Abstract
Background
Although preoperative radiation followed by wide local excision yields excellent local control in soft tissue sarcomas, the risk of wound complications is reported to be higher compared with the incidence in patients who were administered postoperative radiation therapy. Vacuum (vac)-assisted closure may improve wound healing, but it is unknown whether vac-assisted closure during soft tissue sarcoma resection may reduce the risk of wound complications or impair local disease control.
Questions/purposes
(1) Does the use of a wound vac application at the time of soft tissue sarcoma resection reduce the risk of developing wound complications after lower extremity sarcoma resection? (2) Is vac-assisted closure associated with an increased risk of local relapse?
Methods
From 2000 to 2016, 312 patients with stage I to III soft tissue sarcomas were treated. Of these, 123 were treated with preoperative radiation ± chemotherapy followed by limb-sparing resection based on tumor location, size, grade, histology, and patient age. There was a minimum followup of 12 months. Radiation was delivered generally based on tumor size, grade, superficial versus deep nature, and proximity to neurovascular structures. Chemotherapy was administered in patients < 70 years old with high-grade tumors and tumors > 5 cm. Patient, demographic, and treatment variables, including incisional vac application and wound outcomes, were retrospectively evaluated. Incisional vac-assisted closure took place at the time of primary resection in 32% (46 of 123) of patients. Vac-assisted closure was considered when there was a concern for risk of external contamination such as instances in which fixation of adhesives would be difficult or regions where there was a high risk of contamination. Vac-assisted closure may have also been used in instances with increased wound tension at closure or with heightened concern for shearing on the wound such as buttock wounds. Ten patients were lost to followup, two in the vac group and eight in the non-vac group. Potential factors associated with wound complications were evaluated using Fisher’s exact test for univariate analysis and logistic regression for multivariate analysis. Local recurrence-free survival was evaluated using the Kaplan-Meier estimate.
Results
After taking into consideration factors such as tumor size, location, age, and patient comorbidities, it was shown that patients who underwent vac-assisted closure were less likely to experience wound complications compared with patients who did not undergo vac-assisted closure (odds ratio, 0.129; 95% confidence interval [CI], 0.041-0.398; p = 0.004). The local control incidence in the entire cohort was 98%. With the numbers available, Kaplan-Meier survivorship free from local recurrence did not differ between patients treated with or without the vac (100% [95% CI, 154.09-154.09] versus 96% [95% CI, 152.21-169.16]; p = 0.211), respectively.
Conclusions
Vac-assisted closure at the time of resection of proximal lower extremity soft tissue sarcomas is associated with a lower risk of wound complications, and its use apparently did not compromise local control. We show that the use of vac-assisted closure may be worth considering in surgeons’ attempts to reduce the risk of wound complications among patients with soft tissue sarcomas of the proximal lower extremities.
Level of Evidence
Level III, therapeutic study.
Introduction
There are several factors associated with postoperative wound complications in localized soft tissue sarcomas. In addition to preoperative radiation, proximal lower extremity tumors, diabetes, tumor size, volume, and age have all been associated with an increased risk of wound complications [1-3, 12, 16, 18]. Patients with proximal lower extremity soft tissue sarcomas have a much higher risk of postoperative wound complications compared with patients with distal lower extremity and upper extremity soft tissue sarcomas. In fact, many surgeons are reticent to use preoperative radiation in the proximal lower extremity because the incidence of wound complications is reported to be as high as 50% [1, 14, 20]. There are known mitigating factors to the development of wound complications such as the use of intensity-modulated therapy [14, 20], but evidence about surgical factors that may decrease the incidence of wound complications is sparse.
Vacuum-assisted closure (vac) uses subatmospheric pressure to accelerate wound healing and decrease the time to wound closure [9-13]. Negative pressure wound therapy (NPWT) impacts wound healing through both direct and indirect mechanisms. Direct mechanisms include wound apposition and creation of a pressure gradient between the wound and suction. Indirect mechanisms include increased blood flow, reduced bacterial burden, increased production and migration of fibroblastic growth factor, increased collagen organization, and vascular endothelial growth factor expression [6-11, 19]. Each of these events occurs in the wound healing cascade and thus establishes the role of NPWT and wound healing in any surgical intervention.
