include myocardial lipotoxicity and glucotoxicity, damage from glycated end products and reactive oxygen species, impaired calcium homeostasis, mitochondrial dysfunction, activation of the renin–angiotensin–aldosterone system, altered myocardial substrate utilization, and cardiac autonomic neuropathy (Figure 1).70
Figure 1.
Local and systemic perturbations involved in the pathophysiology of diabetic cardiomyopathy.
RAAS, renin–angiotensin–aldosterone system; CAD, coronary artery disease; LV, left ventricle.
Several inverse correlations with diastolic dysfunction in T2D have been identified: HbA1c level,57 age,48 aortic stiffness,26 duration of DM,26 microvascular dysfunction71 and myocardial triglyceride content.72–74 Similarly, multiple associations with LV systolic strain are reported; these include BMI,75 waist circumference,75 HbA1c,76 blood pressure (BP),60 sex,34 presence of microalbuminuria,34,77 LV relative wall thickness,34,77 CAD78 and, again, myocardial triglyceride.33 Importantly, these findings were made on the basis of multivariate analyses, which have been limited by small sample sizes (seldom greater than 100 subjects) and incomplete datasets, with significant risk of overfitting the regression models.79
Myocardial energy metabolism
Impaired myocardial substrate utilization and altered energy metabolism have recently been described in T2D and are likely to be contribute to the development of cardiac impairment. The normal heart derives 70% of its energy from free-fatty-acid metabolism and 30% from glucose metabolism.80 In T2D, there is a shift toward increased free-fatty-acid utilization by the myocardium in T2D due to increased free-fatty-acid availability. This is less energy efficient with lower adenosine triphosphate (ATP) yield81 and leads to metabolic inefficiency in the diabetic heart.82
Myocardial energetics can now be assessed non-invasively by phosphorus magnetic resonance spectroscopy. This enables assessment of the myocardial creatine phosphate (PCr)/ATP ratio, which is a sensitive index of the energetic state of the myocardium.83 Decreased myocardial PCr/ATP ratios have been demonstrated in T2D and suggest that myocardial energetic impairment is a key component in the pathophysiology of diabetic cardiomyopathy.84–86 Impairment in myocardial energetics has also been exacerbated by exercise86 and may reflect metabolic inflexibility in the diabetic heart. Importantly, a decreased PCr/ATP ratio has been linked to contractile dysfunction and is a predictor of mortality, although this was in patients with dilated cardiomyopathy.87
Myocardial steatosis
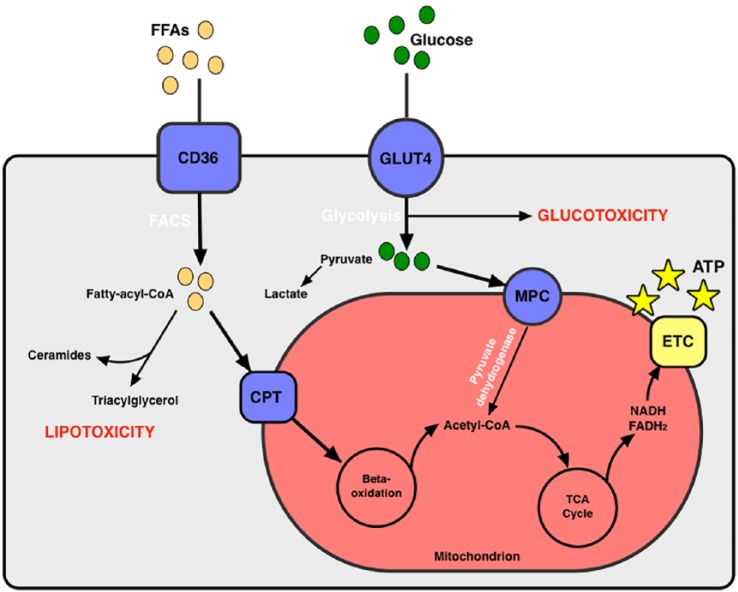
Metabolic dysregulation is central to the pathogenesis of DM. Insulin resistance results in decreased availability of glucose in the myocardium.88 There is an increased supply of free fatty acids and a shift towards fatty-acid oxidation in the myocyte.89 The supply of fatty acids, however, exceeds the oxidative capacity of the heart and nonoxidative lipid metabolism ensues.90 Products of nonoxidative lipid metabolism include ceramide and diacylglycerol, which are toxic to cardiomyocytes and can induce myocardial dysfunction, apoptosis and fibrosis (Figure 2).91 Excessive myocardial triglyceride accumulation (steatosis) was first demonstrated in mouse models of DM92 and has emerged as a contributor to development of diabetic cardiomyopathy. Myocardial steatosis is mediated by microribonucleic acid (microRNA) dysregulation (such as miR-451 and miR-494-3p) and was linked with LV dysfunction in recent animal models of T2D and obesity,93,94 supporting a central role for microRNAs in the pathogenesis of diabetic cardiomyopathy.
Figure 2.
Myocyte energy metabolism and alterations that contribute to lipotoxicity and glucotoxicity.
CoA, coenzyme A; CD36, cluster of differentiation 36; FACS, ; FFAs, free fatty acids; CPT, carnitine palmitoyltransferase; FADH2, flavine adenine dinucleotide; GLUT 4, glucose transporter type 4; MPC, mitochondrial pyruvate carrier; NADH, nicotinamide adenine dinucleotide; TCA, tricarboxylic acid; ETC, electron-transport chain; ATP, adenosine triphosphate.
Magnetic resonance spectroscopy also allows the detection of myocardial steatosis in vivo, and several studies have confirmed elevated myocardial triglyceride content in T2D (Table 2).33,72,74,95 Steatosis has been significantly correlated with diastolic strain rate on speckle tracking echocardiography73 and on CMR,74 and was also an independent predictor of systolic strain in a small study with 66 patients.33 However, all studies to date have involved small numbers. If confirmed in a larger sample size with appropriate adjustment for confounders, it will likely play a central mechanistic role in the pathophysiology of diabetic heart disease.
Table 2.
