Short abstract
Objective
This systematic review aimed to assess the effectiveness, feasibility and acceptability of mobile health (mHealth) technology (including wearable activity monitors and smartphone applications) for promoting physical activity (PA) and reducing sedentary behaviour (SB) in workplace settings.
Methods
Systematic searches were conducted in seven electronic databases (MEDLINE, SPORTDiscus, Scopus, EMBASE, PsycINFO, Web of Science and the Cochrane library). Studies were included if mHealth was a major intervention component, PA/SB was a primary outcome, and participants were recruited and/or the intervention was delivered in the workplace. Study quality was assessed using the Effective Public Health Practice Project (EPHPP) tool. Interventions were coded for behaviour change techniques (BCTs) using the Coventry, Aberdeen and London – Refined (CALO-RE) taxonomy.
Results
Twenty-five experimental and quasi-experimental studies were included. Studies were highly heterogeneous and only one was rated as ‘strong’ methodological quality. Common BCTs included self-monitoring, feedback, goal-setting and social comparison. A total of 14/25 (56%) studies reported a significant increase in PA, and 4/10 (40%) reported a significant reduction in sedentary time; 11/16 (69%) studies reported a significant impact on secondary outcomes including reductions in weight, systolic blood pressure and total cholesterol. While overall acceptability was high, a large decline in technology use and engagement was observed over time.
Conclusions
While methodological quality was generally weak, there is reasonable evidence for mHealth in a workplace context as a feasible, acceptable and effective tool to promote PA. The impact in the longer term and on SB is less clear. Higher quality, mixed methods studies are needed to explore the reasons for decline in engagement with time and the longer-term potential of mHealth in workplace interventions.
Protocol registration: The review protocol was registered with PROSPERO: CRD42017058856
Keywords: Systematic review, mobile health, physical activity, sedentary behaviour, workplace, occupational health, behaviour change
Introduction
Physical inactivity is considered one of the biggest public health problems of the 21st century.1 Failing to meet the recommended guidelines is associated with an increased risk of morbidity due to cardiovascular disease, cancer and metabolic syndrome and general mortality.2–6 There is now also substantial evidence that sedentary behaviour (SB) is an independent predictor of poor health and mortality.7–9
Interventions to increase physical activity (PA) levels and reduce SB are clearly vital. The workplace is viewed as an important setting for health promotion and disease prevention.10 Around half of weekday sitting time is work-related,11,12 and up to 71% of working hours in office workers are spent sedentary.13 Occupational sedentary time is predicted to further increase in future with rises in automation and information technology use.14 Promotion of PA in the workplace has many potential benefits, including improved health and wellbeing of employees and economic benefits for employers.15
Mobile health (mHealth) technology has rapidly gained popularity in the general population. mHealth technology includes wearable PA monitors or trackers and smartphone applications (apps) designed to help people to manage their own health and wellbeing. The potential value of mHealth in health promotion lies in its widespread appeal, accessibility and ability to reach large populations at a low cost.16 It also offers the potential for tailoring of interventions to the needs of individuals or specific groups.
Studies have investigated the use of mHealth to promote PA in a range of settings, including the workplace.16 Whilst the results of clinical and general population studies suggest that mHealth may be a feasible and cost-effective way to promote PA,17 the findings of existing reviews have been inconclusive. Some reviews have reported nonsignificant effects of mobile technology on PA levels,18 and where beneficial effects are reported, effect sizes have generally been small,17,19–21 Additional limitations of previous reviews are the inclusion of studies where mHealth devices were used as a data collection tool rather than as an intervention in their own right,20,22 and a lack of a comprehensive description of interventions and study procedures.19 Furthermore, with two recent exceptions,17,23 few reviews of mHealth interventions have assessed both PA and SB outcomes.
Identification of behaviour change techniques (BCTs) using standardised taxonomies is important for recognition of effective and acceptable components, to allow replication and comparison of interventions, and to facilitate further development and testing of theories.24 There is also evidence that including established BCTs is associated with greater intervention effectiveness.25 Despite this, previous reviews have concluded that many mHealth interventions lack an explicit theoretical basis,19,20 and it remains unclear which components are most effective and accepted.16 Identification or coding of included BCTs, and identifying the theoretical basis of existing studies are therefore important gaps to address.
As mHealth is such a rapidly progressing field due to advances in technology, studies have increased exponentially in a short space of time. Early reviews predominantly comprised studies of text messaging (SMS) interventions, but the emergence of new technologies (e.g. tablets, commercial wearable activity monitors, and ‘exergaming’) means the evidence should be frequently reviewed in order to accurately reflect the current status. Furthermore, the use and effectiveness of mHealth interventions in specific population groups remains unclear.23 It is important to consider setting or context in the evaluation of mHealth interventions as due to their complex nature, various components may produce different outcomes for different individuals in different settings.26 Workplace mHealth interventions may differ from general interventions in terms of both intervention content and timing of effectiveness.27 To the authors’ knowledge, there has been no previous systematic review of mHealth technology for promoting PA and reducing SB in workplace settings. A recent review of general digital health interventions in the workplace concluded that the evaluation of smartphone apps in this context is an important ‘next step’ for future research.28
Employee populations potentially have much to gain from mHealth interventions for PA and SB, yet little is known about the impact of this technology in a workplace context. Feasibility and acceptability are important aspects to consider but remain understudied and underreported.26 This review therefore aims to provide a comprehensive synthesis of current evidence in relation to the effectiveness, feasibility and acceptability of mHealth interventions in the promotion of PA and reduction of SB in the workplace. This includes a description of intervention content in terms of common BCTs using an established behaviour change taxonomy, and a consideration of subgroup differences and the wider impact of interventions on health and related outcomes.
Methods
Protocol and registration
The review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Additional file 1).29 The protocol was registered with the University of York Centre for Reviews and Dissemination PROSPERO database (CRD42017058856).
Data sources and search strategy
Searches were conducted in the following databases: MEDLINE, SPORTDiscus, Scopus, EMBASE, PsycINFO, Web of Science and the Cochrane library (including the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews and Effect (DARE) and Health Technology Assessment (HTA)). Studies with a publication date between January 2007 (around the time smartphones were introduced) and February 2017 were included, with no language restriction. Full updated searches were later conducted to include studies to the end of February 2018, then to the end of December 2018. A master search strategy was developed (Additional file 2) and revised for each database (see Additional file 3 for example search strategy for MEDLINE). Both free text searching and controlled vocabulary were used, including key terms such as ‘mHealth’, ‘smartphone’, ‘application or app’, ‘activity monitor or tracker’, ‘physical activity’, ‘sedentary’, ‘workplace’ and ‘occupation’.
In addition, relevant studies were identified via forward and backward citation searching, including reference lists of included articles and published systematic reviews. A search of grey literature, using the same key terms and for the same time period, included dissertations and theses (ProQuest Dissertations and Theses Global), ‘mHealth Evidence’, and the ‘Fitabase’ research library (studies using the Fitbit® activity tracker).
Inclusion criteria and study selection
Both experimental (e.g. randomised controlled trials, RCTs) and quasi-experimental (e.g. pre-post uncontrolled trials) study designs were included. Studies were included if they: 1) used mHealth (including mobile phone, smartphone apps, personal digital assistants (PDAs), tablets, wearable activity monitors/trackers) as a major component of the intervention, as stated by the authors or apparent from the context of the paper, 2) included a control or comparison group (experimental studies) or pre- and post- exposure data (quasi-experimental and observational studies), 3) recruited participants in the workplace and/or the intervention was delivered in the workplace, and 4) included any measure of PA and/or SB (self-reported or objective) as a primary quantitative outcome.
Pilot and feasibility trials were included if they met the inclusion criteria. Interventions could be either standalone mHealth or multi-component (e.g. facilitated with telephone counselling). The rationale for including multi-component interventions was that many digital workplace interventions for PA and SB, as delivered in the real world, are part of multi-component health promotion programmes,30 and we wanted to maximise the number of studies for inclusion and scope of the review. Interventions could be designed as an exclusive workplace or a wider lifestyle intervention (i.e. where the intervention was initiated or delivered in the workplace but also included activity outside of working hours). Studies using smartphone apps for PA or SB alone or with other behaviours (e.g. diet, weight) were included.
Exclusion criteria were studies reporting web-only interventions or traditional pedometers (i.e. not able to transmit data to a consumer interface), as these fall outside the realm of mHealth technology. Interventions involving basic text messaging (SMS) alone were excluded as these have been more extensively reviewed in the past,19 and are felt to be a different type of intervention than more advanced mHealth tools such as multimedia smartphone apps and activity monitors. Studies using mobile devices for data collection only, and studies with clinical or student populations (i.e. school, college, university) were excluded. Studies reporting only qualitative data, non-human studies, review articles and editorials, and reports published only as conference abstracts or proceedings were also excluded.
All search results were imported into EndNote X7 bibliographic software (Thompson Reuters, San Francisco, CA, USA) and duplicates removed. Two independent reviewers (SAB and AJW) screened papers for eligibility by title and abstract followed by full text screening. Disagreements were resolved through discussion and consulting a third reviewer (KM).
Data extraction
Standardised data extraction forms were completed by one reviewer (SAB) and verified by a second reviewer (AJW). Any disagreements were resolved through discussion and consulting a third reviewer (KM). The following data were captured: author; year; country; setting or workplace; study design; participants (number and characteristics); intervention description (type of mHealth technology or tool, intervention components including whether standalone mHealth or multi-component, theoretical basis, key motivational strategies or BCTs, duration and frequency); control or comparator; study aim (i.e. increase PA and/or reduce SB); primary PA/SB outcome (including method of assessment); secondary outcomes; duration of follow-up; main study results including any relevant subgroup findings; details of acceptability, engagement and attrition. Key within- and between-group quantitative findings were summarised for each study; significant effects were P < 0.05.
Study quality assessment
Included studies were appraised using the Effective Public Health Practice Project (EPHPP) quality assessment tool for quantitative studies.31 This tool was developed for health promotion interventions and was selected for its application to a wide range of study designs (e.g. RCTs, cohort trials and case-control studies). The tool has demonstrated content and construct validity and both intra- and inter-rater reliability.31,32
The EPHPP quality assessment tool assesses six domains: 1) selection bias; 2) study design; 3) confounders; 4) blinding; 5) data collection methods; and 6) withdrawals and dropouts. Each study was given a rating of ‘strong’, ‘moderate’ or ‘weak’ for each domain; based on this, a global rating was then assigned for each study – ‘strong’ (no weak ratings), ‘moderate’ (one weak rating) or ‘weak’ (two or more weak ratings). Intervention integrity (proportion of participants receiving the intended intervention), fidelity of delivery (whether studies measured consistency of intervention) and appropriateness of analysis methods were also separately considered.
Two independent raters (SAB and AJW) used the tool to assess risk of bias and study quality. KM was consulted to resolve any uncertainties.
Coding of BCTs
Interventions in included studies were coded for BCTs using definitions provided in the ‘Coventry, Aberdeen and London – Refined’ taxonomy for PA and healthy eating behaviours.33 This 40-item evidence-based taxonomy was selected as it was specifically designed for PA and healthy eating behaviours, and is widely used including to characterise smartphone apps for PA and wearable activity monitors.34,35 Content was coded for each intervention as a whole (i.e. mHealth and any additional components) using information from relevant results and protocol papers. Coding was completed by two independent reviewers (SAB and AJW) who were trained in Michie et al.’s Behaviour Change Taxonomy v1,36 and consensus was reached through discussion.
Results
Study selection
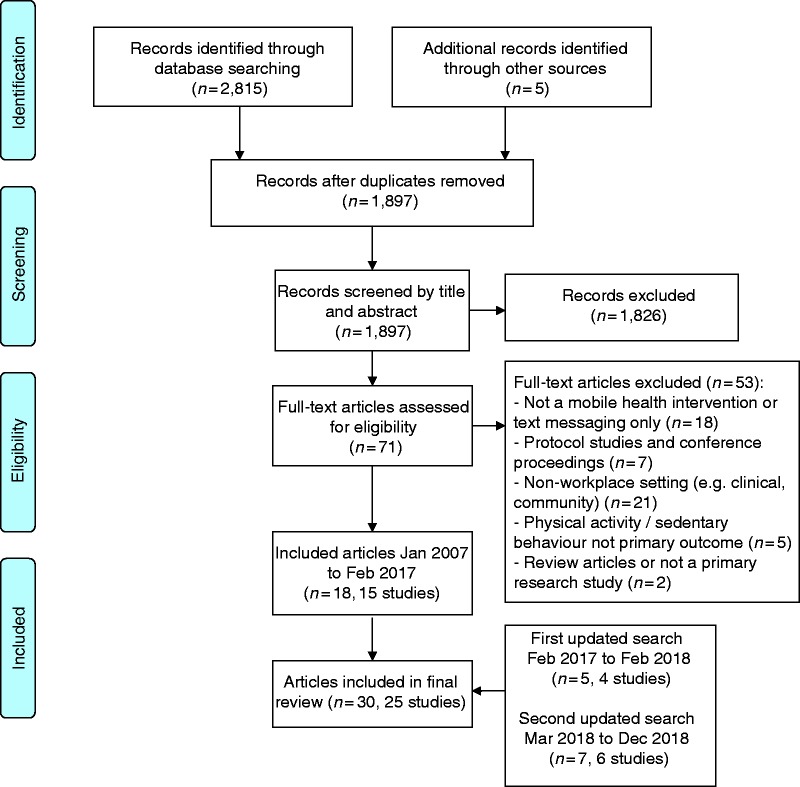
A flow diagram of the study selection process is shown in Figure 1. A total of 2820 publications were identified in the initial searches (2815 from databases and 5 from other sources). After removal of duplicates, 1897 publication titles and abstracts were screened. The full text was obtained for 71 publications; of these, 18 publications describing 15 studies met the criteria for inclusion.37–54 An updated search to February 2018 found an additional five publications describing four studies,55–59 and a second updated search to December 2018 found an additional seven publications for six studies,60–66 resulting in a total of 30 publications (25 studies) for inclusion in the review.
Figure 1.
Flow diagram of study selection process.
Study and intervention characteristics
The characteristics of the 25 included studies are summarised in Table 1. Eleven studies were conducted in the USA,42–46,50,53,57,59,60,62 five in Australia,37,38,49,55,56,61,66 two in Canada,58,63 two in the Netherlands,52,54 one in Belgium,64,65 Singapore,39,40 Finland,47,48 Norway,51 and one in multiple countries.41 Workplace settings included academic and academic medical institutions,42–44,50,53,57,59,60,62 healthcare,63,66 health insurance,45,49 wellbeing improvement,46 property and infrastructure,37,38 pension insurance,47,48 financial services,61 road maintenance,51 and haulage.55,56 Six studies targeted multiple organisations.39–41,52,54,58,64,65 Both public and private sector organisations were represented.
Table 1.