Incisional vac-assisted closure has been shown to decrease postoperative wound complications in both nononcologic and oncologic settings [4, 5, 15, 17, 19], but there have been no data in the use of wound vacs in soft tissue sarcomas and their impact on postoperative wound complications. Moreover, there are data that suggest that subatmospheric pressure promotes malignant tissue growth [13], which might raise concern with the use of a vac in patients with sarcoma. The aim of this study was to determine whether the use of incisional wound vacs in proximal lower extremity soft tissue sarcomas decreases the incidence of wound complications in patients treated with preoperative radiation. In addition, the authors sought to determine if the use of incisional vac affects local control compared with patients who did not undergo vac-assisted closure.
Patients and Methods
All investigators completed training in both human research and patient privacy and obtained approval from the institutional review board for this retrospective study. Patient records from the musculoskeletal oncology database and tumor repository at the Medical College of Wisconsin were then retrospectively reviewed for all patients with soft tissue sarcomas of the extremity or chest wall treated with preoperative radiation between 2000 and 2016. Pathology review was done at the time of initial diagnosis. Patients were staged according to the 2009 American Joint Committee on Cancer (AJCC) system, 7th edition. Patients were followed for a minimum of 12 months. None of the 123 patients were lost to followup.
Exclusion criteria included metastatic disease on initial presentation, age < 18 years old, soft tissue sarcomas in locations other than the proximal lower extremity (defined as thigh, buttock, pelvis, and groin), patients who did not receive preoperative radiation, recurrent sarcomas at first presentation to our sarcoma center, and small subcutaneous tumors. Histopathologic types demonstrating rhabdomyosarcoma, bone sarcomas, extraosseous primitive neuroectodermal tumor, Kaposi’s sarcoma, angiosarcoma, aggressive fibromatosis, and dermatofibrosarcoma protuberans were also excluded.
All patients were discussed at a multidisciplinary tumor board consisting of surgical and orthopaedic oncologists, medical and radiation oncologists, radiologists, and pathologists. Treatment recommendations from this tumor board were presented to the patient.
Radiotherapy was recommended in patients with deep, intermediate- to high-grade tumors, patients who had tumors near neurovascular bundles, and in patients with anticipated close margins. Patients received a median preoperative radiation dose of 50 Gy using three-dimensional conformal radiation or intensity-modulated radiotherapy. Tumor volumes were designated based on CT simulations and, if possible, an MRI acquired in the treatment position. Neoadjuvant chemotherapy was recommended and administered in patients who were typically < 70 years of age with large (> 5 cm), deep, high-grade lesions. Forty-two (34%) patients received chemotherapy and equal proportions of patients in both groups received chemotherapy. Chemotherapy was delivered before radiation using Adriamycin and ifosfamide for two to three cycles. Limb-sparing resection was performed in all patients 4 to 6 weeks after radiation.
Wide surgical resection was performed by fellowship-trained musculoskeletal oncologists grossly through normal tissue planes with sacrifice of arteries or veins that were involved by tumor. Preservation of neurovascular structures was performed when possible. The goal of the operation was to achieve negative margins. Vascular or reconstructive plastic surgeons were involved in the care of patients who underwent vascular reconstruction, difficult wound closures, and free flap reconstructions. Incisional vac-assisted closure data were obtained through medical records and included information on patient, tumor, and treatment characteristics, medical history as well as closure technique. Vac-assisted closure was considered when there was a concern for risk of external contamination such as instances in which fixation of adhesives would be difficult (groin, buttock) or regions where there was a high risk of contamination (proximal thigh, buttock, groin). Vac-assisted closure may have also been used in instances with increased wound tension at closure or with heightened concern for shearing on the wound such as buttock wounds.
After sarcoma resection by the musculoskeletal oncologist, the wound was closed in layers generally with the use of a deep and superficial drain (surgeon-dependent). Skin layer varied in the study patients and included subcuticular stitches, external sutures, and, rarely, staples. After closure, AdapticTM (Acelity, San Antonio, TX, USA) was placed along the incision and then the vac sponge and dressings were applied in a sterile fashion along the incision and put to 75-mmHg continuous suction. The goal of the vac dressing was to seal the wound to prevent any external contamination of the wound and to create a negative pressure environment to control additional fluid and encourage wound healing. Deep and superficial drains were removed at the discretion of the musculoskeletal oncologist. Patients generally kept the vac device on for 5 to 7 days. Although we did not assess length of stay in this study, some patients were discharged to home with the vac in place. The vac was changed at 5 to 7 days and generally discontinued if the surgeon felt the wound was sealed.The postoperative proportion of patients with wound complications was 33% (41 of 123) in all proximal lower extremity tumors and 29% (15 of 52) in patients who had medial compartment tumors. The incidence of positive margins was 7% (nine of 123). The demographics between patients who underwent vac-assisted closure versus those who did not were relatively equal (Table 1). On univariate analysis, incisional vac-assisted closure (p < 0.001) was associated with a decrease in the development of postoperative wound complications (see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A64).
