Abstract
Background
Lower urinary tract symptoms (LUTS) and urinary bother have been reported in adults undergoing surgery and have been associated with urinary tract infections, longer hospital stays, increased surgical costs, and decreased patient satisfaction. Previous reports indicate that up to one in two patients with lumbar spine pathology have moderate-to-severe LUTS, but little is known about LUTS in patients with cervical spine conditions.
Questions/purposes
(1) What is the prevalence of moderate-to-severe LUTS and clinically relevant urinary bother among patients undergoing elective cervical spine surgery? (2) Does the presence of myelopathy affect frequency of moderate-to-severe LUTS or clinically relevant urinary bother among patients undergoing elective cervical spine surgery? (3) Do MRI findings of spinal cord injury or compression correlate with presence and severity of LUTS?
Methods
We performed a cross-sectional study using clinical data collected from adult patients undergoing elective cervical spine surgery. Over an approximately 30-month period, we approached all patients who were evaluated in the preoperative clinic before undergoing elective cervical spine surgery. Of the 257 approached, 242 participated (94%). Study participants ranged in age from 34 to 83 years with a mean age of 58 years (SD 12). There were 108 males (45%) and 134 females (55%). A validated questionnaire, the International Prostate Symptom Score (IPSS), was used to identify LUTS. The IPSS score ranges from 0 to 35 points with LUTS presence defined as a score of ≥ 8 and LUTS severity categorized as mild (IPSS 0-7), moderate (IPSS 8-19), or severe (IPSS 20-35). Quality of life resulting from urinary bother is scored 0 to 6 with scores ≥ 4 considered clinically relevant urinary bother. Patients were grouped into a myelopathy group and a nonmyelopathy group based on diagnosis as assigned by the operating surgeon. MRIs were analyzed by one spine surgeon to identify the presence of cord signal, number of levels with cord compression (mm), and a calculated compression ratio score with cord compression and with compression ratio among patients with myelopathy.
Results
The prevalence of moderate LUTS in our patient sample was 40% (97 of 242; 95% confidence interval [CI], 34%–47%). The prevalence of severe LUTS in our patient sample was 8% (19 of 242; 95% CI, 5%–12%). Clinically relevant urinary bother was reported in 18% of patients (41 of 228; 95% CI, 13%–24%). After adjustment for age and sex, the odds of moderate-to-severe LUTS among patients with myelopathy was greater than that observed in patients without myelopathy (adjusted odds ratio, 2.0; p = 0.015). The prevalence of clinically relevant urinary bother was higher in patients with myelopathy (30% [26 of 88]) compared with those with no myelopathy (11% [15 of 140]; p < 0.001). With the numbers available, among patients with myelopathy, there was no difference in distribution of LUTS symptom severity or IPSS score according to cord signal presence (50% [23 of 46]) and absence (65% [31 of 48]; p = 0.153), number of levels with compression (70% [seven of 10 with four levels]; 59% [13 of 22 with three levels]; 51% [19 of 37] with two levels; and 60% [15 of 25] with one level; p = 0.730), millimeters of cord compression (r = 0.02; p = 0.854), or compression ratio (r = 0.09; p = 0.413).
Conclusions
Nearly half of all patients undergoing elective cervical spine surgery had moderate-to-severe LUTS. This is more than double the prevalence that has been reported in a community-dwelling adult population. These symptoms can impair quality of life, lead to surgical complications (urinary retention or incontinence), and may be mistaken for cauda equina, prompting potentially unnecessary imaging and studies. Given that urinary bother is reported less frequently than LUTS, patients may be less likely to seek care for urinary symptoms before undergoing surgery. Therefore, it is important to increase provider awareness of the high prevalence of LUTS.
Level of Evidence
Level III, prognostic study.