Summary of magnetic resonance spectroscopy studies evaluating myocardial triglyceride content in T2D.
| Study | Patients | n | Mean age (years) | M/F | Mean BMI (kg/m2) | Key outcomes |
|---|---|---|---|---|---|---|
| McGavock 96 | Lean controls | 15 | 35 ± 3 | 7/8 | 23 ± 2 | Myocardial TG elevated in IGT and T2D versus controls (0.95 ± 0.60 versus 1.06 ± 0.62 versus 0.46 ± 0.30 fat/water, p < 0.05) |
| Obese | 21 | 36 ± 12 | 10/11 | 32 ± 5 | ||
| IGT | 20 | 49 ± 9 | 5/15 | 31 ± 6 | ||
| T2D | 78 | 47 ± 10 | 37/41 | 34 ± 7 | ||
| Rijzewijk et al. 72 | Controls | 28 | 54 ± 1 | 28/0 | 26.9 ± 0.5 | Myocardial TG increased in T2D versus controls (0.96 ± 0.07% versus 0.65 ± 0.05%, p < 0.05) |
| T2D | 38 | 57 ± 1 | 38/0 | 28.1 ± 0.6 | ||
| Korosoglou et al. 74 | Controls | 16 | 62 ± 3 | 10/6 | 23.9 ± 2.5 | Myocardial TG in T2D 0.86 ± 0.14 Association between TG and mean diastolic strain rate (r = −0.71, p < 0.001) and peak systolic strain rate (r = 0.41, p = 0.02) |
| T2D | 42 | 62 ± 6 | 26/16 | 31.6 ± 4.8 | ||
| Levelt et al. 33 | Controls | 20 | 54 ± 10 | 9/11 | 28.6 ± 2.8 | Elevated myocardial TG in T2D (1.13 ± 0.78 versus 0.64 ± 0.52, p = 0.017) Negative correlation between TG and systolic strain (r = −0.40, p = 0.003) |
| T2D | 46 | 55 ± 9 | 24/22 | 29.6 ± 5.7 |
BMI, body mass index; IGT, impaired glucose tolerance; TG, triglyceride; T2D, type 2 diabetes.
Coronary microvascular dysfunction
Abnormalities of vascular function are well described in T2D, including endothelial dysfunction. Impaired coronary microvascular function has been demonstrated in older,25,35,74 but not younger patients26 with T2D. The mechanisms contributing to coronary microvascular dysfunction include: endothelial dysfunction, increased myocardial mass with reduced capillary density, myocardial fibrosis and reduced transmyocardial perfusion gradient due to increased LV diastolic pressure.97 Impaired myocardial performance index, a marker of overall systolic and diastolic function, has also shown association with microvascular dysfunction in T2D.98 However, evidence of this relationship between microvascular dysfunction and diastolic impairment in T2D is conflicting. CMR studies have failed to identify an association,26,74 whereas an echo study using tissue Doppler indices found significant associations.71 However, none of these studies angiographically excluded epicardial CAD. The potential link between coronary microvascular dysfunction and diastolic impairment in T2D is, therefore, unclear. Moreover, microvascular dysfunction may coexist with and potentiate the effects of epicardial CAD on cardiac dysfunction.
Myocardial fibrosis
Myocardial fibrosis is thought to be mediated by damage from advanced glycation end products99 and apoptosis caused by lipotoxicity.74 CMR techniques now allow the non-invasive detection of replacement myocardial fibrosis (late gadolinium enhancement) and an estimate of diffuse interstitial fibrosis using T1 mapping and calculation of myocardial extracellular volume.100 Patients with diabetes have shorter global contrast-enhanced myocardial T1 times compared with controls, indicative of a higher burden of diffuse interstitial myocardial fibrosis.101 This is independently associated with myocardial systolic101 and diastolic function.99,101 Subjects with T2D have a higher extracellular volume fraction than controls,102,103 although the differences found are small (1–2%),103 and there is a large degree of overlap when control subjects are well matched to those with diabetes.33 Elevated extracellular volume fraction is associated with increased admissions for HF and mortality, but this was in patients already referred for a clinical CMR with inevitable selection bias.103
Arterial stiffness
The aorta is a conduit for the delivery of blood to peripheral tissues. In addition, the elastic properties of the aorta act to dampen the sudden fluctuations in BP generated by blood ejected from the LV during each cardiac cycle. This transforms the pulsatile stroke volume into continuous blood flow through the peripheral arterial tree.104 This buffering capacity of the thoracic aorta is essential for maintaining normal LV structure and function. Aortic stiffening is an increase in the elastic resistance of the aorta to deformation and naturally occurs with ageing but is additionally accelerated by the traditional cardiovascular risk factors.105,106 Increased aortic stiffness is a strong predictor of adverse cardiovascular events in several cohorts,107–109 including T2D.110,111 Our group has previously shown a modest but significant correlation exists between mean aortic distensibility and peak early diastolic strain rate (r = 0.564, p = 0.023) in young adults with T2D.26 This suggests that increased aortic stiffness is an early change in diabetes, which contributes to subclinical cardiac dysfunction in T2D, independent of age and BP.
Poorer blood-glucose control is associated with accelerated aortic stiffness, particularly in younger adults with T2D.112 Reducing HbA1c levels may attenuate the progression of aortic stiffness and some studies have shown that aortic stiffness is modifiable by diabetes treatment.113–116
Despite the recognition that aortic stiffening is a key determinant of LV dysfunction in several diseases, the precise mechanisms linking aortic stiffness with adverse cardiovascular outcomes are unclear. The most prominent hypothesis for the pathophysiological basis linking aortic stiffness with adverse cardiovascular events in T2D is the development of LV remodelling.117 Aortic stiffening disturbs the arterial–ventricular interaction, augmenting ventricular afterload and supplementing the development of LVH. This results in increased LV filling pressures and impairment in the passive flow of blood from the left atrium to the LV in early diastole. Aortic stiffness therefore likely mediates the development of diabetic cardiomyopathy by stimulating LVH. We have recently confirmed this in a cross-sectional study of 80 young adults with T2D, where we have demonstrated that aortic stiffness is an independent predictor of concentric LV remodelling.118 Given that aortic stiffening is potentially reversible with aggressive BP reduction,119 this may yield a potential therapeutic strategy for preventing or treating diabetic cardiomyopathy.
Pharmacological interventions to reverse cardiovascular dysfunction in T2D
Glycaemic control
The majority of previous, large, multicentre, randomized-controlled trials such as UKPDS,120 ADVANCE,121 ACCORD122 and VADT123 did not demonstrate an improvement in macrovascular outcomes with tight blood-glucose control. A meta-analysis of these four large trials, comprising over 27,000 patients assigned to more-intensive versus less-intensive blood-glucose control showed only modest reduction in major adverse cardiovascular events [hazard ratio (HR) 0.91; 95% CI 0.84–0.99].124 This was primarily driven by a reduction in myocardial infarction, with no overall benefit on all-cause or cardiovascular death.124 Similarly, a meta-analysis of data from eight randomized trials comprising 37,229 people with T2D revealed no observed benefit of intensive glycaemic control on HF-related outcomes.125 Even with intensive glucose control, underlying epigenetic alterations that promote oxidative stress, myocardial inflammation and LV dysfunction persisted in a mouse model of diabetes. This may be a key mechanism driving the persistence of cardiac dysfunction in the context of tight glycaemic control; and targeting epigenetic networks has been proposed as a novel strategy to ameliorate LV dysfunction in T2D.126
Recently, new classes of glucose-lowering therapies, such as glucagon-like peptide-1 (GLP-1) agonists,127 and inhibitors of sodium–glucose cotransporter 2 (SGLT2 inhibitors)128 have shown exciting results with improved glycaemic control, as well as reduced cardiovascular mortality in patients with T2D.129 However, these recent trials were designed to assess the safety and tolerability of these novel drugs, and therefore the mechanisms behind the observed cardiovascular benefits are speculative. Nevertheless, the increased use of these agents has been advocated in recent guidelines of management of hyperglycaemia in T2D.
