Abstract
Background
Bipolar endoprosthetic replacement is an option for reconstruction of the proximal femur to restore a functional extremity and salvage the limb. However, because these patients are young, there is a theoretical risk for long-term degenerative changes of the acetabulum. Currently, there is a paucity of data concerning the proportion of patients who experience degenerative acetabulum changes after reconstruction and whether these changes are associated with Musculoskeletal Tumor Society (MSTS) scores.
Questions/purposes
(1) What proportion of patients develop acetabular cartilage degeneration after bipolar hemiarthroplasty for malignant tumor-related reconstructions? (2) What is the survivorship free from revision for acetabular wear, erosions, or progressive arthritis? (3) Is there an association between the presence of acetabular erosions and lower MSTS scores?
Methods
Between 2000 and 2015, 148 patients underwent endoprosthetic reconstruction of the proximal femur with a bipolar hemiarthroplasty for a malignant tumor and were potentially eligible for this retrospective study. Minimum followup was 1 year except for those who died or were revised earlier; of the 148, no patients were lost to followup before that time who were not known to have died; mean followup on the remainder was 79 months (range, 12-220 months), and the mean time to death after surgery for those who died was 28 months (range, 0-196 months). Over the course of the study, 93 (63%) patients died. The mean (± SD) patient age was 57 ± 17 years, and 55% (81 of 148) of the patients were men. We used magnification-corrected supine AP plain radiographs of the hip to evaluate degenerative acetabulum changes, and we used the 1993 MSTS score to assess function through chart review and a longitudinally maintained institutional database. We used a competing-risks survivorship estimator rather than Kaplan-Meier because of the high proportion of patients who had died during the surveillance period.
Results
Nineteen patients (13%) developed cartilage erosion > 2 mm in the acetabulum, with two also developing protrusio after proximal femoral replacement with a bipolar endoprosthesis. Three additional patients also developed signs of protrusio. The mean acetabular wear after bipolar replacement was 1.2 mm. Patients with longer followup (p = 0.001) were at higher risk for developing acetabular wear. Six patients underwent conversion to THA to treat hip pain. At 10 years the cumulative incidence for conversion to THA for acetabular wear is 5% (95% confidence interval [CI], 0%-11%), whereas the cumulative incidence of death was 70% (95% CI, 61%-79%). There was no difference in mean MSTS scores between patients who developed > 2 mm of acetabular erosion (65% ± 25%) and those who did not (67% ± 20%; p = 0.77).
Conclusions
Wear was uncommon among patients with malignant hip tumors treated with bipolar endoprostheses, but the followup here was short, and some patients indeed developed wear and underwent wear-related revisions to THA. Patients expected to survive more than a few years should have periodic radiographic surveillance and should be followed for a longer period to get a better sense for whether the problem worsens with time, as we expect it may, among patients who survive for longer periods.
Level of Evidence
Level III, therapeutic study.
Introduction
The proximal femur is a common location for malignant and benign bone tumors as well as metastatic disease [26, 33]. Adjuvant treatments, improved surgical techniques, and better imaging modalities have allowed limb salvage to become the treatment of choice for malignant tumors and benign aggressive lesions of the proximal femur [6, 15, 37]. Endoprosthetic replacements have become the primary treatment for reconstructing the proximal femur after tumor resection because they allow immediate weightbearing and are relatively cost-effective, widely available, and durable [8, 10, 11, 16, 18, 21, 38].
Historically, a bipolar hip component sometimes is used to reduce the dislocation rate and to preserve the acetabulum after proximal femoral replacement [5, 11, 26, 30]. Because most patients with metastatic disease typically do not live longer than 2 years after surgery [8, 19, 28, 34], it was thought that the conversion rate to THA because of acetabular erosions would be low. However, these data come from smaller series and combine endoprosthetic replacements from multiple locations [9, 11, 17, 18, 20, 21, 23, 30, 33, 38]. Concerns that are largely unanswered include a more precise estimate of how often patients with orthopaedic tumors treated with bipolar endoprostheses develop deep acetabular erosions or progressive acetabular wear, how often these patients undergo THA to treat symptoms from acetabular cartilage wear, and to what degree those patients who have acetabular cartilage wear in this setting notice it in terms of pain or impairment.