Introduction
Lower urinary tract symptoms (LUTS) are common in adults, affecting approximately one in five people between the ages of 30 and 79 years [16, 29]. The prevalence of LUTS increases with age in both male and female patients, and LUTS has been shown to negatively impact quality of life [7, 8, 10, 16, 19, 29, 32]. An increased prevalence of LUTS has been reported in patients with lumbar spine pathology and back pain compared with the general population; however, little has been reported about the prevalence of LUTS in patients with cervical spine pathology [7, 9, 12, 18, 22, 27, 28, 35]. The prevalence of LUTS can be estimated using the International Prostate Symptom Score (IPSS), a questionnaire based on the American Urologic Association symptoms index for benign prostate hyperplasia [3]. The IPSS contains seven questions addressing voiding symptoms (incomplete emptying, intermittency, weak stream, and straining to void) and storage symptoms (frequency, urgency, and nocturia). There is an additional urinary bother question that addresses quality of life resulting from urinary symptoms. This tool is widely used in urologic and other medical evidence and has been validated for use in quantifying LUTS in both males and females [4, 5, 11, 16, 17, 25, 30]. Numerous population-based studies have been performed investigating the prevalence of LUTS in general populations. The reported rates of LUTS vary from 19% in a group of US males and females to 43% in a group of Asian males and females [2, 4, 6, 16, 36]. In a study looking specifically at patients undergoing elective lumbar spine surgery, the prevalence of LUTS was found to be 46%, higher than reported rates in the general population [18].
However, we do not have data on the prevalence of LUTS in patients undergoing cervical spine surgery. Such data would be important to acquire because these symptoms may influence preoperative decision-making, have a substantial impact on quality of life, and are associated with potentially avoidable postoperative complications such as urinary retention and urinary tract infections [1, 10, 14-16]. Additionally, it would be beneficial to identify if there are any associations between specific cervical spine pathologies and LUTS to better identify patients who may be at higher risk for these symptoms. In patients with cervical myelopathy, MRI findings, including cord signal and compression ratio, have been correlated with disease severity and neurologic prognosis [23, 24, 31]. As far as we are aware, a relationship between MRI findings pertaining to cord compression and prevalence of LUTS has not been investigated.
We therefore used the IPSS survey to answer the following questions: (1) What is the prevalence of moderate-to-severe LUTS and clinically relevant urinary bother among patients undergoing elective cervical spine surgery? (2) Does the presence of myelopathy impact frequency of moderate-to-severe LUTS or clinically relevant urinary bother among patients undergoing elective cervical spine surgery? (3) Do MRI findings of spinal cord injury or compression correlate with presence and severity of LUTS?
Patients and Methods
Institutional review board approval was obtained before the initiation of this study. Adult patients undergoing elective cervical spine surgery with one of four orthopaedic spine surgeons at a single academic institution were prospectively enrolled from October 2, 2015 to April 23, 2018. A cross-sectional study of data collected at the preoperative visit was performed.
All adult patients undergoing elective cervical spine surgery were considered to be eligible. Not eligible were those who were younger than 18 years of age at the time of surgery, those undergoing emergent surgery, pregnant females, incarcerated patients, and patients with a preoperative appointment who did not undergo surgery.
Patient demographic data including age, sex, cervical spine diagnosis, and location of pathology were obtained through a review of electronic medical records. The IPSS survey with the supplemental urinary bother question was obtained as part of the clinical standard of care for all patients at their preoperative clinic appointment within 1 month of scheduled surgery. Answers to the IPSS were scored on a scale of 0 to 5 with response options ranging from “not at all” (0) to “almost always” (5) per IPSS guidelines [3]. The final score was computed as the sum scores to the first seven questions and used to define presence of LUTS as a score of ≥ 8 points [3, 16]. For descriptive assessments, we further categorized symptom severity as mild (IPSS 0-7), moderate (IPSS 8-19), or severe (IPSS ≥ 20) [35]. Urinary quality of life, known as urinary bother, was assessed with a separately scored question asking “if you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?” Answers were scored on a scale of 0 to 6 with options ranging from “delighted” (0) to “terrible” (6). Scores ≥ 4 are considered to represent clinically relevant urinary bother [16]. Patients underwent surgery as planned without additional intervention.
