Abstract
Positive immunohistochemistry (IHC) controls are intended to detect problems in both immunostaining and heat-induced epitope retrieval (HIER). However, it is not known what features in a control are important for verifying HIER. Contrary to expectation, the fact that a tissue is formalin-fixed does not necessarily render it suitable in verifying proper HIER. Some tissue controls, for some immunostains, strongly stain even without HIER. Consequently, the control may verify the immunostain but provide little or no information regarding the HIER step. To sort this out, we used formalin-fixed peptide epitopes, a model that provides for precise definition of analyte concentration, epitope composition, and degree of fixation. Our data demonstrate that formalin fixation generates a variable level of protein epitope masking, depending on the epitope recognized by the primary antibody. Some epitopes are highly masked while others hardly at all. Furthermore, the ability of amino acids in the epitope to react with formaldehyde can, at least in part, account for this variability. Most important, we demonstrate the importance of selecting a positive control with a low or intermediate analyte concentration (relative to the immunostain’s analytic sensitivity). High analyte concentrations can be insensitive in verifying the HIER step.
Keywords: antigen retrieval, control, epitope, formaldehyde, IHC, immunohistochemistry, immunostain, peptide
Introduction
Before the initial description of heat-induced epitope retrieval (HIER, also known as “antigen retrieval”) in 1991,1 the need for formalin fixation often frustrated attempts to develop clinically useful immunohistochemical stains. Formalin fixation provides the benefit of tissue preservation but was known to abrogate immunoreactivity. The advent of HIER overcame that limitation, fostering explosive growth in Diagnostic Immunohistochemistry (IHC) testing.2 However, the molecular explanation for why HIER restores immunoreactivity was slow to emerge. The fact that boiling restores protein immunoreactivity was initially counterintuitive. Boiling is typically considered a denaturing process, contrary to what might be expected for restoring immunoreactivity.
As a greater understanding emerged over the years describing why HIER restores immunoreactivity, there is now an opportunity to focus on the optimal parameters for verifying the proper performance of HIER in a Diagnostic IHC laboratory. This report focuses on quality control (QC) for the HIER step. Like the subsequent immunostaining steps that comprise the analytic phase of the test, proper HIER is verified with the benefit of an external control. There is perhaps an unspoken assumption that formalin-fixed cells and tissues, by their very nature, control for HIER. According to this assumption, the fact that they are fixed in formalin automatically renders them as sensitive controls for the HIER step. However, we are not aware of controlled studies testing that assumption. In fact, the literature possibly suggests the opposite. There are now some widely used primary antibodies that strongly stain even without HIER.3–7 Tissue sections that strongly stain without the need for HIER are incapable of controlling for HIER. Although there is a large body of literature describing HIER, comparatively little attention has been given to characterizing which features are important for verifying the HIER step. That is the purpose of this report.
We previously described the use of peptide epitopes (attached to a glass surface) as a model system for studying HIER.8–11 Peptide epitopes are substantially simpler models than biological samples such as cells and tissues. Consequently, well-defined model systems comprised of proteins12–16 or peptides17–20 offer advantages as experimental models. For example, it is difficult to examine the role of analyte concentration in a tissue section because there are no readily available methods of measurement (in absolute units such as molecules per cell). Peptide epitopes, however, can be readily quantified in traceable units of measure. For tissue sections, it is impractical to dissect out which of the many potential formaldehyde-mediated chemical reactions are relevant to restoring immunoreactivity after HIER. Peptide epitopes, on the contrary, are an easier model with which to study the effect of amino acid composition on immunoreactivity after formalin fixation. Using the peptide epitope model, we previously demonstrated that HIER can reverse a cross-linking reaction between tyrosine and arginine, thereby restoring immunoreactivity after formalin fixation.10 These two amino acids can interact in a Mannich reaction.
In this study, we used this peptide epitope model to investigate the role of three variables on HIER. Those variables are as follows: analyte concentration, epitope composition, and degree of fixation.
Materials and Methods
Peptide Epitope-coated Microbeads
The peptide epitope-coated microbeads were previously described.21–23 Briefly, cell-sized glass microbeads (Cospheric, Santa Barbara, CA) serve as a solid surface on which peptides corresponding to the native sequence of human epidermal growth factor receptor type 2 (HER2), estrogen receptor (ER), or progesterone receptor (PR) are anchored. The microbead suspension is comprised of two different types of microbeads: analyte-coated glass test microbeads (7–8 µm diameter) and color standard microbeads (4.5 µm diameter). The analyte-coated microbeads bear covalently linked peptide epitopes for HER2, ER, and/or PR. The microbeads are suspended in a proprietary clear liquid that hardens after application to the glass microscope slide, thereby retaining the microbeads on the glass slide during baking, deparaffinization, antigen retrieval, and IHC staining. Once dried, the droplet can be treated as one would treat a tissue sample. Each dried microliter droplet on the slide incorporates approximately 5000 analyte-coated (test) microbeads. Among these 5000 microbeads are some that bear the relevant analyte and others that have an antigenically irrelevant peptide, as a negative internal control.
The microbead suspension also includes smaller color standard microbeads, which are permanently colored dark brown regardless of the IHC staining procedure. The color standard microbeads serve as a color intensity reference for standardizing color intensity measurements of the peptide epitope-coated microbeads by image analysis.
The peptide epitope-coated test microbeads are manufactured at a series of different analyte (peptide) concentrations that differ from each other by approximately one log, ranging from approximately 106 molecules/microbead (the highest concentration) to 102 (the lowest concentration). The method of analyte quantification with the peptide epitope-coated microbeads was previously described.21 After peptide conjugation, some groups of microbeads are fixed in formaldehyde in the presence of casein, as previously described.22 The commercial casein preparation contains all four casein subtypes (α, β,, γ, and κ). We selected casein as an inexpensive protein that is readily available.
Cleavage of the ivDde Protecting Group on Lysines
By design, peptide epitopes are anchored to a chemically activated microbead via a terminal amine. This design requires that each peptide has only one amine for covalent linkage to microbeads. However, two peptide epitopes contain an internal lysine residue, each of which has an epsilon amine. These peptides are for the HER2 SP3 and PR 1E2 immunostains.
To prevent the epsilon amine from binding to the glass surface, we block the epsilon amine of epitope lysine residues by incorporating 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3 methylbutyl-D-lysine (“ivDde”) during synthesis.
The immunostains function properly with peptide epitopes without removing the ivDde group. The ivDde group does not appear to interfere with primary antibody binding. Nonetheless, the ivDde group can be cleaved after the peptide is coupled to the glass microbead, restoring the epsilon amine to the lysine. We removed the ivDde group in certain experiments (as indicated in the Results), to evaluate the importance of lysine residues in the epitope during formalin fixation and HIER.
The ivDde group is cleaved with 4% hydrazine (Sigma-Aldrich, St. Louis, MO) in dimethyl formamide (DMF, Thermo-Scientific, Waltham, MA) after the peptide was coupled to glass microbeads. An aliquot of peptide-coated test microbeads was incubated with 4% hydrazine in DMF for 9 min at room temperature. The microbeads were then sedimented by centrifugation in a tabletop mini centrifuge at 2000 × g for 2 min and the supernatant aspirated out. The microbeads were then rinsed twice more with DMF. Each rinse was performed by adding 1 ml DMF, vortex mixing, and sedimenting the microbeads. Next, the microbeads were rinsed three times with 0.2 M potassium phosphate buffer (KH2PO4, pH 8.6, Sigma-Aldrich Corp., St. Louis, MO) with 0.02% casein (Sigma-Aldrich, St. Louis, MO). These microbeads, after cleavage of the ivDde group, were then used in experiments designed to evaluate the role of lysine residues in formalin fixation and HIER. The fact that the epsilon amine was restored after cleavage of the ivDde group rendered the (epitope’s) lysine residue potentially reactive during a subsequent formalin fixation step. The effect of restoring the epsilon amine was examined by measuring immunoreactivity with and without HIER.
Photomicroscopy
Images were acquired as previously described.21 Photomicroscopy was performed with a Zeiss Axioskop microscope fitted with a Spot Imaging Solutions Insight Gigabit charge-coupled device (CCD) camera (Diagnostic Instruments Inc., Sterling Heights, MI). Before photomicroscopy, the camera was white-balanced and a flat-field correction was performed. For brightfield photomicroscopy of peptide epitope-coated microbeads, the microscope optics are first set for Köhler illumination. Stained tissue sections are photographed with Köhler illumination. For peptide epitope-coated microbeads, once Köhler illumination is established, the condenser aperture is opened wide because the microbeads have more than sufficient contrast. With this adjustment, unstained test microbeads are faintly visible alongside stained microbeads. The camera software was set at a gamma of 1.0, using manual (fixed) photographic exposure times. Whole slide imaging was not used. Each slide’s color intensity was measured by averaging three images per peptide epitope microbead spot (slide). Each data point in the Results represents the mean ± SD of triplicate slides.