GLP-1 agonists
GLP-1 agonists exert their effects by suppressing appetite, glucagon secretion and gastric emptying, and by stimulating the release of insulin.130 In the randomized-controlled LEADER trial, patients with T2D and high cardiovascular risk treated with liraglutide had lower rates of cardiovascular death compared with those having placebo.127 Similarly, in high-risk T2D patients, cardiovascular event rates (death, nonfatal myocardial infarction and nonfatal stroke) were found to be significantly lower with semaglutide131 and albiglutide than for placebo.132 However, in the EXSCEL trial of the GLP-1, exenatide versus placebo, there was no overall cardiovascular risk benefit with the study drug, although this study included patients with or without a prior history of CVD133 (Table 3). In the PIONEER-6 study, the cardiovascular safety and efficacy of the first oral formulation of a GLP-1 agonist (semaglutide) will be compared with placebo in over 3000 high-risk patients with T2D.134 Although the complete results of this trial are yet to published, early data indicate a 51% relative risk reduction in cardiovascular death and a 49% relative risk reduction in all-cause mortality with semaglutide, but no difference in the risk of nonfatal myocardial infarction or stroke compared with placebo.135 Treatment with semaglutide was also associated with a 13.8% reduction in weight in obese people in a phase II clinical trial.136 Notably, the beneficial effects of GLP-1 agonists appear to be on atherosclerotic CVD, rather than lowering the risk of HF development. Agents with costimulatory effects on GLP-1 and glucose-dependent insulinotropic polypeptide receptors have recently emerged, which have demonstrated superior glucose and weight-lowering properties than GLP-1 analogues alone.137 The cardiovascular benefits of these drugs have yet to be demonstrated.
Table 3.
Cardiovascular outcome trials of sodium–glucose cotransporter-2 inhibitors and glucagon-like peptide–receptor analogues.
| Study | Agent | Sample size, n | Key inclusion criteria | Mean age, years | Median follow-up duration, years | Key findings |
|---|---|---|---|---|---|---|
| Sodium–glucose-cotransporter-2 inhibitors | ||||||
| EMPA-REG OUTCOME138 | Empagliflozin | Total: 7020 Drug: 4687 Placebo: 2333 |
T2D and CVD, HbA1c 7–10% | 63.2 | 3.1 | 14% ↓ in primary outcome, 38% ↓ CV death, 13% ↓ MI, 24% ↑ stroke, 35% ↓ heart-failure hospitalization |
| CANVAS139 | Canagliflozin | Total: 10,142 Drug: 5795 Placebo: 4347 |
T2D and history of or high risk for CVD, HbA1c 7–10.5% | 63.3 | 2.4 | 14% ↓ in primary outcome, 13% ↓ CV death, 15% ↓ MI, 10% ↓ stroke, 33% ↓ heart-failure hospitalization |
| DECLARE-TIMI 58140 | Dapagliflozin | Total: 17160 Drug: 8582 Placebo: 8578 |
T2D with and without history of CVD, HbA1c | 64.0 | 4.2 | 7% ↓ in primary outcome, 17% ↓ CV death, 11% ↓ MI, 27% ↓ heart-failure hospitalization |
| Glucagon-like peptide–receptor analogues | ||||||
| LEADER127 | Liraglutide | Total: 9340 Drug: 4668 Placebo: 4672 |
T2D and CVD, HbA1c ⩾ 7.0% | 64.3 | 3.8 | 13% ↓ in primary outcome, 22% ↓ CV death, 12% ↓ MI, 11% ↓ stroke, 13% ↓ heart-failure hospitalization |
| SUSTAIN-6131 | Semaglutide | Total: 3297 Drug: 1648 Placebo: 1649 |
T2D and CVD, HbA1c ⩾7.0% | 64.5 | 2.1 | 26% ↓ in primary outcome, 2% ↓ CV death, 26% ↓ MI, 39% ↓ stroke, 11% ↑ heart-failure hospitalization |
| EXSCEL133 | Exenatide | Total: 14752 Drug: 7356 Placebo: 7396 |
T2D, 70% with CVD and 30% without, HbA1c 6.5–10% | 62.0 | 3.2 | Non-inferior but not superior to placebo for primary outcome No significant difference in rates of CV death, MI, stroke or heart-failure hospitalization between groups |
| HARMONY OUTCOMES132 | Albiglutide | Total: 9463 Drug: 4731 Placebo: 4732 |
T2D and CVD, HbA1c >7% | 64.1 | 1.5 | 22% ↓ in primary outcome, 7% ↓ CV death, 25% ↓ MI, 14% ↓ stroke |
CV, cardiovascular; CVD, cardiovascular disease; HbA1c, glycosylated haemoglobin; MI, myocardial infarction; T2D, type 2 diabetes.
SGLT2 inhibitors
SGLT2 inhibitors have emerged as glucose-lowering therapies showing improved cardiovascular outcomes in T2D (Table 3). In the first of these, the EMPA-REG OUTCOME138 and CANVAS139 studies, there was a relative risk reduction in cardiovascular mortality and hospitalization for HF in patients with T2D and established, or at high risk of, CVD. More recently, in the largest of the SGLT2 inhibitor trials with the longest follow-up duration, the DECLARE-TIMI 58, a study of the SGLT2 inhibitor dapagliflozin versus placebo, reduced rates of hospitalization for HF were also observed in lower-risk subjects with T2D.140 Furthermore, when participants of the EMPA-REG OUTCOME were stratified according to risk of HF development at baseline, the beneficial effects of empagliflozin on reducing incident HF-related events were observed in patients at low, intermediate and high risk of HF.141 These latter studies suggest a potential role for SGLT2 inhibitors in the prevention of HF development in low-risk patients with T2D.