We attempted to collect information on all eligible patients, and most (94% [242 of 257]) participated, which should have mitigated selection bias. Patient diagnosis was assigned by the operating surgeon. All patients had MRI studies, which were obtained preoperatively and included T1- and T2-weighted axial, sagittal, and coronal images of the full cervical spine. The cervical spine diagnosis and location of pathology were determined from clinical symptoms, including subjective patient complaints and physical examination findings from an examination performed during a clinic visit with a spine surgeon. These symptoms were confirmed with MRI, which included a radiology report and review by one of our spine surgeons (JUY). Our study patients were separated into a myelopathy group and nonmyelopathy group based on diagnosis. Diagnoses in the nonmyelopathy group included radiculopathy and axial pain. All MRI studies for patients in the myelopathy group were reviewed by a single fellowship-trained spine surgeon (JUY). Consistent with published methods, we defined cord signal as intramedullary hyperintensity on T2 images and recorded it if present at one or more cervical levels [24]. We identified cord compression as narrowing of the intramedullary space on T2 axial and midsagittal images. For each patient, we recorded the number of levels with compression. The area of maximal compression was defined as the MRI cut with the smallest cord diameter (mm) measured on axial images. At this level, we recorded the anterior-to-posterior diameter of the spinal cord and width of the above adjacent noncompressed segment (mm). If additional compressed levels were identified above the level of maximal compression, we recorded the anterior-to-posterior diameter of the spinal cord and width of the below adjacent noncompressed segment. A compression ratio (CR), bound by 0 and 1 where values closer to 1 represent more compression, was then calculated as CR = anterior to posterior/width (CR = AP/W).
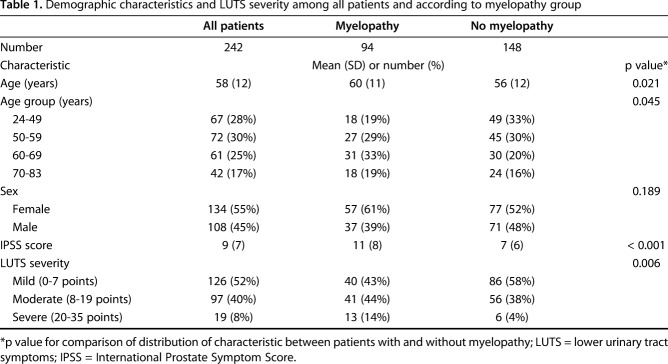
The mean age of our study population was 58 years (SD 12) and ranged from 24 to 83 years (Table 1). Just more than half the patients were females (55% [134 of 242]). Myelopathy was observed in 94 patients (39% [94 of 242]). The average age in the myelopathy group was 60 years (SD 11), which was higher than the average of 56 years (SD 12) in the nonmyelopathy group. Patients with myelopathy were more likely to be 60 years or older (52% with myelopathy [49 of 94] versus 36% without myelopathy [54 of 148]; p = 0.017).
Table 1.
Demographic characteristics and LUTS severity among all patients and according to myelopathy group
Data were collected from 242 patients, which was 94% of eligible patients (242 of 257) with 108 males (45% [108 of 242]) and 134 females (55% [134 of 242]). The 6% of eligible patients (15 of 257) who were not included in the study were excluded because of incomplete or absent survey data.
Statistical Analysis
We estimated prevalence and 95% confidence intervals (CIs) of LUTS and urinary bother using methods for binomial proportions. Distributions of age, sex, IPSS scores, and LUTS severity are presented in the sample using means and SDs or number and percent and were compared between patients with and without myelopathy with t-tests for continuous variables or chi-square or Fisher’s exact tests for categorical variables. Multiple logistic regression was used to estimate odds ratios (ORs) and their 95% CIs for the association of myelopathy with presence of moderate-to-severe LUTS before and after adjustment for age and sex. In analyses restricted to the 94 patients with myelopathy, the prevalence of moderate-to-severe LUTS was compared descriptively according to the presence or absence of cord signal and in categories of cervical spine levels compressed and Pearson’s correlation coefficients (r) were estimated for the association of IPSS score with compression (mm) and with compression ratio. Analyses were conducted using SAS version 9.4 software (SAS Institute Inc, Cary, NC, USA).