Stain Intensity Image Quantification
Stain intensity of peptide epitope microbeads was quantified with a custom algorithm embedded in MatLab, as previously described.22 The algorithm measures image intensity of the test microbeads’ rims relative to the color standard microbeads’ rims. Consequently, stain intensity is expressed as a ratio. A score of 1.0 means that the test microbeads, stained for HER2, ER, or PR, are equally intense in color (expressed in mean pixel intensity) as the color standard microbeads. A score ≥1 represents strong stain intensity.
Tissue Sections
For ER tissue immunostaining, archival formalin-fixed, paraffin-embedded tissue blocks were obtained from the Tissue Biorepository of the Department of Pathology and Laboratory Medicine, Maine Medical Center, under an approved Institutional Review Board protocol.
Immunohistochemistry Staining
Slides were initially baked at approximately 57C to 60C for 40 min, deparaffinized in xylene, and then hydrated in decreasing grades (percentages) of ethanol. IHC staining was performed using the Dako Corp./Agilent Autostainer Plus (Carpinteria, CA). The open architecture of the instrument simplified the use of immunostains from multiple manufacturers. Several immunostains were purchased from Dako/Agilent Corp. (Carpinteria, CA): HercepTest, PR 636, PR 1294, and ER 1D5/2-123. The HER2 and ER/PR PharmDx kits are sold with prediluted solutions and reagents, and were stained according to the manufacturer’s instructions. Two immunostains were purchased from Leica Corp. (Newcastle Upon Tyne, UK): ER 6F11 and PR 16. We used the Leica ER 6F11 and PR 16 antibodies with the Leica kit detection reagents. Four immunostains were purchased from Ventana Medical/Roche Corp. (Tucson, AZ): ER SP1, PR 1E2, HER2 4B5, and HER2 SP3. These Ventana primary antibodies were coupled with the Ventana kit detection reagents. The slides were immunostained on the Dako Autostainer, modeling the protocol that occurs on the Benchmark XT. As the Autostainer does not warm the slides as the Benchmark XT does, the incubations were slightly extended, from 16 min on the Benchmark XT to 30 min on the Autostainer Plus. Moreover, we diluted the Ventana reagents 1:3 in Tris-buffered saline with 0.05% Tween-20. This dilution simulates the dilution that occurs on the slide when dispensed in a Benchmark XT. At the end of each immunostaining protocol, the slides were counterstained with Hematoxylin, dehydrated through increasing grades (percentages) of ethanol, immersed in xylene, and coverslipped using Permount (ThermoFisher Corp., Waltham, MA).
HIER Conditions
Three different HIER protocols were used depending on the immunostain. HIER for HER2 immunostains (HercepTest, 4B5, and SP3) were performed using Dako’s HIER solution (provided with the HercepTest kit), heated in a water bath to 97C to 99C for 40 min. For ER/PR HIER using monoclonal antibodies (MAbs) ER 1D5/2-123, ER SP1, PR 636, PR 1294, and PR 1E2, the slides were processed for 25 min in a Biocare Medical Decloaking Chamber pressure cooker using the solution provided with the Dako PharmDx kit. For ER 6F11 and PR 16 MAbs, HIER was performed using the Leica Bond Polymer Refine Detection kit HIER solution for 40 min at 97C to 99C (in a water bath). For experimental groups that were not subjected to HIER, the slides remained in water after deparaffinization and hydration. Before loading onto the Autostainer for staining, they were immersed in Tris-buffered saline with 0.05% Tween-20.
Statistical Analysis
Each data point represents the mean ± SD from triplicate slides. Each slide bears a peptide epitope control spot containing approximately 5000 analyte-coated microbeads. To quantify a single peptide epitope control spot, we photographically sampled three different microscopic areas. This is analogous to photographically sampling three fields of a patient’s breast carcinoma for assessment of HER2 or ER/PR. From these three fields, we calculated the mean stain intensity per spot (slide). Each peptide-coated microbead stain intensity data point represents the mean ± SD of three separate peptide-coated microbead spots, each of which was sampled in triplicate.
Results
Analyte Concentration in HIER Controls
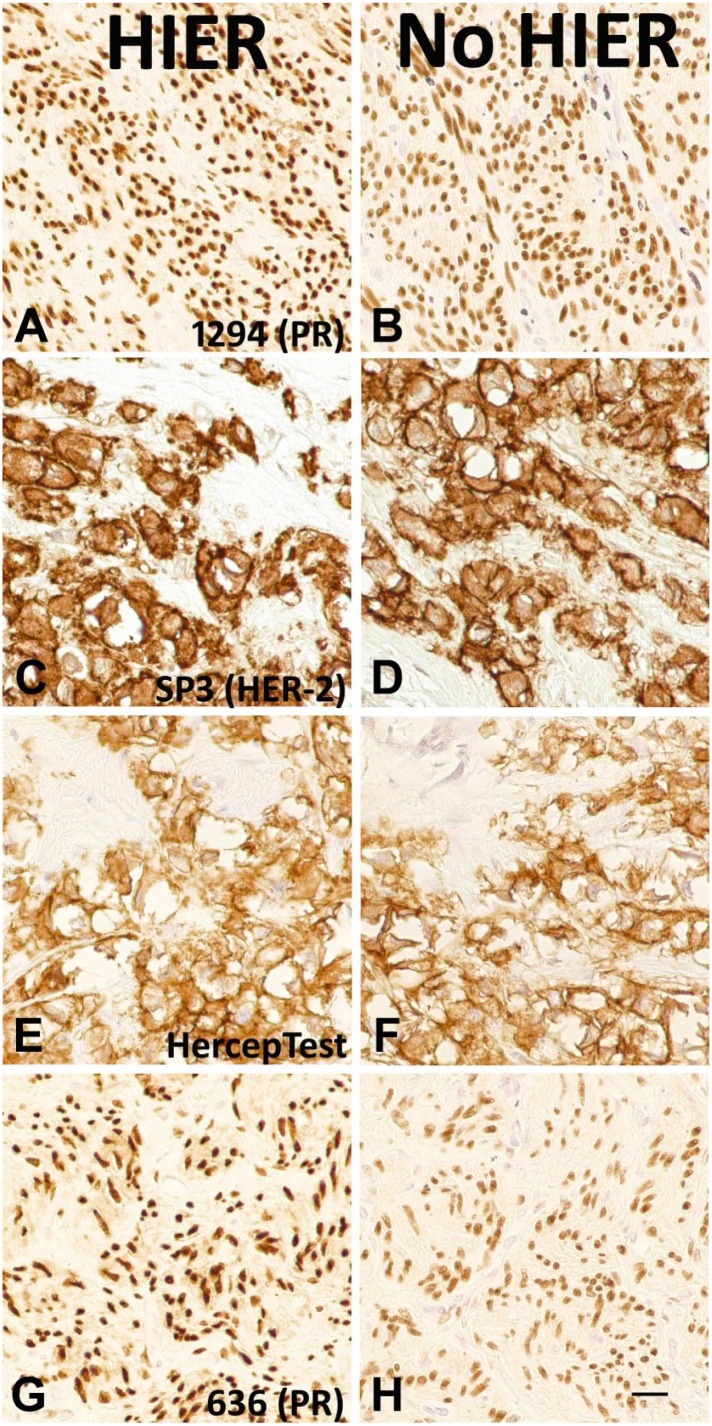
As an analytic component of the test, HIER requires a control to verify proper performance. In selecting an HIER control, a substantial increase in immunoreactivity after HIER is a sine qua non for detecting potential problems with HIER. Surprisingly, we recently learned that some diagnostically important MAbs stain strongly even without HIER.3–7 There is either no increase, or only a mild increase, in stain intensity after HIER. We replicated these surprising findings in-house. Figure 1 depicts representative tissue stains with and without HIER for the PR 1294 (Panels A and B), HER2 SP3 (Panels C and D), HER2 HercepTest (Panels E and F), and PR 636 (Panels G and H) immunostains. We did not use a counterstain. The images show that the stains are fairly strong even without HIER. The improvement in stain intensity after HIER is small. Consequently, we believe that it would be difficult to detect the absence of HIER using these tissue sections as controls.
Figure 1.