SGLT2 inhibitors lower blood-glucose levels by promoting urinary glucose excretion. Secondary effects include weight loss, a modest diuretic effect and BP reduction.142 The precise mechanisms linking SGLT2 inhibitors to lower risk of HF and favourable cardiovascular outcomes are unclear. The most popular hypothesis is that the increased fluid losses (driven by urinary glucose and sodium excretion) lead to a reduction in intravascular volume and systolic BP. This in turn reduces preload and afterload, leading to improvements in myocardial oxygen supply and vascular function.142 A mediation analysis of the EMPA-REG OUTCOME trial has indeed demonstrated that changes in plasma volume are central to the CV risk benefits observed with empagliflozin.143 Furthermore, in a randomized trial of empagliflozin versus placebo in patients with T2D and uncontrolled nocturnal hypertension, empagliflozin was associated with significant 24 h ambulatory BP reductions compared with placebo (−10.0 versus −2.4 mmHg, respectively, over 12 weeks, p < 0.001).144 Others suggest that SGLT2 inhibitors, through a shift in myocardial metabolism towards ketones, have favourable effects on cardiac energetics.145 The results of these studies, while promising, should be viewed with a degree of caution. HF risk reduction was not the primary endpoint of either study and was based on investigator-reported HF events rather than objective measures (such as echocardiography or measurement of B-type natriuretic peptide levels). Nevertheless, several mechanistic studies are now underway to identify the specific cardiovascular effects of SGLT2 inhibitors. Whether the same cardiovascular benefits of SGLT2 inhibitors are seen in earlier stages of diabetic cardiomyopathy remains to be established.
Blood pressure reduction
Patients with diabetes are twice as likely to suffer from hypertension than nondiabetics.146 Coexistence of these two conditions confers a greater risk of CVD, including CAD, LV hypertrophy, stroke and HF, compared with either diabetes or hypertension in isolation.147 Despite the high prevalence of hypertension in diabetes, there are inconsistencies in the recommended BP targets in these patients.148–150 Previous recommendations favoured an intensive approach to BP management in diabetes given the high CVD risk profile of these patients. Indeed, in nondiabetic patients at high risk of CVD, intensive BP lowering (to a systolic BP < 120 mmHg) dramatically lowers cardiovascular risk and all-cause mortality.151 It has therefore been suggested that tighter BP control be targeted in patients with T2D,152 although there are limited data to support this strategy as a means of overall cardiovascular risk reduction.
In the ACCORD BP study, which specifically addressed the issue of intensive BP lowering in T2D, there was no demonstrable survival benefit with intensive BP reduction (systolic BP < 120 mmHg) compared with a standard BP reduction target (<140 mmHg) over a median follow-up period of 4.7 years in 4733 patients with T2D.153 The annual incidence of all-cause mortality was similar with either BP target (1.28 and 1.19%, respectively; HR 1.07; 95% CI 0.85–1.35, p = 0.52). Intensive BP treatment was in fact harmful and led to increased incidence of syncope and hyperkalaemia.154 Even after longer-term follow up (median duration 8.8 years) was carried out for 3957 patients from the ACCORD study, there remained no reduction in the rate of a composite of fatal and nonfatal major cardiovascular events or mortality with intensive versus standard BP control.154 Similarly, patients with T2D and a history of CAD do not appear to benefit from an intensive BP-lowering-treatment strategy.155 Recent data suggest a U-shaped relationship between BP and cardiovascular outcomes in T2D, where systolic BP over 150 mmHg or less than 110 mmHg portends a poorer prognosis.156 Maintenance of BP within this range appears to be the most appropriate strategy in T2D. In view of these and other large prospective studies evaluating BP-lowering targets in DM, current National Institute for Health and Care Excellence (NG28) recommendations are that BP be maintained below 140/80 mmHg in uncomplicated T2D or below 130/80 mmHg if there is a history of kidney, eye or cerebrovascular disease.148
Lifestyle interventions to reverse cardiovascular dysfunction
Weight loss
T2D has long been regarded as a chronic condition capable of being ameliorated but not cured. However, proof that T2D is a reversible condition has been firmly established in patients undergoing bariatric surgery157,158 and in a primary-care-led administration of a 825–853 kcal/day meal-replacement diet.159 The extent of weight loss is strongly linked to reversal of T2D. Insulin use, diabetes duration and high HbA1c levels reduce the chances of reversal.158 None of these reports, however, have assessed changes in cardiovascular function.
In obese subjects without T2D, sustained weight loss, either with diet or after surgery, has resulted in favourable reductions in CMR-measured LV mass, volumes, arterial stiffness and diastolic function.160 Improved diastolic function following weight loss in obesity has been associated with improved energetics161 and with reduced myocardial triglyceride content.162
In patients with insulin-treated T2D, a 471 kcal/day very-low-energy diet has also been shown to reduce myocardial steatosis (0.88 ± 0.12% to 0.64 ± 0.14%, p = 0.02) in a small (n = 12) single-group study and was associated with improved diastolic filling on CMR.163 Interestingly, a recent brief report from the same group, suggests that in the first few days after commencing a very-low-energy diet, there may actually be an increase in steatosis and reduced diastolic mitral filling.164 However, it should be noted that there was a dramatic decrease in ventricular volumes, with a nonsignificant decrease in estimated filling pressure, which are both likely to have affected the diastolic filling rate.
Recently, the selective-serotonin-2C-receptor agonist lorcaserin, which suppresses appetite, has been shown to cause sustained weight loss, reduce hyperglycaemia and reduce the risk of microvascular complications in high-risk overweight and obese patients with and without diabetes.165,166 Whether this leads to lower macrovascular complications remains to be demonstrated. Alter-natively Lorcarserin may be used as an adjunct to surgical or dietary strategies as a means to supplement or maintain weight reduction.
Exercise programmes
Large cohort studies have shown that increased aerobic exercise capacity is associated with significantly lower cardiovascular and overall mortality in men167 and women168 with DM. Peak exercise capacity (maximal volume of oxygen uptake) is a recognized prognostic marker in subjects with CVD169 and in T2D.170 In a small study (n = 19), exercise capacity was significantly reduced in subjects with T2D having diastolic dysfunction compared with those having normal diastolic function.171 This suggests that improvements in exercise capacity may yield improvements in cardiac dysfunction in T2D; at the very least, in the early stages of diabetic cardiomyopathy. This is supported by a recent position paper from the European Association of Preventive Cardiology, which advocates the promotion of individualized exercise training programmes in people with T2D to improve both cardiovascular and metabolic function.172
Intervention studies have demonstrated a strong causal link between exercise training and glycaemic control in those with T2D. Exercise training has consistently been found to lower HbA1c by 0.6–0.7%, with greater effects seen with higher volumes of exercise.173,174 Importantly, these substantial benefits are maintained when exercise training does not result in weight loss.173,174 This is consistent with experimental studies that have elucidated key insulin-dependent and insulin-independent pathways linking physical activity to improved glucose regulation that do not act through adiposity.175,176
While the benefits of exercise training on glycaemic control are well established, the effects on diastolic function are less well known, primarily due to insufficient data, differences in measurement and poor study design. However, encouraging data are starting to emerge in those with obesity and chronic disease. For example, an 8-week exercise training programme in obese men improved diastolic function to levels seen in lean controls, despite no weight loss.177 Similarly, just 4 weeks of exercise training has been shown to improve diastolic function in those with HF with similar improvements also observed in a matched cohort without HF.178
Only two studies have been conducted in T2D assessing the effects of exercise training on diastolic function. In a small pilot study, 3 months of aerobic exercise training reversed diastolic dysfunction in almost half (45%) of individuals with T2D and grade 1 diastolic dysfunction,179 while another study found no overall effect.180 However in the latter study, a post hoc analysis revealed that change in moderate-intensity physical activity was significantly associated with change in myocardial strain rate, although it is unclear whether this was systolic, diastolic or both.180 This mirrors the wider evidence where light-to-moderate aerobic exercise training has repeatedly been demonstrated to improve diastolic function across a number of groups.177,179 The effectiveness of vigorous-intensity exercise or combined aerobic and resistance training is less well established, with at least one study showing the latter approach is not effective.181 Given this evidence base, it is important the efficacy of aerobic exercise training is investigated further.