Results
The prevalence of moderate LUTS was 40% (97 of 242; 95% CI, 34%–47%) and of severe LUTS was 8% (19 of 242; 95% CI, 5%–12%) among patients undergoing elective cervical spine surgery. Overall, 48% of patients (116 of 242; 95% CI, 42%–54%) had moderate-to-severe LUTS and 18% had clinically relevant urinary bother (41 of 228; 95% CI, 13%–24%). The mean IPSS score was 9 (SD 7) among the patients in our sample (Table 1).
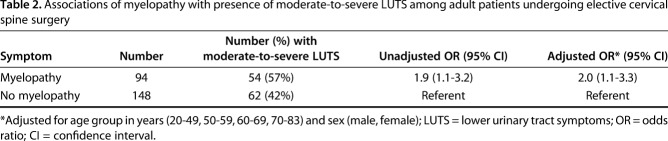
After adjustment for age and sex, the odds of moderate-to-severe LUTS among patients with myelopathy was greater than that observed in patients without myelopathy (adjusted OR, 2.0; 95% CI, 1.1–3.3; p = 0.015, nonmyelopathy referent; Table 2). There were 94 patients (39% [94 of 242]) with myelopathy and 148 patients (61% [148 of 242]) without myelopathy in the study sample.
Table 2.
Associations of myelopathy with presence of moderate-to-severe LUTS among adult patients undergoing elective cervical spine surgery
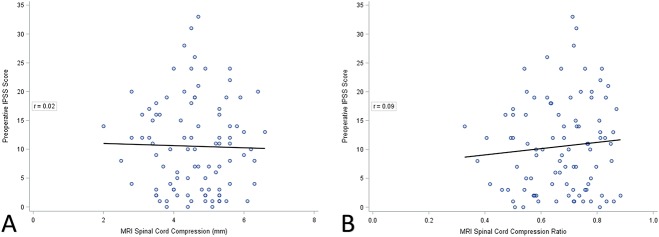
The prevalence of moderate-to-severe LUTS did not differ among patients with myelopathy and cord signal present compared with those without (50% [23 of 46] with cord signal and 65% [31 of 48] without cord signal; p = 0.153; Table 3). Similarly, the prevalence of moderate-to-severe LUTS was not higher in patients with more compared with fewer compressed levels (70% [seven of 10] with four levels; 59% [13 of 22] with three levels; 51% [19 of 37] with two levels; and 60% [15 of 25] with one level; p = 0.730; Table 3). Finally, there was no correlation between IPSS and millimeters of cord compression (r = 0.02; p = 0.854; Fig. 1A) or compression ratio (r = 0.09; p = 0.413; Fig. 1B).
Table 3.
Associations of cord signal and number of levels with compression with presence of moderate-to-severe lower urinary tract symptoms (LUTS) among adult patients undergoing cervical spine surgery
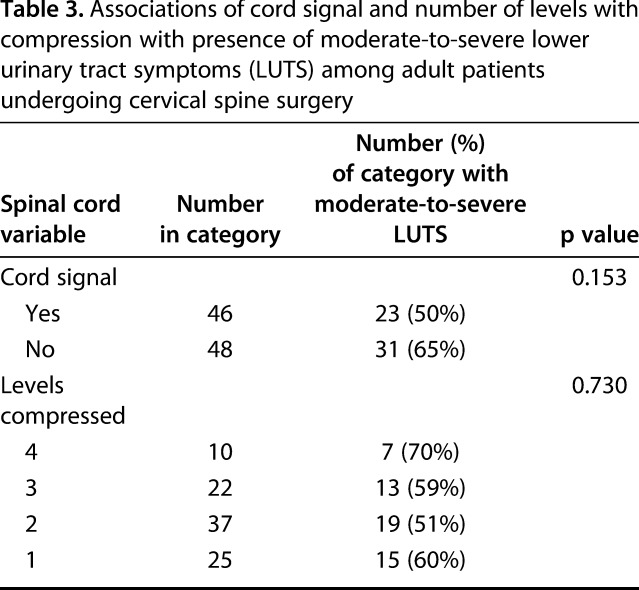
Fig. 1 A-B.