Characteristics of included studies.
| Author Year Country |
Setting/workplace | Study design | Participants | Type of mHealth technology/toola | Intervention | Control/comparison group(s) | Aim | Primary PA/SB outcome(s) (OB or SR) | Secondary outcome(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Brakenridge et al. 2016 (protocol)37 Brakenridge et al. 2016 (results)38 Australia |
International property and infrastructure group (Lendlease) | Cluster RCT |
n = 153 54% M, 46% F Age IG: 37.6±7.8 CG: 40.0±8.0 Office workers (at least 0.5 FTE) |
Wearable activity monitor and smartphone app | Waist-worn ‘LUMOback’ activity monitor (LUMO Bodytech, USA) and associated smartphone app with organisational support. | Organisational support only – e.g. manager support, e-mails and educational materials. | SB | Average time per day spent sitting (work hours and overall) (OB, activPAL3™ accelerometer) |
Average time per day spent in prolonged sitting bouts (30 min or more), standing and stepping Daily steps Average time period between sitting bouts Job performance, job control and work satisfactionStress, physical and mental health-related QoL Activity monitor usage |
12 months |
| Finkelstein et al. 2015 (protocol and baseline data)39 Finkelstein et al. 2016 (results)40 Singapore |
13 organisations (various industries and government sectors) | RCT (4-arm) |
n = 800 46% M, 54% F Age IG1: 35.4±8.3 IG2: 35.5±8.6 IG3: 35.5±8.4 CG: 35.6±8.6 Mostly desk-based employees (full-time) |
Wearable activity monitor (and website) | Waist-worn Fitbit Zip activity monitor (Fitbit, USA) and associated website.Monetary incentives: IG1 = Fitbit only IG2 = Fitbit and charity donation IG3 = Fitbit and cash Educational booklets on PA. |
No activity monitor or incentives. Educational booklets on PA only. |
PA | MVPA bout minutes per week (OB, ActiGraph™ GT-3x+ accelerometer) |
Mean daily step count % of participants meeting 70,000 weekly step goal Weight Systolic BP Cardiorespiratory fitness Quality of life Weekly step count Sedentary, light, moderate and vigorous PA (min/week) Participants meeting 150 min per week moderate PA Participants meeting 10,000 daily step target |
12 months |
| Ganesan et al. 201641 64 countries (majority of participants from India (90.2%), Australia (5%), New Zealand (1.1%) and Singapore (0.6%)) |
481 employers (private and public sector organisations) in 1481 cities | Prospective cohort (pre- and post- uncontrolled) |
n = 69,219 76% M, 24% F Age 36.0±8.4 Adult employees |
Smartphone app | Non-interactive pedometer and ‘Stepathlon’ mobile app (also available as website). | No control or comparison group | PA + SB | Daily steps (SR, pedometer data entered by participants) |
Number of exercise days/week Exercise duration (<30 or ≥30 min/day) Sitting duration (hours/day) Weight in kilograms |
100 days (approx.) |
| Gilson et al. 2016 (baseline data and smartphone use)55 Gilson et al. 2017 (results)56 Australia |
Two large Australian haulage companies | Prospective cohort (pre- and post- uncontrolled feasibility study) |
n = 44 100% M, 0% F Age 47.0±10.1 Truck drivers |
Wearable activity monitor and smartphone app | Wrist-worn Jawbone UP activity monitor (Jawbone, USA) used with associated smartphone app. Monetary incentives (vouchers for attaining step goals and logging diet) |
No control or comparison group | PA + SB | Proportions of work time and non-work time spent physically active, sedentary and stationary + (i.e. sitting with upper limb movement or standing) (OB – GENEActiv™ wrist accelerometer) |
Workday diet (fruit, vegetable, saturated fat and sugar intake) Engagement with the intervention Qualitative outcomes (interviews) –driver and depot manager experiences; perceived impact of the intervention; barriers to PA |
28 weeks (approx.) |
| Gremaud et al. 201860 USA | Academic organisation (university) | RCT |
n = 146 25% M, 75% F Age IG: 40.6±11.7 CG: 40.3±11.1 Sedentary office workers (full-time) |
Wearable activity monitor and smartphone app (web-based) | Waist-worn Fitbit Zip activity monitor (Fitbit, USA) used with ‘MapTrek’ app for gamified walking. | Activity monitor only | PA + SB | Daily steps Daily active minutes (minutes with ≥100 steps/min) (OB – data from Fitbit) |
Bouts of sedentary behaviour (consecutive minutes with 0 steps) | 10 weeks |
| Jones 201642 USA | Academic medical centre (Wake Forest Baptist Health) | Prospective cluster trial (with asynchronous control group) |
n = 47 18% M, 82% F Age Overall mean = 50.8, range 25 to 74 years (SD not reported) Sedentary employees |
Wearable activity monitor (and computer software) | Clip-on Fitbit One activity monitor (Fitbit, USA) and associated software, with wellness education. IG1 = Fitbit only IG2 = Fitbit and shared active workstations |
Usual treatment (blinded activity monitors for data collection) | PA + SB | Daily steps Daily sedentary time BMI (OB – steps and sedentary time from Fitbit. BMI assessed objectively) |
Life satisfaction Anxiety (state and trait) Health-related quality of life Self-reported sleep patterns |
6 months |
| Koyle 201343 USA | Academic medical centre (University of Utah Health Care) | RCT |
n = 73 0% M, 100% F Age 46.5±7.6 Physically inactive employees (<150 min exercise per week) |
Smartphone app with integrated accelerometer (and motivational text messages) | ‘Adidas miCoach’ smartphone app to track walking exercise. Educational materials on PA. Tailored motivational text messages. |
Smartphone app and educational materials (same as intervention group). No motivational text messages. | PA | Walking distance and duration (OB – smartphone app-integrated accelerometer for collection of PA data) |
Walking for exercise self-efficacy beliefs Likeliness of participating in other forms of PA beyond walking Height and weight (BMI) Resting pulse rate Systolic BP Qualitative experiences of the intervention (survey) |
6 weeks |
| Losina et al. 201757 USA | Academic medical centre (Brigham and Women’s Hospital, Boston, Massachusetts) | Prospective cohort (pre- and post- uncontrolled feasibility study) |
n = 292 17% M, 83% F Age 38±11 Sedentary, non-clinician hospital employees |
Wearable activity monitor (linked with websites) | Wrist-worn Fitbit Flex activity monitor (Fitbit, USA) used with Fitbit website and study website for monitoring PA and progress. Monetary incentives (individual and team) for meeting PA goals. |
No control or comparison group | PA | Average weekly minutes of MVPA Proportion of participants meeting weekly MVPA goals and CDC PA guidelines (OB – step data from Fitbit converted to weekly minutes of MVPA) |
Fitbit wear adherence (number of weeks wearing Fitbit for ≥10 h/day and ≥4 days/week) Participant satisfaction with programme |
26 weeks (including two pre-intervention weeks) |
| Neil-Sztramko et al. 201758 Canada | Multiple workplaces in Greater Vancouver (nursing, emergency services, casinos and airport) | Prospective cohort (pre- and post- uncontrolled feasibility study) |
n = 20 0% M, 100% F Age 42.2±8.6 Female shift workers |
Wearable activity monitor and smartphone app (or website) | Wrist-worn Fitbit Flex activity monitor (Fitbit, USA) used with Fitbit app and/or website. Distance-based behavioural counselling (telephone/online) |
No control or comparison group | PA | MVPA (total min/week and min/week bouts ≥10 mins) (OB - ActiGraph™ GT-3x+ accelerometer) |
Daily steps Sedentary time (min/week bouts ≥10 mins) Self-reported PA and sedentary timeBody weight and BMI Physical and mental health-related QoL Sleep quantity and quality Feasibility outcomes: demand (reach and recruitment), implementation (delivery and resources) and acceptability (attrition and adherence to intervention, participant satisfaction). |
12 weeks |
| Olsen et al. 201861 Australia | Financial services organisation (Brisbane) | Prospective cohort (pre- and post- uncontrolled pilot) |
n = 49 31% M, 69% Fb Age 39.5±8.7 Flexible workers (e.g. work from home 1 day/week) |
Wearable activity monitor and smartphone app | Wrist-worn Jawbone activity monitor (Jawbone, USA) used with associated app. Group-based action planning session. Weekly e-mail reminders and resources. Healthy living seminar |
No control or comparison group | SB | Sitting time (including overall and occupational, min/day) (OB - ActiGraph™ GT-3x+ accelerometer, also self-reported sitting time assessed using adapted version of Workforce Sitting Questionnaire) |
Light PA and MVPA (min/day, accelerometer-assessed) Self-reported PA (min/week, assessed using adapted version of Active Australia survey) Acceptability of the intervention (survey-assessed) |
6 weeks |
| Patel et al. 201644 (study 1) USA | Academic organisation (University of Pennsylvania) | RCT (4-arm) |
n = 281 (279 completed baseline assessment) 22% M, 78% F Age IG1: 37.1±10.9 IG2: 40.3±11.2 IG3: 41.9±11.6 CG: 39.4±12.2 Overweight and obese employees (BMI ≥27 kg/m2) |
Smartphone app with integrated accelerometer | ‘Moves’ smartphone app (Proto Geo Oy, Finland) for step tracking. Daily feedback on steps. Monetary incentives: IG1 = gain incentive IG2 = lottery incentive IG3 = loss incentive |
Smartphone app and daily feedback (as intervention group). No financial incentives. | PA | Proportion of participant-days 7000 step goal achieved during intervention (OB – smartphone app-integrated accelerometer) |
Proportion of participant-days 7000 step goal achieved during follow-up Daily steps – intervention and follow-up |
26 weeks |
| Patel et al. 201645 (study 2) USA | Health insurance organisation (Independence Blue Cross) | RCT (4-arm) |
n = 304 23% M, 77% F Age IG1: 39.3±10.2 IG2: 38.7±10.2 IG3:41.2±10.8 CG: 43.2±10.0 Mostly sedentary employees |
Smartphone app with integrated accelerometer | ‘Moves’ smartphone app (Proto Geo Oy, Finland) for step tracking. Daily feedback on steps. Monetary incentives: IG1 = individual IG2 = team IG3 = combined |
Smartphone app and daily feedback (as intervention group). No financial incentives. | PA | Proportion of participant-days 7000 step goal achieved during intervention (OB – smartphone app-integrated accelerometer) |
Proportion of participant-days 7000 step goal achieved during follow-up Daily steps – intervention and follow-up |
26 weeks |
| Patel et al. 201862 USA | Academic organisation (University of Pennsylvania) | RCT (4 arm) |
n = 209 23% M, 77% F Age IG1: 41.2±11.1 IG2: 40.6±10.5 IG3: 42.9±10.3 CG: 40.0±11.0 Overweight and obese employees (BMI ≥27 kg/m2) |
Smartphone app with integrated accelerometer | ‘Moves’ smartphone app (Proto Geo Oy, Finland) for step tracking. Daily feedback on steps. Monetary incentives: IG1 = higher frequency, smaller reward lottery IG2 = jackpot lottery IG3 = combined lottery |
Smartphone app and daily feedback (as intervention group). No financial incentives. | PA | Proportion of participant-days 7000 step goal achieved during intervention (OB – smartphone app-integrated accelerometer) |
Proportion of participant-days 7000 step goal achieved during follow-up Daily steps – intervention and follow-up |
26 weeks |
| Poirier et al. 201646 USA | Wellbeing improvement company (Healthways Inc) | RCT |
n = 265 34% M, 66% F Age IG: 40.3±11.4 CG: 39.6±12.0 Headquarter-based employees |
Wearable activity monitor (linked with website, and optional text messages) | Hip- or shoe-worn Pebble+ activity monitor (Fitlinxx Inc, USA) used with ‘Walkadoo’ internet-based program. Electronic messaging. | One week of blinded activity monitor wear, then instructed to maintain their usual activity routine. | PA | Daily steps (OB – activity monitor and website) |
Proportion of participants increasing steps by 1000/day Engagement with intervention – wear time, e-mail opening and website visits |
6 weeks |
| Reijonsaari et al. 2009 (protocol)47 Reijonsaari et al. 2012 (results)48 Finland |
Insurance company | RCT |
n = 544 (521 included in analysis) 36% M, 64% F Age IG: 43±10.0 CG: 44±10.0 Mainly clerical employees (working ≥8 h per week) |
Wearable activity monitor (linked with website) | Belt-worn ‘AM 200’ activity monitor/ accelerometer (PAM BV, Netherlands) used with associated website. Educational materials on PA. Written results of fitness tests. Distance counselling (telephone/online) |
Educational materials on PA. Written results of fitness tests. | PA | Weekly MET-minutes of total activity Work productivity Sickness absence (SR – MET-minutes from IPAQ, productivity from QQ instrument but objective sickness absence data) |
Body weight (kg) Waist circumference (cm) Body fat percentage Systolic and diastolic BP (mmHg) Aerobic fitness (maximal oxygen uptake, VO2 max, ml/kg/min) |
12 months |
| Reed et al. 201863 Canada | Tertiary care cardiovascular institute (University of Ottawa Heart Institute) | Parallel-group randomised trial (3-arm) |
n = 76 3% M, 97% F Age 46.3±10.9 Nurses |
Wearable activity monitor (linked with website) | Ankle-worn Tractivity® activity monitor (Tractivity®, Vancouver, BC) linked with website for monitoring PA and taking part in challenges: IG1 = individual challenge IG2 = friend challenge IG3 = team challenge |
No control or comparison group | PA | MVPA (weekly minutes in bouts ≥10 mins) Daily steps (OB – data from Tractivity® activity monitor) |
Body mass (kg) BMI Waist circumference Body fat % Resting systolic BP |
6 weeks |
| Rowe-Roberts et al. 201449 Australia | Private healthcare and insurance company (Australian Unity group) | Prospective cohort (uncontrolled pilot) |
n = 212 38% M, 62% F Age 42% under 35 35% 35-44 15% 45-54 8% 55+ Adult employees |
Wearable activity monitor | Waist-worn Fitbit Ultra activity monitor (Fitbit, USA) | No control or comparison group | PA | Daily steps (OB – step data from Fitbit) |
AUSDRISK (Australian Type 2 Diabetes Risk Assessment Tool) score Engagement with intervention (activity monitor wear) Qualitative outcomes (survey and focus groups) – experiences, engagement and activity, preferred motivational strategies |
7 months |
| Schrager et al. 201750 USA | Academic emergency medicine residency | Prospective cohort (pre- and post- uncontrolled pilot) |
n = 30 53% M, 47% F Age Median 28 years (IQR = 4.0) Physicians on a single site |
Wearable activity monitor and smartphone app (or website) | Wrist-worn Fitbit Flex activity monitor (Fitbit, USA) used with Fitbit app and/or website | No control or comparison group | PA | Days per week with ≥30 min PA (SR) |
Days per week with ≥10,000 steps or ≥30 min of active time (as measured by Fitbit at one month) Qualitative outcomes (survey) – adoption and use of device, measures of wellness, changes in PA behaviour |
6 months |
| Simons et al. 2018 (app development and feasibility)64 Simons et al. 2018 (results of RCT)65 Belgium |
Multiple workplaces in Flanders, Belgium (including retail, catering, social employment and factories) | Study 2, 2018b = Cluster RCT (study 1, 2018a was a qualitative evaluation and impact on PA/SB not reported) |
n = 130 (29 clusters) 49% M, 51% F Age IG: 24.8±3.1 CG: 25.1±3.0 Lower educated (i.e. no university or college degree) working young adults, not meeting PA guidelines at baseline (<150 min MVPA/week) |
Wearable activity monitor and smartphone app | Wrist-worn Fitbit Charge activity monitor (Fitbit, USA) used with ‘Active Coach’ app for monitoring PA | Educational brochure on PA only (generic information) | PA | Daily minutes of light, moderate and vigorous intensity PA (OB - ActiGraph™ GT-3x+ accelerometer) |
Daily steps (from Fitbit) Self-reported context-specific PA (IPAQ) Psychosocial variables: social support, attitude (perceived benefits and barriers), self-efficacy, knowledge and intentions Engagement: usage statisticsProcess evaluation interviews: Opinions on Fitbit and app (e.g. usability, preferred features) |
21 weeks |
| Skogstad et al. 201651 Norway | Road maintenance enterprise | Prospective cohort (pre- and post- uncontrolled) |
n = 121 64% M, 36% F Age M = 41.8±12.0 F = 42.6±12.5 24% road workers, 76% office workers |
Wearable activity monitor (linked with website) | Wrist-worn Tappa® activity monitor/ accelerometer used with associated website (Dytt®) for step tracking.Rewards given for best performances. | No control or comparison group | PA | Weekly exercise frequency and duration (SR) |