Finally, in the randomized-controlled LookAHEAD trial, the effects of intensive-lifestyle intervention (which included a combined dietary weight loss and exercise programme) versus a diabetes support programme were evaluated in 5145 overweight or obese (mean age 58.7 years, BMI 36 kg/m2) people with T2D over a median follow-up duration of 9.6 years. Disappointingly, there was no difference in the rate of cardiovascular events in the intensive-lifestyle-intervention arm, despite a greater extent of weight loss, increased fitness and improved glycaemic control.182 However, the mean weight loss achieved in the intensive-lifestyle-intervention arm was only 6% by the end of the trial, and both study groups had intensive medical management of cardiovascular risk factors, which may have limited the treatment effect in the intervention arm.
Conclusions
The rapid increase in prevalence of T2D now represents a global pandemic. These patients are at high risk of developing HF and dying prematurely, but the prevalence of subclinical cardiac dysfunction and the causes are uncertain. Improved glycaemic control per se does not reduce the risk of developing HF, but newer pharmacologic agents reduce CV complications and SGLT2 inhibitors have been shown to decrease HF-related hospitalizations. Weight loss, either with low-calorie diets or bariatric surgery, is also an attractive option for reversing diabetes and the risk of HF, but further studies are needed.
Footnotes
Authors’ Note: GPM conceived the idea for the manuscript and decided the overall theme and content. GSG and LA drafted the manuscript. All authors critically reviewed and approved the final submission.
Funding: Professor Gerry McCann is funded by the National Institute for Health Research (NIHR) through a career development fellowship (G McCann, CDF 2014-07-045) and directly supported by the NIHR Leicester Biomedical Research Centre.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: Ethical approval was not required for this review.
ORCID iD: McCann GP  https://orcid.org/0000-0002-1740-9270
https://orcid.org/0000-0002-1740-9270
Contributor Information
Gaurav S. Gulsin, Department of Cardiovascular Sciences, University of Leicester and the Leicester NIHR Biomedical Research Centre, Leicester, UK
Lavanya Athithan, Department of Cardiovascular Sciences, University of Leicester and the Leicester NIHR Biomedical Research Centre, Leicester, UK.
Gerry P. McCann, Department of Cardiovascular Sciences, University of Leicester and the Leicester NIHR Biomedical Research Centre, Glenfield Hospital, Groby Road, Leicester LE3 9QP, UK.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138: 271–281. [DOI] [PubMed] [Google Scholar]
- 2. Wilmot EG, Edwardson CL, Biddle SJ, et al. Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk’ UK adults: insights from the STAND programme of research. Diabet Med 2013; 30: 671–675. [DOI] [PubMed] [Google Scholar]
- 3. Haines L, Wan KC, Lynn R, et al. Rising incidence of type 2 diabetes in children in the UK. Diabetes Care 2007; 30: 1097–101. [DOI] [PubMed] [Google Scholar]
- 4. Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grinstein G, Muzumdar R, Aponte L, et al. Presentation and 5-year follow-up of type 2 diabetes mellitus in African-American and Caribbean-Hispanic adolescents. Horm Res 2003; 60: 121–126. [DOI] [PubMed] [Google Scholar]
- 6. Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol 2018; 17: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Partnership HQI. National diabetes audit, 2015–2016. NHS Digital. Leeds, UK, 2017. [Google Scholar]
- 8. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379: 633–644. [DOI] [PubMed] [Google Scholar]
- 9. Centre HSCI. Are diabetes services in England and Wales measuring up? National Diabetes Audit 2011–12. Leeds, UK, 2012. [Google Scholar]
- 10. Boonman-de Winter LJ, Rutten FH, Cramer MJ, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012; 55: 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kannel W, Hjortland M, Castelli W. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 12. Aronow W, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest 1999; 115: 867–868. [DOI] [PubMed] [Google Scholar]
- 13. Bertoni A, Tsai A, Kasper E, et al. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care 2003; 26: 2791–2795. [DOI] [PubMed] [Google Scholar]
- 14. Maisch B, Alter P, Pankuweit S. Diabetic cardiomyopathy–fact or fiction? Herz 2011; 36: 102–115. [DOI] [PubMed] [Google Scholar]
- 15. Lundbaek K. Diabetic angiopathy: a specific vascular disease. Lancet 1954; 266: 377–379. [DOI] [PubMed] [Google Scholar]
- 16. Tillquist MN, Maddox TM. Update on diabetic cardiomyopathy: inches forward, miles to go. Curr Diab Rep 2012; 12: 305–313. [DOI] [PubMed] [Google Scholar]
- 17. Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014; 64: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacDonald MR, Petrie MC, Hawkins NM, et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J 2008; 29: 1224–1240. [DOI] [PubMed] [Google Scholar]
- 19. National Diabetes Audit, 2015–16. Report 2b: Complications and mortality. (Associations between disease outcomes and preceding care.) Leeds, UK, 2017. [Google Scholar]
- 20. Norhammar A, Schenck-Gustafsson K. Type 2 diabetes and cardiovascular disease in women. Diabetologia 2013; 56: 1–9. [DOI] [PubMed] [Google Scholar]
- 21. Rubler S, Dlugash J, Yuceoglu YZ, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972; 30: 595–602. [DOI] [PubMed] [Google Scholar]
- 22. Galderisi M. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (The Framingham Heart Study). Am J Cardiol 1991; 68: 85–89. [DOI] [PubMed] [Google Scholar]
- 23. Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk in the Community study. Circ Heart Fail 2015; 8: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Marco M, De Simone G, Roman MJ, et al. Cardiac geometry and function in diabetic or prediabetic adolescents and young adults: the Strong Heart Study. Diabetes Care 2011; 34: 2300–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larghat AM, Swoboda PP, Biglands JD, et al. The microvascular effects of insulin resistance and diabetes on cardiac structure, function, and perfusion: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2014; 15: 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan JN, Wilmot EG, Leggate M, et al. Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging 2014; 15: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 27. Bertoni A, Goff D, D’Agostino R, et al. Diabetic cardiomyopathy and subclinical cardiovascular disease. Diabetes Care 2006; 29: 588–594. [DOI] [PubMed] [Google Scholar]
- 28. Gottdiener J, Arnold A, Aurigemma G, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000; 35: 1628–1637. [DOI] [PubMed] [Google Scholar]
- 29. Savage DD, Levy D, Dannenberg AL, et al. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am J Cardiol 1990; 65: 371–376. [DOI] [PubMed] [Google Scholar]
- 30. Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound 2005; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992; 20: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 32. Armstrong AC, Gidding S, Gjesdal O, et al. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging 2012; 5: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levelt E, Mahmod M, Pichnik SK, et al. Relationship between left ventricular structural and metabolic remodelling in type 2 diabetes mellitus. Diabetes 2015; ePub: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ernande L, Bergerot C, Girerd N, et al. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr 2014; 27: 479–488. [DOI] [PubMed] [Google Scholar]