(A) Preoperative IPSS score is compared with compression (mm) in patients with myelopathy and its correlation coefficient is presented. (B) Preoperative IPSS score is compared with compression ratio in patients with myelopathy and its correlation coefficient is presented.
Discussion
It is important to be aware of the high frequency of LUTS in patients undergoing elective cervical spine surgery because these symptoms may put patients at risk for postoperative complications and may raise red flags for cauda equina syndrome, prompting unnecessary imaging and other studies [14, 15, 27]. In this study, we found that LUTS was common in adult patients undergoing elective cervical spine surgery with nearly half the patients we surveyed reporting moderate or severe symptoms. The frequency of LUTS and of urinary bother was higher in patients with myelopathy compared with those who did not have myelopathy, even after controlling for age and sex. However, among patients with myelopathy, we found no association between spinal cord compression as diagnosed on MRI and LUTS.
There were several limitations to our study. First, LUTS were determined by patient self-evaluation and reporting in the form of a survey questionnaire. We did not obtain clinical urologic metrics such as post-void residual volume or urodynamic assessments. However, the IPSS has been well validated and is commonly used in clinical practice to assess the presence and severity of LUTS. Moreover, the IPSS is the standard instrument used in clinical and epidemiologic research to estimate LUTS prevalence. Therefore, we did not believe that urodynamic data would add to the goal of this study, which was to evaluate the prevalence of LUTS and urinary bother as reported by patients. Second, we did not grade severity of myelopathy or calculate the Japanese Orthopaedic Association (JOA) score or modified JOA score for myelopathy [13, 33]. The purpose of these scoring systems is to grade the severity of myelopathy, which can be helpful in making surgical recommendations and predicting functional outcomes. However, in this study we were investigating a population of patients who had symptoms that indicated surgical intervention was needed. As such, we did not feel that JOA scores would add to our results [13, 33]. Additionally, we did not evaluate patients for lumbar spine disease. It is common for patients with spinal stenosis to have narrowing in more than one region with occurrence of concomitant cervical and lumbar diseases as high as 59% [26]. Thus, the high prevalence of LUTS found in our patients with cervical spine disease may be confounded by less symptomatic lumbar stenosis leading to LUTS. A single surgeon evaluated the MRI studies with no intraobserver assessment, which is another possible limitation to this study. Although there is likely to be error in the surgeon’s classifications, any errors would not be expected to have been associated with LUTS status, because the evaluator was blinded to that variable at the time of MRI review. Random error would tend to underestimate any association of the MRI findings with LUTS. Finally, our data were collected from a sample of patients at a single institution, which may decrease the generalizability of our results. Our study included adult patients undergoing elective cervical spine surgery at an academic institution in the United States. Thus, our results may not be consistent with the prevalence of LUTS in younger patients, patients having surgery in a community hospital setting, or patients in different geographic regions.
The prevalence of LUTS in patients undergoing cervical spine surgery appears to be greater than the prevalence among adults more generally. Published prevalence estimates of LUTS range from 19% to 43% [2, 4, 6, 16, 36] and this variation may be the result of differences in sample selection on sex, age, ethnicity, or medical comorbidities. Therefore, the impact of LUTS in clinical samples is best evaluated in comparison to LUTS prevalence from population-based samples. The Boston Area Community Health (BACH) Survey is an observational prospective cohort study of urologic symptoms in a large, racially and ethnically diverse US population-based sample of males and females [16]. This population is similar in both age (30-79 years) and sex distribution to our study sample. In the BACH study, the prevalence of moderate-to-severe LUTS was 19%. In comparing IPSS results, we found the prevalence of moderate-to-severe LUTS was nearly 2.5 times greater in patients undergoing cervical spine surgery as compared with the general population. In reviewing the evidence regarding LUTS in patients undergoing elective lumbar spine surgery, prevalence estimates range from 27% to 55% [28, 35]. A study examining patients undergoing elective lumbar spine surgery reported the prevalence of moderate-to-severe LUTS as 46% [18].