Photomicrographs of paired, stained tissue samples with (left column) and without (right column) HIER. The images show strong immunostaining even without HIER. The immunostains include PR 1294 (Panels A and B), HER2 SP3 (Panels C and D), HercepTest (Panels E and F), and PR 636 (Panels G and H). Scale bar, 20 µm. Abbreviations: HIER, heat-induced epitope retrieval; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
This observation complicates the selection of positive controls. For some tissues, or for some immunostains, HIER appears to have only a small or negligible effect. It is unclear whether this is a property of the tissue samples, the immunostain, or possibly a combination of both. To better understand, we repeated the observation using a well-defined experimental model comprised of peptide epitopes conjugated to cell-sized glass microbeads. An advantage of this model over the use of tissue sections is that it facilitates better characterization of certain experimental variables such as analyte concentration. We tested whether HIER is needed for ER SP1 immunostaining because of reports that ER SP1 stains even without HIER.3,5
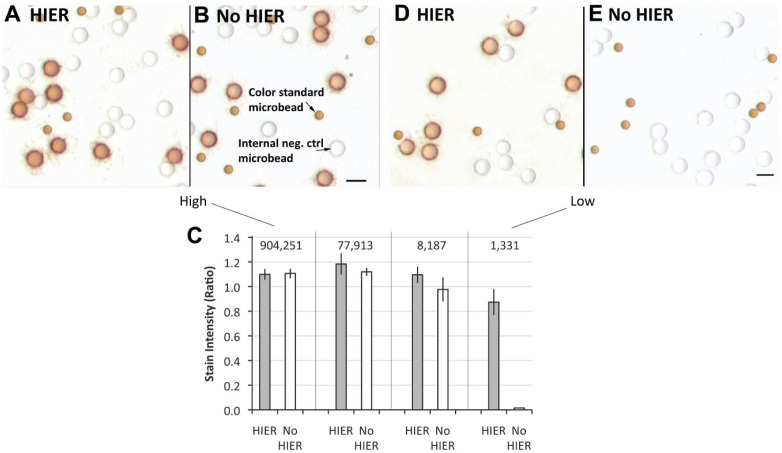
Figure 2A and B show the peptide epitope-coated microbeads at the highest analyte concentration after immunostaining with the ER SP1 MAb, with (Figure 2A) and without (Figure 2B) HIER. The images show that both conditions result in strong staining. To clarify the interpretation, it may be helpful to explain that in addition to the stained microbeads, there are also two other types of microbeads: (1) unstained microbeads bearing an antigenically irrelevant peptide (“Internal neg. ctrl microbead”) and (2) smaller optical “color standard microbeads” that are permanently brown. These optical standard microbeads serve to normalize the optics for quantification of stain intensity. We quantified stain intensity by image analysis using a previously described method.22 The stain intensity scores associated with Fig. 2A and B are shown to the far left in Fig. 2C. There is no statistically significant difference in stain intensity when comparing the HIER and no HIER groups. In summary, these data confirm previously published reports3,5 that the ER SP1 MAb does not require HIER.
Figure 2.
Effect of analyte concentration on ER immunostaining using the ER SP1 MAb, with and without HIER. Panels A and B depict immunostaining of peptide epitopes (coated onto glass microbeads) with (A) and without (B) HIER. These microbeads bear an average of 904,251 ER peptides per microbead. In Panel C, the ER peptide concentrations per microbead are listed above the vertical bars. The unstained test microbeads (labeled “Internal neg. ctrl microbead” in Panel B) bear an unrelated ER peptide epitope. They represent an internal negative control. The “Color standard microbead” (labeled in Panel B) is smaller and permanently brown, regardless of immunostaining. It is used as an internal optical reference standard for image quantification, normalizing variability in optical settings. Panels D and E depict immunostaining of the same peptide epitopes with (D) and without (E) HIER, except that these microbeads bear an average of 1331 peptide epitopes per microbead. The data (Panel C) represent the mean ± SD of triplicate slides. Scale bar, 10 µm. Abbreviations: ER, estrogen receptor; HIER, heat-induced epitope retrieval; PR, progesterone receptor.
A completely different conclusion emerges when a single experimental parameter is changed. Figure 2D and E illustrate the results of the exact same experiment but, in this case, the microbeads bear an average of 1331 molecules of ER. This is in contrast to the experiment in Fig. 2A and B, which was performed with microbeads bearing an average of 904,251 molecules of ER peptide epitope. The ER concentration of the microbeads used in Fig. 2D and E have 0.15% the concentration of the microbeads in Fig. 2A and B. Figure 2D and E illustrate that, in this case, the ER SP1 immunostain is entirely dependent on HIER. There is no detectable stain on the test microbeads without HIER. Figure 2D and E show that the ER SP1 MAb epitope is, in fact, affected by formaldehyde fixation, masking immunoreactivity with the ER SP1 MAb. The small colored microbeads in Fig. 2D and E are the optical reference standards, not stained test microbeads. Stain intensity of these test microbeads is quantified and shown at the far right in Fig. 2C.
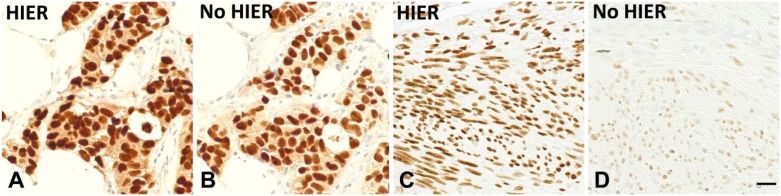
These findings show that the determination as to whether HIER significantly improves immunostain intensity depends on the analyte concentration in the sample. High analyte concentrations are less improved by HIER. Samples expressing low analyte concentrations experience a much greater proportional increase in stain intensity after HIER. This finding is not an artifact of the peptide epitope model because we replicated the ER SP1 findings using formalin-fixed, paraffin-embedded tissue sections. Figure 3A and B show the appearance of a tumor specimen after ER SP1 immunostaining with (Fig. 3A) and without (Fig. 3B) HIER. The stain intensities of the two are almost equal. This pattern mirrors the analyte-high concentration pattern using peptide epitopes (Fig. 2A and B). Figure 3C and D show the appearance of normal uterine endometrial stromal cells after staining with the ER SP1 MAb. These cells appear to express comparatively lower levels of ER. Figure 3D shows that without HIER, the stain intensity is significantly lower than with HIER (Fig. 3C). Like the peptide epitopes, the SP1 immunostain requires HIER in tissue samples with low ER concentrations.
Figure 3.
Staining of tissue sections using the ER SP1 immunostain, with (A and C) and without HIER (B and D). Panels A and B depict a strongly staining carcinoma with almost equal stain intensity regardless of whether HIER was used. Panels C (with HIER) and D (without HIER), on the contrary, illustrate a significant difference in stain intensity. Scale bar, 20 µm. Abbreviations: ER, estrogen receptor; HIER, heat-induced epitope retrieval.
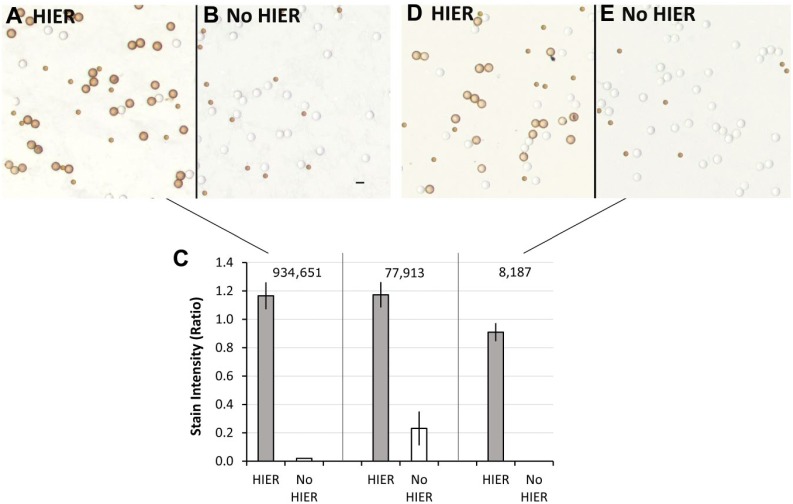
The ER SP1 immunostain represents one end of a spectrum in its need for HIER. Namely, it stains well without HIER except at the low end of analyte concentration. At the opposite end of this spectrum is the ER 1D5 immunostain. Figure 4 demonstrates the same type of data as in Fig. 2 but for the ER 1D5 immunostain. Unlike the ER SP1 data, the ER 1D5 immunostain requires HIER for strong staining across all analyte concentrations that we tested. The ER 1D5 data exemplify an immunostain that is highly dependent on HIER regardless of the analyte concentration. We believe this relates to the degree of formaldehyde-induced cross-linking at the epitope (see the section “Discussion”). In conclusion, the selection of appropriate HIER controls depends on both the properties of the immunostain and the analyte concentration. The reasons that may account for this are described in the section “Discussion.”
Figure 4.