Aerobic fitness (maximal oxygen uptake, VO2 max, ml/kg/min) Systolic and diastolic BP (mm Hg) Resting heart rate Lipids (total, HDL and LDL cholesterol) C-reactive protein (CRP) Glycosylated haemoglobin (HbA1c) |
8 weeks (approx.) |
| Slootmaker et al. 200952 Netherlands | 8 worksites surrounding Amsterdam (mainly office settings) | RCT |
n = 102 40% M, 60% F Age IG: 32.5±3.4 CG: 31.2±3.5 Mainly office workers |
Wearable activity monitor (linked with website) | Belt-worn ‘AM 101’ activity monitor/ accelerometer (PAM BV, Netherlands) used with associated website (PAM COACH). | Educational booklet on PA only | PA + SB | Weekly PA and sedentary time – weekly minutes of light, moderate and vigorous intensity activity and sedentary minutes (SR – assessed by the AQuAA questionnaire) |
Self-reported determinants of PA – including behavioural intention, attitude, social influence, self-efficacy, knowledge of PA recommendations Aerobic fitness (maximal oxygen uptake, VO2 max, ml/kg/min) Body composition – body weight and height (BMI), waist and hip circumference, skin fold thickness (% body fat) |
8 months |
| Thorndike et al. 201453 USA | Healthcare organisation (Massachusetts General Hospital) | Phase 1 = RCT Phase 2 = team-based prospective cohort (pre- and post- uncontrolled) |
n = 104 46% M, 54% F Age Mean and range (SD not reported) IG: 29 (23–36) CG: 29 (25–37) Physicians-in-training |
Wearable activity monitor (linked with website) | Fitbit activity monitor (Fitbit, USA) used with Fitbit website. Gift card lottery for wearing device (phase 1) and highest steps (phase 2). Workplace initiatives: access to fitness centres, personal training and nutritionists, weekly healthy lunch |
Phase 1 – blinded activity monitor (no access to website). Gift card lottery and workplace initiatives (as intervention group). Phase 2 – no control or comparison group |
PA | Daily step count (phase 1 median and mean steps/day, phase 2 mean steps/day) (OB – step data from Fitbit) |
Proportion of days activity monitor was worn (i.e. compliance) Weight BMI Waist circumference Systolic and diastolic BP Lipids (total, HDL and LDL cholesterol) Use/engagement with the wider wellness programme (e.g. fitness centre, nutrition) |
12 weeks |
| Torquati et al. 201866 Australia | Two metropolitan hospitals in Brisbane (private and public) | Prospective cohort (pre- and post- uncontrolled pilot) |
n = 47 13% M, 87% F Age 41.4±12.1 Nurses and nursing managers |
Smartphone app | Smartphone app for PA and diet with non-interactive pedometer and dedicated Facebook group | No control or comparison group | PA + SB | Time spent sedentary and in light activity and MVPA Daily steps (OB - ActiGraph™ GT-3x+ accelerometer) |
Diet behaviour: Food Frequency Questionnaire (FFQ) Weight BMI Waist circumference Blood pressure Self-rated health PA and diet self-efficacy Social support Feasibility outcomes (including qualitative interviews): reach, adoption (use) and implementation |
6 months |
| van Dantzig et al. 201354 Netherlands |
Offices at various companies in Netherlands (no further detail given) | Experiment 2 = RCT (experiment 1 was a small qualitative evaluation and impact on PA/SB not reported) |
n = 86 60% M, 40% F Age IG: 44.5±7.9 CG: 44.3±8.0 Sedentary office workers |
Wearable activity monitor (linked with website, and persuasive text messages) | Clip-on commercial activity monitor (tri-axial accelerometer, model not stated) linked with personal web page for viewing PA data. Timely persuasive text messages on smartphones. |
Activity monitor only. No text messages. |
SB | Computer activity (minutes, proxy for SB) Physical activity (minutes) (OB – computer activity from computer software; PA from activity monitor) |
Engagement with the intervention (proportion of text messages read) | 6 weeks |
| Yeung et al. 201759 USA | Academic hospital residency (Cincinnati, Ohio) | Prospective cohort (pre- and post- uncontrolled crossover study) |
n = 86 38% M, 62% Fb Ageb 62% 21–30 31% 31–40 5% 41–50 Internal medicine residents |
Wearable activity monitor and smartphone app (or website) | Wrist-worn Fitbit Flex (Fitbit, USA) used with Fitbit app and/or website for monitoring steps (weeks 1–4 blinded, weeks 5–8 unblinded). Optional in-app activity tracking group for weeks 5–8. | No control or comparison group | PA | Daily steps (comparison of blinded vs. unblinded periods) (OB – step data from Fitbit) |
Proportion of participants achieving ≥10,000 steps/day | 8 weeks |
M: male; F: female; IG: intervention group; CG: control/comparison group; FTE: full time equivalent; PA: physical activity; SB: sedentary behaviour; OB: objective; SR: self-reported; QoL: quality of life; RCT: randomised controlled trial; ± or SD: standard deviation; MVPA: moderate to vigorous physical activity; BP: blood pressure; BMI: body mass index; MET: metabolic equivalent; IPAQ: international physical activity questionnaire; QQ: Quantity and Quality questionnaire; IQR: Interquartile Range; HDL: high-density lipoprotein; LDL: low-density lipoprotein; AQuAA: Activity Questionnaire for Adolescents and Adults; CDC: Centers for Disease Control and Prevention
aTools may be referred to as activity monitors or trackers in the literature; the term ‘monitor’ is used here for consistency.
bYeung et al. and Olsen et al. report gender and age of study completers only.
The number of participants ranged from 20 in a feasibility cohort study,58 to over 69,000 in a large international cohort study.41 The majority of studies targeted sedentary, office-based employees. Of the 25 studies, 16 had a markedly higher proportion of female (≥60%) than male participants.42–46,48,49,52,57–63,66
The most common study designs were individual RCTs (n = 10)39,40,43–48,52,54,60,62 and pre-post prospective cohort studies (n = 10).41,49–51,55–59,61,66 One study used a combination of these designs in two phases.53 Other designs included cluster RCTs,37,38,65 a parallel group uncontrolled randomised trial,63 and a prospective cluster trial with an asynchronous control group.42 Study duration varied greatly, with length of follow-up ranging from 6 weeks to 12 months.
Assessing the effectiveness, feasibility and/or acceptability of mHealth technology for PA promotion was the primary aim of 16 studies.39,40,43–51,53,57–59,62,63,65 Six studies targeted both PA and SB in a single intervention41,42,52,55,56,60,66 and three studies aimed to reduce SB (but also included PA as an outcome measure).37,38,54,61 Although recruitment and/or delivery of the intervention took place in the workplace in all cases, 24 of the 25 studies used mHealth as a wider lifestyle intervention, including both workplace and non-workplace activity. Only one study, designed to reduce SB, was based exclusively in the workplace.54
The main mHealth tools used were wearable activity monitors or trackers (n = 11),39,40,42,46–49,51–54,57,63 smartphone apps (n = 6),41,43–45,62,66 or a combination of the two (n = 8).37,38,50,55,56,58–61,65 Some studies had additional mHealth and technology intervention components, including motivational or persuasive text messaging43,46,54 or e-mails,61 computer software or websites linked to the activity monitor,39,40,42,46–48,51–54,57–59,63 and dedicated social media groups.66 Eleven studies assessed mHealth as a standalone intervention,41,46,49,50,52,54,59,60,63,65,66 whereas 14 studies used mHealth in the context of a multi-component workplace health or PA programme.37–40,42–45,47,48,51,53,55–58,61,62 Among the multi-component programmes in particular, interventions were diverse and additional components included educational materials on health and PA,37–40,43,47,48,55,56,58,61 managerial support,37,38 financial incentives or rewards,39,40,44,45,51,53,55–57,62 shared active workstations,42 online or telephone counselling,47,48,58 personalised feedback on activity,55–57 wellness education delivered in the workplace,42,53,61 group-based action planning,61 and access to personal training and nutritionists.53 Further detail on intervention content is given in Table 2.
Table 2.
Summary of intervention components.
| Intervention description Standalone mHealth (SA) or multi-component programme (MC)? |
Duration of intervention | Frequency of intervention (if applicable) | Theoretical basis of intervention | Behaviour change techniques (BCTs) included (whole intervention) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Goal-setting (behaviour or outcome) | Self-monitoring (behaviour or outcome) | Prompts/cues | Feedback(behaviour or outcome) | Rewards/incentives (virtual or real, progress towards or achieving behaviour) | Social support(online or offline) | Social comparison |
Information on consequences of behaviour (general or individual) |
Other BCTs | ||||
| Brakenridge et al. 201637; Brakenridge et al. 201638 | Wearable activity monitor and smartphone app for feedback on sitting, standing, stepping, sitting breaks, posture and sleep. MC: organisational support (e-mails, educational materials) |
12 months (although main focus, e.g. e-mails was in the first 12 weeks) |
Ad lib wear of activity monitor and use of smartphone app. Fortnightly e-mails for first 12 weeks. |
None stated | ? | Y | Y | Y | N | N | N | Y | Information on where and when to perform behaviour |
| Finkelstein et al. 201539; Finkelstein et al. 201640 | Wearable activity monitor used with website including social components. MC: 2 of the 3 intervention groups earned weekly incentives for step count (cash vs. charity) Educational booklets on PA. |
6 months | Daily wear of activity monitor. Incentives for weekly step counts distributed every 4-6 weeks. |
Economic theory and Theory of Reasoned Action | Y | Y | N | Y | Y | Y | Y | Y | |
| Ganesan et al. 201641 | ‘Stepathlon’ mobile app (also available as website). Participants self-entered daily activity data and steps from pedometers. Included personalised tools for self-monitoring PA and dietary intake, quizzes, motivational e-mails, online community and chat for interactive advice/expert guidance, social networking, competitions and health information. Also gamification – race around a virtual world map. SA |
100 days | Daily e-mails to encourage daily activity data entry. | None stated | ? | Y | Y | Y | N | Y | Y | Y | |
| Gilson et al. 201655;Gilson et al. 201756 | Wearable activity monitor used with associated smartphone app for monitoring steps and diet. MC: Part of the ‘Shifting Gears’ programme. Earning points and financial rewards for attaining step goals and logging diet. Educational materials on PA and diet. Personalised feedback and guidance from researchers. |
20 weeks |
Ad lib wear of activity monitor and use of smartphone app. Personalised feedback and guidance from researcher every 4 weeks. Rewards at end of programme. |
None stated | Y | Y | N | Y | Y | Y | N | Y | Information on where and when to perform behaviour Action planning Graded tasks |
| Jones 201642 | Wearable activity monitor used with software installed on work computers. Included monitoring progress towards step and activity goals, competing against colleagues and earning incentives and awards for meeting step targets. MC: One intervention group used shared active workstations.Group wellness education delivered in the workplace. |
6 months | Daily wear of activity monitor.Use of active desks for at least 30 min a day, 5 days a week. Monthly wellness education. | Socio-Ecological Model | Y | Y | N | Y | Y | Y | Y | Y | Environmental restructuring |
| Koyle 201343 | ‘Adidas miCoach’ smartphone app with integrated accelerometer. Motivational text messages tailored based on initial app-delivered fitness test and walking logs. MC: Part of a health promotion programme including educational materials on PA. |
6 weeks | Ad lib use of smartphone app. Weekly motivational text messages. | Self-Efficacy Theory (part of Social Cognitive Theory) | Y | Y | Y | Y | N | N | N | Y | Action planning |
| Losina et al. 201757 | Wearable activity monitor used with websites to monitor steps and PA, including individual and team progress. MC: Monetary incentives (individual and team) for meeting PA goals. Personalised weekly e-mails for feedback on MVPA. |
24 weeks | Daily wear of activity monitor. Weekly financial rewards. Weekly feedback e-mails. |
Behavioural economic theory | Y | Y | N | Y | Y | Y | N | N | Graded tasks Shaping |
| Neil-Sztramko et al. 201758 | Wearable activity monitor used with associated app and/or website to monitor steps and PA. MC: Individualised behavioural counselling (telephone/online – all participants chose telephone). |
12 weeks |
Ad lib wear of activity monitor and use of smartphone app. 8 counselling sessions over 12 weeks. |
Health Action Process Approach (HAPA) | Y | Y | N | Y | N | Y | N | Y | Barrier identification/problem solving |
| Patel et al. 201644 | ‘Moves’ smartphone app with integrated accelerometer. Feedback on steps. MC: The three intervention groups received differently framed financial incentives for achieving step goals (gain, lottery and loss). |
13 weeks |
Ad lib use of smartphone app (instructed to carry phone when active). Daily feedback and incentives. |
Behavioural economics – immediate vs. delayed gratification, prospect theory and regret aversion. | Y | ? | N | Y | Y | N | N | N | Action planning Prompt anticipated regret |
| Patel et al. 201645 | ‘Moves’ smartphone app with integrated accelerometer. Feedback on steps. MC: The three intervention groups received differently framed financial incentives for achieving step goals (individual, team and combined). |
13 weeks |
Ad lib use of smartphone app (instructed to carry phone when active). Daily feedback and incentives. |
Behavioural economics; variable reinforcement; social behaviour change theories | Y | ? | N | Y | Y | N | Y | N | Action planning |
| Poirier et al. 201646 | Wearable activity monitor used with website (data transmitted wirelessly) for monitoring steps. Tailored goals based on activity. Motivational electronic messages (optional). Virtual rewards, social messaging and competitions. SA |
6 weeks | Daily wear of activity monitor. Messages up to 4 times/day (optional). |
Tailored, adaptive goals based on behavioural economics and operant shaping. | Y | Y | Y | Y | Y | Y | Y | N | Shaping Information about others’ approval |
| Reijonsaari et al. 200947; Reijonsaari et al. 201248 | Wearable activity monitor used with website for goal-setting and PA monitoring. MC: Personalised distance counselling and support (online and telephone). Written information on fitness test results, PA and health. |
12 months | Daily wear of activity monitor. Phone call or message from coach if did not log on to website every 2 weeks to upload PA data. |
None stated | Y | Y | Y | Y | N | Y | N | Y | |
| Rowe-Roberts et al. 201449 | Wearable activity monitor for monitoring steps. Participants were offered an optional additional device for friends/family midway through the intervention for social support and competition. SA |
7 months | Daily wear of activity monitor. | None stated (although mentioned Transtheoretical Model in discussion) | N | Y | N | N | N | Y | Y | N | |
| Schrager et al. 201750 | Wearable activity monitor used with associated app and/or website to view and monitor data. SA |
1 month intervention/active monitoring and optional use up to 6 months | Daily wear of activity monitor. | None stated (although key behaviour change techniques reported in discussion) | ? | Y | ? | ? | ? | ? | ? | N | |
| Skogstad et al. 201651 | Wearable activity monitor used with associated website to monitor PA and compete against colleagues individually and in teams. Gamification – virtual internet mountain track. MC: Part of an organised 8-week workplace PA programme (‘Dytt®’). Rewards given for best performances. |
8 weeks | Daily wear of activity monitor and ad lib use of website. Weekly PA results posted on intranet. Rewards at end of programme. |
None stated | N | Y | N | N | Y | N | Y | N | |
| Slootmaker et al. 200952 | Wearable activity monitor used with website to monitor PA (data uploaded via docking station and software on work computers). Website provided tailored goal-setting and PA advice. Comparison of PA scores with peers. SA |
3 months | Daily wear of activity monitor. Ad lib use of website. |
