Abstract
Background
Excision of bone tumors and endoprosthetic reconstruction allow patients early weightbearing and a potential functional advantage compared with amputation. These reconstructions do not restore the limb to normal status, however, and patients are subject to complications that may result in revision or loss of the limb. Because better understanding of these complications based on current information might help the patient and surgeon in decision-making, we undertook a systematic review of studies published on this topic.
Questions/purposes
(1) What are the primary modes and proportion of failure of tumor endoprostheses in patients undergoing reconstruction after excision of primary extremity bone sarcomas?
Methods
We systematically searched MEDLINE, Embase, and the Cochrane Library for all studies published from April 15, 1998, to April 15, 2018. Three reviewers independently reviewed studies reporting endoprosthetic reconstruction survival and events requiring revision for primary extremity bone tumors treated with endoprosthetic reconstruction for inclusion and performed independent data extraction. We excluded all studies with fewer than five patients, any systematic review/meta-analyses, and any study not reporting on primary extremity bone tumors. All discrepancies were resolved by the study’s senior author. Data extracted from included studies were any reoperation event for wound dehiscence, any operative fixation for a pathologic fracture, and any revision of the primary endoprosthesis for implant wear or breakage, deep infection not amenable to prosthesis retention, or for local recurrence. We assessed the overall quality of the evidence with the Methodological Index for Non-Randomized Studies (MINORS) approach with a higher MINORS score representative of a more methodologically rigorous study with a total possible score of 16 points for noncomparative and 24 points for comparative studies. Forty-nine studies met criteria for inclusion from an initial search return of 904 studies, of which no studies were randomized controlled trials. From a total patient population of 2721, there was a mean followup of 93 months (range, 1-516 months) with loss to followup or death occurring in 447 of 2118 (21%) patients with six studies not providing loss to followup data. The mean MINORS score was 14 for prospective studies and 11 for retrospective studies.
Results
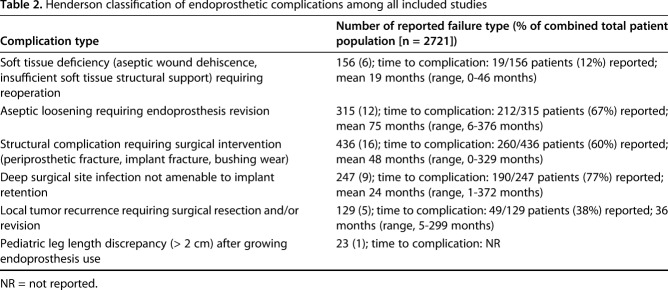
Overall, there were 1283 reoperations among the 2721 (47%) patients. Reoperation for mechanical endoprosthetic events (soft tissue dehiscence or periarticular soft tissue instability, aseptic loosening, or implant wear/fracture) occurred in 907 of 2721 (33%) patients. Aseptic loosening occurred at a mean of 75 months (range, 1-376 months) in 212 of 315 patients (67%). Deep infection requiring removal of the initial prosthesis occurred in 247 of 2721 (9%) patients with deep infection occurring at a mean of 24 months (range, 1-372 months) in the 190 infections (77%) with time to infection data available. Local recurrence rates requiring revision or amputation occurred in 129 (5%) of all patients. There was an overall primary endoprosthesis survival rate without any surgical reintervention of 63% among reporting studies at a mean of 79 months followup.
Conclusions
Failures of endoprosthetic reconstructions after extremity tumor surgery are common, most often resulting from implant wear or fracture, aseptic loosening, and infection. Importantly, the aggregated data are the first to attempt to quantify the time to specific complication types within this patient population. Deep infection not amenable to endoprosthesis retention appears to occur approximately 2 years postoperatively in most patients, with aseptic loosening occurring most commonly at 75 months. Although endoprosthetic reconstruction is one of the most common forms of reconstruction after bone tumor resection, the quality of published evidence regarding this procedure is of low quality with high loss to followup and data quality limiting interstudy analysis. The quality of the evidence is low with high loss to followup and inconsistent reporting of times to reintervention events. Although the most common modes of endoprosthetic failure in this population are well known, creation of quality prospective, collaborative databases would assist in clarifying and informing important elements of the followup process for these patients.
Level of Evidence
Level IV, therapeutic study.