Effect of analyte concentration on ER immunostaining using the ER 1D5 MAb, with and without HIER. It is presented as a contrast to the data of Fig. 2, showing dependence on HIER at all analyte concentrations. Panels A and B depict immunostaining of peptide epitopes (coated onto glass microbeads) with (A) and without (B) HIER. These microbeads bear an average of 934,651 ER peptides per microbead. In Panel C, the ER peptide concentrations per microbead are listed above the vertical bars. Panels D and E depict immunostaining of the same peptide epitopes with (D) and without (E) HIER, except that these microbeads bear an average of 8187 peptide epitopes per microbead. The 1331 molecules per microbead group is not shown because it is below the limit of detection regardless of HIER. The data (Panel C) represent the mean ± SD of triplicate slides. Scale bar, 10 µm. Abbreviations: ER, estrogen receptor; HIER, heat-induced epitope retrieval.
Role of Epitope Composition
It would be helpful to understand why some MAb epitopes (such as the ER SP1) are less dependent on HIER while others (such as the ER 1D5) are highly dependent on HIER. In the course of developing positive controls using peptide epitopes, we encountered two peptides with a lysine residue in the epitope. These peptides include the epitopes for the HER2 SP3 MAb and the PR 1E2 MAb. We synthesized peptides with sequences that exactly match the epitopes as found in the native protein (along with flanking sequences). Normally, we block the epsilon amine of lysines during peptide synthesis with a cleavable ivDde group. By blocking the epsilon amine that lysine is no longer chemically reactive. For example, it cannot undergo formaldehyde-induced cross-linking reactions. The peptides are designed so that the only chemically reactive amine is at the peptide terminus, which is used for anchoring the peptide to a glass microbead. This ensures a desired peptide orientation on the glass surface.
This situation presents a unique opportunity to test the importance of epitope composition as it relates to fixation and HIER. The ivDde group prevents formaldehyde from reacting with lysine. Cleaving the ivDde group (after the peptide is attached to the glass surface) restores the epsilon amine, permitting reactivity with formaldehyde. By comparing the immunoreactivity of peptides before and after cleavage of the ivDde group, we can test the effect of formaldehyde reactivity in a controlled experiment. We can experimentally test if the variability in the need for HIER among different immunostains is related to the formaldehyde reactivity of the epitope to which the MAb binds. We predicted that epitopes with amino acids that are reactive with formaldehyde will generate protein or peptide cross-links, sterically masking immunoreactivity, and therefore require HIER to a greater degree. Epitopes with less formaldehyde reactivity will stain well even without HIER.
The peptide for the HER2 SP3 MAb is shown in Fig. 5. Each sphere represents an amino acid using the single letter amino acid code. The figure illustrates that the peptide is covalently linked to a microbead. The PIWKF sequence comprises the epitope, where the antibody binds. The other amino acids flanking the epitope are an exact match to the native protein, starting at amino acid 601 (numbering from the original protein). This particular peptide has two lysines (K). At the lysine (K) corresponding to amino acid 605, outside the epitope, we synthesized the peptide with an attached fluorescein group at the epsilon amine. Fluorescein is pictorially depicted as a yellow sphere emitting light. Fluorescein is used to measure peptide concentration, as previously described21 As the epsilon position is occupied with a fluorescein, the lysine is not chemically reactive with formaldehyde. A second lysine that is located at the epitope (corresponding to amino acid position 615) has a cleavable ivDde group at the epsilon amine. The ivDde group blocks the epsilon amine, preventing formaldehyde-mediated cross-linking at this position. These two modifications, attaching a fluorescein and an ivDde on the lysines, leave only one free amine on the peptide at the amino terminus. This single free amine is where the peptide attaches to aminosilane-coated glass microbeads.
Figure 5.
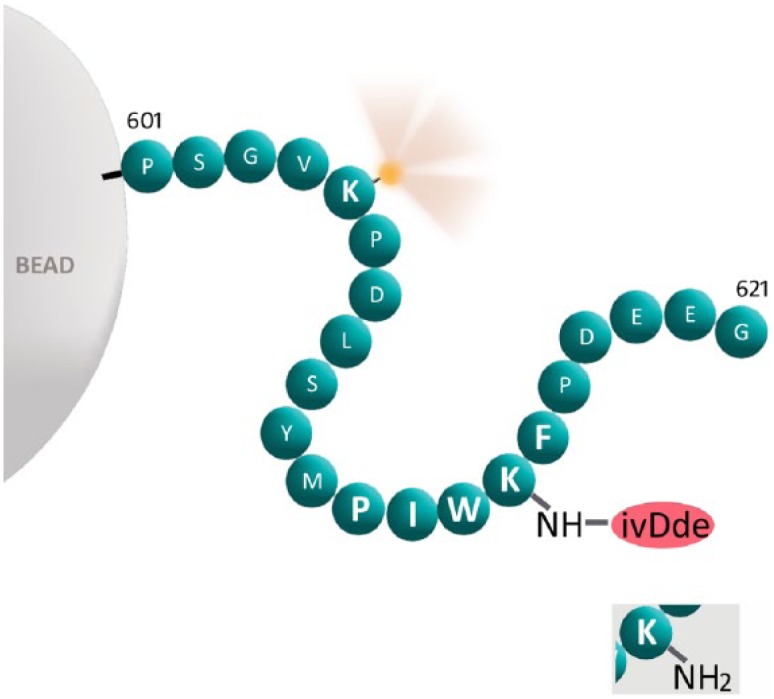
Schematic representation of the peptide for the HER2 SP3 immunostain covalently bound to a glass microbead. Each sphere represents an amino acid, represented by the single letter amino acid code. The peptide is covalently linked to a microbead at the left. The “601” and “621” designations refer to the positions in the native ER protein where the sequence corresponds to. Fluorescein is pictorially depicted as a yellow sphere emitting light. The PIWKF epitope (for the HER2 SP3 MAb) is in a larger font. The peptide is synthesized with an ivDde blocking group at the epsilon position of lysine (K) in the epitope. With the ivDde blocking group, the epsilon amine is non-reactive with formaldehyde. If cleaved, then the original epsilon amine is regenerated, as shown in the inset at the lower right. Abbreviations: HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; HIER, heat-induced epitope retrieval.
To test the role of epitope lysine (K) residues in fixation and antigen retrieval, we examined whether removing the ivDde group affects immunoreactivity after formaldehyde fixation. We first attached the peptide to the aminosilane-coated glass microbeads in the usual manner. Next, we cleaved off the ivDde group (see “Materials and Methods”), restoring the epsilon amine while maintaining the peptide orientation on the glass microbead. A representation of the lysine with its regenerated epsilon amine is shown in the Fig. 5 inset. If lysine plays a role in masking the epitope and abrogating immunoreactivity after formaldehyde fixation, then cleaving off the ivDde group should affect immunoreactivity. As ivDde cleavage regenerates a chemically reactive lysine in the epitope, formaldehyde fixation should cause protein cross-linking and sterically block immunoreactivity. HIER should reverse those cross-links and restore immunoreactivity.
The data confirm the hypothesis. Cleaving the ivDde group and restoring an epsilon amine renders the peptide epitope susceptible to formaldehyde fixation. This is demonstrated in Fig. 6. Figure 6A compares immunoreactivity of the HER2 SP3 immunostain under four conditions. In the left-most group, a control, the epitope lysine is blocked with an ivDde group (“ivDde+”) and the peptide is not exposed to formaldehyde fixation (“Unfixed”). This group shows strong immunoreactivity with (blue bars) and without (orange bars) HIER. HIER makes no difference because, without formaldehyde fixation, there are no cross-links to reverse. In the next group to the right (“ivDde+” and “Fixed”), the lysine again incorporates the ivDde blocking group. The peptide is formaldehyde-fixed but immunoreactivity remains intact. This is also as expected. The ivDde blocking group prevents formaldehyde-induced protein cross-linking. For the final two (right-most groups), the ivDde group is cleaved off (“ivDde–”), restoring the epsilon amine. In the unfixed group, there is still strong immunoreactivity with the HER2 SP3 immunostain regardless of whether HIER was performed. This group again serves as a control, verifying that the cleavage of the ivDde group does not independently affect immunoreactivity. Finally, in the group shown at the far right of Fig. 6A, the data show a significant diminution of immunoreactivity after fixation and without HIER (orange bar, p<0.001). HIER restores some of the immunoreactivity (blue bar). The fact that HIER does not restore 100% immunoreactivity was observed with other peptide epitopes as well. In summary, this experiment demonstrates that the epsilon amine of lysine residues in an epitope affects immunoreactivity during formalin fixation.
Figure 6.