None stated | Y | Y | N | Y | N | N | Y | Y | Action planning Graded tasks |
| Thorndike et al. 201453 | Wearable activity monitor used with website to monitor steps, PA, weight and diet. Included gamification – virtual ‘avatar’ on activity monitor screen that changed size with varying level of PA/SB. Phase 1 = individual monitoring Phase 2 = team-based steps competition MC: Part of a 10-week, team-based worksite wellness programme (‘BeFit’) – included access to personal training and nutritionists. Lottery to reward device wear and attainments (highest steps). |
12 weeks (phase 1 = 6 weeks, phase 2 = 6 weeks) | Daily wear of activity monitor and ad lib use of website. Weekly e-mail reminders to charge device and with details of gift card lottery. |
None stated | N | Y | N | Y | Y | N | Y | N | |
| van Dantzig et al. 201354 | Wearable activity monitor linked with personal web page to monitor activity patterns. Timely, persuasive text messages on smartphone during prolonged periods of sitting (detected by computer software). SA |
6 weeks | Daily wear of activity monitor. Text messages sent every 30 min of uninterrupted computer activity (up to a maximum of three messages/ day). |
Intervention based on four of six social influence strategies72 – authority, commitment, consensus and scarcity | N | Y | Y | Y | N | N | N | N | |
| Yeung et al. 201759 | Wearable activity monitor used with associated app and/or website for monitoring steps (weeks 1–4 blinded, weeks 5–8 unblinded). Optional resident-only activity tracking group to ‘connect and compete’ with peers for weeks 5–8. SA |
8 weeks | Daily wear of activity monitor. Ad lib use of app/website. |
None stated | Y | Y | N | Y | N | N | Y optional | N | Action planning |
| Gremaud et al. 201860 | Wearable activity monitor used with ‘MapTrek’ app (smartphone or web-based) for gamification of walking, including virtual ‘avatar’ and races, including a social competitive element and automated text messages based on PA. SA |
10 weeks | Daily wear of activity monitor. Ad lib use of smartphone app. |
Cognitive evaluation/self-determination theories and Social Cognitive Theory | Y | Y | Y | Y | Y | N | Y | N | |
| Olsen et al. 201861 | Wearable activity monitor used with associated app for self-monitoring, social support and prompts to reduce SB. MC: Group-based goal-setting, action planning and problem solving session delivered in workplace. Weekly e-mail reminders and information resources. Healthy living seminar delivered in workplace (week 4). |
6 weeks |
Ad lib wear of activity monitor and use of smartphone app. Weekly e-mails. One group-based session and one seminar. |
Social Cognitive Theory | Y | Y | Y | Y | N | Y | Y | Y | Action planning Barrier identification/problem solving Information on where and when to perform behaviour Instruction on how to perform behaviour |
| Patel et al. 201862 | ‘Moves’ smartphone app with integrated accelerometer. Feedback on steps. MC: The three intervention groups received differently framed financial incentives for achieving step goals (high frequency small reward lottery, jackpot lottery and combined lottery). |
13 weeks |
Ad lib use of smartphone app (instructed to carry phone when active). Daily feedback and incentives. |
Behavioural economics – immediate vs. delayed gratification, prospect theory and regret aversion. | Y | ? | N | Y | Y | N | N | N | Action planning Prompt anticipated regret |
| Reed et al. 201863 | Wearable activity monitor linked with website for monitoring PA and steps and taking part in one of three challenges (individual, friend or team) SA |
6 weeks | Daily wear of activity monitor. Ad lib use of website. |
Social behaviour change theories including self-presentation theory | ? | Y | N | Y | N | N | Y | N | |
| Simons et al. 201864; Simons et al. 201865 | Wearable activity monitor used with ‘Active Coach’ smartphone app for goal-setting, self-monitoring, and tailored information to promote PA SA |
9 weeks | Daily use of activity monitor and app encouraged | Self-determination theory (and BCTs identified during app development) | Y | Y | Y | Y | N | Y | N | Y | Barrier identification/problem solving Graded tasks Information on where and when to perform behaviour Instruction on how to perform behaviour |
| Torquati et al. 201866 | Smartphone app for PA and diet (goal-setting) with non-interactive pedometer (self-monitoring) and dedicated Facebook group (social support) SA |
3 months | Ad lib use of smartphone app and Facebook group | Social Cognitive Theory, goal-setting theory and control theory | Y | Y | N | N | N | Y | Y | N | |
BCT: Behaviour Change Technique; SA: Standalone mHealth; MC: Multi-Component programme; Y: Yes, included in intervention; N: No, not included in intervention;?: unclear or difficult to determine whether included from available intervention description; PA: Physical Activity; SB: Sedentary Behaviour; MVPA: Moderate to Vigorous Physical Activity
Intervention duration ranged from 1 to 12 months. Frequency of delivery of the intervention components was variable but daily wear of activity monitors was encouraged in most studies. Fifteen studies reported that the intervention was based on a named behaviour change theory and/or principles of behavioural economics.39,40,42–46,54,57,58,60–66 A further two studies alluded to behaviour change techniques or theory in their discussion,49,50 and eight studies had no clear theoretical basis.37,38,41,47,48,51–53,55,56,59 The most frequently cited behaviour change theories were the Theory of Reasoned Action,67 the Socio-Ecological Model,68 Social Cognitive Theory and Self-Efficacy,69 Self-Determination Theory,70 other social influence theories such as self-presentation theory and Cialdini’s social influence strategies,71,72 and the Health Action Process Approach.73
A control or comparator group was present in 14 of the 25 studies.37–40,42–48,52–54,60,62,65 Of these, six could not be classed as a ‘true’ control group as the participants received at least a partial mHealth intervention,43–45,54,60,62 and another three studies supplied controls with wearable activity monitors for data collection.42,46,53
Outcome measures of PA and SB were heterogeneous. The most frequently used outcome measures for PA included daily step count, daily or weekly minutes or metabolic equivalent (MET) minutes of total activity or moderate to vigorous PA (MVPA). Other outcomes included exercise frequency and proportion of participants meeting step or PA goals. Studies that assessed SB commonly reported daily or weekly sedentary time, although the one study using an exclusive workplace intervention used computer activity as a proxy for SB.54 Objective PA/SB outcomes were used in 20 studies,37–40,42–46,49,53–63,65,66 whilst four studies relied on self-report for the primary measure of PA or SB.47,48,50–52 Ganesan and colleagues used pedometer data that was self-entered by participants.41
Study quality
A summary of the risk of bias and quality assessment for the included studies is presented in Table 3. Using the EPHPP tool, only one study was judged as ‘strong’ quality.53 Nine studies were assigned a ‘moderate’ quality rating,39,40,43,44,46,52,60,62,63,65 and 15 studies were given a ‘weak’ rating.37,38,41,42,45,47–51,54–59,61,66 All except two studies were judged as ‘weak’ in terms of selection bias; participants were typically self-selected employees who volunteered to take part in a wellness programme.50,53 Representativeness and level of participation were unclear in many of the included studies.
Table 3.
Summary of risk of bias assessment.
| Selection bias | Study design | Confounders | Blinding | Data collection methoda | Withdrawals and dropouts | Attrition rateb | Global rating | |
|---|---|---|---|---|---|---|---|---|
| Brakenridge et al. 201637; Brakenridge et al. 201638 | W | S | S | W | S | W | IG: 62% CG: 47% Overall: 54% | Weak |
| Finkelstein et al. 201539; Finkelstein et al. 201640 | W | S | S | M | S | S | IG1: 21%, IG2: 24%, IG3: 14% CG: 17% Overall: 19% | Moderate |
| Ganesan et al. 201641 | W | M | S | M | M | W | IG: 47% | Weak |
| Gilson et al. 201655; Gilson et al. 201756 | W | M | W | M | S | W | IG: 57% | Weak |
| Gremaud et al. 201860 | W | S | S | M | S | S | IG: 0% CG: 1% Overall: 1% | Moderate |
| Jones 201642 | W | M | W | M | S | M | IG1: 32%, IG2: 16% CG: 25% Overall: 24% | Weak |
| Koyle 201343 | W | S | S | M | M | S | IG: 17% CG: 11% Overall: 14% | Moderate |
| Losina et al. 201757 | W | M | W | M | M | S | IG: 3% | Weak |
| Neil-Sztramko et al. 201758 | W | M | W | M | S | S | IG: 0% | Weak |
| Olsen et al. 201861 | W | M | W | M | S | M | IG: 39% | Weak |
| Patel et al. 201644 | W | S | S | M | M | S | IG1: 3%, IG2: 8%, IG3: 4% CG: 4% Overall: 5% | Moderate |
| Patel et al. 201645 | W | S | W | M | M | S | IG1: 3%, IG2: 4%, IG3: 3% CG: 6% Overall: 4% | Weak |
| Patel et al. 201862 | W | S | S | M | M | S | IG1: 5%, IG2: 5%, IG3: 7% CG: 8% Overall: 6% | Moderate |
| Poirier et al. 201646 | W | S | S | M | S | S | IG: 20% CG: 17% Overall: 18% | Moderate |
| Reed et al. 201863 | W | S | S | M | M | S | IG1: 8%, IG2: 0%, IG3: 4% Overall: 4% | Moderate |
| Reijonsaari et al. 200947; Reijonsaari et al. 201248 | W | S | S | W | S | M | IG: 35% CG: 32% Overall: 33% | Weak |
| Rowe-Roberts et al. 201449 | W | M | W | M | S | M | IG: 34% | Weak |
| Schrager et al. 201750 | M | M | W | M | W | M | IG: 35% | Weak |
| Simons et al. 201864; Simons et al. 201865 | W | S | S | M | S | S | IG: 12% CG: 19% Overall: 15% | Moderate |
| Skogstad et al. 201651 | W | M | S | M | W | S | IG: 15% | Weak |
| Slootmaker et al. 200952 | W | S | S | M | M | M | IG: 26% CG: 18% Overall: 22% | Moderate |
| Thorndike et al. 201453 | S | M | S | M | S | S | Phase 1 IG: 4% CG: 6% Phase 2 IG: 8% Whole trial: 13% | Strong |
| Torquati et al. 201866 | W | M | W | M | S | W | IG: 74% | Weak |
| van Dantzig et al. 201354 | W | S | S | M | W | S | Attrition not reported but 85/86 participants appear to have completed the trial based on final data (i.e. 1.2% attrition). | Weak |
| Yeung et al. 201759 | W | M | W | M | S | S | IG: 14% | Weak |
S: strong; M: moderate; W: weak; IG: Intervention Group; CG: Control/Comparison Group
aValidity and reliability of primary outcome. For data collection method, studies were rated as ‘strong’ if the measure had known validity and reliability, ‘moderate’ if the measure had reasonable validity or reliability, and ‘weak’ if validity and reliability were unknown.
bPercentage of participants failing to provide data at final follow-up (to nearest whole percentage)
All 25 studies used robust experimental or quasi-experimental designs. Of the 25 studies, 15 reported controlling for important confounders in their design and/or analysis. Ten studies were assigned a ‘weak’ rating in this domain due to lack of reporting or poor control of confounders in analysis.42,45,49,50,56–59,61,66 No studies received a ‘strong’ rating for blinding due to the difficulty and impracticality in blinding participants to this type of mHealth intervention. Blinding of outcome assessors was often not described, and two studies were rated as ‘weak’ in this domain as outcome assessors were reported to be unblinded.37,38,47,48
A ‘strong’ data collection method for the main PA/SB outcome was used by 14 studies; this included research-grade accelerometers (e.g. activPAL™, Actigraph™, GENEActiv™)37–40,55,56,58,61,65,66 and commercial activity monitors with high validity and reliability for the particular measure (e.g. Fitbit® used to monitor steps),42,46,49,53,59,60,74 and the International Physical Activity Questionnaire (IPAQ) with reasonable validity and reliability.47,48,75 Eight studies used ‘moderate’ data collection tools with either acceptable validity or reliability, including smartphone-integrated accelerometers,43–45,62 the Activity Questionnaire for Adolescents and Adults (AQuAA),52,76 the Tractivity® activity monitor,63 self-entered pedometer data,41 and step data from the Fitbit® converted to MVPA.57 Two studies used self-reported data in non-validated questionnaires,50,51 and one study54 used computer software and an activity monitor with unreported validity and reliability; these were therefore given a ‘weak’ data collection rating.
Withdrawals and dropouts were reported by the majority of studies (n = 24). Definitions of attrition varied between studies but it was possible to calculate attrition rates based on the number of participants failing to provide data at the final follow-up, which ranged from 0% to 74%. Only four studies38,66,41,56 were rated as ‘weak’ in this domain due to having particularly high attrition rates of greater than 40%.
Regarding intervention integrity, most studies reported the proportion of participants receiving the allocated intervention, which was most frequently in the range of 80–100%. Approximately two-thirds of studies reported measuring consistency of delivery or use of the intervention, with outcomes such as device wear time and interaction with technology. In the majority of studies, it was judged to be possible that participants had received an unintended intervention or this could not be determined from the reports.
Data analysis methods were generally deemed appropriate. Of the 13 RCTs, 8 used intention-to-treat analysis.37–40,44,45,52,53,60,62
BCTs
Due to the relatively small number of studies and BCTs identified, it was not possible to determine which techniques were associated with intervention efficacy. In many cases it was difficult to determine intervention content and specific BCTs used from the available descriptions. The most frequently identified BCTs (or categories of BCTs) are shown in Table 2. These included self-monitoring of the behaviour or outcome (n = 22, 88% of studies), provision of feedback on the behaviour or outcome (n = 21, 84%), goal-setting for the behaviour or outcome (n = 17, 68%), social comparison (n = 14, 56%), social support (n = 12, 48%), rewards and incentives contingent on progress towards or achieving the behaviour (n = 11, 44%), and provision of information on consequences of PA and SB to the individual or in general (n = 11, 44%). Prompts and cues (n = 9, 36%) were also a common intervention component. Action planning was identified in eight studies (32%), graded tasks were described by four studies (16%), information on where and when to perform the behaviour was given in four studies (16%), and barrier identification/problem solving was used in three studies (12%). Instruction on how to perform the behaviour, shaping, and prompting anticipated regret were each used in two studies (8%). Information about others’ approval and environmental restructuring were each found in only one study (4%). Individual or team competitions, and various types of gamification (such as virtual avatars and racing around a virtual landscape) were not part of the CALO-RE taxonomy but were used in several studies with smartphone apps and websites. Of the 40 BCTs listed in the CALO-RE taxonomy, 16 were not identified in any of the coded interventions.
Prompts and cues were used more frequently in interventions for SB; these were found in 5 of 9 studies (56%) that aimed to reduce SB compared with 6 of 22 (27%) aiming to promote PA. Rewards and incentives were more frequently part of interventions targeting PA (11/22 studies, 50%) compared with 3 of 9 (33%) studies that aimed to reduce SB.
Effects of interventions
Statistical methods of combining the results were not considered feasible for several reasons. There was high methodological heterogeneity with a range of different study designs, outcome measures (particularly for PA) and outcome time points. Incomplete reporting of outcome data and standard deviations precluded the calculation of reliable effect sizes. Some studies reported change in PA while others reported absolute values. In addition, several studies were either uncontrolled or did not have a ‘true’ control group (i.e. the comparison group received an mHealth intervention), which would have resulted in an underestimation of effect sizes. The data were therefore summarised narratively and visually. A summary of the main results for each included study is shown in Table 4. Impact on PA, SB and health and other related outcomes is reported separately.
Table 4.