Introduction
Advanced endoprosthetic implant designs and fixation methods have given orthopaedic oncologists increased options to reconstruct osseous defects after tumor excision [45]. Endoprosthetic reconstruction has several advantages compared with other reconstruction techniques, including wide availability, versatility, improvement of ambulatory status, and early rehabilitation, all while enabling a surgeon to remove all gross disease to minimize recurrence rates [3, 49, 62]. Complications of endoprosthetic reconstruction include infection, aseptic loosening, structural/hardware failure, and local recurrence. Any of these complications can diminish prosthesis survival [53, 62].
Infection of tumor endoprostheses is a major complication that complicates somewhere between 2% and 20% of these reconstructions [18, 40, 47, 48]. Endoprosthesis survival rates are widely variable and affected by both malignancy type and study sample size with some reports quoting 5- and 10-year survival rates > 80% with overall malignancy survival rates ranging from 61% to 92% [23, 62]. Despite improvements in implant design, implant-related complications and infections continue to complicate endoprosthetic reconstructions [23]. Reported reintervention for oncologic endoprosthesis reconstructions is higher than that for primary joint arthroplasty for all anatomic sites [23]. Racano et al. [53] previously conducted a systematic review on infection events in the endoprosthesis reconstruction population after long-bone tumor surgery. This review reports infection rates of 10% in the tumor endoprosthesis reconstruction population while not clearly identifying important intervals of when infections present [51]. Furthermore, there is currently no aggregated data on accepted complication values for all possible modes of postoperative complications. Most concerning, there is a wide discrepancy among orthopaedic oncologists with regard to followup and surveillance practices with 95% of recently surveyed surgeons admitting to following nonevidence-based surveillance protocols, calling for improved surveillance guidelines [21]. Thus, we sought to conduct a systematic review of the available evidence on modes of complications for primary extremity tumor endoprosthetic reconstructions, because this information may also aid surgeons in their discussions with patients about potential complications and longevity of these implants when deciding on limb salvage options.
Materials and Methods
We followed the protocol outlined in the Cochrane Handbook for Systematic Reviews of Interventions [34]. We report our findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [46].
Search Strategy and Criteria
We searched MEDLINE (1946 to the present), Embase (1974 to the present), and the Cochrane Library (no date limit) for articles published from April 15, 1998, up to and including April 15, 2018. Various combinations of subject headings and subheadings were utilized and supplementation with free text increased our sensitivity (see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A128). Additionally, we performed a manual review of the reference lists of all included full-text studies to identify any potential additional studies for inclusion. The “related articles” function in PubMed was also used to identify any potential further applicable studies.
Inclusion and Exclusion Criteria
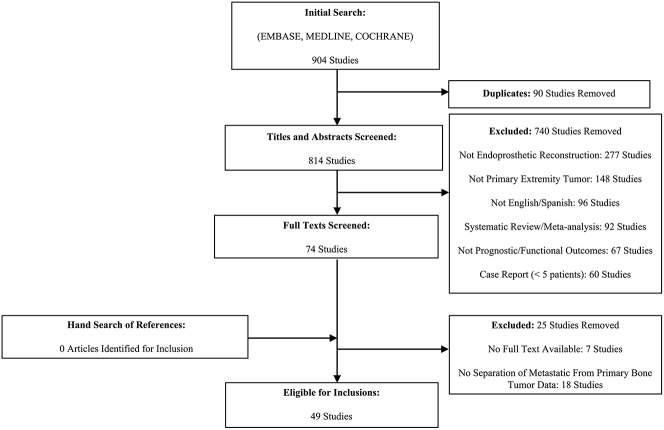
Three unblinded reviewers (AM, MV, PT) performed triplicate independent study title and abstract screening for eligibility from an initial search return of 904 studies (Fig. 1). Any disagreements identified were resolved by the study’s senior author (RV). We included all English language studies that reported postoperative complications with endoprosthetic reconstruction in the setting of a primary extremity sarcoma. Given that functional outcomes in endoprosthetic reconstruction are strongly correlated to and directly relate to a number of implant and soft tissue-related complications requiring surgical reintervention, we included those studies that provided prognostic and/or functional patient-reported outcomes for a primary extremity bone tumor population that had undergone endoprosthetic reconstruction [32]. We excluded all case reports with less than five patients to minimize the risk of falsely under- or overrepresenting complications of endoprosthesis complications as a percentage of included patient numbers. In addition, we excluded all review articles and systematic reviews/meta-analyses. We also excluded studies with < 12 months of followup or if they reported endoprosthetic reconstructions for bony metastatic disease without reporting endoprosthetic survival or reoperation events for primary bony extremity sarcomas separate of metastatic tumor reconstructions. Additionally, given the expected high rates of loss to followup from death resulting from the patient’s primary diagnoses, any study not reporting on reintervention events among patients who died during a study followup period were excluded. Ultimately, after reviewing 74 studies in full, 49 studies with a total of 2721 patients met criteria for inclusion (Fig. 1).