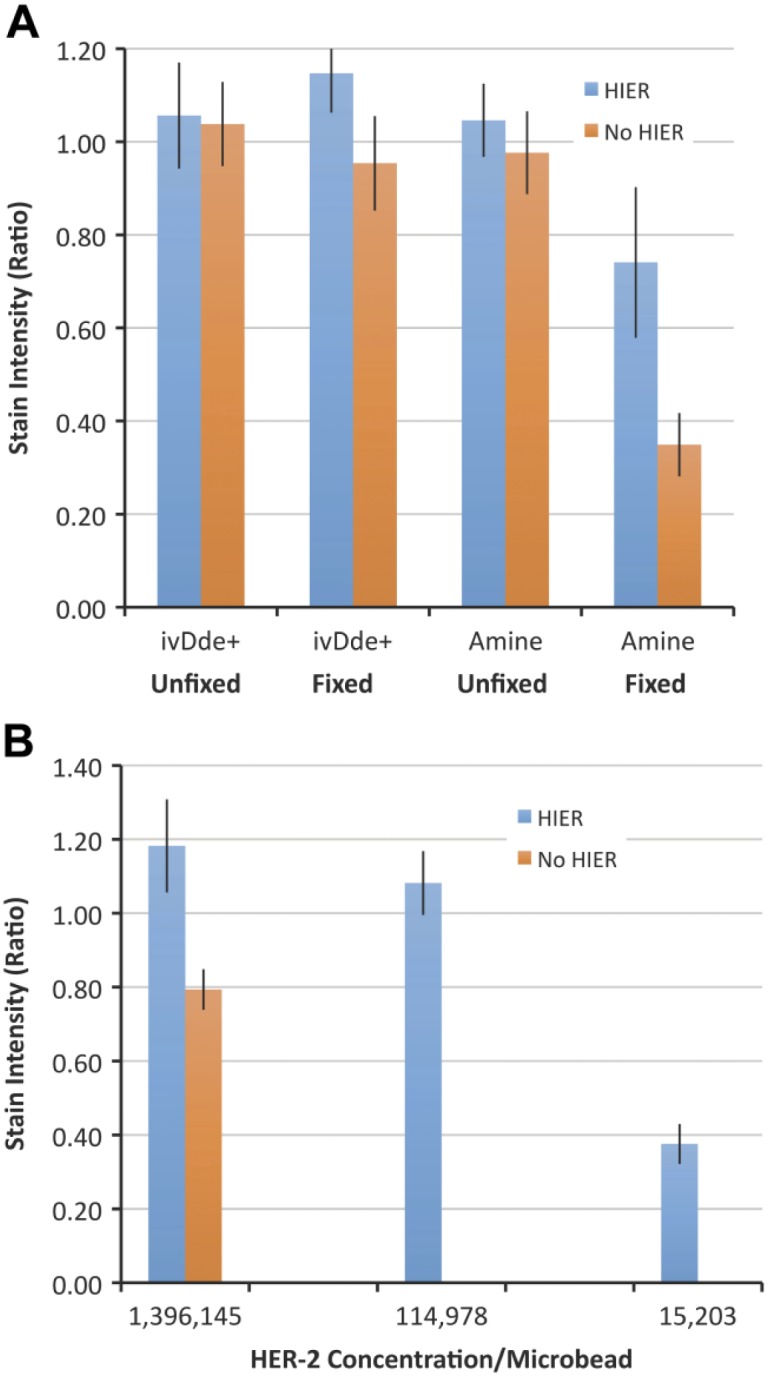
Graphical representation of stain intensity (y-axis) after immunostaining with the HER2 SP3 immunostain. Panel A shows that the loss of immunoreactivity after exposure to formaldehyde only occurs if the ivDde blocking group is removed. In Panel A, stain intensity of the ivDde-blocked peptide (“ivDde+”) is compared with the same peptide after regeneration of the epsilon amine (“Amine”). Each peptide (ivDde+ and Amine) was tested after formaldehyde fixation (“Fixed”) or without formaldehyde fixation (“Unfixed”). Each experimental group was stained with (blue) or without (orange) HIER. In Panel A, all of the peptides were at a concentration of 1,396,145 peptide epitopes per microbead. In Panel B, we examine the effect of various peptide concentration on HER2 SP3 immunostain intensity. In Panel B, all three groups (at various peptide concentrations) are formaldehyde-fixed and are after regeneration of the epsilon amine. The group to the far left (1,396,145) is under the same conditions as the group to the far right in Panel A. The data represent the mean ± SD of triplicate slides. Abbreviations: HIER, heat-induced epitope retrieval; HER2, human epidermal growth factor receptor 2.
Fig. 6B is a second, follow-on study of the same peptide but this experiment is performed at three different analyte concentrations. In this figure, all of the experimental groups have a restored epsilon amine (after ivDde cleavage) and are all formaldehyde-fixed. Whereas Fig. 6A shows data using the highest HER2 concentration, Fig. 6B shows the effect of HIER (blue bars) compared with “No HIER” groups (orange bars) at lower HER2 peptide epitope concentrations. The left-most group is at the same HER2 concentration as used in Fig. 6A (1,396,145 molecules per microbead). The data show that the effect of HIER is best observed at lower analyte concentrations. This is exemplified by the middle HER2 peptide epitope concentration (114,978 molecules per microbead). The reactive lysine (after cleavage of the ivDde group) masks the peptide epitope after formaldehyde fixation, abrogating immunoreactivity without HIER. The fact that the effect of formaldehyde is most pronounced at the lower analyte concentrations matches the previously described conclusion (Fig. 2). The lowest HER2 concentration (15,203 molecules per microbead, far right) is approaching the limit of detection. Consequently, there is low stain intensity even with HIER. Overall, these HER2 SP3 immunostain data confirm the expected role of lysines (during formalin fixation) within an epitope.
We observed similar findings with the peptide that contains the PR 1E2 epitope. This epitope also contains a lysine. Figure 7A depicts stain intensity of the formaldehyde-fixed peptide with the ivDde protecting group after immunostaining with the PR 1E2 MAb. Regardless of the PR concentration, HIER causes only a small increase in stain intensity. The effect of formaldehyde is quite small because of the ivDde blocking group on lysine. The ivDde protecting group mostly preserves immunoreactivity despite exposure to formaldehyde. This is as expected because the ivDde group blocks the epsilon amine, preventing chemical reactivity with formaldehyde. The small increase in stain intensity in Fig. 7A after HIER can be explained as a formaldehyde-independent effect, as described in Fig. 8.
Figure 7.
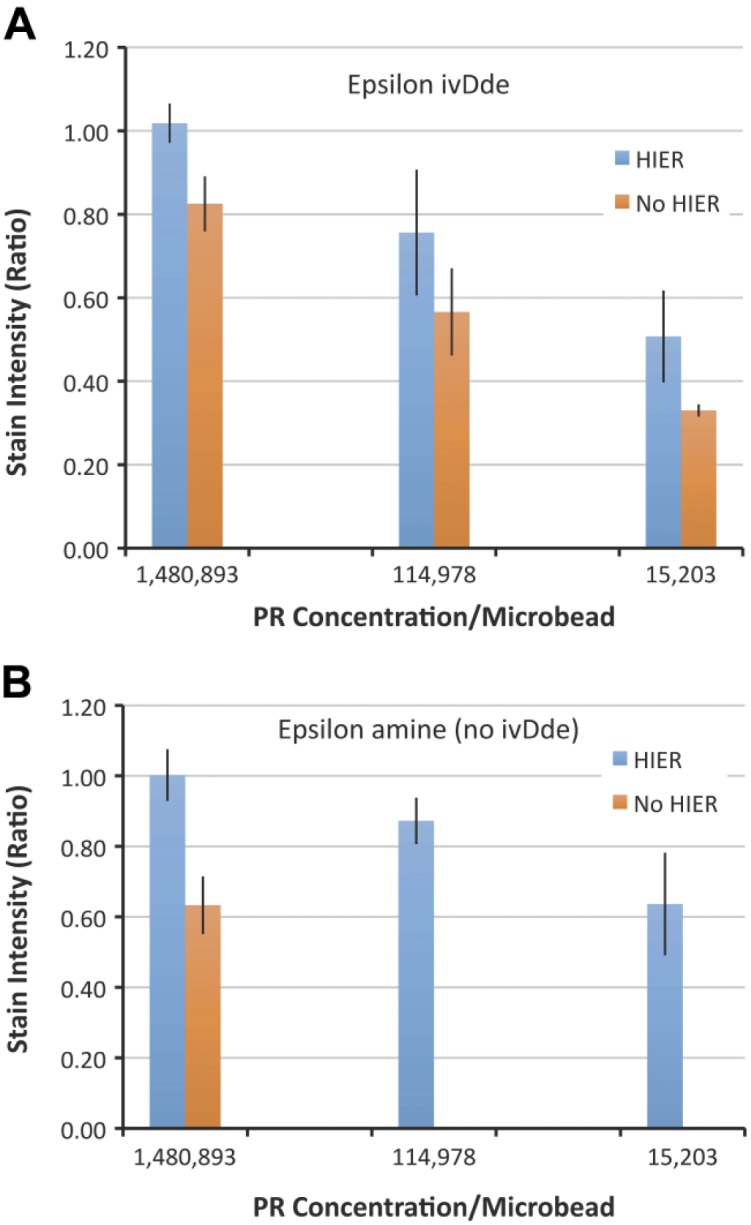
Graphical representation of stain intensity with and without HIER after immunostaining with the PR 1E2 antibody. The peptides in Panel A incorporate an ivDde blocking group at the epsilon amine of a lysine in the epitope. There is only a mild increase in stain intensity after HIER (blue) across all concentrations of peptide. The conditions depicted in Panel A are mirrored in Panel B, except after cleavage of the ivDde blocking group. Cleaving off the ivDde group and regenerating the epsilon amine (Panel B) renders the peptide dependent on HIER at the moderate (114,978) and lower (15,203) peptide concentrations. At the 114,978 concentration, for example, there is no staining without HIER. The data represent the mean ± SD of triplicate slides. Abbreviations: HIER, heat-induced epitope retrieval; PR, progesterone receptor.