Summary of main results.
| Study | Key findings |
Subgroup findings and sensitivity analysesa (if applicable) | Feasibility and additional findings (including engagement, attrition and acceptability) | |||
|---|---|---|---|---|---|---|
| Impact on PA/SBa | Impact on related health/other outcomes | Impact on PA/SB | Impact on other outcomes | |||
| Brakenridge et al. 201637; Brakenridge et al. 201638 | No significant between-group difference in PA or SB at 3 months (although the organisational support group only showed an increase in overall standing time, +14.6 min/day, 95% CI 2.5–26.8, P = 0.018). Significant between-group adjusted mean difference (MD) in overall daily stepping time (+20.6 min, 95% CI 3.1–38.1, P = 0.021) and number of daily steps (+846.5 steps, 95% CI 67.8–1625.2, P = 0.033) at 12 months – favouring the intervention group. |
No significant within- or between-group findings for any health or work-related outcomes. |
↑ PA
↑ SB (favoured control) |
0 | Sensitivity analysis: study completers were more likely to show larger and statistically significant changes in activity at 3 months (bias from dropout of healthy participants?). | Engagement: 70.5% of participants provided with the activity monitor used it in the first 12 weeks, with mean usage of 12.1±11.6 days. Use had ceased by 12 months in all intervention participants. Acceptability: 41/153 (26.8%) participants reported at least one adverse event (e.g. reactions to activity monitor or accelerometer wear). |
| Finkelstein et al. 201539; Finkelstein et al. 201640 | No between-group difference in MVPA (P = 0.0854) or steps (P = 0.1362) between the Fitbit only and control groups at 6 months. Cash and charity incentive groups showed higher MVPA compared with control at 6 months (cash group MD = +29 MVPA bout mins/week, 95% CI 10–47, P = 0.0024; charity group MD = +21 MVPA bout mins/week, 95% CI 2–39, P = 0.0310). At 12 months, the Fitbit and charity groups showed higher MVPA than the control (Fitbit group MD = +37 MVPA bout mins/week, 95% CI 19–56, P = 0.0001; charity group MD = +32 MVPA bout mins/week, 95% CI 12–51, P = 0.0013). The cash incentive group did not differ from the control (P = 0.1363). The only significant within-group increase in MVPA at 12 months was for the Fitbit only group (+16 mins/week, 95% CI 2–30, P = 0.0301). The only significant between-group differences in mean daily steps were for the cash vs. control group at 6 months (+1050 steps, 95% CI 600–1490, P < 0.0001) and 12 months (+500 steps, 95% CI 50–960, P = 0.0289). |
No evidence for improvement in health outcomes - all intervention groups showed improvement in cardiorespiratory fitness (NET-FVO2 max) at 6 and 12 months, but control group showed improvement at 12 months. | ↑ PA |
0
(improved aerobic fitness in control and intervention groups) |
Subgroup analysis by baseline activity level found some differences – e.g. those in the cash and charity groups who were insufficiently active at baseline showed a significant increase in MVPA from baseline to 6 months (+22 mins/week, 95% CI 5–38, P = 0.0096 and +17 mins/week, 95% CI 2–32, P = 0.0231 respectively). For those who were sufficiently active at baseline, changes in MPVA were non-significant in both groups. | Engagement: 40% abandoned the Fitbit within 6 months, and by month 12 only around 10% of all participants in the intervention groups were still wearing the device. Attrition: predictors of loss to follow-up at 12 months included gender (higher attrition in females) and ethnicity (lower attrition in Chinese). Higher adherence was seen in the cash incentive group compared with the other groups (particularly at 6 months). |
| Ganesan et al. 201641 | Significant increase pre- to post-intervention in mean daily steps (+3519 steps, 95% CI 3484–3553, P < 0.0001), exercise days/week (+0.89 days, 95% CI 0.87–0.92, P < 0.0001) and odds of exercising ≥30 min/day (1.65, 95% CI 1.61–1.68, P < 0.0001). Significant decrease pre- to post-intervention in mean sitting duration (−0.74 h/day, 95% CI –0.78 to –0.71, P < 0.0001). |
Significant pre- to post-intervention reduction in weight (−1.45kg, 95% CI –1.53 to –1.38, P < 0.0001). |
↑ PA
↓ SB |
↓ weight | Subgroup analysis by gender, year cohort, geographic region and income group – no significant differences for any of the main outcomes. Men showed greater weight loss than women (−1.63kg, 95% CI –1.72 to –1.54 compared with –0.74kg, 95% CI –0.91 to –0.57). Predictors of weight loss included increase in step count, increase in exercise days and decrease in sitting duration. |
|
| Gilson et al. 201655; Gilson et al. 201756 |
Non-significant increase in mean proportions of work time spent physically active from baseline to post-intervention and follow-up (+1%, 7 min/day). Non-significant decrease in mean proportions of work time spent sedentary at post-intervention (−6%) and follow-up (−9%). Significant increase in mean proportion of workday non-work time spent sedentary baseline to follow-up (P = 0.007) and decrease in mean proportion of workday non-work time stationary+ baseline to post-intervention (P = 0.037) and follow-up (P < 0.033). 65% of participants showed positive changes in PA (and at least one dietary choice) at follow-up. |
Significant increase in workday fruit (P = 0.023) and vegetable (P= 0.024) consumption by one serving/day at end of programme. |
0 PA
↑ SB (workday non-work time only) |
↑ diet(fruit and vegetable intake) | N/A | Engagement: 26/44 (59%) participants used the activity monitor. Use for step count monitoring remained constant but dietary logging significantly declined from baseline to study completion. Attrition: moderately high - only 19/44 (43%) participants completed the study. Acceptability: Barriers to technology use included technical issues, data usage costs and privacy concerns. From qualitative interviews, the overall intervention was perceived as feasible, acceptable and as having positive impact on PA by drivers and depot managers. The mHealth component was perceived to have a greater impact on behaviour than financial incentives. |
| Gremaud et al. 201860 | Relative to control (Fitbit-only group) after the start of the intervention, the smartphone app users showed an immediate increase in mean daily steps of 2183 (95% CI 992–3344). Daily active minutes similarly increased by 12.8 (95% CI 6.3–19.3). However, participants’ steps declined during the study period overall. The mean longest bouts of sedentary time decreased by 26.6 min (95% CI –70.9 to–17.3) in the intervention group relative to control. |
Not studied |
↑ PA
↓ SB (but control group received mHealth intervention) |
Not studied | Sensitivity analyses accounting for wear compliance, and excluding data collected following a bug in the app platform did not alter the significance of the findings. | Engagement: compliance with Fitbit wear declined over time, but app users were more likely to wear the Fitbit daily than the Fitbit-only group. Low attrition: 144/146 (99%) participants provided follow-up data (although short-term study). Acceptability: participants rated the app as easy and enjoyable to use and useful for increasing PA. The main reported barrier to technology use was Fitbit battery issues (8/48, 17% of respondents). |
| Jones 201642 | Between-group difference in % change in mean daily steps neared significance: IG1 (activity monitor only) = +9%IG2 (activity monitor and active desk) = -17% CG = –15% (P = 0.06, favouring the activity monitor only group) Significant increase in sedentary time in intervention groups compared with control:IG1 = +255.5 min/day, 95% CI 127.5–383.5 IG2 = +353.2 min/day, 95% CI 219.1–487.3 CG = 0 min/day, 95% CI –58.6 to 58.5 (P < 0.0001) |
No significant within- or between-group findings for BMI, sleep or any psychosocial outcomes. |
0 PA
↑ SB (favoured control) |
0 | N/A | Engagement: participants reporting follow-up data wore the Fitbit activity monitor for a mean of 177 of 210 days (84% adherence). Attrition: dropouts were similar to study completers in terms of baseline activity, weight, gender and ethnicity. |
| Koyle 201343 | The ‘control’ group showed a significant within-group reduction in mean weekly walking duration from week 1 to week 6 (−50.3 min, P < 0.001). The reduction in the intervention group was non-significant (−20.8 min/week, P = 0.99). After controlling for baseline activity level, the intervention group walked more minutes than the control but a significant difference was found only at week 6 (β = 38.21, P = 0.03). |
Significant pre- to post-intervention increase in self-efficacy beliefs (walking self-efficacy scale) for both groups: Intervention 85.6±12.1 to 90.0±10.8 (P = 0.0003) and control 78.3±14.7 to 87.3±11.6 (P = 0.0288). Significant decrease in resting mean pulse rate for intervention group only: 70.0±11.0 to 63.2±10.2 (P = 0.038). No increase in likeliness to participate in other PA. No significant within- or between-group findings for weight, BMI or systolic BP. |
↑ PA
(but control group received mHealth intervention) |
↑ self-efficacy
↓ resting pulse rate |
N/A | Acceptability: Participants in both groups commented positively on their experiences of taking part and found the study motivating. Text messages were reported as helpful.Many participants felt stronger and/or reported having lost weight as a result of taking part.At the end of the trial, all but one person chose to continue using the app. |
| Losina et al. 201757 | Average weekly duration of MVPA increased from 54±64 min in 2 weeks pre-intervention to 62±89 min post-intervention (statistical significance of changes not reported). 86% of participants met either their weekly PA goal or CDC PA guidelines for ≥6/24 weeks. 52% met either their PA goal or CDC guidelines for ≥12/24 weeks. |
Not studied | ?(statistical significance of PA changes not reported and pre-intervention measure was contaminated by use of Fitbit) | Not studied | Subgroup analysis by baseline physical ability, ethnicity and obesity found some differences. Those able to walk a mile at baseline (vs. those unable, P = 0.010), African Americans (vs. all other ethnicities, P = 0.016) and non-obese participants (vs. obese, P = 0.018) met PA guidelines more consistently throughout the programme. | Engagement: 63% of participants were classed as adherent Fitbit wearers (i.e. wearing Fitbit ≥4 days/week for ≥20 weeks). Wear declined over time (e.g. 94% adherent wearers after 1 month vs. 62% after 6 months). Acceptability: two-thirds of participants were satisfied with the programme; 79% indicated they would participate again. |
| Neil-Sztramko et al. 201758 | Significant increase in mean total MVPA (+110.3 min/week, P < 0.01) and significant increase in mean daily steps (+1488.7, P < 0.01) from baseline to post-intervention. Significant reductions in objective mean sedentary time (−405.5 min/week, bouts ≥10 mins, P = 0.02) and self-reported mean sedentary time (−425.3 min/week, P < 0.01) from baseline to post-intervention. |
Significant pre- to post-intervention reductions in weight (mean change –0.9kg, P = 0.03) and BMI (mean change –0.3kg/m2, P = 0.04). Significant improvements in some domains of health-related quality of life: energy/fatigue (P = 0.01) and emotional wellbeing (P = 0.04). Significant improvements in sleep disturbances (P = 0.04) and day dysfunction due to sleepiness (P = 0.04). |
↑ PA
↓ SB |
↓ weight and BMI
↑energy/fatigue and emotional wellbeing score ↓ sleep disturbance and day dysfunction due to sleepiness |
N/A | High engagement with Fitbit: all participants reported using the Fitbit. Of the 18 who provided Fitbit data, the device was worn 94.5% of the total study period. Attrition: all participants completed the study. High acceptability: 16/19 (84.2%) participants were very or somewhat satisfied with the intervention. Demand: high demand for participation. Recruitment to time and target was exceeded. Implementation: technical issues were common; 5 (25%) Fitbits were returned. A further two devices were lost. |
| Olsen et al. 201861 | No significant changes in sedentary time from pre- to post-intervention: Mean change in accelerometer-assessed sedentary min/day +0.08 (95% CI –30 to +30). Mean change in total self-reported sitting time in office –56 mins/day (95% CI –128.5 to +17.0) and when working at home +20.5 (95% CI –64.5 to 105.5). No significant changes in accelerometer-assessed or self-reported PA. |
Not studied |
0 PA
0 SB |
Not studied | N/A | Moderate attrition: only 30 of the 49 (61%) recruited participants provided some post-intervention data. Acceptability: overall acceptability of the intervention was high. 90% of participants were satisfied or very satisfied with the programme and 83% agreed that the tracker was a useful tool for behaviour change. |
| Patel et al. 201862 | Compared with control during the intervention period, the (unadjusted) mean proportion achieving the 7000 step goal was significantly higher for the combined lottery incentive group only (0.38 vs. 0.26). The adjusted odds ratio for achieving the goal (combined vs. control) was 3.00 (95% CI 1.28–7.02, P = 0.012). No significant differences were sustained at follow-up, after incentives were removed. No significant between-group differences in mean daily steps (within-group changes not reported). |
Not studied |
↑ PA
(but control group received mHealth intervention) |
Not studied | Sensitivity analysis: adjusting for device and missing data did not change the significance of the findings. | Low attrition: only 6% of participants did not complete the study. |
| Patel et al. 201644 | No significant between-group differences in mean daily steps (within-group changes not reported). Proportion of participant-days 7000 step goal was achieved was significantly higher for loss incentive group compared with control for the 13 week intervention period (MD = +0.16, 95% CI 0.06–0.26, P = 0.001). This effect was not sustained at follow-up after incentives were removed. |
Not studied | ↑ PA(but control group received mHealth intervention) | Not studied | Sensitivity analysis: adjusting for device and different methods of accounting for missing data did not affect the significance of the findings. | Low attrition: only 5% of participants did not complete the study. |
| Patel et al. 201645 | Compared with control during the intervention period, the mean proportion achieving the 7000 step goal was significantly higher for the combined incentive group only (MD = +0.17, 95% CI 0.07–0.28, P < 0.001). The combined incentive group also had higher mean daily steps than the control at the end of the intervention period (MD = +1446 steps, 95% CI 448–2444, P = 0.005). No significant differences were sustained at follow-up. |
Not studied |
↑ PA
(but control group received mHealth intervention) |
Not studied | Sensitivity analysis: adjusting for device and missing data did not affect the significance of the findings. | Low attrition: only 4% of participants failed to complete the study. |
| Poirier et al. 201646 | Mean daily steps pre- to post-intervention increased for the intervention group (+309 steps/day, ±1874) and decreased for the control group (−661 steps/day, ±1824). MD = 970 steps/day, P < 0.001. The proportion of participants achieving an increase of 1000 steps/day was significantly greater in the intervention group (29.9%) than the control (16.4%), P = 0.018. |
Not studied | ↑ PA | Not studied | Subgroup analysis by baseline activity level: Sedentary group ( < 5000 steps/day): Mean change +594±1558 steps/day in intervention group vs. +47±1299 steps/day in control group, P = 0.04. Low-to-somewhat active group (5000–9999 steps/day): Mean change of –110±2106 steps/day in intervention group vs. –1286±1783 steps/day in control group, P = 0.004. Sensitivity analysis: included some study non-completers – statistically significant between-group difference in mean daily steps remained (P < 0.001). |
High engagement with intervention: Participants wore the activity monitor on 78.6% of days (33/42) on average; e-mails were opened on 21.9% of days (9.2/42); website visits occurred every 3.6 days (11.8/42). 130/133 (97.7%) intervention participants still wore the activity monitor, opened e-mails and/or visited the website after 6 weeks. Attrition: moderately low (around 82% provided complete follow-up data) although short term study. Participants with complete outcome data were similar to those without in terms of baseline PA level, ethnicity, income and education. |
| Reijonsaari et al. 200947; Reijonsaari et al. 201248 | No significant within- or between-group differences in PA: 6-month between-group adjusted MD = –365 weekly MET-minutes, 95% CI –733 to 3; 12-month between-group adjusted MD = –207 weekly MET-minutes, 95% CI –531 to 116 (negative values favour control). | No significant between-group difference in productivity (adjusted MD in QQ score at 6 months = 1.3, 95% CI –2.0 to 4.7 and adjusted MD at 12 months = –1.1, 95% CI –4.9 to 2.8). Between-group difference for change in weight and % body fat favoured control (adjusted MD for weight change at 12 months = –0.5kg, 95% CI –1.0 to 0.0; adjusted MD for change in % body fat = –0.6%, 95% CI –1.0 to –0.2). |