Fig. 1.
Depiction of the flow of selection of studies for inclusion and exclusion is shown.
Assessment of Study Quality
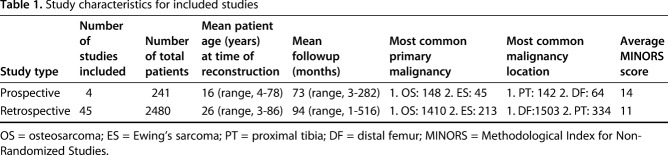
The bulk of included trials centered on average age at index surgery of approximately 20 to 30 years ranging from patients as young as 3 years to 86 years (see Appendices, Supplemental Digital Content 2, http://links.lww.com/CORR/A129, and Supplemental Digital Content 3, http://links.lww.com/CORR/A130). Among 47 studies reporting gender demographics, there were 1401 of 2526 (55%) male patients with a mean followup of 94 months (range, 1-516 months). Most studies were retrospective in nature with population sizes highly variable (5-278 patients). Nearly all included studies were single-center reporting univariate statistics without comparators. Across all included studies, the overall quality of reporting was low and highly heterogeneous.
A quality assessment analysis of all included studies was performed using the Methodological Index for Non-Randomized Studies (MINORS) criteria [61]. Each of the 12 items of the MINORS criteria is scored 0 to 2 for an ideal score of 16 for noncomparative studies and 24 for comparative studies with higher scores representing better study quality [61]. The MINORS criteria were applied to each included study in triplicate by the reviewing authors (AM, MV, PT) with discrepancies again resolved by the senior author (RV).
We found no published, completed randomized controlled trials reporting on prognostic factors for reintervention among endoprosthetic reconstructions for extremity tumor surgery. Additionally, the types of studies reporting endoprosthetic survival and complications are primarily medium-sized (55 [range, 5-278]) patient retrospective (45 studies) studies with few prospective studies (four studies) (Table 1). The most frequently reported site of malignancy and endoprosthetic reconstruction was the distal femur (1567 patients) and proximal tibia (476 patients), with osteosarcoma (1558 patients), and Ewing’s sarcoma (258 patients) as the most commonly reported primary extremity malignancies. Across the included studies, the average MINORS score was 14 for prospective studies and 11 for retrospective studies (ideal score of 16 for noncomparative studies and 24 for comparative studies). The bulk of the included studies was rated down for inconsistent followup reporting and loss to followup as well as retrospective sample size calculations.
Table 1.
Study characteristics for included studies
Data Collection and Extraction
For consistency as well as completeness of data extraction, triplicate data extraction (AM, MV, PT) was used with comparison of all results. Any discrepancies with respect to extracted data were resolved by the senior author (RV). From each study, the age at the time of surgery, site of primary malignancy, tumor type, and length of followup were extracted. To determine the modes of complications among endoprosthetic reconstructions for the included studies, complication modalities were classified according to a modification of the Henderson classification with a focus on only those complications requiring surgical intervention or prosthesis removal in the case of infection and local recurrence [32]. Henderson et al. [32] previously identified six primary modes of endoprosthetic “failure” after tumor excision and reconstruction including mechanical failures (soft tissue failure [Type 1]; aseptic loosening [Type 2]; structural failure [Type 3]) and nonmechanical failures (infection [Type 4]; tumor progression [Type 5]; pediatric failures [Type 6]). When studies reported complications without utilizing the Henderson classification, the data extraction authors determined the most applicable complication type by the Henderson classification definition with any discrepancies clarified by the senior author (RV).
Data Synthesis
We calculated interobserver agreement for the assessments of study eligibility using the Cohen κ coefficient and interpreted the κ values according to Landis and Koch as poor (κ value 0), slight (0.01-0.20), fair (0.21-0.40), moderate (0.41-0.60), substantial (0.61-0.80), or near perfect (0.81-1.00) [44]. From the initial screening, the three review authors achieved a Fleiss κ of 0.78, corresponding to high substantial agreement [16].