Table 1.
VAC versus no VAC demographics
Patients at the institution were monitored with the following protocol. Followup care was coordinated through the musculoskeletal oncologists in a multidisciplinary clinic in conjunction with medical oncology, surgical oncology, radiation oncology, and interventional radiology. Followup consisted of imaging of the primary site, typically with an MRI and surveillance of distant disease. Once therapies were initiated, followup included an interval history, physical examination, and imaging with CT of the chest, abdomen, and pelvis every 4 to 6 months to monitor for disease progression up to 5 years. MRI of the primary tumor was also acquired every 4 to 6 months up to 5 years or if any localized symptoms ensued.
Followup was lengthened to yearly between Year 5 and up to Year 10. Followup occurred until the patient died, decided to pursue hospice measures, or generally at 10 years.
Postoperative wound complications were defined by the Canadian Multicenter Trial and were recorded if they occurred within 6 months after limb salvage surgery. In general, patients who required reoperation, prolonged wound care, or antibiotics after resection were considered to have wound complications [1]. Patient variables evaluated included age, Karnofsky Performance Status, presence or absence of cardiovascular disease and/or diabetes, body mass index (BMI), and smoking history. BMI was assessed as a continuous variable. Tumor variables included size and location. Treatment variables included the use of neoadjuvant chemotherapy, nonstaged flap reconstruction, and incisional vac-assisted closure at the time of resection. The investigators attempted to reduce bias by evaluating patients with soft tissue sarcomas who were at the highest risk of postoperative wound complications, those with proximal lower extremity tumors who received preoperative radiation.
Staging of patients was performed using the AJCC guidelines. Median followup was 3.9 years (range, 12-180 months). Median followup in the vac group was 3.3 years (range, 15-98 months) and 4.2 years (range, 12-180) in the non-vac group. Two patients were lost to followup in the vac group and eight were lost to followup in the non-vac group. There were 51 women and 72 men. Before 2006, besides those who were lost to followup, all patients have been seen within 18 months.
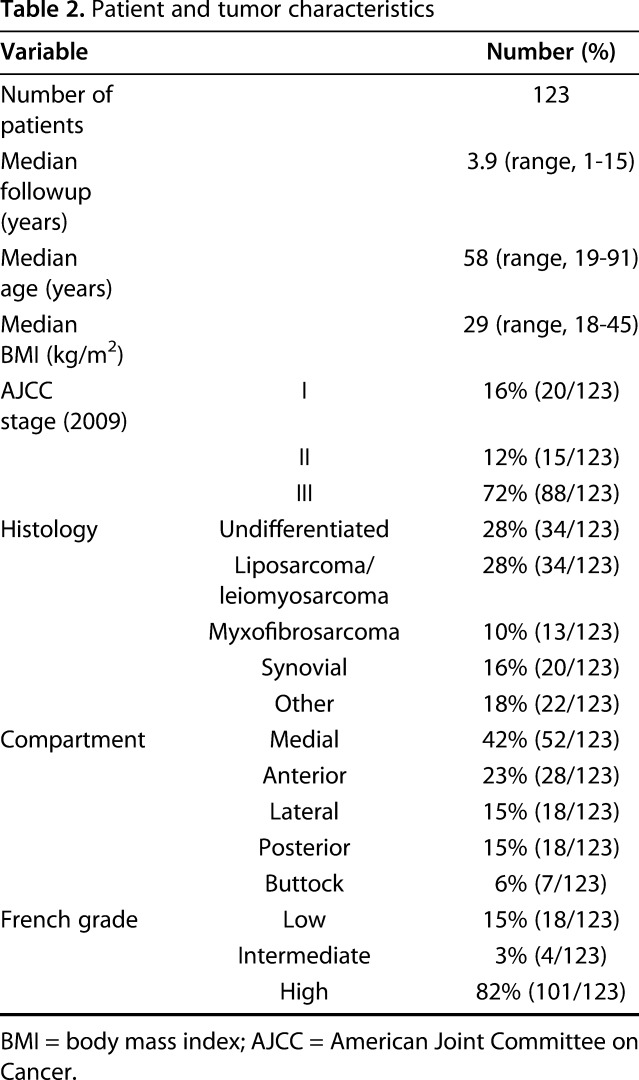
The mean age at diagnosis for patients presenting with stage I to III disease was 58 years (range, 19-92 years). The majority of patients had high-grade tumors (82%) (Table 2). A multidisciplinary review of our institutional soft tissue sarcomas registry identified 312 patients from 2000 to 2016. Of these, 123 presented with proximal lower extremity tumors that were treated with preoperative radiation and were eligible for analysis. All 123 patients underwent definitive resection and were followed at the treating institution, per the previously described protocol.