0 PA |
↑ weight (favoured control)
↑ % body fat (favoured control) |
Subgroup analyses by gender, job characteristics, age, self-rated baseline PA level and sickness absence days in the past year did not modify the results. Adherence to the intervention did not mediate sickness absence (MD between adhering and non-adhering subgroups was 0.0 days, 95% CI –1.2 to 0.9). Sensitivity analysis: adjusting for missing data did not affect the results. |
Engagement: decline in engagement (use of website, communication with coaches) over time, particularly in the last 6 months. |
| Reed et al. 201863 | Initial increase in MVPA but significant decline from week 2 to week 6 (i.e. post-intervention), P < 0.05. Significant decline in daily steps from baseline to week 6 (P < 0.05). There were no significant between-group differences in either MVPA (P = 0.292) or steps (P = 0.333). |
Within-group significant reductions in % body fat (−0.8±4.8, P = 0.015) and resting systolic BP (−2.6±8.8 mm Hg, P = 0.019). No significant within- or between-group changes in body mass, BMI or waist circumference. |
↓ PA(steps only, and no control group) |
↓ % body fat
↓ systolic BP |
N/A | Engagement: Participants wore the activity monitor for at least 10 hours/day for 31/42 intervention days on average (overall compliance rate 74%). Wear declined over time (e.g. average of 6.0±1.9 days per week at baseline compared with 3.5±3.0 days in week 6). Low attrition: 72/75 (96%) participants completed the study. |
| Rowe-Roberts et al. 201449 | Findings only reported descriptively. Average daily steps reported by AUSDRISK score at beginning and end of trial; ‘high’ baseline score participants that moved to ‘low’ risk at the end of the study had the highest average daily steps at the end of the study (12,294). Average overall daily steps were: High risk group: 8588 Medium risk group: 7836 Low risk group: 7878 |
23% of participants reduced their AUSDRISK score over 7 months. | ?(pre- to post- change in PA unclear and no control group) | ↓ AUSDRISK score | Results stratified by AUSDRISK score – device seemed more effective for those at high risk of diabetes (see left). | Engagement: overall low engagement with the activity monitor – average monthly dropout rate was 15% and only 36% of participants were still using the device at the end of the study. High baseline diabetes risk participants showed the highest level of engagement: Mean number of months engaged with the activity monitor = 5.7 for high risk; 4.4 for medium risk; 4.2 for low risk Acceptability: low engagement was predominantly driven by device issues, e.g. broken, lost, forgotten devices. Individual differences in preferred motivational strategies, e.g. inactive/unengaged participants preferred games whereas active/engaged participants preferred ‘goal-oriented functionalities’, e.g. smart reminders and normative information about appropriate PA levels. |
| Simons et al. 201864; Simons et al. 201865 | No significant between-group differences in any of the objective or self-reported PA outcomes at post-intervention or follow-up (PA decreased over time in the intervention and control groups). | No significant impact on any self-reported psychosocial variables. | 0 PA | 0 (improved knowledge of PA guidelines in control and intervention groups) | N/A | Engagement: Decline in engagement over time, with significant reductions in Fitbit wear, viewing graphs in the app and reading notifications. Attrition: 110/130 (85%) participants provided primary outcome data at follow-up. Acceptability: The majority of participants rated the Fitbit and app as self-explanatory (36/51, 71%), user friendly (40/51, 78%), and interesting (34/51, 67%), but few found the tips and facts motivating (10/41, 24%), used them to be physically active (8/41, 20%) and believed they were tailored to their lifestyle (7/41, 17%). Barriers to technology use included technical problems and forgetting to wear or charge the Fitbit. |
| Schrager et al. 201750 | No significant overall change in PA level. Self-reported median (IQR) days/week of ≥30 min PA: Baseline 2.5 (1.9) 1 month 2.8 (1.5) 6 months 3.0 (2.0) (P = 0.67 for change baseline to 1 month; P = 0.36 for change baseline to 6 months) PA monitor-measured median (IQR) days/week ≥10,000 steps or ≥30 min PA: Baseline 2.5 (1.9) 1 month 2.5 (2.7)(P = 0.69 for change baseline to 1 month) |
18/30 (60%) participants described a positive impact on their wellness after one month of activity monitor use. |
↑ PA
(low baseline activity level only) |
↑ wellness (qualitative report only) | Subgroup analysis by baseline activity level and device use. Significant increase in self-reported median (IQR) days/week of ≥30 min PA for the most inactive (n = 10): Baseline 1.5 (0.9) 1 month 2.4 (1.2) 6 months 2.0 (2.0) (P = 0.04 for change baseline to 1 month; P = 0.04 change baseline to 6 months) No significant between- or within-group differences in PA level for those who used the activity monitor for 6 months and those who discontinued use prior to the study end. |
Engagement: decline in engagement over time – 67% continued to use the device after one month, but only 33% still used their device after 6 months. Acceptability: barriers to use included forgetfulness, not wanting to wear the device, boredom, beliefs it was not accurate, technical issues and fashion. |
| Skogstad et al. 201651 | Significant increase in self-reported frequency of PA from baseline to follow-up (P = 0.001). % exercising at baseline:37% ≤1 day/week 47% 2–3 times/week 15% ≥4 times/week % exercising at follow-up: 13% ≤1 day/week 58% 2–3 times/week 28% ≥4 times/week Half of participants reported increased PA frequency at follow-up. Mean increase in daily low intensity PA (e.g. walking): 13.7±29.4 min for men 13.7±17.2 min for women. Mean increase in daily high intensity PA (e.g. jogging: 8.3±18.2 min for men 8.6±14.6 min for women |
Significant improvement in maximal oxygen uptake (+2.8 ml/kg/min, 95% CI 1.4–4.3, P = 0.00022). Significant reduction in total cholesterol (−0.12 mmol/L, 95% CI –0.22 to –0.01, P = 0.032) and LDL cholesterol (−0.13 mmol/L, 95% CI –0.22 to –0.04, P = 0.0034). Significant increase in diastolic BP (+1.67 mm Hg, 95% CI 0.23–3.12, P = 0.024). Despite the significant improvements in health outcomes (aerobic fitness, total and LDL cholesterol), further analysis could not attribute these to the change in individual PA levels. |
↑ PA |
↑ aerobic fitness
↓ total cholesterol ↓ LDL cholesterol ↑ diastolic BP |
Subgroup analysis by education. Education was an effect modifier for diastolic BP, total and LDL cholesterol: Only workers with low education showed a significant increase in diastolic BP (+4.4 mm Hg, 95% CI 2.03–6.86, P = 0.0004). Only workers with high education showed a significant decrease in total cholesterol (−0.21 mmol/L, 95% CI –0.08 to –0.34, P = 0.0015) and LDL cholesterol (−0.22 mmol/L, 95% CI –0.12 to –0.32, P = 0.0001). |
Attrition: Participants lost to follow-up differed from study completers – younger, lower HDL and higher CRP (and mostly men and blue collar workers). Acceptability: perceived impact on other outcomes – 12 participants reported improved nutritional habits at follow-up, 3 increased quality of sleeping, 4 reduced or quit smoking and 2 reduced alcohol intake. |
| Slootmaker et al. 200952 | No overall significant between-group difference in sedentary time or PA (mins/week) at 3 months or 8 months follow-up. 3 month between-group difference (adjusted for gender, age, education and BMI), β and 95% CI: Sedentary time: 10 (−435 to 455) Light intensity PA: –129 (−337 to 79) Moderate intensity PA: -13 (−89 to 63) Vigorous intensity PA: –6 (−75 to 62) |
No overall significant intervention effect on any secondary outcomes (aerobic fitness, determinants of PA and body composition) at 3 or 8 months. |
↓ PA
(light-intensity, highly educated only) 0 SB |
↑ awareness of PA level (overweight only)
↓ body weight (lower educated only) |
Subgroup analysis by education, adherence to programme and BMI. Education was an effect modifier for PA; higher educated intervention participants showed a significant reduction in light intensity PA at 3 months compared with control: adjusted difference in mins/week, β and 95% CI = –349 (−632 to –66), P = 0.02. The proportion of participants aware of their adherence to PA recommendations increased among overweight participants in the intervention group compared with control at 3 months (adjusted OR = 16.4, 95% CI 1.3–214, P = 0.02). There was a reduction in body weight among the lower educated intervention participants compared with control at 3 months (adjusted difference, β = –1.6kg, 95% CI –2.8 to –0.4, P = 0.01). Higher engagement/adherence to the programme did not result in increased PA. |
Engagement: majority of participants engaged with the intervention; 73% wore the activity monitor regularly and the website was used almost once a week. Acceptability: barriers to technology use included lack of interest and difficulty finding items on the website.74% of activity monitor users read the tailored advice, of whom 39% found it unappealing. |
| Thorndike et al. 201453 | At the end of phase 1, there was no significant difference between the intervention and control groups in median daily steps overall (intervention 6369 vs. control 6063, P = 0.16) or mean daily steps on days the monitor was worn (intervention 7886±3622 vs. control 7600±3492, P = 0.63). Mean daily steps were significantly higher in phase 2 (team competition) than phase 1 (individual monitoring) for those assigned to the control group in phase 1 (7971 vs. 7567, P = 0.002) but not for those in the intervention group (7832 vs. 7739, P = 0.13). |
Significant overall reduction in systolic BP from baseline to end of study: 121±15.4 mm Hg to 117±12.6 mm Hg (P = 0.004). Significant overall increase in HDL cholesterol from baseline to end of study: 57±14.7 mg/dL to 61±15.7 mg/dL (P < 0.001). No significant change in diastolic BP, weight, BMI, waist circumference, total or LDL cholesterol. |
↑ PA(team-based competition only) |
↓ systolic BP
↑ HDL cholesterol |
The authors compared mean daily steps during inpatient rotations with outpatient rotations during the whole study; physicians were significantly more active during outpatient rotations (difference of 648 steps, P < 0.001). | Engagement: compliance with wearing the activity monitor was significantly higher in phase 2 than phase 1: 77% vs. 60%, P < 0.001. |
| Torquati et al. 201866 | Significant reduction in daily MVPA from baseline to 3 months, median (IQR): 19.1 (24.6) to 13.3 (13.9) min/day (P = 0.01). Near significant reduction in daily MVPA from 3 months to 6 months: median (IQR): 13.3 (13.9) to 12.5 (13.4) min/day (P = 0.07). Significant reduction in mean daily steps from baseline to 3 months (8496±2528 to 8136±2395, P = 0.04). No significant changes in sedentary time from baseline to 3 months (P = 0.17) or from 3 months to 6 months (P = 0.64). |
Significant increase in daily fruit and vegetable intake from baseline to 3 months (P = 0.04). Significant improvement in self-rated health from month 3 to month 6 (P < 0.05). No significant changes in weight, BMI, waist circumference, blood pressure, PA or diet self-efficacy or social support. |
↓ PA
0 SB |
↑ diet(fruit and vegetable intake)
↑ self-rated health |
Subgroup analysis with participants with complete data only did not change the significance of the findings (although MVPA increased at month 6 following an initial reduction). | Engagement: Low engagement with the smartphone app, with 68.4% using it less than once per month or never. PA goals were set infrequently, and social components were not used. Attrition was high, with only 12/47 (26%) attending the 6 month follow-up. Acceptability: Participants reported that changing both PA and diet at the same time was challenging. Interviews revealed low perceived usefulness of the smartphone app. Overall reach was poor (13% of potential participants were reached and 9.4% were willing to take part). Implementation: Participants required more frequent contacts with researchers or a workplace champion. |
| van Dantzig et al. 201354 | A significantly higher reduction in computer activity (mean minutes of activity, 30 min before and after receiving (virtual) text message) was observed in the intervention group compared with control: Intervention group reduction of 10 min vs. control group reduction of 5.9 min, P < 0.001. Non-significant within- or between-group change in PA (average value during 5 min interval before and after a message) (P-value not reported). |
Not studied |
0 PA
↓ SB (but control group received mHealth intervention) |
Not studied | Engagement with the intervention was explored with subgroup analysis. There was no significant impact of proportion of text messages read on computer activity (P>0.10) and no significant interaction between proportion read and time (P>0.10), i.e. receiving messages led to breaks but content was not important. | Engagement: an average of 46% (SD = 34.6) of the total number of text messages sent were read. |
| Yeung et al. 201759 | Significant increase in median daily steps from blinded to unblinded intervention period (P = 0.001): Median (IQR) Weeks 1–4 (blinded): 7260 (2410) steps/day Weeks 5–8 (unblinded): 8266 (3306) steps/day Significant increase in number of participants achieving an average of ≥10,000 steps/day (P = 0.04): Weeks 1–4 (blinded): n = 9 (12%) Weeks 5–8 (unblinded): n = 17 (23%) |
Not studied | ↑ PA | Not studied | Subgroup analysis by occupation. Surgical residents had significantly higher steps than non-surgical residents (P = 0.018), however differential impact of the intervention was unclear. Participants who elected to join the activity tracking group had higher median daily steps than those who did not (7938 vs. 7442, P = 0.042), however the direction of the effect is unclear (may be due to prior increased motivation). |
Moderate engagement with Fitbit: device wear ranged from 91% in those volunteering for the activity tracking group to 51% in those not volunteering (unblinded period). A decline in engagement was seen over the 12 weeks in the latter group only. Attrition: high adherence with 74/86 (86%) participants completing the study. Acceptability: The most common barrier to participation was loss of the Fitbit (7/86, 8%). |
IG: intervention group; CG: control/comparison group; PA: physical activity; SB: sedentary behaviour; MVPA: moderate to vigorous physical activity; MD: mean difference; BMI: body mass index; BP: blood pressure; ±: standard deviation; N/A: non-applicable; AUSDRISK: Australian Type 2 Diabetes Risk Assessment Tool; IQR: interquartile range; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; OR: odds ratio; CDC: Centers for Disease Control.
↑ = significant increase in outcome ↓ = significant decrease in outcome 0 = no significant change in outcome (between-group or within-group)
aSignificant P-values (where reported in included papers) are in bold
Impact on PA
A significant increase in one or more measures of PA, over time or relative to the control or comparison group, was reported by 14 of the 25 studies (56%).38,40,41,43–46,50,51,53,58–60,62 These outcomes included mean (or median) daily steps, frequency and/or duration of activity, and odds of meeting step goals. Schrager and colleagues reported a significant impact of the intervention only in participants with a low baseline activity level.50 Six studies (24%) reported no significant impact on any PA outcome.42,48,54,56,61,65 Three studies (12%) reported reductions in PA; two uncontrolled studies reported reductions in daily steps,63 and MVPA66 over time, and one RCT found a reduction in light intensity PA relative to the control group, but only in a highly educated subgroup.52 It was not possible to determine the impact of the intervention in two studies; in one the pre- to post- change in PA was unclear,49 and another (a feasibility study) did not report the statistical significance of changes as there was no reliable baseline measure of PA.57 It should be noted that five of the 14 studies that found a relative increase in PA did not have a true (i.e. non-mHealth) control group (see Table 4); the results suggested that one or more mHealth or complementary components had contributed to this increase, including a smartphone app,60 motivational text messages43 and financial incentives.44,45,62
Of the 10 studies rated as ‘high’ or ‘moderate’ quality, 7 (70%) reported a significant impact of the intervention on PA.40,43,44,46,53,60,62 Only 4 of the 11 studies (36%) using a wearable activity monitor as a single mHealth tool reported a significant absolute or relative increase in PA, compared with 10 of the 14 studies (71%) using smartphone apps or activity monitors combined with apps. Of the 14 studies (64%) using multi-component interventions, 9 reported a significant impact on PA,38,40,43–45,51,53,58,62 compared with five of the 11 studies (45%) that assessed standalone mHealth interventions.41,46,50,59,60 There were no other discernible associations between type or length of intervention, type of workplace and impact on PA.