Figure 8.
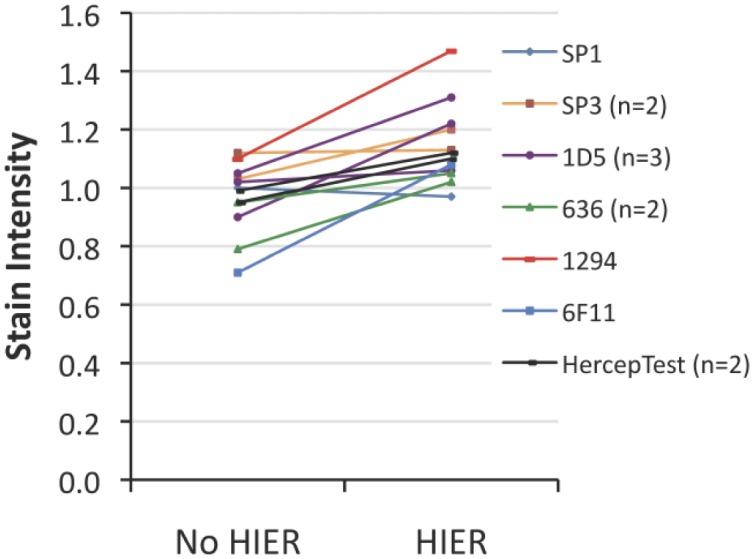
Stain intensity of unfixed peptide epitope-coated microbeads with (right) and without (left) HIER. Stain intensity often, but not always, mildly increases after HIER even though the peptide epitopes were not exposed to formaldehyde. The legend shows the primary antibody used for the immunostain. The experiment was repeated more than once (as indicated in the parentheses) on separate days for four immunostains. Each of the peptide epitopes in the figure had high analyte concentrations, generally 700,000 to 1,200,000 molecules per microbead. The data represent single slides. Abbreviation: HIER, heat-induced epitope retrieval.
Fig. 7B depicts immunoreactivity of the same formaldehyde-fixed peptide after cleavage of the ivDde protecting group and regeneration of the epsilon amine. With the regenerated epsilon amine, the peptide is now sensitive to formaldehyde-induced cross-linking. Without HIER, there is complete abrogation of immunoreactivity at 114,978 and 15,203 molecules (Molecules of Equivalent Fluorochrome) per microbead (p<0.001 for both groups). This finding supports the hypothesis. The presence of a chemically reactive lysine in the epitope leads to a loss of immunoreactivity after fixation with formaldehyde. As noted previously, higher analyte concentrations are less dependent on HIER for staining. The group bearing 1,480,893 molecules PR peptide per microbead moderately stained even without HIER (orange column). This finding is consistent with our previous findings; high analyte concentrations can still be immunoreactive (see the section “Discussion” for further explanation).
A Formaldehyde-independent Effect of HIER
HIER is traditionally viewed as a process to reverse the deleterious side effects of formalin fixation. It is widely believed that the recovery of immunostaining after HIER is mainly due to the reversal of formaldehyde-induced cross-links.20,24,25 We also previously presented data supporting this mechanism.10,19 During the course of this work, we had reason to test peptide epitopes that were never exposed to formaldehyde. We expected unfixed peptide epitope controls to be unaffected by HIER. If HIER acts by reversing formaldehyde-induced cross-links, and there are no formaldehyde-induced cross-links, then HIER should be an irrelevant treatment. As peptide epitope controls do not require fixation for reasons of preservation, the peptide epitope controls provide the opportunity to test this prediction.
Figure 8 depicts the stain intensity of unfixed peptide epitope-coated microbeads with and without HIER. Contrary to expectation, we usually observe a mild to moderate increase in immunostain intensity after HIER of unfixed peptide epitope-coated microbeads. This was a surprising finding that we repeated with a variety of peptide analytes and immunostains (Fig. 5). The data show that most of the time, there is a small increase in stain intensity after HIER. From all of these data points, there is an average increase in stain intensity of 18% (range: –3% to 53%). These data indicate that a small part of the increase in stain intensity after HIER is unrelated to formaldehyde fixation.
Discussion
This study aims to identify the important features required of a positive IHC control if it is to verify proper HIER. Using a peptide epitope model, we analyzed the roles of analyte concentration, epitope composition, and degree of fixation. We explored how these variables affect the sensitivity of a control for HIER. The peptide epitope model uniquely allows us to control these experimental variables, which is otherwise difficult or impossible to accomplish using conventional tissue samples. The key conclusions are as follows:
The masking of epitopes after formalin fixation is not a binary event whereby there is immunoreactivity without formalin fixation but an absence of immunoreactivity afterwards. Instead, we find that formalin fixation induces a variable degree of epitope masking. Some primary MAb/epitope combinations are highly dependent on HIER whereas staining of others is only minimally enhanced with HIER.
Analyte concentration is an important variable in selecting controls that verify the HIER step was properly performed. Controls with lower analyte concentrations that still produce easily detectable immunostaining are more sensitive than high analyte concentration controls. This is especially important for primary MAbs/epitopes that are incompletely masked by formalin fixation.
The chemical reactivity of amino acids in the epitope with formaldehyde can, at least in part, account for differences in formalin-induced epitope masking. This was demonstrated by rendering lysine residues located in the epitope as reactive or not reactive and then testing the effect of fixation and HIER. We previously demonstrated a similar correlation with amino acids arginine and tyrosine.10
HIER often augments stain intensity even when peptide epitopes are unfixed.
Variability in the Effect of HIER
Our data highlight the observation that a positive IHC control is not automatically capable of controlling for the HIER step solely by virtue of being formalin-fixed. In fact, formalin-fixed controls can be profoundly insensitive in detecting the absence of HIER. This situation arises when the sample stains strongly with or without HIER. If HIER has little or no effect on the staining of a tissue sample, then the sample is an insensitive indicator of HIER problems.
This is not the first report describing strong immunostaining of formalin-fixed tissue sections without HIER.3–7 Previously, however, the focus was on the primary antibody. Namely, the observation that some immunostains stain fairly well even without HIER was perceived as a property of the primary antibody. We find this to be true but with an important caveat. In measuring the need for HIER, analyte concentration must be accounted for. Some primary antibodies may initially appear to stain fairly well without HIER but it may only be at certain analyte concentrations. At high analyte concentrations, HIER may appear to have minimal or no effect on immunostaining. At low analyte concentrations, in the concentration-dependent region of the immunostain’s analytic response curve, the immunostain may require HIER. For example, staining with the ER SP1 MAb depends on HIER, but only at the lowest analyte concentration (Fig. 2). Otherwise, HIER had little or no effect on stain intensity. In contrast, the ER 1D5 MAb (Fig. 4) requires HIER regardless of the analyte concentration. The reasons for this differential susceptibility to formaldehyde are discussed later. In summary, there is a varying need for HIER among primary antibodies. The ER SP1 and ER 1D5 MAbs used in this study represent two primary antibodies at opposite ends of this spectrum. The important conclusion of our findings is that one must account for analyte concentration when evaluating an antibody’s need for HIER. Also, when selecting positive IHC controls, lower analyte concentrations are more sensitive.