We therefore combined data from two tertiary sarcoma centers that consistently used bipolar endoprosthetic replacements for reconstruction in the setting of malignant disease in the proximal femur to answer the following questions: (1) What proportion of patients develop acetabular cartilage degeneration after bipolar hemiarthroplasty for malignant tumor-related reconstructions? (2) What is the survivorship free from revision for acetabular wear, erosions, or progressive arthritis? (3) Did patients with acetabular erosions > 2 mm deep have lower Musculoskeletal Tumor Society (MSTS) scores than those patients without such acetabular erosions?
Patients and Methods
After obtaining approval from our institutional review boards for this retrospective study, we reviewed our institutions’ total joint and tumor databases to obtain the records of all patients who underwent modular endoprosthetic reconstruction of the proximal femur with a bipolar hemiarthroplasty after en bloc resection of a malignant tumor between 2000 and 2015 because electronic radiographs were available for these patients. The study was chosen to provide a convenience sample of patients for whom electronic radiographs were available.
We identified 148 patients who met these criteria and were potentially eligible for this retrospective study. Minimum followup was 1 year except for those who died or were revised earlier; of the 148, no patients were lost to followup before that time and not known to have died; mean followup on the remainder was 79 months (range, 12-220 months), and the mean time to death after surgery for those who died was 28 months (range, 0-196 months). Of the surviving patients, four had not been seen in the clinic for the past 5 years and are not known to have died. The mean followup for this cohort was 59 months (range, 37-91 months). Of these patients, two had evidence of metastatic disease at their most recent followup.
This cohort consisted of 67 females (45%) and 81 males (55%) with a mean age at the time of surgery of 57 years (range, 11–88 years). Three patients were < 18 years of age at the time of surgery. No expandable tumor endoprostheses and no unipolar hemiarthroplasties were used in any of the study patients. Mean body mass index was 28 kg/m2 (range, 14–45 kg/m2). More patients had primary bone sarcomas (74 patients [50%]) than metastatic disease (65 patients [44%]) or hematologic malignancies (nine patients [6%]; Table 1). The mean resection length of the femur was 19 cm (range, 5–47 cm). Forty-eight patients (32%) received radiation therapy (19 postoperative, 28 preoperative, and three pre- and postoperative), and 74 patients (50%) were treated with chemotherapy.
Table 1.
Diagnosis at the time of proximal femoral replacement
Of the 74 patients with primary bone sarcoma, five (7%) had metastases at diagnosis so oncologic outcome was analyzed in the remaining 69 patients. Chondrosarcoma (28 patients) and osteosarcoma (15 patients) were the most common primary tumor pathologies. The mean tumor size was 11 cm (range, 3–34 cm) and the mean tumor volume was 513 cm3 (range, 17–3691 cm3). Forty-four of the primary tumors (59%) were considered high grade. A negative surgical margin (R0) was obtained in 73 patients (99%). One patient with dedifferentiated chondrosarcoma had a microscopically positive surgical margin and received postoperative chemotherapy but still developed a local recurrence. Thirty-five patients (47%) with bone sarcomas underwent chemotherapy within 3 months of their arthroplasty. Five patients received neoadjuvant radiation, one patient received adjuvant radiation, and two patients received combined neoadjuvant and adjuvant radiation. The mean proximal femoral resection length was 21 cm (range, 6–47 cm).
Patients were followed at regular intervals during the study using the total joint and tumor registries at their respective institutions to evaluate for death, implant revision and reoperation, complications, tumor recurrence, or amputation. Patients were followed for tumor recurrence every 3 months for the first 2 years postoperatively with a physical examination and plain radiographs of the pelvis, hip, and femur along with chest CT scans or chest radiographs, depending on the tumor histology. The patients were then seen in the clinic at 6-month intervals for postoperative years 2 to 5 and annually until 10 years after tumor resection.