Table 2.
Patient and tumor characteristics
Statistical Analysis
Wound outcome was a dichotomous variable. Statistical software MedCalc (Version 15.6; MedCalc Software bvba, Ostend, Belgium) was used for all data analysis. Fisher’s exact test was used for univariate analysis. Local recurrence-free survival was evaluated using the Kaplan-Meier estimate. If a variable had a p value < 0.25, then it was used in the multivariate model. A logistic regression analysis was used for multivariate analysis. For all analyses, a type I error was maintained at 0.05 and all tests were two-sided. A probability of < 0.05 was accepted as statistically significant.
Results
After controlling for potential confounding variables like tumor size, location, age, and patient comorbidities, we found that patients with vac-assisted closure were less likely to experience a wound complication after surgery than were patients treated without the vac (odds ratio [OR], 0.129; 95% confidence interval [CI], 0.041-0.398; p = 0.004). Postoperative wound complications occurred in 47% (39 of 84) of patients who did not undergo vac-assisted closure versus 8% (three of 39) of patients who received the incisional vac (p < 0.001). Of the medial compartment tumors, wound complications occurred in 46% (13 of 28) of patients who did not undergo vac-assisted closure versus 9% (two of 24) in patients who received the incisional vac at the time of closure (p = 0.001). An inverse relationship was demonstrated between vac-assisted closure from 2000 to 2016 and the incidence of wound complications in all proximal lower extremity soft tissue sarcomas and medial compartment soft tissue sarcomas (Fig. 1 A-B).
Fig. 1 A-B.

An inverse relationship was demonstrated between vac-assisted closure from 2000 to 2016 and the rates of wound complications in all (A) proximal lower extremity soft tissue sarcomas and (B) medial compartment soft tissue sarcomas. STS = soft tissue sarcoma; WC = wound complications.
With the numbers available, Kaplan-Meier survivorship free from local recurrence did not differ between patients treated with or without the vac (100% [mean: 154.1 ± 1.4; 95% CI, 154.09-154.09] versus 96% [mean: 161.3 ± 4.0, 95% CI, 152.21-169.16]; p = 0.276), respectively (Fig. 2).
Fig. 2.

Local recurrence-free survival in patients with vac and non-vac assisted closure is shown.
The local control incidence of the entire cohort was 98%. Of the patients who underwent vac-assisted closure, the local control incidence was 100% and 96% in patients who did not undergo vac-assisted closure.
Discussion
Incisional vac-assisted closure has been associated with a decreased risk of postoperative wound complications in both nononcologic and oncologic resections; however, no data speak to the impact of incisional vac use in the setting of soft tissue sarcomas [4, 5, 11, 15, 17]. Patients with stage I through III proximal lower extremity soft tissue sarcomas who undergo preoperative radiation are at a high risk of postoperative wound complications [1-3, 12]. In this retrospective study, incisional vac-assisted closure was associated with a decreased risk of wound complications after soft tissue sarcoma resections, and we observed no difference in local recurrence or distant metastasis.