Significant effects on PA were reported from 1 month to 12 months after beginning the intervention, although only three studies reported a significant increase in PA at a time point of 6 months or later.38,40,50 In some cases an initial increase in PA was not sustained at later follow-up.44,45,62,63 In contrast, Brakenridge and colleagues reported a significant impact of the intervention at 12 months but not 3 months.38
There was wide variation in effect sizes. For example, for studies reporting a significant positive impact of the intervention on mean daily step count, this ranged from a between-group difference of around 847 (95% CI 68–1625)38 to 2183 (95% CI 992–3344)60 steps per day. The large international cohort study reported the largest effect, with a mean pre–post increase of 3519 (95% CI 3484–3553) steps per day.41
Impact on sedentary behaviour
Of the 10 studies reporting impact of their intervention on sedentary time, only 4 (40%) found a significant reduction; these were a short-term wearable activity monitor and text messaging intervention in the workplace;54 an activity monitor and smartphone app intervention;60 an activity monitor, app and behavioural counselling intervention;58 and a standalone smartphone app intervention.41 Van Dantzig and colleagues found a mean between-group difference in reduction in computer activity (a proxy for sedentary time) of 4.1 min, 30 min before and after receiving a persuasive text message.54 Gremaud et al. reported a reduction of 26.6 min (95% CI –70.9 to –17.3) in the mean longest sedentary time in those with an activity monitor and app compared with the activity monitor only group.60 Neil-Sztramko et al. found a mean reduction in both objective and self-reported weekly sedentary time of 405.5 and 425.3 min, respectively, from baseline to 12 weeks post-intervention.58 Ganesan and colleagues reported a mean reduction in self-reported daily sitting duration of 0.74 h (95% CI 0.78–0.71) after 100 days of the smartphone app intervention.41
Two studies using objective measures of sedentary time showed no significant impact of a smartphone app, pedometer and social media intervention,66 and a multi-component programme including an activity monitor and smartphone app combined with group-based action planning and a healthy living seminar.61 Another study found no impact of an activity monitor on self-reported sedentary time at either 3 or 8 months follow-up.52 A further two studies using objective measures showed significantly higher daily standing time and lower daily sedentary time respectively in controls relative to the intervention group.38,42 Another study using accelerometer data reported a significant increase in the mean proportion of time spent sedentary from baseline to follow-up, but only in workday non-work time (there was a slight reduction in proportion of work time spent sedentary).56
Impact on other outcomes
Of the 25 studies, 16 (64%) assessed the impact of the mHealth intervention on secondary outcomes including health and fitness, wellbeing and determinants of PA.37–43,47–53,55,56,58,63,65,66 Of these 16 studies, 11 (69%) found an improvement in at least one outcome over time or relative to the control or comparison group.41,43,49–53,56,58,63,66 Significant beneficial effects included weight or BMI reduction,41,58 reduced body fat percentage,63 reduced systolic blood pressure,53,63 reduced resting pulse rate,43 reduced total and low-density lipoprotein (LDL) cholesterol and increased high-density lipoprotein (HDL) cholesterol,51,53 improved ‘AUSDRISK’ (Australian Type 2 Diabetes Risk) score,49 improved diet,56,66 improved aerobic fitness,51 improved self-reported health or wellness,50,66 greater self-reported energy and emotional wellbeing,58 reduced sleep disturbance,58 and improved self-efficacy for walking.43 However, the study by Skogstad and colleagues could not attribute the changes in aerobic fitness and cholesterol levels to changes in individual PA levels.51
Slootmaker and colleagues reported a significant impact on secondary outcomes in subgroups only. This included increased awareness of PA level in overweight participants only (after 3 months) and reduced body weight in lower educated participants only (after 8 months).52 Four studies found no impact on any secondary outcomes,38,40,42,65 and one RCT found a significant between-group difference in weight loss and percentage body fat, but in favour of the control group.48 Only two studies assessed work-related outcomes including work productivity and sickness absence,48 and job performance, job control and work satisfaction;38 there was no significant effect on these outcomes in the short or long term.
Subgroup findings
The most important subgroup and sensitivity findings (where applicable) for each study are reported in Table 4. Potential effect modifiers associated with intervention effectiveness were low baseline activity level,40,46,50 lower education level,52 African American ethnicity,57 non-obesity,57 and high risk of diabetes.49
Feasibility and acceptability/additional findings
Three studies were designed primarily to assess feasibility of the intervention and/or trial methods, including measures of engagement, acceptability, attrition, demand (i.e. reach and recruitment) and implementation (i.e. delivery of the intervention).55–58,66 Many effectiveness studies also reported some of these outcomes, with engagement and attrition measured most frequently. Definitions of engagement and acceptability were variable. Engagement with interventions tended to be measured quantitatively using outcomes such as activity monitor wear time, usage time for apps, features used or proportion of text messages read. Acceptability was a broader concept incorporating both quantitative and qualitative measures such as participant satisfaction, perceived usability, perceived effectiveness and usefulness of the intervention (for PA/SB/other outcomes), preferred components, intentions to continue technology use, barriers to use/engagement, and adverse events. Only a small number of studies assessed qualitative experiences as reported by the participants as a measure of acceptability. For example, Rowe-Roberts and colleagues used focus groups to gain further insight into employee experiences of using the activity monitor,49 while Gilson and colleagues interviewed drivers and depot managers to capture experiences, insights into perceived impact of the intervention and barriers to PA.55,56
The main findings in relation to engagement, acceptability and attrition are summarised in Table 4. A clear decline in technology usage and engagement over time was reported by all longer duration studies (i.e. more than 12 weeks) that assessed these outcomes. Schrager and colleagues reported that only 33% of participants used their activity monitor after 6 months,50 Brakenridge and colleagues reported that activity monitor use had ceased in all participants by 12 months,38 and Finkelstein and colleagues found that only around 10% of participants still wore their activity monitor after 12 months.40 Common reasons for lack of engagement were broken or lost devices,49,59 forgotten devices,49,50,65 lack of interest or boredom,50,52 beliefs the device was not accurate,50 technical issues,50,60,65 fashion,50 privacy concerns,56 data usage costs,56 and usability issues such as difficulty navigating the website.52
Overall, participant satisfaction was high, and employees perceived wearable activity monitor and smartphone app interventions to be an acceptable and useful method to improve PA. Of the studies aiming to reduce SB, only two included qualitative measures of acceptability.56,66 In one study, the activity monitor and smartphone app were perceived by drivers and depot managers as feasible, acceptable and as having a positive impact on PA and SB.56 In contrast, a study of a smartphone app for improving diet and PA (and reducing SB) in nurses found low perceived usefulness, and interviews revealed difficulties in changing more than one behaviour at a time, and the desire for a workplace champion to implement the intervention.66 Additional findings in relation to acceptability included individual differences in preferred motivational strategies according to levels of activity and engagement,49 and higher compliance with activity monitor wear with team-based competition as opposed to individual monitoring.53 It is also important to consider adverse events associated with technology use; in one study around 27% of participants reported at least one adverse event, typically related to reactions to wearing the activity monitor or accelerometer.38 Due to the relatively small number of studies reporting measures of acceptability, and the heterogeneity of interventions and outcomes, no associations between acceptability and intervention type or length or type of workplace were evident.
Attrition rates ranged from 0% to 74%. Predictors of loss to follow-up included female gender,40 younger age51 and ethnicity, with lower attrition in Chinese participants.40
Discussion
While methodological quality of many of the included studies was weak, based on this review there is reasonable evidence that mHealth interventions in workplace settings are a potentially effective and feasible method for increasing PA. There is some evidence that they may also be effective in reducing SB. However, findings are mixed and effect sizes are small, particularly for the impact on SB and in the longer term.
A significant increase in PA, either over time or relative to the control or comparison group, was observed in 56% (14/25) of studies, and in a higher proportion of studies rated as ‘high’ or ‘moderate’ quality (7/10, 70%). The findings in relation to SB were less clear, with only 40% (4/10) of studies reporting a significant reduction in sedentary time, and a further three studies reporting relative increases in certain measures of sedentary time. It may be that reducing sedentary time at work leads to corresponding increases in time spent sedentary outside of work; this demonstrates the importance of holistic interventions that take both work and non-work contexts into account.56
It is important to assess feasibility in addition to effectiveness of complex interventions such as mHealth.77,78 Many studies included measures of engagement with the intervention, and the vast majority showed a decline in engagement over time. It is not yet clear whether this disengagement from the technology is detrimental to behaviour change or if sustained behaviour change can be achieved without continued used of the mHealth tool. Future studies could draw comparisons with, and learn from, eHealth interventions to reduce SB and increase PA in the workplace, such as the studies by Mainsbridge et al. (2014),79 Pedersen et al. (2014)80 and Irvine et al. (2011).81 Only a small number of studies included qualitative measures of acceptability such as interviews to explore participants’ experiences, mechanisms of behaviour change and reasons for the decline in engagement. Future studies should focus on these areas. There also appears to be a need for more standardised definitions, assessment and reporting of engagement and acceptability in the field of mHealth.26
The findings generally concur with the existing evidence for potential effectiveness and acceptability, most prominent in the short term, reported in reviews of mHealth interventions for PA and SB in non-workplace contexts.17,19–21,23 Due to considerable heterogeneity and the small number of high quality studies, it was not possible to draw any definitive conclusions on the relative effectiveness or acceptability of different types of interventions, although there was some evidence that wearable activity monitors alone, and standalone mHealth interventions with no additional ‘offline’ components, were less likely to result in increased PA. Similarly, previous reviews have suggested that multi-component interventions may be more effective than standalone mHealth interventions.23,30
This review is the first to focus on mHealth technology for the promotion of PA and reduction of SB in workplace interventions. A recent systematic review by Stephenson and colleagues that assessed the impact of computer-based, mobile and wearable technologies on SB suggested that the effects of workplace interventions may be more prominent than non-workplace interventions at medium-term follow-up.27 While there was insufficient data to test this hypothesis in the present review, this highlights the potential importance of setting and the possibility of differential results.
There was a small amount of evidence to suggest that mHealth for PA and SB may be more effective for more sedentary employees,40,46,50 and those with lower levels of education.52 There may also be differential effectiveness according to health status at baseline,49 BMI and ethnicity.57 Future studies should aim to clarify which subgroups are likely to benefit most from workplace mHealth interventions. The acceptability and impact of mHealth for underrepresented groups such as shift workers, who experience unique barriers to PA and may have an elevated risk of cardiovascular disease,82 diabetes83 and obesity,84 should also be explored further.
The review found some evidence for a positive impact on health and wellbeing outcomes (physiological and psychological) of mHealth interventions for PA and SB. It is recommended that future studies investigate the wider impact on health and wellbeing in addition to measures of ‘organisational wellness’ such as productivity, sickness absence and economic analyses, which were included as outcomes in only a small minority of studies. Most studies included in this review focused on workplaces in developed countries, with many based in academic and healthcare organisations. There will be a need for more diverse samples in a greater range of workplace settings as mHealth becomes more prevalent.
A ‘weak’ quality rating was assigned to a high proportion (15/25, 60%) of studies. Selection bias and lack of blinding were the weakest areas overall, although these are common issues in workplace and mHealth interventions.21,85 Many studies lacked a true control group or did not include a reliable measure of baseline activity. Studies were highly heterogeneous in terms of methodology and outcomes, and some studies used data collection methods for the primary PA or SB outcome with below satisfactory validity and reliability. The mHealth tool itself may be an efficient method for data collection, for example most commercial activity monitors provide a real-time, objective, valid and reliable measure of step count.74 This will be an important advance for studies that currently rely on self-reported data. There is also a need for improved reporting and consistent use of outcome measures to facilitate future synthesis of findings and meta-analyses. Combined with the relatively small number of included studies and mostly small sample sizes, these factors make it difficult to draw definitive conclusions regarding the impact of mHealth on PA and SB.
The most frequently used mHealth interventions were wearable activity monitors or trackers and/or smartphone apps. However, interventions were highly heterogeneous in terms of both mHealth and additional content, frequency, duration and mode of delivery. Similar to previous reviews of mHealth for PA and SB, the most commonly identified BCTs included self-monitoring, feedback, goal-setting and social comparison.16,23,27 Several studies incorporated rewards (virtual or real) and social support in their interventions. Prompts and cues were more frequently used to target SB; this BCT was also frequent in the workplace interventions reviewed by Stephenson et al., compared with non-workplace interventions.27 However, descriptions of interventions and BCTs were unclear or incomplete in many cases and it was not possible to determine with confidence which specific techniques were incorporated.
Future studies should aim for more transparent reporting of intervention content and specification of embedded BCTs, to facilitate identification of the most impactful and acceptable intervention components. There may also be a need for new behaviour change taxonomies specifically for mHealth interventions, for example to include in-app competitions, various types of gamification, virtual avatars, and to distinguish between virtual and real rewards. It was also apparent that many interventions did not have a strong theoretical or evidence basis. It has been suggested that new behaviour change models and theories may be needed to account for the interactive, dynamic and adaptive nature of mHealth interventions.86
Long-term impact and acceptability of mHealth technology is still unclear. There is a need for studies with longer duration of follow-up, further qualitative investigation of reasons for the substantial decline in engagement over time and subsequently how engagement may be maximised. Mixed methods studies will be particularly valuable to elucidate the feasibility and acceptability of mHealth to promote PA and reduce SB in a workplace setting, as well as determining the longer-term impact on outcomes.
Strengths and limitations
Strengths of this review are that it was conducted in accordance with PRISMA guidelines,29 the robust nature of the search strategy, study selection and data extraction process, and the systematic assessment of study quality using the EPHPP tool.31 The review comprehensively included a range of study types, with a combination of quantitative and mixed methods studies. This enabled synthesis of findings related to acceptability and engagement in addition to intervention effectiveness. The identification of BCTs using an established taxonomy will facilitate comparison of interventions and possible future replication. The review is the first to consider studies of mHealth for PA and SB that were conducted in a workplace setting.
The main limitations are that meta-analysis could not be performed due to the relatively small number of included studies, heterogeneity of methods and outcomes and incomplete reporting. The high proportion of studies rated ‘weak’ for methodological quality limits confidence in the findings. Furthermore, the possibility of publication bias should be recognised.
Summary of recommendations for future research
According to the findings of this review, it is recommended that future studies:
Use larger samples in more diverse workplace settings (outside of academia and healthcare), include underrepresented groups such as shift workers, and consider behaviour both within and outside of the workplace.
Report more fully intervention components (including the identification of BCTs using established taxonomies such as CALO-RE) and outcomes.
Focus on SB in addition to PA, and use objective and efficient data collection methods (including the mHealth tool itself) to capture this data.
Where practicable, include a no-intervention control (experimental studies) or at least a reliable baseline measure of PA/SB (for quasi-experimental studies).
Consider the wider impact on health and wellbeing, and work-related outcomes such as productivity and sickness absence.
Use mixed and qualitative methods to explore short- and long-term impact, feasibility and acceptability, including participants’ experiences, reasons for the decline in engagement with mHealth technology, mechanisms of behaviour change, and the relationship between engagement and intervention effectiveness.
Capture data on adverse events associated with mHealth technology use.