Results
Overall there is a high proportion of surgical reintervention among endoprosthetic reconstructions for primary extremity tumor reconstructions with an overall reintervention proportion of 1283 of 2721 (47%) for all modes of complication (Table 2). Among included prospective and retrospective studies, the most common mode of surgical reintervention was mechanical complications (periprosthetic fracture, implant fracture, bushing/implant wear) (436 of 2721 [16%]) followed closely by revision for aseptic loosening (315 of 2721 [12%]). The time to mechanical complication requiring reoperation was available for only 260 of 436 patients (60%), highlighting a time to failure mean of 48 months (range, 0-329 months), whereas aseptic loosening failures were reported in 212 of 315 patients (67%) at a mean of 75 months (range, 6-376 months). Deep surgical site infection not amenable to primary implant retention occurred in 247 of 2721 (9%) patients at a mean followup of 24 months (range, 1-372 months) in the 190 of 247 (77%) patients with available time to infection data. Interestingly, the overall combined incidence of tumor progression/recurrence at the reconstruction site was reported in only 129 of 2721 (5%) of all patients. Time to local recurrence data were only available for 49 of 129 patients (38%) with reported time to local recurrence of 36 months (range, 5-299 months). Across all included trials, the proportion of pediatric complications according to the Henderson classification (pediatric “failures” of limb length discrepancy or angular deformity about a joint) were poorly reported. Although Type 6 “failures” can incorporate all pediatric complications according to the Henderson classification, many authors classified pediatric complications as one of Types 1 to 5 instead, whereas other studies reported these failures as pediatric (Type 6) regardless of complication type when a patient had a complication when younger than age 18 years. It was difficult to determine postoperative complication types universally among any studies including a broader age range incorporating both adults and pediatric patients. Additionally, functional outcome scores among pediatric patients with a surviving primary endoprosthesis were inconsistently reported across the included studies. However, 29 included studies (59%) in which reported functional outcome scores were available reported outcome scores using the Musculoskeletal Tumor Rating Scale (MSTS) with a mean of 25 out of a possible score of 30 (83%) (range, 19-30).
Table 2.
Henderson classification of endoprosthetic complications among all included studies
Discussion
Endoprosthetic reconstruction continues to be widely used for primary extremity tumor reconstruction. We have provided the first comprehensive aggregate data on overall endoprosthesis reconstruction outcomes and when these complications can be most commonly expected to occur. There is a high reported proportion of reoperations for endoprosthetic reconstructions, primarily implant-related complications requiring surgical intervention (436 patients [16%]) and aseptic loosening (315 patients [12%]) with very low reported proportions of local tumor recurrence (129 patients [5%]). The most accurately reported complication for revision of endoprostheses was in the setting of deep surgical site infection not amenable to implant retention with an event proportion of 247 (9%) patients at an average of 24 months postoperatively.
This study had a number of limitations. There was wide variability among included studies with respect to functional patient-reported outcomes and reasons for complications. The associated heterogeneity limited a formal comparative statistical analysis. Given the retrospective nature of the majority of included studies and relatively high mortality among this population, followup was inconsistent among the included studies. This raises the concern for transfer bias. Although our review demonstrated a mean followup of 94 months, it was not possible to determine losses to followup nor the time at which patients were lost to followup in many studies. Importantly, six studies did not report a loss to followup at all, whereas only 18 studies provided a mean time to loss of followup. Of studies reporting a loss to followup, a mean of 21% of patients was lost to followup within these studies. This finding may artificially decrease our complication rate because it is possible that many of these patients, who were lost to followup (death from disease in most instances), may have had one or more complication during their postoperative course had they survived longer. Furthermore, few studies provided SDs for any mean data points reported. With the inconsistency within the provided data among the included studies, we were limited in our ability to provide any statistical comparisons across studies. This prevented our analysis comparing complication rates for given endoprostheses, sites of reconstruction, and tumor type. There was minimal selection bias within the included studies because operative indication, reconstructive technique, and endoprosthesis type were typically clearly detailed. However, every included study is at risk of assessment bias because all studies describe the operative surgeon determining when a complication had occurred. It was often difficult to determine within soft tissue complications what reoperation was performed, whereas conversely, revision surgery for deep prosthetic infections was easily determined. Furthermore, with regard to mechanical complications requiring revision surgery, no studies quantified the degree of bushing wear leading to bushing revision. This is important considering bushing revision represents significantly less morbid revision surgery than mechanical complications from periprosthetic fracture or implant fracture. Additionally, we did not perform a detailed search of nonpeer-reviewed studies, which may limit our ability to identify any forthcoming unpublished studies, including any potential randomized trials [65]. The discrepancy among the lengths of followup (mean, 94 months [range, 1-516 months]) among included studies potentially underreports failures, most probably aseptic loosening, within studies with shorter followups. Finally, the inconsistently reported pediatric complications (limb length discrepancy most importantly) limit our ability to reliably conclude what factors most predict a pediatric complication because often these results were not reported separately of adult complications in the same study.