The most important limitation on this study is selection bias. We did not assign treatment with the vac randomly, and in retrospective studies, this shortcoming can cause an overestimate of the benefit of treatment. However, we do not believe that is likely in this case, because we generally chose to use vac-assisted closure on patients with more complex and more at-risk wounds; the fact that despite this, there were fewer wound complications suggests to us that indeed the vac-assisted closure likely is effective. In addition, we controlled for potential confounding variables such as tumor size, location, age, and patient comorbidities, giving us more confidence in the main conclusion drawn here about the efficacy of vac at preventing wound complications. Another limitation is loss to followup; in general, patients lost to followup are at increased risk of having experienced complications. Although only two patients in the vac group were lost to followup, this still may impact the findings, because there were only 34 patients in the vac-assisted closure group. In addition, unaccounted factors that were not ascertained included the flap used during the study period, surgical technique and surgeon experience, implementation of postoperative antibiotics, use of drains as well as preoperative radiation methods. However, these factors among surgeons at the treating institution are relatively equal and unlikely to impact the outcome. Lastly, there were 30 patients with 12- to 24-month followup who may still be at risk for local recurrence. A prospective study incorporating the aforementioned and additional factors that may impede wound healing would better assess the role incisional vac-assisted closure plays in the development of postoperative wound complications, especially in this high-risk subset of tumors.
In the present study, vac-assisted closure in soft tissue sarcomas was correlated with a decreased risk of postoperative wound complications in all proximal lower extremity tumors and medial compartment soft tissue sarcomas. There are relatively few studies on the use of NPWT in oncologic resections. Blackham et al. [4] retrospectively reviewed patients who endured operations for pancreatic, colorectal, or peritoneal surface malignancies. Patients who received incisional NPWT were compared with those who received standard sterile dressing changes postoperatively. In a subgroup analysis of clean, contaminated procedures, patients treated with NPWT developed fewer superficial incisional infections (6.0% versus 27.4%), decreased total surgical site infections (16.0% versus 35.5%), and decreased wound openings (16% versus 35.5%) compared with patients with sterile dressings [4]. In the nononcologic setting, subatmospheric pressure improves the wound environment by decreasing the time to wound closure and accelerating wound healing. Through the application of a semipermeable dressing, which controls the pressure gradient between the wound and suction, vac will directly promote wound healing through apposition, fluid transport from the wound bed and interstitial space, and decrease edematous buildup [6-10]. The use of wound vacs has been well established in nononcologic sites. O’Leary et al. [14] randomized 50 patients undergoing open abdominal laparotomies to sterile wound dressings or NPWT through an incisional vac. In this study, the 30-day incidence of infection was decreased from 32% to 8% in patients who received sterile wound dressings versus vac-assisted closure. In addition, the length of hospital stay was decreased by 8.6 days [14]. NPWT has also been demonstrated to decrease the incidence of postoperative wound complications in orthopaedic procedures. Stannard et al. [17] randomized 249 patients with high-risk lower extremity fractures (heel, tibial plateau) to incisional NPWT versus sterile wound dressings. In this study, NPWT was correlated with a reduced frequency of infection, 19% versus 10%, and dehiscence, 16.5% versus 8.6%. Additionally, the relative risk of developing postoperative infection was approximately two times higher in patients who underwent sterile dressing changes than in patients treated with incisional NPWT (95% CI, 1.03–3.55) [17].
In the current study, the local control rates did not differ between the incisional and nonincisional vac cohorts. In fact, no patient who underwent vac-assisted closure developed a local recurrence, demonstrating the safety of this procedure. Moreover, the incidence of positive margins at the time of primary resection was 7%. Although the number of patients is low, it does suggest that in this setting, vac-assisted closure may be considered if there is a high level of confidence that negative margins have been achieved until there is more abundant data on the safety of this technique. Although vac-assisted closure has been shown to be beneficial in numerous studies, there are contraindications to its practice. It has been reported that subatmospheric pressure may promote malignant growth, and malignancy is generally a contraindication to the use of a vac. This has led to the cautious use of this intervention in oncologic resections [13, 18]. In the few studies that have randomized or retrospectively reviewed NPWT, there are no reported data on the development of local recurrence, distant metastasis, or progression of disease [14, 17, 19].
Patients with proximal lower extremity soft tissue sarcomas treated with preoperative radiation are at high risk of postoperative wound complications. This study has revealed that vac-assisted closure at the time of resection of proximal lower extremity soft tissue sarcomas is associated with a lower risk of wound complications, but does not appear to compromise local control rates. Although careful attention to the soft tissues while obtaining a wide margin and postoperative wound management is warranted in any patient with soft tissue sarcomas, the use of vac-assisted closure may play an important role in reducing wound complications for patients with soft tissue sarcomas of the proximal lower extremity. Future studies may elucidate the significance of vac-assisted closure in patients with localized soft tissue sarcoma who are at high risk of wound complications.