Modeling the Effect of HIER at Various Analyte Concentrations
The explanation for these observations is accounted for by three factors: analyte concentration, analytic sensitivity of the immunostain, and the percentage of epitopes that are blocked after fixation (and therefore inaccessible to the primary antibody). Consider a hypothetical strongly ER-positive breast carcinoma cell that expresses 1,000,000 molecules of ER. Moreover, assume for the purpose of this explanation that formaldehyde-induced cross-linking causes steric blockade and loss of immunoreactivity for 99% of the available epitopes. In this circumstance, the formalin-fixed tumor cell will have 990,000 sterically blocked ER epitopes and 10,000 unblocked, immunoreactive epitopes. As 99% of the epitopes are sterically blocked and therefore non-immunoreactive, one might reasonably expect that the ER SP1 epitope requires HIER. As it turns out, the ER SP1 immunostain is so sensitive that the residual 10,000 molecules still produce a strong immunostain. Strong staining will occur (without HIER) if the remaining 1% of unaffected epitopes can still be detected in a sensitive immunostain. Our previously published data for the ER SP1 immunostain21 show that it is exceptionally sensitive. There is little difference in stain intensity with the ER SP1 immunostain for 10,000 versus 1,000,000 molecules of ER per microbead. Consequently, even after cross-linking and blocking 99% of the epitopes, an immunostain can be strongly positive despite the absence of HIER.
The data in Fig. 2 suggest that the actual percentage of ER SP1 antibody epitopes rendered inaccessible after formaldehyde fixation is probably far lower than 99%. We reach this conclusion because the immunostain (without HIER) even detects the peptide epitope control with 8187 molecules per microbead. If only 1% of the epitopes at the 8187 molecules per microbead level were immunoreactive, then the immunostain would be detecting 100th of 8187, or 82 molecules. The ER SP1 immunostain is not that sensitive; 82 molecules of ER is below the limit of detection. In fact, the actual percentage of ER SP1 epitopes rendered non-immunoreactive after formaldehyde fixation is probably well under 50%. For example, our data can be accounted for if formaldehyde-induced cross-linking blocks 40% of the epitopes and the limit of detection is 800 molecules per microbead. With these parameters, the lowest peptide epitope group will have only 60% of the 1331 peptide epitopes as immunoreactive (798 peptide epitopes per microbead). The number of remaining immunoreactive epitopes (798) falls below the limit of detection (800). After HIER, the number of immunoreactive peptide epitopes rises well above the (800) threshold, resulting in strong staining. Whatever the percentage of sterically blocked epitopes after formaldehyde fixation, it must account for the observation that the immunostain requires HIER at 1331 molecules per microbead concentration but not at 8187. This model fits with our previously published data, which shows that the ER SP1 analytic response curve is steep in this concentration range.21
The ER 1D5 MAb, on the contrary, is highly dependent on HIER across all concentrations. It is at the opposite end of the spectrum in regard to HIER. Formaldehyde fixation appears to render a far higher percentage of epitopes to be inaccessible to the primary antibody. Consequently, at any analyte concentration, the number of immunoreactive epitopes falls below the immunostain’s limit of detection (Fig. 4). This feature mandates the use of HIER. We propose that the key distinguishing feature between these two opposite extremes (as represented by the ER SP1 and ER 1D5 MAbs) is the percentage of epitopes that are rendered non-immunoreactive after formaldehyde fixation.
A Molecular Explanation for Variability in Epitope Masking
The existence of an HIER spectrum among MAbs begs for an explanation. Why does formaldehyde appear to dramatically affect some antibody epitopes but not others? What factors determine the extent to which an antibody’s epitope is blocked by formaldehyde cross-linking? We speculate that the difference between MAbs such as ER SP1 and ER 1D5, accounting for their differential needs for HIER, will primarily relate to their epitope compositions. Both MAbs are immunoreactive with estrogen receptor (alpha) but at different epitopes. We predict that the amino acid composition of those sites is the principal determinant affecting where antibodies fall on the spectrum of HIER dependence. The presence of formaldehyde-reactive amino acids (summarized in the next section) in or near the epitope will render the epitope more susceptible to steric interference after formaldehyde fixation. Consequently, formaldehyde-induced protein cross-links will, to a greater extent, sterically prevent primary antibody binding. Such MAbs (such as the ER 1D5 MAb) require HIER to reverse those cross-links and restore immunoreactivity.
Conversely, the absence of formaldehyde-reactive amino acids in the epitope will allow the epitope to remain accessible to primary antibody binding even after fixation. Such MAbs will more closely resemble the behavior of the ER SP1 MAb, being largely independent of HIER. In support of this hypothesis, the ER 1D5 MAb epitope contains three formaldehyde-reactive amino acids: one glutamine and two tyrosines.10 The ER SP1 MAb epitope, however, has no formaldehyde-reactive amino acids. The ER SP1 MAb epitope comprises the sequence GEAEGFP (single letter amino acid abbreviations), none of which are chemically reactive with formaldehyde. Formaldehyde-reactive amino acids include lysine (K), tyrosine (Y), arginine (R), glutamine (Q), cysteine (C), histidine (H), asparagine (N), and tryptophan (W).17,26 Confirming this hypothesis will require analyzing larger numbers of epitopes and correlating them to where they fall on the spectrum of HIER dependence. Moreover, if the hypothesis is true, then it is also plausible that other protein-related factors can potentially modulate the extent of protein cross-linking. Such factors may include protein conformation, steric blocking by adjacent macromolecules and epitope hydration. Even if there are formaldehyde-reactive amino acid side chains at the epitope, these other factors may nonetheless inhibit formaldehyde-induced cross-linking, decreasing the need for HIER.
The Role of Epitope Composition: Lysine Residues
If our hypothesis is true, that is, that epitope composition affects where a primary antibody falls on the spectrum of HIER dependence, then we can directly test a prediction. The model predicts that adding or subtracting formaldehyde-reactive amino acids from an epitope will influence the need for HIER. Adding formaldehyde-reactive amino acids should increase the need. Conversely, removing formaldehyde-reactive amino acids should diminish the need for HIER. From an experimental standpoint, however, there is a constraint. Any amino acid additions or deletions from the epitope will likely drastically alter the affinity of the MAb. It would then be impossible to assess the need for HIER. Despite this constraint, there is a way to test the prediction.
Lysine is chemically reactive with formaldehyde. Two recently identified peptides (for MAbs SP3 and 1E2, Figs. 5 and 6) contain a lysine in their respective epitopes. It is possible to selectively block or unblock the epsilon amine group of that lysine. If blocked (with ivDde), the lysine cannot chemically react with formaldehyde. Unblocked, the epsilon is restored and the lysine is chemically reactive. In the presence of formaldehyde, an unblocked epsilon amine of lysine (in our peptides) is predicted to cross-link to other proteins (casein) during fixation, sterically interfering with subsequent primary antibody binding.
The data fit the prediction. When the epsilon amine was blocked by the ivDde group, the peptide retained immunoreactivity after formaldehyde fixation. The ivDde group prevented formaldehyde-induced cross-linking. After the ivDde group was removed, formaldehyde fixation led to a loss of immunoreactivity. Cleavage of the ivDde group restores the epsilon amine, rendering the lysine chemically reactive with formaldehyde. These findings are the first demonstration to our knowledge that the chemical reactivity of lysine (in an epitope) correlates with immunoreactivity.
A Formaldehyde-independent Effect of HIER
A large body of data support the view that HIER improves stain intensity by reversing formaldehyde-induced cross-links.20,24,25 In this hypothesis, formaldehyde plays a central role. Therefore, it was a surprise to repeatedly observe increases in stain intensity after HIER even when the peptide epitope was unfixed. As the peptides in those experiments were not exposed to formaldehyde, the mechanism causing the increased stain intensity must be something else. The same phenomenon observed with peptide epitopes may also apply to tissue sections. Kakimoto et al.27 reported similar findings after analyzing unfixed frozen tissue sections. These findings suggest that HIER may also act through other mechanisms in addition to reversing protein cross-linking. Possible mechanisms include dissociating peptide aggregates or removing weakly (non-covalently) associated proteins that may sterically interfere with antibody binding. Another possible explanation relates to sample hydration. Treatments that help prevent dehydration are described as improving stain intensity.28,29 Therefore, the mechanism of increased staining on unfixed peptide epitopes may be that HIER improves hydration. Finally, another possibility is that a small amount of peptide cross-linking occurs during the conjugation of peptide to aminosilane-coated glass microbeads, regardless of the presence of formaldehyde. HIER may reverse it, exposing epitopes that would have otherwise been inaccessible.
A final observation from these experiments relates to the peptide epitope model itself. The ability to precisely control analyte concentration, epitope composition, and fixation enabled the performance of these studies. These are the advantages of a synthetic model. A limitation of the peptide epitope model is that the concentrations of analyte per microbead listed in the figures will not exactly correlate with concentrations per cell. The microbeads are perfectly spherical objects, approximately 7 to 8 µm diameter. Tissue sections, on the contrary, are often approximately 4 to 5 µm thick. Another difference is that microbeads have analyte only on the glass surface. This may fairly mimic the distribution of analyte expressed on the cell membrane but not if it is a nuclear or cytoplasmic protein. Despite these small numerical differences, the themes are the same. Whenever possible, similar findings were demonstrated with tissue sections (Figs. 1 and 3) or are described for tissue sections in the published literature.3–7 This correlation supports the proposition that peptide epitopes accurately represent fixation and antigen retrieval processes as they occur in tissue samples.