Our study demonstrates the majority of patients (63%) have a primary endoprosthesis surviving at a mean of 79 months. This represents the largest compilation of all endoprosthesis data for primary bone tumor reconstructions. However, the proportion of complications after endoprosthetic repair is still high despite increased use of endoprostheses over the past several decades [20, 25, 45]. The majority of included studies report on patients with endoprosthetic reconstruction about the knee. Sharil et al. [59] demonstrated that functional patient-reported outcomes after endoprostheses around the knee are good to excellent with good ROM, allowing for daily activities [7, 10, 17, 43, 59] with patients who do predictably well. However, important distinctions in endoprosthesis survival are still unknown for specific reconstruction locations and remain so after this analysis given the data-reporting limitations of the available studies. Jeys et al. [41] report a 10-year survival rate of upper limb endoprostheses of 85%, differing from their 53% lower limb reconstruction survival rates. Overall, they reported limb salvage maintenance after 20 years in 84% of patients [41]. Song et al. [62] reported a 10-year prosthetic survival of their 117 implanted endoprostheses for tumor reconstruction. In their analysis, bone resection > 40% was associated with mechanical prosthesis failure with an optimal cutoff of 43% for resection. Our study showing a proportion of aseptic loosening of approximately 12% is comparable to previously reported event rates of aseptic loosening on a large scale [22, 62].
Concerning for all orthopaedic oncologists, the quality of the available evidence on endoprosthetic reconstructions limits any meaningful interstudy comparison. This draws particular attention to the fragility of all followup protocols within this population. Uncertainty still exists with regard to specific site endoprosthetic reconstructions because the largest aggregate body of data on endoprosthetic reconstructions, which is presented in this study, highlights an overall high proportion of all-cause complications (47%) with a mean 21% loss to followup. We are unable to provide followup recommendations specific to surgical reconstruction site, bone resection amount, implant type, nor primary malignancy given the heterogeneity within the data. The evidence base that informs the reasons for all-cause failures postendoprosthetic reconstruction has not previously been synthesized. Of important clinical relevance is the lack of evidence-based guidelines to inform orthopaedic oncologists of ideal surveillance protocols for their patients after endoprosthetic reconstruction. All studies were rated down by the MINORS score for high loss to followup and biases within their data reporting. Although it is commonly accepted that the quality of evidence surrounding endoprosthesis reconstruction for primary malignancy is low, we wished to demonstrate the paucity of quality data that currently exists to guide practice management for a difficult patient population.
Although it is known that the current level of evidence on survival rates of endoprostheses in this population is low, our combined results highlight a much more concerning phenomenon within orthopaedic oncology, the reliance of clinical management on poorly conducted observational studies. Our systematic review has demonstrated that the myriad of endoprosthetic failures that can occur is stratified with infection requiring revisions typically at the 24-month postoperative period and local recurrence reported at an average of 36 months postoperatively with revision for implant wear/fracture or aseptic loosening at 48 and 75 months postoperatively, respectively. However, these rates are subject to the frailty of the reported data quality. The inconsistency within the endoprosthesis reconstruction population with respect to loss to followup and time to complications highlights the importance of collaborative efforts that ideally should be established to create a prospective database of all endoprosthetic reconstructions. It is only through the creation of such large-scale databases that we will be able to delineate the true complication event rates and better understand how to manage this patient population postoperatively. In the meantime, we hope that the results of this study can help guide surgeons when they discuss potential complications of endoprosthetic reconstructions to their patients.
Footnotes
One of the authors (MG) reports grants from the Canadian Institutes of Health Research and grants from the Canadian Cancer Society, outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
This work was performed at McMaster University, Hamilton, Ontario, Canada, and the Hospital Universitario Vall d’Hebron, Barcelona, Spain.