Footnotes
One or more of the authors (DAH, JCN) is on the Board of the Musculoskeletal Transplant Foundation, outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Baldini EH, Lapidus MR, Wang Q, Manola J, Orgill DP, Pomahac B, Marcus KJ, Bertagnolli MM, Devlin PM, George S, Abraham J, Ferrone ML, Ready JE, Raut CP. Predictors for major wound complications following preoperative radiotherapy and surgery for soft-tissue sarcoma of the extremities and trunk: importance of tumor proximity to skin surface. Ann Surg Oncol. 2013;20:1494–1499. [DOI] [PubMed] [Google Scholar]
- 2.Barkley HT, Jr, Martin RG, Romsdahl MM, Lindberg R, Zagars GK. Treatment of soft tissue sarcomas by preoperative irradiation and conservative surgical resection. Int J Radiat Oncol Biol Phys. 1988;14:693–699. [DOI] [PubMed] [Google Scholar]
- 3.Bedi M, King DM, Shivakoti M, Wang T, Zambrano EV, Charlson J, Hackbarth D, Neilson J, Whitfield R, Wang D. Prognostic variables in patients with primary soft tissue sarcoma of the extremity and trunk treated with neoadjuvant radiotherapy or neoadjuvant sequential chemoradiotherapy. Radiat Oncol. 2013;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg. 2013;205:647–664. [DOI] [PubMed] [Google Scholar]
- 5.Chadi SA, Kidane B, Britto K, Brackstone M, Ott MC. Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon Rectum. 2014;57:999–1006. [DOI] [PubMed] [Google Scholar]
- 6.Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006;56:418–422. [DOI] [PubMed] [Google Scholar]
- 7.Ichioka S, Watanabe H, Sekiya N, Shibata M, Nakatsuka T. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen. 2008;16:460–465. [DOI] [PubMed] [Google Scholar]
- 8.Kairinos N, Hudson D, Solomons M. Depth of penetration of negative pressure wound therapy into underlying tissues. Wound Repair Regen. 2009;17:456. [DOI] [PubMed] [Google Scholar]
- 9.Kairinos N, Solomons M, Hudson DA. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg. 2009;123:589-598; discussion 599–600. [DOI] [PubMed] [Google Scholar]
- 10.Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg. 2009;123:601–612. [DOI] [PubMed] [Google Scholar]
- 11.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen OS, Cummings B, O'Sullivan B, Catton C, Bell RS, Fornasier VL. Preoperative and postoperative irradiation of soft tissue sarcomas: effect of radiation field size. Int J Radiat Oncol Biol Phys. 1991;21:1595–1599. [DOI] [PubMed] [Google Scholar]
- 13.Novak A, Wasim KS, Palmer I. The evidence-based principles of negative pressure wound therapy in trauma & orthopedics. Open Orthop J. 2014;8:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Leary DP, Peirce C, Anglim B, Burton M, Concannon E, Carter M, Hickey K, Coffey JC. Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the PICO Trial. Ann Surg. 2017;265:1082–1086. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan B, Griffin AM, Dickie CI, Sharpe MB, Chung PW, Catton CN, Ferguson PC, Wunder JS, Deheshi BM, White LM, Kandel RA, Jaffray DA, Bell RS. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119:1878–1884. [DOI] [PubMed] [Google Scholar]
- 17.Stannard JP, Volgas DA, McGwin G, 3rd, Stewart RL, Obremskey W, Moore T, Anglen JO. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26:37–42. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JF, Ballo MT, Langstein HN, Wayne JD, Cormier JN, Hunt KK, Feig BW, Yasko AW, Lewis VO, Lin PP, Cannon CP, Zagars GK, Pollock RE, Pisters PW. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13:1209–1215. [DOI] [PubMed] [Google Scholar]
- 19.Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6:185–194. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhang Q, Eisenberg BL, Kane JM, Li XA, Lucas D, Petersen IA, DeLaney TF, Freeman CR, Finkelstein SE, Hitchcock YJ, Bedi M, Singh AK, Dundas G, Kirsch DG. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]