- 35. Moir S, Hanekom L, Fang ZY, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart 2006; 92: 1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ofstad AP, Urheim S, Dalen H, et al. Identification of a definite diabetic cardiomyopathy in type 2 diabetes by comprehensive echocardiographic evaluation: a cross-sectional comparison with non-diabetic weight-matched controls. J Diabetes 2015; 7: 779–790. [DOI] [PubMed] [Google Scholar]
- 37. Saglam H, Seyfeli E, Gul I, et al. Index of myocardial performance in patients with type 2 diabetes without hypertension and its relationship with clinical and echocardiographic parameters. J Diabetes 2009; 1: 50–56. [DOI] [PubMed] [Google Scholar]
- 38. Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level–risk factors, screening, and outcomes. Nat Rev Cardiol 2011; 8: 673–685. [DOI] [PubMed] [Google Scholar]
- 39. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008; 52: 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salton CJ, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventircular anatomy in an adult population free of hypertension. J Am Coll Cardiol 2002; 39: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez CJ, Sciacca RR, Diez-Roux AV, et al. Relation between socioeconomic status, race-ethnicity, and left ventricular mass: the Northern Manhattan study. Hypertension 2004; 43: 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rider OJ, Francis JM, Ali MK, et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2009; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verdecchia P, Carini G, Circo A, et al. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38: 1829–1835. [DOI] [PubMed] [Google Scholar]
- 44. Jorgensen PG, Jensen MT, Biering-Sorensen T, et al. Burden of uncontrolled metabolic risk factors and left ventricular structure and function in patients with type 2 diabetes mellitus. J Am Heart Assoc 2018; 7: e008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011; 58: 1733–1740. [DOI] [PubMed] [Google Scholar]
- 46. Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000; 101: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 47. Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004; 93: 870–875. [DOI] [PubMed] [Google Scholar]
- 48. Yazici M, Ozdemir K, Gonen MS, et al. Is there any relationship between metabolic parameters and left ventricular functions in type 2 diabetic patients without evident heart disease? Echocardiography 2008; 25: 675–682. [DOI] [PubMed] [Google Scholar]
- 49. Seyfeli E, Duru M, Saglam H, et al. Association of left ventricular diastolic function abnormalities with aortic elastic properties in asymptomatic patients with type 2 diabetes mellitus. A tissue doppler echocardiographic study. Int J Clin Pract 2008; 62: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 50. Kadappu KK, Boyd A, Eshoo S, et al. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging 2012; 13: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 51. Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci 2004; 106: 53–60. [DOI] [PubMed] [Google Scholar]
- 52. Andersen NH, Poulsen SH, Eiskjaer H, et al. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci 2003; 105: 59–66. [DOI] [PubMed] [Google Scholar]
- 53. Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104: 1398–1401. [DOI] [PubMed] [Google Scholar]
- 54. Roos CJ, Scholte AJ, Kharagjitsingh AV, et al. Changes in multidirectional LV strain in asymptomatic patients with type 2 diabetes mellitus: a 2-year follow-up study. Eur Heart J Cardiovasc Imaging 2014; 15: 41–47. [DOI] [PubMed] [Google Scholar]
- 55. Liu JH, Chen Y, Yuen M, et al. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2016; 15: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jorgensen PG, Biering-Sorensen T, Mogelvang R, et al. Predictive value of echocardiography in type 2 diabetes. Eur Heart J Cardiovasc Imaging 2018: 1–7. [DOI] [PubMed] [Google Scholar]
- 57. Ceyhan K, Kadi H, Koc F, et al. Longitudinal left ventricular function in normotensive prediabetics: a tissue Doppler and strain/strain rate echocardiography study. J Am Soc Echocardiogr 2012; 25: 349–356. [DOI] [PubMed] [Google Scholar]
- 58. Stefanidis A, Bousboulas S, Kalafatis J, et al. Left ventricular anatomical and functional changes with ageing in type 2 diabetic adults. Eur J Echocardiogr 2009; 10: 647–653. [DOI] [PubMed] [Google Scholar]
- 59. Muranaka A, Yuda S, Tsuchihashi K, et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 2009; 26: 262–271. [DOI] [PubMed] [Google Scholar]
- 60. Enomoto M, Ishizu T, Seo Y, et al. Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. Circ J 2015; 79: 1749–155. [DOI] [PubMed] [Google Scholar]
- 61. Zoroufian A, Razmi T, Taghavi-Shavazi M, et al. Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography 2014; 31: 456–463. [DOI] [PubMed] [Google Scholar]
- 62. Ernande L, Bergerot C, Rietzschel ER, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 2011; 24: 1268–1275.e1. [DOI] [PubMed] [Google Scholar]
- 63. Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018; 19: 380–386. [DOI] [PubMed] [Google Scholar]
- 64. Gaggin HK, Januzzi JL., Jr. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta 2013; 1832: 2442–2450. [DOI] [PubMed] [Google Scholar]
- 65. Gormus U, Ozmen D, Ozmen B, et al. Serum N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) and homocysteine levels in type 2 diabetic patients with asymptomatic left ventricular diastolic dysfunction. Diabetes Res Clin Pract 2010; 87: 51–56. [DOI] [PubMed] [Google Scholar]
- 66. Ciftel S, Icagasioglu S, Yildiz G, et al. Association of left ventricular diastolic dysfunction with elevated NT-proBNP in type 2 diabetes mellitus patients with preserved ejection fraction: the supplemantary role of tissue doppler imaging parameters and NT-proBNP levels. Diabetes Res Clin Pract 2012; 96: 179–186. [DOI] [PubMed] [Google Scholar]
- 67. NICE. Chronic Heart Failure: management of chronic heart failure in adults in primary and secondary care. London, UK, 2010. [Google Scholar]
- 68. Ohkuma T, Jun M, Woodward M, et al. Cardiac stress and inflammatory markers as predictors of heart failure in patients with type 2 diabetes: the ADVANCE trial. Diabetes Care 2017; 40: 1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harmancey R, Taegtmeyer H. The complexities of diabetic cardiomyopathy: lessons from patients and animal models. Curr Diab Rep 2008; 8: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ernande L, Derumeaux G. Diabetic cardiomyopathy: myth or reality? Arch Cardiovasc Dis 2012; 105: 218–225. [DOI] [PubMed] [Google Scholar]
- 71. Brooks BA, Franjic B, Ban CR, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab 2008; 10: 739–746. [DOI] [PubMed] [Google Scholar]
- 72. Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008; 52: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 73. Ng AC, Delgado V, Bertini M, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010; 122: 2538–2544. [DOI] [PubMed] [Google Scholar]
- 74. Korosoglou G, Humpert PM, Ahrens J, et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging 2012; 35: 804–11. [DOI] [PubMed] [Google Scholar]