References
- 1.Abudu A, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int Orthop. 1999;23:291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldlyami E, Abudu A, Grimer RJ, Carter SR, Tillman RM. Endoprosthetic replacement of diaphyseal bone defects: long-term results. Int Orthop. 2005;29:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvi HM, Damron TA. Prophylactic stabilization for bone metastases, myeloma, or lymphoma: do we need to protect the entire bone? Clin Orthop Relat Res . 2013;471:706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batta V, Coathup MJ, Parratt RC, Aston WJ, Cannon SR, Skinner JA, Briggs TW, Blunn GW. Uncemented, custom-made, hydroxyapatite-coated collared distal femoral endoprostheses: up to 18 years’ follow-up. Bone Joint J. 2014;96:263-269. [DOI] [PubMed] [Google Scholar]
- 5.Belthur MV, Grimer RJ, Suneja R, Carter SR, Tillman RM. Extendible endoprostheses for bone tumors of the proximal femur in children. J Pediatr Orthop. 2003;23:230-235. [PubMed] [Google Scholar]
- 6.Benevenia J, Patterson F, Beebe K, Tucker K, Moore J, Ippolito J, Rivero S. Results of 20 consecutive patients treated with the Repiphysis expandable prosthesis for primary malignant bone. Springerplus. 2015;4:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney K, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term follow-up study. Clin Orthop Relat Res. 2002;400:225-235. [DOI] [PubMed] [Google Scholar]
- 8.Bus MP, van de Sande MA, Fiocco M, Schaap GR, Bramer JA, Dijkstra PD. What are the long-term results of MUTARS® modular endoprostheses for reconstruction of tumor resection of the distal femur and proximal tibia? Clin Orthop Relat Res . 2017;475:708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan A, Joshi GR, Chopra BK, Ganguly M, Reddy GR. Limb salvage surgery in bone tumors: a retrospective study of 50 cases in a single center. Indian J Surg Oncol. 2013;4:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choong PF, Sim FH, Pritchard DJ. Megaprosthesis after resection of distal femoral tumours: a rotating hinge design in 30 patients followed for 2-7 years. Acta Orthop Scand. 1996;67:345-351. [DOI] [PubMed] [Google Scholar]
- 11.Coathup MJ, Batta V, Pollock RC, Aston WJ, Cannon SR, Skinner JA, Briggs TW, Unwin PS, Blunn GW. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. J Bone Joint Surg Am. 2013;95:1569-1575. [DOI] [PubMed] [Google Scholar]
- 12.Decilveo AP, Szczech BW, Topfer J, Wittig JC. Reconstruction using expandable endoprostheses for skeletally immature patients with sarcoma. Orthopedics. 2017;40:157-163. [DOI] [PubMed] [Google Scholar]
- 13.Dieckmann R, Henrichs MP, Gosheger G, Holl S, Hardes J, Streitburger A. Short-stem reconstruction for megaendoprostheses in case of an ultrashort proximal femur. BMC Musculoskeletal Disord. 2014;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dotan A, Dadia S, Bickels J, Nirkin A, Flusser G, Issakov J, Neumann Y, Cohen I, Ben-Arush M, Kollender Y, Meller I. Expandable endoprosthesis for limb-sparing surgery in children: long-term results. J Child Orthop. 2010;4:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckardt JJ, Kabo JM, Kelley CM, Ward WG, Sr, Asavamongkolkul A, Wirganowicz PZ, Yang RS, Eilber FR. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;373:51-61. [DOI] [PubMed] [Google Scholar]
- 16.Freelon D. ReCal: intercoder reliability calculation as a web service. International Journal of Internet Science. 2010;5:20-33. [Google Scholar]
- 17.Frink JS, Rutledge J, Lewis VO, Lin PP, Yasko AW. Favorable long-term result of prosthetic arthroplasty of the knee for distal femur neoplasms. Clin Orthop Relat Res. 2005;438:55-70. [DOI] [PubMed] [Google Scholar]
- 18.Ghert M; PARITY Investigators. Prophylactic antibiotic regimens in tumour surgery (PARITY): a pilot multicentre randomised controlled trial. Bone Joint Res. 2015;4:154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilg MM, Gaston CL, Parry MC, Jeys L, Abudu A, Tillman RM, Carter SR, Grimer RJ. What is the morbidity of a non-invasive growing prosthesis. Bone Joint J. 2016;98:1697-1703. [DOI] [PubMed] [Google Scholar]
- 20.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164-171. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg DD, Crawford B. Surveillance strategies for sarcoma: results of a survey of members of the Musculoskeletal Tumor Society. Sarcoma. 2016;2016:8289509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79. [DOI] [PubMed] [Google Scholar]
- 23.Grimer RJ, Aydin BK, Wafa H, Carter SR, Jeys L, Abudu A, Parry M. Very long-term outcomes after endoprosthetic replacement for malignant tumours of bone. Bone Joint J. 2016;98:857-864. [DOI] [PubMed] [Google Scholar]