Innervation of the urinary system comes from nerves located in the lumbar and sacral spine [20]. Therefore, we were surprised to find no difference in the prevalence of LUTS and urinary bother in this population of patients undergoing cervical spine surgery. Clinically relevant urinary bother was reported less frequently than moderate-to-severe LUTS. If patients are less bothered, they may be less likely to mention these symptoms, especially when being evaluated for spine surgery [37]. Additionally, LUTS has been associated with comorbidities, including anxiety and depression, which are independent risk factors for a smaller degree of improvement in function and quality of life after spine surgery [7, 8, 10, 19, 21, 34]. In patients undergoing elective lumbar spine surgery, the prevalence of urinary bother was reported to be 18% in females and 10% in males [18]. We did not find the same sex difference in our patients undergoing elective cervical spine surgery.
The lower prevalence of LUTS and urinary bother in patients without myelopathy compared with myelopathy is consistent with what we expected given the micturition reflex, which involves signal transmission from the lumbosacral plexus to the brain through the spinal cord [20]. However, we do not have a neurologic explanation for the high prevalence of LUTS in patients with nonmyelopathic cervical pathology.
Although we cannot fully explain the etiology of LUTS in patients undergoing cervical spine surgery, we found that these symptoms were common. None of the patients included in this study had cauda equina syndrome or other diagnoses requiring emergent surgical intervention. Urinary symptoms may continue to be considered “red flags” for cauda equina syndrome; however, the commonness of this problem should encourage providers to have a higher threshold for ordering more studies over rushing to surgery. Future studies may focus on evaluating pain, narcotic use, psychologic diagnoses, or other nonspine factors that might be associated with LUTS. MRI findings including cord signal and compression ratio have been correlated with severity of myelopathy; however, we do not know of any reports correlating MRI findings with severity of LUTS [24, 31]. In this study, we did not find any association between MRI findings such as cord signal or compression ratio and severity of LUTS. Thus, we cannot use this information to add to algorithms that might help providers decide which patients should be recommended to have surgery. In our cohort, the number of spinal levels involved did not have any influence on LUTS. It remains unknown if the number of compressed levels has any prognostic implication on postoperative urologic symptoms.
To conclude, using the IPSS, we found the prevalence of LUTS in patients undergoing elective cervical spine surgery to be approximately 50%. This is more than double the reported prevalence in the community and is approximately the same as the reported prevalence in patients undergoing elective lumbar spine surgery [16, 18]. LUTS is more frequently reported in patients with myelopathy than in patients with radiculopathy. After adjusting for age and sex, odds of LUTS remain higher in the myelopathy group. Patients with myelopathy also report clinically relevant urinary bother more frequently than those without myelopathy. Overall, clinically relevant urinary bother was reported less frequently than LUTS, which may influence how likely patients are to report or seek treatment for these symptoms.
Acknowledgments
We thank Ryan Boone, Huy Hoang, and Valentina Haj for their assistance with data collection.