- 75. Wierzbowska-Drabik K, Hamala P, Kasprzak JD. Delayed longitudinal myocardial function recovery after dobutamine challenge as a novel presentation of myocardial dysfunction in type 2 diabetic patients without angiographic coronary artery disease. Eur Heart J Cardiovasc Imaging 2015; 16: 676–683. [DOI] [PubMed] [Google Scholar]
- 76. Zhang X, Wei X, Liang Y, Liu M, Li C, Tang H. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose: a three-dimensional speckle-tracking echocardiography-based study. J Am Soc Echocardiogr 2013; 26: 499–506. [DOI] [PubMed] [Google Scholar]
- 77. Nakai H, Takeuchi M, Nishikage T, et al. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr 2009; 10: 926–932. [DOI] [PubMed] [Google Scholar]
- 78. Scholte AJ, Nucifora G, Delgado V, et al. Subclinical left ventricular dysfunction and coronary atherosclerosis in asymptomatic patients with type 2 diabetes. Eur J Echocardiogr 2011; 12: 148–155. [DOI] [PubMed] [Google Scholar]
- 79. Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004; 66: 411–421. [DOI] [PubMed] [Google Scholar]
- 80. Maack C, Lehrke M, Backs J, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur Heart J 2018; 39: 4243–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins, Leukot Essent Fatty Acids 2004; 70: 309–319. [DOI] [PubMed] [Google Scholar]
- 82. Mazumder PK, O’Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004; 53: 2366–2374. [DOI] [PubMed] [Google Scholar]
- 83. Neubauer S. The failing heart — an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 84. Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003; 107: 3040–3046. [DOI] [PubMed] [Google Scholar]
- 85. Shivu GN, Phan TT, Abozguia K, et al. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation 2010; 121: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 86. Levelt E, Rodgers CT, Clarke WT, et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 2016; 37: 3461–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997; 96: 2190–2196. [DOI] [PubMed] [Google Scholar]
- 88. Garvey WT, Maianu L, Huecksteadt TP, et al. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest 1991; 87: 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Robertson RP, Harmon J, Tran PO, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004; 53(Suppl. 1): S119–S124. [DOI] [PubMed] [Google Scholar]
- 90. Zhang J, Duncker DJ, Ya X, et al. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation 1995; 92: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 91. Brindley DN, Kok BP, Kienesberger PC, et al. Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. Am J Physiol Endocrinol Metab 2010; 298: E897–E908. [DOI] [PubMed] [Google Scholar]
- 92. Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004; 18: 1692–1700. [DOI] [PubMed] [Google Scholar]
- 93. Costantino S, Akhmedov A, Melina G, et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur Heart J 2019: 1–13. [DOI] [PubMed] [Google Scholar]
- 94. Kuwabara Y, Horie T, Baba O, et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res 2015; 116: 279–288. [DOI] [PubMed] [Google Scholar]
- 95. Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 2007; 55: 230–236. [DOI] [PubMed] [Google Scholar]
- 96. McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007; 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 97. Steadman CD, Jerosch-Herold M, Grundy B, et al. Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis. JACC Cardiovasc Imaging 2012; 5: 182–189. [DOI] [PubMed] [Google Scholar]
- 98. Kalkan GY, Gur M, Sahin DY, et al. Coronary flow reserve and myocardial performance index in newly diagnosed diabetic patients. Echocardiography 2013; 30: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 99. Jellis C, Wright J, Kennedy D, et al. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circulation Cardiovasc Imaging 2011; 4: 693–702. [DOI] [PubMed] [Google Scholar]
- 100. Ugander M, Oki AJ, Hsu LY, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 2012; 33: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ng AC, Auger D, Delgado V, et al. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging 2012; 5: 51–59. [DOI] [PubMed] [Google Scholar]
- 102. Rao AD, Shah RV, Garg R, et al. Aldosterone and myocardial extracellular matrix expansion in type 2 diabetes mellitus. Am J Cardiol 2013; 112: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wong TC, Piehler KM, Kang IA, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014; 35: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Catalano M, Scandale G, Carzaniga G, et al. Increased aortic stiffness and related factors in patients with peripheral arterial disease. J Clin Hypertens (Greenwich) 2013; 15: 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Van der Meer RW, Diamant M, Westenberg JJ, et al. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson 2007; 9: 645–651. [DOI] [PubMed] [Google Scholar]
- 106. Stacey RB, Bertoni AG, Eng J, et al. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 2010; 55: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 108. Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99: 2434–2439. [DOI] [PubMed] [Google Scholar]
- 109. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002; 106: 2085–2090. [DOI] [PubMed] [Google Scholar]
- 111. Cardoso CR, Ferreira MT, Leite NC, et al. Prognostic impact of aortic stiffness in high-risk type 2 diabetic patients: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care 2013; 36: 3772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ferreira MT, Leite NC, Cardoso CR, et al. Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care 2015; 38: 897–904. [DOI] [PubMed] [Google Scholar]
- 113. Bibra H, Siegmund T, Ceriello A, et al. Optimized postprandial glucose control is associated with improved cardiac/vascular function - comparison of three insulin regimens in well-controlled type 2 diabetes. Horm Metab Res 2009; 41: 109–115. [DOI] [PubMed] [Google Scholar]
- 114. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kiyici S, Ersoy C, Kaderli A, et al. Effect of rosiglitazone, metformin and medical nutrition treatment on arterial stiffness, serum MMP-9 and MCP-1 levels in drug naive type 2 diabetic patients. Diabetes Res Clin Pract 2009; 86: 44–50. [DOI] [PubMed] [Google Scholar]
- 116. Nakamura T, Matsuda T, Kawagoe Y, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism 2004; 53: 1382–1386. [DOI] [PubMed] [Google Scholar]
- 117. Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart 2004; 90: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gulsin GS, Swarbrick DJ, Hunt WH, et al. Relation of aortic stiffness to left ventricular remodeling in younger adults with type 2 diabetes. Diabetes 2018; 67: 1395–1400. [DOI] [PubMed] [Google Scholar]
- 119. Ripley DP, Negrou K, Oliver JJ, et al. Aortic remodelling following the treatment and regression of hypertensive left ventricular hypertrophy: a cardiovascular magnetic resonance study. Clin Exp Hypertens 2015; 37: 308–316. [DOI] [PubMed] [Google Scholar]
- 120. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 121. Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 122. Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 124. Control G, Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52: 2288–2298. [DOI] [PubMed] [Google Scholar]
- 125. Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011; 162: 938–948.e2. [DOI] [PubMed] [Google Scholar]