- 24.Grimer RJ, Belthur M, Carter SR, Tillman RM, Cool P. Extendible replacements of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2000;82:255-260. [PubMed] [Google Scholar]
- 25.Groundland JS, Ambler SB, Houskamp LD, Orriola JJ, Binitie OT, Letson GD. Surgical and functional outcomes after limb-preservation surgery for tumor in pediatric patients: a systematic review. JBJS Rev. 2016;4. [DOI] [PubMed] [Google Scholar]
- 26.Ham SJ, Schraffordt Koops H, Veth RP, van Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Ann Surg Oncol. 1998;5:423-436. [DOI] [PubMed] [Google Scholar]
- 27.Hanna SA, Sewell MD, Aston WJS, Pollock RC, Skinners JA, Cannon SR, Briggs TWR. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J Bone Joint Surg Br. 2010;92:867-874. [DOI] [PubMed] [Google Scholar]
- 28.Hardes J, Henrichs MP, Gosheger G, Guder W, Nottrott M, Andreou D, Bormann E, Eveslage M, Hauschild G, Streitburger A. Tumour endoprosthesis replacement in the proximal tibia after intra-articular knee resection in patients with sarcoma and recurrent giant cell tumour. Int Orthop. 2018;42:2475-2481. [DOI] [PubMed] [Google Scholar]
- 29.Hardes J, Henrichs MP, Gosheger G, Holl S, Dieckmann R, Hauschild G, Streitburger A. Endoprosthetic replacement after extra-articular resection of bone and soft-tissue tumours around the knee. Bone Joint J. 2013;95:1425-1431. [DOI] [PubMed] [Google Scholar]
- 30.Hardes J, Henrichs MP, Hauschild G, Nottrott M, Guder W, Streitburger A. Silver-coated megaprosthesis of the proximal tibia in patients with sarcoma. J Arthroplasty. 2017;32:2208-2213. [DOI] [PubMed] [Google Scholar]
- 31.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389-395. [DOI] [PubMed] [Google Scholar]
- 32.Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system including biological and expandable reconstructions. Bone Joint J. 2014;96:1436-1440. [DOI] [PubMed] [Google Scholar]
- 33.Henderson ER, Pepper AM, Marulanda G, Binitie OT, Cheong D, Letson GD. Outcome of lower-limb preservation with an expandable endoprosthesis after bone tumor resection in children. J Bone Joint Surg Am. 2012;94:537-547. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions . Version 5.1.0. Oxford, UK: Cochrane Collaboration; 2011. [Google Scholar]
- 35.Holm CE, Bardram C, Riecke AF, Horstmann P, Petersen MM. Implant and limb survival after resection of primary bone tumors of the lower extremities and reconstruction with mega-prostheses fifty patients followed for a mean of fourteen years. Int Orthop. 2018;42:1175-1181. [DOI] [PubMed] [Google Scholar]
- 36.Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24:1079-1085. [DOI] [PubMed] [Google Scholar]
- 37.Ilyas I, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for distal femoral tumors. Int Orthop. 2001;25:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilyas I, Pant R, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for proximal femoral tumors. Int Orthop. 2002;26:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilyas I, Younge D, Pant R, Moreau P. Limb salvage for proximal tibial tumours using a modular prosthesis. Int Orthop. 2000;24:208-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87:842–849. [DOI] [PubMed] [Google Scholar]
- 41.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265-1271. [DOI] [PubMed] [Google Scholar]
- 42.Kabukcuoglu Y, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement for primary malignant tumors of the proximal femur. Clin Orthop Relat Res. 1999;358:8-14. [PubMed] [Google Scholar]
- 43.Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon DG. A rotating-hinge knee replacement for malignant tumours of the femur and tibia. J Arthroplasty. 1999;14:187-196. [DOI] [PubMed] [Google Scholar]
- 44.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 45.Levin AS, Arkader A, Morris CD. Reconstruction following tumor resections in skeletally immature patients. J Am Acad Orthop Surg. 2017;25:204-213. [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [DOI] [PubMed] [Google Scholar]
- 47.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526. [DOI] [PubMed] [Google Scholar]
- 48.Peel T, May D, Buising K, Thursky K, Slavin M, Choong P. Infective complications following tumour endoprosthesis surgery for bone and soft tissue tumours. Eur J Surg Oncol. 2014;40:1087-1094. [DOI] [PubMed] [Google Scholar]