Footnotes
One of the authors certifies that she (EGL) received research support, during the study period, an amount of USD 10,000 to USD 100,000, from the Cervical Spine Research Society Resident/Fellow grant.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Altschul D, Kobets A, Nakhla J, Jada A, Nasser R, Kinon MD, Yassari R, Houten J. Postoperative urinary retention in patients undergoing elective spinal surgery. J Neurosurg Spine . 2017;26:229–234. [DOI] [PubMed] [Google Scholar]
- 2.Andrades M, Paul R, Ambreen A, Dodani S, Dhanani RH, Qidwai W. Distribution of lower urinary tract symptoms (LUTS) in adult women. J Coll Physicians Surg Pak. 2004;14:132-135. [PubMed] [Google Scholar]
- 3.Barry MJ, Fowler FJ, Jr, O’leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol . 1992;148:1549-1557. [DOI] [PubMed] [Google Scholar]
- 4.Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R, Kiemeney L, Lee C; UrEpik Study Group. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409-414. [DOI] [PubMed] [Google Scholar]
- 5.Chai TC, Belville WD, McGuire EJ, Nyquist L. Specificity of the American Urological Association voiding symptom index: comparison of unselected and selected samples of both sexes. J Urol . 1993;150:1710-1713. [DOI] [PubMed] [Google Scholar]
- 6.Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, Wang J, Yoo TK, Chu R, Sumarsono B. Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based study. Adv Ther . 2017;34:1953-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne K, Kvasz M, Ireland AM, Milson I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol . 2011;61:88-95. [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Wein AJ, Tubaro A, Sexton CC, Thompson CL, Kopp ZS, Aiyer LP. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103:4-11. [DOI] [PubMed] [Google Scholar]
- 9.Eisenstein SM, Englebrecht DJ, el Masry WS. Low back pain and urinary incontinence. A hypothetical relationship. Spine. 1994;19:1148-1152. [DOI] [PubMed] [Google Scholar]
- 10.Girman CJ, Jacobsen SJ, Tsukamoto T, Richard F, Garraway WM, Sagnier PP, Guess HA, Rhodes T, Boyle P, Lieber MM. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 1998;51:428–436. [DOI] [PubMed] [Google Scholar]
- 11.Groutz A, Blaivas JG, Fait G, Sassone AM, Chalkin DC, Gordon D. The significance of the American Urological Association symptom index score in the evaluation of women with bladder outlet obstruction. J Urol. 2000;163:207-211. [PubMed] [Google Scholar]
- 12.Hellström P, Tammela TLJ, Niinimäki TJ. Voiding dysfunction and urodynamic findings in patients with lumbar spinal stenosis and the effect of decompressive laminectomy. Scand J Urol Nephrol. 1995;29:167-172. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Orthopaedic Association Scoring system for cervical myelopathy. J Jpn Orthop Assoc. 1994;68:490-503. [Google Scholar]
- 14.Jung HJ, Park JB, Kong CG, Kim YY, Park J, Kim JB. Postoperative urinary retention following anterior cervical spine surgery for degenerative cervical disc diseases. Clin Orthop Surg. 2013;5:134-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalik U, Plante MK. Urinary retention in surgical patients. Surg Clin North Am. 2016;96:453-567. [DOI] [PubMed] [Google Scholar]
- 16.Kupelian V, Wei JT, O’Leary MP, Kusek JW, Litman HJ, Link CL, McKinlay JB; BACH Survey Investigators. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381-2387. [DOI] [PubMed] [Google Scholar]
- 17.Lepor H, Machi G. Comparison of AUA symptom index in unselected males and females between fifty-five and seventy-nine years of age. Urology. 1993;42:36-40. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman EG, Boone RM, Radoslovich SS, Haj V, Hiratzka J, Marshall LM, Yoo JU. Prevalence of pre-operative lower urinary tract symptoms in patients undergoing elective lumbar spine surgery. Spine. 2018;43:E1152-E1156. [DOI] [PubMed] [Google Scholar]