- 126. Costantino S, Paneni F, Mitchell K, et al. Hyperglycaemia-induced epigenetic changes drive persistent cardiac dysfunction via the adaptor p66(Shc). Int J Cardiol 2018; 268: 179–186. [DOI] [PubMed] [Google Scholar]
- 127. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 129. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metabol 2002; 87: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 131. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 132. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 133. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab 2018; 21: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nordisk N. Oral semaglutide demonstrates favourable cardiovascular safety profile and significant reduction in cardiovascular death and all-cause mortality in people with type 2 diabetes in the PIONEER 6 trial. 2018. Published on tctmd. URL: https://www.tctmd.com/news/pioneer-6-oral-semaglutide-passes-test-cardiovascular-outcomes-study
- 136. Nordisk N. Novo Nordisk reports up to 13.8% weight loss in people with obesity receiving semaglutide in phase 2 trial. 2017. Press release, published on Globenewswire website. URL: https://www.globenewswire.com/news-release/2017/06/23/1028468/0/en/Novo-Nordisk-reports-up-to-13-8-weight-loss-in-people-with-obesity-receiving-semaglutide-in-phase-2-trial.html
- 137. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018; 392: 2180–2193. [DOI] [PubMed] [Google Scholar]
- 138. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 139. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and Renal Events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 140. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 141. Fitchett D, Butler J, Van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J 2018; 39: 363–370. [DOI] [PubMed] [Google Scholar]
- 142. Sattar N, Petrie MC, Zinman B, et al. Novel diabetes drugs and the cardiovascular specialist. J Am Coll Cardiol 2017; 69: 2646–2656. [DOI] [PubMed] [Google Scholar]
- 143. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 144. Kario K, Okada K, Kato M, et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation 2018: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016; 39: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 146. Sowers JR. Recommendations for special populations: diabetes mellitus and the metabolic syndrome. Am J Hypertens 2003; 16: 41S–45S. [DOI] [PubMed] [Google Scholar]
- 147. Grossman A, Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc Diabetol 2017; 16: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Excellence NIfHaC. NICE guideline [NG28] Type 2 diabetes in adults: management. London: NICE, 2015. [Google Scholar]
- 149. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 150. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016; 32: 569–588. [DOI] [PubMed] [Google Scholar]
- 151. Group SR, Wright JT, Jr., Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003; 42: 1206–1252. [DOI] [PubMed] [Google Scholar]
- 153. Group AS, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. ACCORDION. ACCORDION: long-term follow-up of ACCORD patients. American Heart Association (AHA) 2015 Scientific Sessions. Orlando, Florida, 2015. [Google Scholar]
- 155. Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010; 304: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Navar AM, Gallup DS, Lokhnygina Y, et al. Hypertension control in adults with diabetes mellitus and recurrent cardiovascular events: global results from the trial evaluating cardiovascular outcomes with sitagliptin. Hypertension 2017; 70: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville gastric bypass. Ann Surg 1987; 206: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Meijer RI, Van Wagensveld BA, Siegert CE, et al. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: a systematic review. Arch Surg 2011; 146: 744–750. [DOI] [PubMed] [Google Scholar]
- 159. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018; 391: 541–551. [DOI] [PubMed] [Google Scholar]
- 160. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol 2009; 54: 718–726. [DOI] [PubMed] [Google Scholar]
- 161. Rider OJ, Francis JM, Tyler D, et al. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging 2013; 29: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 162. Utz W, Engeli S, Haufe S, et al. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int J Cardiol 2013; 167: 905–909. [DOI] [PubMed] [Google Scholar]
- 163. Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008; 52: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 164. Jonker JT, Djaberi R, van Schinkel LD, et al. Very-low-calorie diet increases myocardial triglyceride content and decreases diastolic left ventricular function in type 2 diabetes with cardiac complications. Diabetes Care 2014; 37: e1–e2. [DOI] [PubMed] [Google Scholar]
- 165. Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med 2018; 379: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 166. Bohula EA, Scirica BM, Inzucchi SE, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet 2018; 392: 2269–2279. [DOI] [PubMed] [Google Scholar]
- 167. Wei M, Gibbons LW, Kampert JB, et al. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000; 132: 605–611. [DOI] [PubMed] [Google Scholar]
- 168. Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med 2001; 134: 96–105. [DOI] [PubMed] [Google Scholar]
- 169. Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346: 793–801. [DOI] [PubMed] [Google Scholar]
- 170. Nylen ES, Kokkinos P, Myers J, et al. Prognostic effect of exercise capacity on mortality in older adults with diabetes mellitus. J Am Geriatr Soc 2010; 58: 1850–1854. [DOI] [PubMed] [Google Scholar]
- 171. Poirier P, Garneau C, Bogaty P, et al. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol 2000; 85: 473–477. [DOI] [PubMed] [Google Scholar]
- 172. Kemps H, Krankel N, Dorr M, et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A position paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2019: 2047487318820420. [DOI] [PubMed] [Google Scholar]
- 173. Boule NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 174. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011; 305: 1790–1799. [DOI] [PubMed] [Google Scholar]
- 175. Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes-Metab Res 2004; 20: 383–393. [DOI] [PubMed] [Google Scholar]
- 176. Telford RD. Low physical activity and obesity: causes of chronic disease or simply predictors? Med Sci Sports Exerc 2007; 39: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 177. Schuster I, Vinet A, Karpoff L, et al. Diastolic dysfunction and intraventricular dyssynchrony are restored by low intensity exercise training in obese men. Obesity 2012; 20: 134–140. [DOI] [PubMed] [Google Scholar]
- 178. Sandri M, Kozarez I, Adams V, et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: The Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) diastolic dysfunction study. Eur Heart J 2012; 33: 1758–1768. [DOI] [PubMed] [Google Scholar]
- 179. Brassard P, Legault S, Garneau C, et al. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc 2007; 39: 1896–1901. [DOI] [PubMed] [Google Scholar]
- 180. Hordern MD, Coombes JS, Cooney LM, et al. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart 2009; 95: 1343–1349. [DOI] [PubMed] [Google Scholar]
- 181. Smart N, Haluska B, Jeffriess L, et al. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J 2007; 153: 530–536. [DOI] [PubMed] [Google Scholar]
- 182. Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]