Acknowledgments
We are grateful to Dr. Ron Zeheb for his ongoing advice during the course of this project and review of this manuscript, Ms. Drorit Bogen for administrative support, and to the National Cancer Institute, National Institutes of Health (NIH), for funding this work.
Footnotes
Competing Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Three of the authors (S.R.S., K.V., and S.A.B.) have a patent (ownership) interest in the peptide epitope-coated microbeads technology. The other authors (A.K.S. and A.B.) declare they have no competing interests.
Author Contributions: SRS contributed to the conception and manufacture of the peptide epitope-coated microbeads, epitope mapping, and execution of experiments. KV contributed to the manufacture of the peptide epitope-coated microbeads, epitope mapping, execution of the experiments, and image quantification. AKS performed the photomicroscopy and image quantification. AB contributed to the experimental design and development of quality systems for manufacture. SAB contributed to the conception, experimental design, data review, and drafting of the manuscript. All authors have read and approved the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (NIH), National Cancer Institute (grant numbers R44CA183203 and R44CA213476 to SAB), and the National Center for Advancing Translational Sciences (also NIH) (grant number UL1TR001064).
Contributor Information
Seshi R. Sompuram, Medical Discovery Partners LLC, Boston, Massachusetts
Kodela Vani, Medical Discovery Partners LLC, Boston, Massachusetts.
Anika K. Schaedle, Medical Discovery Partners LLC, Boston, Massachusetts
Anuradha Balasubramanian, Medical Discovery Partners LLC, Boston, Massachusetts.
Steven A. Bogen, Medical Discovery Partners LLC, Boston, Massachusetts; Department of Pathology and Laboratory Medicine, Tufts Medical Center, Boston, Massachusetts.
Literature Cited
- 1. Shi S, Key M, Kalra K. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–8. [DOI] [PubMed] [Google Scholar]
- 2. Shi S-R, Shi S, Taylor C. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59(1):13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang Z, Zhu W, Szekeres G, Xia H. Development of new rabbit monoclonal antibody to estrogen receptor: immunohistochemical assessment on formalin-fixed, paraffin-embedded tissue sections. Appl Immunohistochem Mol Morphol. 2005;13(1):91–5. [DOI] [PubMed] [Google Scholar]
- 4. Huang Z, Zhu W, Meng Y, Xia H. Development of new rabbit monoclonal antibody to progesterone receptor (Clone SP2): no heat pretreatment but effective for paraffin section immunohistochemistry. Appl Immunohistochem Mol Morphol. 2006;14(2):229–33. [DOI] [PubMed] [Google Scholar]
- 5. Rossi S, Laurino L, Furlanetto A, Chinellato S, Orvieto E, Canal F, Facchetti F, DeiTos A. Rabbit monoclonal antibodies: a comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal antibodies. Amer J Clin Pathol. 2005;124:295–302. [DOI] [PubMed] [Google Scholar]
- 6. Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, Christensen K, Edwards D. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids. 2002;67(9):799–813. [DOI] [PubMed] [Google Scholar]
- 7. Thomson T, Hayes M, Spinelli J, Hilland E, Sawrenko C, Phillips D, Dupuis B, Parker R. HER-2/neu in breast cancer: interobserver variability and performance of immunohistochemistry with 4 antibodies compared with fluorescent in situ hybridization. Mod Pathol. 2001;14(11):1079–86. [DOI] [PubMed] [Google Scholar]
- 8. Sompuram S, Kodela V, Ramanathan H, Wescott C, Radcliffe G, Bogen S. Synthetic peptides identified from phage-displayed combinatorial libraries as immunodiagnostic assay surrogate quality control targets. Clin Chem. 2002;48(3):410–20. [PubMed] [Google Scholar]
- 9. Sompuram S, Kodela V, Zhang K, Ramanathan H, Radcliffe G, Falb P, Bogen S. A novel quality control slide for quantitative immunohistochemistry testing. J Histochem Cytochem. 2002;50:1425–34. [DOI] [PubMed] [Google Scholar]
- 10. Sompuram S, Vani K, Bogen S. A molecular model of antigen retrieval using a peptide array. Am J Clin Pathol. 2006;125(1):91–8. [PubMed] [Google Scholar]
- 11. Sompuram S, Vani K, Hafer L, Bogen S. Antibodies immunoreactive with formalin-fixed tissue antigens recognize linear protein epitopes. Am J Clin Pathol. 2006;125(1):82–90. [PubMed] [Google Scholar]
- 12. Metz B, Kersten G, Baart G, de Jong A, Meiring H, ten Hove J, van Steenbergen MJ, Hennink W, Crommelin D, Jiskoot W. Identification of formaldehyde-induced modification in proteins: reactions with insulin. Bioconj Chem. 2006;17:815–22. [DOI] [PubMed] [Google Scholar]
- 13. O’Leary T, Fowler C, Evers D, Mason J. Protein fixation and antigen retrieval: chemical studies. Biotech Histochem. 2009;84(5):217–21. [DOI] [PubMed] [Google Scholar]
- 14. Rait V, Xu L, O’Leary T, Mason J. Modeling formalin fixation and antigen retrieval with bovine pancreatic RNase A II. Interrelationship of cross-linking, immunoreactivity, and heat treatment. Lab Invest. 2004;84(3):300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fowler C, O’Leary T, Mason J. Modeling formalin fixation and histological processing with ribonuclease A: effects of ethanol dehydration on reversal of formaldehyde cross-links. Lab Invest. 2008;88(7):785–91. [DOI] [PubMed] [Google Scholar]
- 16. Fowler C, Evers D, O’Leary T, Mason J. Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J Histochem Cytochem. 2011;59(4):366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metz B, Kersten G, Hoogerhout P, Brugghe H, Timmermans H, de Jong A, Meiring H, ten Hove J, Hennink W, Crommelin D, Jiskoot W. Identification of formaldehyde-induced modifications in proteins. J Biol Chem. 2004;279(8):6235–43. [DOI] [PubMed] [Google Scholar]
- 18. Toews J, Rogalski J, Clark T, Kast J. Mass spectrometric identification of formaldehyde-induced peptide modifications under in vivo protein cross-linking conditions. Anal Chim Acta. 2008;618:168–83. [DOI] [PubMed] [Google Scholar]
- 19. Bogen S, Vani K, Sompuram S. Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced crosslinks. Biotech Histochem. 2009;84(5):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vollert C, Moree W, Gregory S, Bark S, Eriksen J. Formaldehyde scavengers function as novel antigen retrieval agents. Sci Rep. 2015;5:17322. doi: 10.1038/srep17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vani K, Sompuram S, Schaedle A, Balasubramanian A, Bogen S. Analytic ranges of commercial breast cancer IHC tests: quantification with IHControls. J Histochem Cytochem. 2017;65(5):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sompuram S, Vani K, Tracey B, Kamstock D, Bogen S. Standardizing immunohistochemistry: a new reference control for detecting staining problems. J Histochem Cytochem. 2015;63(9):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vani K, Sompuram S, Naber S, Goldsmith J, Fulton R, Bogen S. Levey-Jennings analysis uncovers unsuspected causes of immunohistochemistry stain variability. Appl Immunohistochem Mol Morphol. 2016;24(10):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leong T, Leong A. How does antigen retrieval work? Adv Anat Pathol. 2007;14(2):129–31. [DOI] [PubMed] [Google Scholar]
- 25. O’Rourke M, Padula M. Analysis of formalin-fixed, paraffin-embedded (FFPE) tissue via proteomic techniques and misconceptions of antigen retrieval. Biotechniques. 2016;60:229–38. [DOI] [PubMed] [Google Scholar]
- 26. Shi S-R, Gu J, Turrens J, Cote R, Taylor C. editors. Development of the antigen retrieval technique: philosophical and theoretical bases. Natick: Eaton Publishing; 2000. [Google Scholar]
- 27. Kakimoto K, Takekoshi S, Miyajima K, Osamura R. Hypothesis for the mechanism for heat-induced antigen retrieval occurring on fresh frozen sections without formalin-fixation in immunohistochemistry. J Mol Histol. 2008;39(4):389–99. [DOI] [PubMed] [Google Scholar]
- 28. Boi G, Scalia C, Gendusa R, Ronchi S, Cattoretti G. Disaccharides protect antigens from drying-induced damage in routinely processed tissue sections. J Histochem Cytochem. 2016;64(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scalia C, Boi G, Bolognesi M, Riva L, Manzoni M, DeSmedt L, Bosisio F, Ronchi S, Leone B, Cattoretti G. Antigen masking during fixation and embedding, dissected. J Histochem Cytochem. 2017;65(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]