- 19.Marshall LM, Holton KF, Parsons JK, Lapidus JA, Ramsey K, Barrett-Connor E; Osteoporotic Fractures in Men (MrOS) Study Group. Lifestyle and health factors associated with progressing and remitting trajectories of untreated lower urinary tract symptoms among elderly men. Prostate Cancer Prostatic Dis. 2014;17:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAninch JW, Lue TF. Smith & Tanagho’s General Urology. 18th ed. New York, NY, USA: McGraw-Hill; 2012. [Google Scholar]
- 21.Miller JA, Derakhshan A, Lubelski D, Alvin MD, McGirt MJ, Benzel EC, Mroz TE. The impact of preoperative depression on quality of life outcomes after lumbar surgery. Spine J. 2015;15:58-64. [DOI] [PubMed] [Google Scholar]
- 22.Mosdal C, Iversen P, Iversen Hansen R. Bladder neuropathy in lumbar disc disease. Acta Neurochir (Wien). 1979;46:281-286. [DOI] [PubMed] [Google Scholar]
- 23.Nagai T, Takahashi Y, Endo K, Ikegami R, Ueno R, Yamamoto K. Analysis of spastic gait in cervical myelopathy: linking compression ratio to spatiotemporal and pedobarographic parameters. Gait Posture. 2018;59:152-156. [DOI] [PubMed] [Google Scholar]
- 24.Nemani VM, Kim HJ, Piyaskulkaew C, Nguyen JT, Riew KD. Correlation of cord signal change with physical examination findings in patients with cervical myelopathy. Spine. 2015;40:6-10. [DOI] [PubMed] [Google Scholar]
- 25.Okamura K, Nojiri Y, Osuga Y, Tange C. Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology. 2009;73:1199-1202. [DOI] [PubMed] [Google Scholar]
- 26.Overley SC, Kim JS, Gogel BA, Merrill RK, Hecht AC. Tandem spinal stenosis: a systematic review. JBJS Rev. 2017;5:e2. [DOI] [PubMed] [Google Scholar]
- 27.Perner A, Andersen JT, Juhler M. Lower urinary tract symptoms in lumbar root compression syndromes: a prospective survey. Spine. 1997;22:2693-2697. [DOI] [PubMed] [Google Scholar]
- 28.Rosomoff HL. The neurogenic bladder of lumbar disc syndromes. Trans Am Neurol Assoc. 1964;89:249-251. [PubMed] [Google Scholar]
- 29.Sarma AV, Wei JT, Jacobson DJ, Dunn RL, Roberts RO, Girman CJ, Lieber MM, Cooney KA, Schottenfeld D, Montie JE, Jacobsen SJ; Olmsted County Study of Urinary Symptoms and Health Status; Flint Men’s Health Study. Comparison of lower urinary tract symptom severity and associated bother between community-dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men’s Health Study. Urology. 2003;61:1086-1091. [DOI] [PubMed] [Google Scholar]
- 30.Scarpero HM, Fiske J, Xue X, Nitti VW. American Urological Association Symptom Index for lower urinary tract symptoms in women: correlation with degree of bother and impact on quality of life. Urology. 2003;61:1118-1122. [DOI] [PubMed] [Google Scholar]
- 31.Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY. Reevaluation of the Pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg. 2009;1:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor BC, Wilt TJ, Fink HA, Lambert LC, Marshall LM, Hoffman AR, Beer TM, Bauer DC, Zmuda JM, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Study Research Group. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68:804-809. [DOI] [PubMed] [Google Scholar]
- 33.Tetreault L, Kopjar B, Nouri A, Arnold P, Barbagallo G, Bartels R, Qiang Z, Singh A, Zileli M, Vaccaro A, Fehlings M. The modified Japanese Orthopaedic Association Scale: establishing criteria for mild, moderate, and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26:78-84. [DOI] [PubMed] [Google Scholar]
- 34.Trief PM, Grant W, Fredrickson B. A prospective study of psychological predictors of lumbar surgery outcome. Spine. 2000;25:2616-2621. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CH, Chou EC, Chou LW, Chen YJ, Chang CH, Tsou HK, Chen HT. The evaluation of bladder symptoms in patients with lumbar compression disorders who have undergone decompressive surgery. Spine. 2010;35:E849-E854. [DOI] [PubMed] [Google Scholar]
- 36.Wang JY, Liao L, Liu M, Sumarsono B, Cong M. Epidemiology of lower urinary tract symptoms in a cross-sectional, population-based study: the status in China. Medicine (Baltimore). 2018;97:e11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welliver C, Sulaver R, Whittington A, Helfand BT, Cakir OO, Griffith JW, McVary KT. Analyzing why men seek treatment for lower urinary tract symptoms and factors associated with nonimprovement. Urology. 2015;86;862-867. [DOI] [PubMed] [Google Scholar]