- 49.Peterson JR, Decilveo AP, O’Connor IT, Golub I, Wittig JC. What are the functional results and complications with long stem hemiarthroplasty in patients with metastases to the proximal femur? Clin Orthop Relat Res. 2017;475:698-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picardo NE, Blunn GW, Shekkeris AS, Meswania J, Aston WJ, Pollock RC, Skinner JA, Cannon SR, Briggs TW. The medium-term results of the Stanmore non-invasive extendible endoprosthesis in the treatment of paediatric bone tumours. J Bone Joint Surg Br. 2012;94:425-430. [DOI] [PubMed] [Google Scholar]
- 51.Puri A, Gulia A. The results of total humeral replacement following excision for primary bone tumour. J Bone Joint Surg Br. 2012;94:1277-1281. [DOI] [PubMed] [Google Scholar]
- 52.Puri A, Gulia A, Chan WH. Functional and oncologic outcomes after excision of the total femur in primary bone tumors: results with a low cost total femur prosthesis. Indian J Orthop. 2012;46:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Racano A, Pazionis T, Farrokhyar F, Deheshi B, Ghert M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res. 2013;471:2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubio D, Serrano M, Wang E. Tumour endoprosthetic reconstruction for primary aggressive and malignant bone tumours of the distal femur. Malays Orthop J. 2013;7:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saghieh S, Abboud MR, Muwakkit SA, Saab R, Rao B, Haidar R. Seven-year experience of using Repiphysis expandable prosthesis in children with bone tumors. Pediatr Blood Cancer. 2010;55:457-463. [DOI] [PubMed] [Google Scholar]
- 56.Sanjay BK, Moreau PG. Limb salvage surgery in bone tumour with modular endoprosthesis. Int Orthop. 1999;23:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schinhan M, Tiefenboeck T, Funovics P, Sevelda F, Kotz R, Windhager R. Extendible prostheses for children after resection of primary malignant bone tumor: twenty-seven years of experience. J Bone Joint Surg Am. 2015;97:1585-1591. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented endoprosthetic reconstruction of the proximal tibia: how long do they last? Clin Orthop Relat Res. 2010;468:2875-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharil A, Nawaz A, Nor Azman M, Zulmi W, Faisham W. Early functional outcome of resection and endoprosthesis replacement for primary tumor around the knee. Malays Orthop J. 2013;7:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shekkeris AS, Hanna SA, Sewells MD, Spiegelberg BG, Aston WJ, Blunn GW, Cannon SR, Briggs TW. Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours. J Bone Joint Surg Br. 2009;91:1378-1382. [DOI] [PubMed] [Google Scholar]
- 61.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [DOI] [PubMed] [Google Scholar]
- 62.Song WS, Kong CB, Jeon DG, Cho WH, Kim JR, Cho Y, Lee SY. The impact of amount of bone resection on uncemented prosthesis failure in patients with a distal femoral tumor. J Surg Oncol. 2011;104:192-197. [DOI] [PubMed] [Google Scholar]
- 63.Staals EL, Colangeli M, Ali N, Casanova JM, Donati DM, Manfrini M. Are complications associated with the Repiphysis® expandable distal femoral prosthesis acceptable for its continued use? Clin Orthop Relat Res. 2015;473:3003-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan PK, Tan MH. Functional outcome study of mega-endoprosthetic reconstruction in limbs with bone tumour surgery. Ann Acad Med Singapore. 2009;38:192-196. [PubMed] [Google Scholar]
- 65.Thornley P, Evaniew N, Riediger M, Winemaker M, Bhandari M, Ghert M. Postoperative antibiotic prophylaxis in total hip and knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. CMAJ Open. 2015;3:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tillman RM, Grimer RJ, Carter SR, Cool WP, Sneath RS. Growing endoprostheses for primary malignant bone tumors. Semin Surg Oncol. 1997;13:41-48. [DOI] [PubMed] [Google Scholar]
- 67.Torner F, Segur JM, Ullot R, Soldado F, Domenech P, DeSena L, Knorr J. Non-invasive expandable prosthesis in musculoskeletal oncology paediatric patients for the distal and proximal femur. First results. Int Orthop. 2016;40:1683-1688. [DOI] [PubMed] [Google Scholar]
- 68.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64-74. [PubMed] [Google Scholar]
- 69.Yan TQ, Zhou WH, Guo W, Yang RL, Dong S, Liang WM, Sun YF. Endoprosthetic reconstruction for large extremity soft-tissue sarcoma with juxta-articular bone involvement: functional and survival outcome. J Surg Res. 2014;187:142-149. [DOI] [PubMed] [Google Scholar]
- 70.Yang P, Evans S, Khan Z, Abudu A, Jeys L, Grimer R. Reconstruction of the distal tibia following resection of aggressive bone tumours using a custom-made megaprosthesis. J Orthop. 2017;14:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]