Explore further the relative impact and feasibility of standalone mHealth and multi-component interventions, including those combined with other online and offline components.
Explore subgroup differences, including which interventions and components/BCTs are most acceptable and impactful, and for whom.
Conclusion
There is reasonable evidence to support the use of mHealth in the promotion of PA in workplace interventions. Despite low methodological quality, early studies have demonstrated feasibility, acceptability and potential effectiveness of mHealth based interventions in a workplace context. The longer-term impact, and the impact on SB, are less clear. There is a clear need for new high quality, mixed methods studies with better reporting of interventions and outcomes, in order to explore the reasons for decline in engagement over time and the longer-term potential of mHealth in workplace interventions for promoting PA and reducing SB.
Supplemental Material
Supplemental material, Supplemental Material1 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health
Supplemental Material
Supplemental material, Supplemental Material2 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health
Supplemental Material
Supplemental material, Supplemental Material3 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health
Acknowledgements
We would like to thank the PenCLARHC Evidence Synthesis Team at University of Exeter Medical School for their support and assistance.
Contributorship
SAB conceptualised and designed the study with support from AJW, KM, LP and JH. SAB conducted the searches. SAB and AJW completed screening, data extraction, quality assessment and BCT coding. SAB drafted and wrote the manuscript; AJW, KM, LP and JH provided critical feedback. All authors read and approved the final version.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SAB is supported by a PhD studentship from the University of Exeter Medical School and Devon and Cornwall Police.
Guarantor
SAB
Peer Review statement
This manuscript was reviewed by Corneel Vandelanotte, Institute for Health and Social Science Research, Central Queensland University, Australia; Ana Howarth, Cigna Global Well-being Solutions Ltd, USA; and S. Abdin, University of the West of England, United Kingdom.
Supplementary Material
Supplementary material is available for this article online.
References
- 1.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 2009; 43: 1–2. [PubMed] [Google Scholar]
- 2.Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: an epidemiological perspective. Sports Med 2001; 31: 101–114. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Shi Y, Li T, et al. Leisure time physical activity and cancer risk: evaluation of the WHO's recommendation based on 126 high-quality epidemiological studies. Br J Sports Med 2016; 50: 372–378. [DOI] [PubMed] [Google Scholar]
- 4.Metzger J, Catellier D, Evenson K, et al. Associations between patterns of objectively measured physical activity and risk factors for the metabolic syndrome. Am J Health Promot 2010; 24: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015; 175: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped Prospective Cohort Study. PloS one 2015; 10: e0141274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen N, Healy G, Matthews C, et al. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev 2010; 38: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunstan DW, Thorp AA, Healy GN. Prolonged sitting: is it a distinct coronary heart disease risk factor? Curr Opin Cardiol 2011; 26: 412–419. [DOI] [PubMed] [Google Scholar]
- 9.Wilmot E, Edwardson C, Achana F, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012; 55: 2895–2905. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Occupational Safety and Health. Working well: Guidance on promoting health and wellbeing at work, www.iosh.co.uk/workingwell (2015, accessed 1 October 2017).
- 11.Miller R, Brown W. Steps and sitting in a working population. Int J Behav Med 2004; 11: 219–224. [DOI] [PubMed] [Google Scholar]
- 12.Kazi A, Duncan M, Clemes S, et al. A survey of sitting time among UK employees. Occup Med (Lond) 2014; 64: 497–502. [DOI] [PubMed] [Google Scholar]
- 13.Clemes SA, O'Connell SE, Edwardson CL. Office workers' objectively measured sedentary behavior and physical activity during and outside working hours. J Occup Environ Med 2014; 56: 298–303. [DOI] [PubMed] [Google Scholar]
- 14.Hendriksen IJ, Bernaards CM, Steijn WM, et al. Longitudinal relationship between sitting time on a working day and vitality, work performance, presenteeism, and sickness absence. J Occup Environ Med 2016; 58: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriksen IJ, Snoijer M, de Kok BP, et al. Effectiveness of a multilevel workplace health promotion program on vitality, health, and work-related outcomes. J Occup Environ Med 2016; 58: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health 2016; 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Direito A, Carraça E, Rawstorn J, et al. mHealth technologies to influence physical activity and sedentary behaviors: behavior change techniques, systematic review and meta-analysis of randomized controlled trials. Ann Behav Med 2017; 51: 226–239. [DOI] [PubMed] [Google Scholar]
- 18.Flores Mateo G, Granado-Font E, Ferré-Grau C, et al. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res 2015; 17: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanning J, Mullen SP, McAuley E. Increasing physical activity with mobile devices: a meta-analysis. J Med Internet Res 2012; 14: e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bort-Roig J, Gilson ND, Puig-Ribera A, et al. Measuring and influencing physical activity with smartphone technology: a systematic review. Sports Med 2014; 44: 671–686. [DOI] [PubMed] [Google Scholar]
- 21.Muntaner A, Vidal-Conti J, Palou P. Increasing physical activity through mobile device interventions: A systematic review. Health Inform J 2016; 22: 451–469. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly GA, Spruijt-Metz D. Current mHealth technologies for physical activity assessment and promotion. Am J Prev Med 2013; 45: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act 2016; 13: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology 2008; 27: 379–387. [DOI] [PubMed] [Google Scholar]
- 25.Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011; 11: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCallum C, Rooksby J, Gray CM. Evaluating the impact of physical activity apps and wearables: Interdisciplinary review. JMIR mHealth uHealth 2018; 6: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson A, McDonough SM, Murphy MH, et al. Using computer, mobile and wearable technology enhanced interventions to reduce sedentary behaviour: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 2017; 14: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howarth A, Quesada J, Silva J, et al. The impact of digital health interventions on health-related outcomes in the workplace: A systematic review. Digit Health 2018; 4: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 30.Nuffield Health. A Healthier Workplace: How employers can reduce physical inactivity, https://www.sportspartnershiphw.co.uk/uploads/a-healthier-workplace-nuffield-health-whitepaper.pdf (2018, accessed 13 December 2018).
- 31.Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004; 1: 176–184. [DOI] [PubMed] [Google Scholar]
- 32.Armijo-Olivo S, Stiles CR, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012; 18: 12–18. [DOI] [PubMed] [Google Scholar]
- 33.Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011; 26: 1479–1498. [DOI] [PubMed] [Google Scholar]
- 34.Conroy D, Yang C, Maher J. Behavior change techniques in top-ranked mobile apps for physical activity. Am J Prev Med 2014; 46: 649–652. [DOI] [PubMed] [Google Scholar]
- 35.Mercer K, Li M, Giangregorio L, et al. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR mHealth uHealth 2016; 4: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013; 46: 81–95. [DOI] [PubMed] [Google Scholar]
- 37.Brakenridge CL, Fjeldsoe BS, Young DC, et al. Organizational-level strategies with or without an activity tracker to reduce office workers' sitting time: rationale and study design of a pilot cluster-randomized trial. JMIR Res Protoc 2016; 5: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brakenridge CL, Fjeldsoe BS, Young DC, et al. Evaluating the effectiveness of organisational-level strategies with or without an activity tracker to reduce office workers' sitting time: a cluster-randomised trial. Int J Behav Nutr Phys Act 2016; 13: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkelstein EA, Sahasranaman A, John G, et al. Design and baseline characteristics of participants in the TRial of Economic Incentives to Promote Physical Activity (TRIPPA): a randomized controlled trial of a six month pedometer program with financial incentives. Contemp Clin Trials 2015; 41: 238–247. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein EA, Haaland BA, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 983–995. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan AN, Louise J, Horsfall M, et al. International mobile-health intervention on physical activity, sitting, and weight: The Stepathlon Cardiovascular Health Study. J Am Coll Cardiol 2016; 67: 2453–2463. [DOI] [PubMed] [Google Scholar]
- 42.Jones CA. Examining the efficacy and feasibility of digital activity monitors and shared active desks to reduce employee sedentary behavior PhD Thesis, The University of North Carolina at Chapel Hill, Ann Arbor, 2016.
- 43.Koyle AE. The value of infusing self-efficacy theory with smartphone technology to sustain walking for exercise in a worksite population PhD Thesis, The University of Utah, Ann Arbor, 2013.
- 44.Patel MS, Asch DA, Rosin R, et al. Framing financial incentives to increase physical activity among overweight and obese adults: a randomized, controlled trial. Ann Intern Med 2016; 164: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel MS, Asch DA, Rosin R, et al. Individual versus team-based financial incentives to increase physical activity: a randomized, controlled trial. J Gen Intern Med 2016; 31: 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirier J, Bennett WL, Jerome GJ, et al. Effectiveness of an activity tracker- and internet-based adaptive walking program for adults: a randomized controlled trial. J Med Internet Res 2016; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reijonsaari K, Vehtari A, van Mechelen W, et al. The effectiveness of physical activity monitoring and distance counselling in an occupational health setting – a research protocol for a randomised controlled trial (CoAct). BMC Public Health 2009; 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reijonsaari K, Vehtari A, Kahilakoski OP, et al. The effectiveness of physical activity monitoring and distance counseling in an occupational setting – Results from a randomized controlled trial (CoAct). BMC Public Health 2012; 12: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe-Roberts D, Cercos R, Mueller F. Preliminary results from a study of the impact of digital activity trackers on health risk status. Stud Health Technol Inform 2014; 204: 143–148. [PubMed] [Google Scholar]
- 50.Schrager JD, Shayne P, Wolf S, et al. Assessing the influence of a fitbit physical activity monitor on the exercise practices of emergency medicine residents: a pilot study. JMIR mHealth and uHealth 2017; 5: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skogstad M, Lunde LK, Skare O, et al. Physical activity initiated by employer and its health effects; an eight week follow-up study. BMC Public Health 2016; 16: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slootmaker SM, Chinapaw MJM, Schuit AJ, et al. Feasibility and effectiveness of online physical activity advice based on a personal activity monitor: randomized controlled trial. J Med Intern Res 2009; 11: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorndike AN, Mills S, Sonnenberg L, et al. Activity monitor intervention to promote physical activity of physicians-in-training: randomized controlled trial. PloS one 2014; 9: e100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dantzig S, Geleijnse G, van Halteren AT. Toward a persuasive mobile application to reduce sedentary behavior. Pers Ubiquitous Comput 2013; 17: 1237–1246. [Google Scholar]
- 55.Gilson ND, Pavey TG, Vandelanotte C, et al. Chronic disease risks and use of a smartphone application during a physical activity and dietary intervention in Australian truck drivers. Aust N Z J Public Health 2016; 40: 91–93. [DOI] [PubMed] [Google Scholar]
- 56.Gilson ND, Pavey TG, Wright OR, et al. The impact of an m-Health financial incentives program on the physical activity and diet of Australian truck drivers. BMC Public Health 2017; 17: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Losina E, Smith SR, Usiskin IM, et al. Implementation of a workplace intervention using financial rewards to promote adherence to physical activity guidelines: a feasibility study. BMC Public Health 2017; 17: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neil-Sztramko SE, Gotay CC, Sabiston CM, et al. Feasibility of a telephone and web-based physical activity intervention for women shift workers. Transl Behav Med 2017; 7: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeung J, Mazloomdoost D, Crisp CC, et al. Impact of electronic feedback and peer comparisons on residents' physical activity level. J Grad Med Educ 2017; 9: 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gremaud AL, Carr LJ, Simmering JE, et al. Gamifying accelerometer use increases physical activity levels of sedentary office workers. J Am Heart Assoc 2018; 7: e007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsen HM, Brown WJ, Kolbe-Alexander T, et al. A brief self-directed intervention to reduce office employees' sedentary behavior in a flexible workplace. J Occup Environ Med 2018; 60: 954–959. [DOI] [PubMed] [Google Scholar]
- 62.Patel MS, Volpp KG, Rosin R, et al. A randomized, controlled trial of lottery-based financial incentives to increase physical activity among overweight and obese adults. Am J Health Promot 2018; 32: 1568–1575. [DOI] [PubMed] [Google Scholar]
- 63.Reed JL, Cole CA, Ziss MC, et al. The impact of web-based feedback on physical activity and cardiovascular health of nurses working in a cardiovascular setting: a randomized trial. Front Physiol 2018; 9: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simons D, De Bourdeaudhuij I, Clarys P, et al. A smartphone app to promote an active lifestyle in lower-educated working young adults: development, usability, acceptability, and feasibility study. JMIR mHealth uHealth 2018; 6: e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simons D, De Bourdeaudhuij I, Clarys P, et al. Effect and process evaluation of a smartphone app to promote an active lifestyle in lower educated working young adults: cluster randomized controlled trial. JMIR mHealth uHealth 2018; 6: e10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torquati L, Kolbe-Alexander T, Pavey T, et al. Changing diet and physical activity in nurses: a pilot study and process evaluation highlighting challenges in workplace health promotion. J Nutr Educ Behav 2018; 50: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 67.Azjen I, Fishbein M. Understanding attitudes and predicting social behaviour. London: Pearson, 1980. [Google Scholar]
- 68.Robinson T. Applying the socio-ecological model to improving fruit and vegetable intake among low-income African Americans. J Community Health 2008; 33: 395–406. [DOI] [PubMed] [Google Scholar]
- 69.Bandura A. Self-efficacy: the exercise of control. New York: Freeman, 1997. [Google Scholar]
- 70.Deci E, Ryan R. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychol Inq 2000; 11: 227–268. [Google Scholar]
- 71.Leary MR. Self-presentational processes in exercise and sport. J Sport Exerc Psychol 1992; 14: 339–351. [Google Scholar]
- 72.Cialdini R. Influence, science and practice. Boston: Allen & Bacon, 2001. [Google Scholar]
- 73.Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (HAPA). Rehabil Psychol 2011; 56: 161–170. [DOI] [PubMed] [Google Scholar]
- 74.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015; 12: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 76.Chinapaw MJ, Slootmaker SM, Schuit AJ, et al. Reliability and validity of the Activity Questionnaire for Adults and Adolescents (AQuAA). BMC Med Res Methodol 2009; 9: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med 2009; 36: 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mainsbridge CP, Cooley PD, Fraser SP, et al. The effect of an e-health intervention designed to reduce prolonged occupational sitting on mean arterial pressure. J Occup Environ Med 2014; 56: 1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pedersen SJ, Cooley PD, Mainsbridge C. An e-health intervention designed to increase workday energy expenditure by reducing prolonged occupational sitting habits. Work 2014; 49: 289–295. [DOI] [PubMed] [Google Scholar]
- 81.Irvine AB, Philips L, Seeley J, et al. Get moving: a web site that increases physical activity of sedentary employees. Am J Health Promot 2011; 25: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puttonen S, Härmä M, Hublin C. Shift work and cardiovascular disease – pathways from circadian stress to morbidity. Scand J Work Environ Health 2010; 36: 96–108. [DOI] [PubMed] [Google Scholar]
- 83.Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 2015; 72: 72–78. [DOI] [PubMed] [Google Scholar]
- 84.Sun M, Feng W, Wang F, et al. Meta-analysis on shift work and risks of specific obesity types. Obes Rev 2018; 19: 28–40. [DOI] [PubMed] [Google Scholar]
- 85.Cancelliere C, Cassidy JD, Ammendolia C, et al. Are workplace health promotion programs effective at improving presenteeism in workers? A systematic review and best evidence synthesis of the literature. BMC Public Health 2011; 11: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riley WT, Rivera DE, Atienza AA, et al. Health behavior models in the age of mobile interventions: are our theories up to the task? Trans Behav Med 2011; 1: 53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health
Supplemental material, Supplemental Material2 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health
Supplemental material, Supplemental Material3 for Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review by Sarah Ann Buckingham, Andrew James Williams, Karyn Morrissey, Lisa Price and John Harrison in Digital Health