Surgical Procedures
Limb salvage was performed when preoperative imaging suggested that it was possible to achieve a negative surgical margin and provide the patient with a functional extremity.
All surgical procedures were performed by orthopaedic oncology subspecialty surgeons. Of the reconstructions, 110 (74%) were cemented. If the hip capsule remained after tumor resection and endoprosthetic reconstruction, the surgeon repaired it using a pursestring suture around the neck of the prosthesis. Alternatively, if the capsule was deficient and could not be repaired, as was the case for 29 patients (20%), it was augmented with synthetic mesh (Marlex; BARD Davol, Minneapolis, MN, USA) as previously described [25]. In addition, based on the amount of remaining hip abductor and quadriceps musculature, the muscles were repaired to the proximal portion of the prosthesis or to the iliotibial band with either Ethibond (Ethicon, Somerville, NJ, USA) or FiberWire® (Arthrex, Naples, FL, USA) sutures as previously described [35]. Greater trochanter fragments were included in the abductor repair if present. All hip reconstructions during the study period at the participating institutions were performed using bipolar endoprostheses. No unipolar hemiarthroplasties or THAs were performed during this period.
Assessment of Study Outcomes
We defined revision as any surgery resulting in removal and/or replacement of the hip components because of acetabular wear. We defined reoperation as any surgical procedure performed on the hip where the arthroplasty components were retained. Data were drawn from institutional databases and chart review (by MTH and AMG).
We defined acetabular erosion as the change in acetabular cartilage thickness based on the magnification-corrected supine radiographs using templating software (Merge OrthoCase™, IBM Watson Health Imaging, Chicago, IL, USA; and OrthoView, Carestream Health Inc, Rochester, NY, USA) postoperative and most recent AP hip radiographs as previously described [24] before review of the patient’s status (MTH, OSD); protrusio was defined as medial migration of the acetabulum > 3 mm in males and > 6 mm in females past the ilioischial line [1, 24]. Kappa value was not assessed.
The MSTS scores were collected through a combination of chart review and a review of a longitudinally maintained institutional database (MTH, AMG). A comparison of the MSTS score was made between patients with an erosion > 2 mm and those without erosion.
Statistical Analysis
We used unpaired Student’s t-tests to assess continuous variables and compared categorical variables with Fisher’s exact test and odds ratios (ORs). We used competing risk analysis in which death was considered as a competing risk to implant revision for acetabular wear, erosions, or progressive arthritis to analyze revision over time. Cumulative incidence functions and Gray’s test were used for comparing competing risks by age group. MSTS scores [12] were calculated for patients at their last clinical followup. All tests were two-sided. Probability values < 0.05 were considered statistically significant. Analyses were performed using R 3.4.3 (R Core Team, Vienna, Austria).
Results
Proportion of Patients Developing Acetabular Erosions
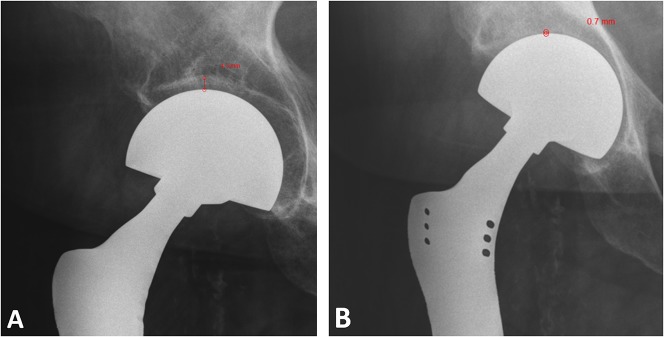
Overall, postoperative radiographic evaluation of 148 patients revealed acetabular erosion (Fig. 1A-B) in 52 patients (35%). The mean change in acetabular cartilage thickness was 1.2 mm (range, 0–49 mm). However, 21 patients (14%) had > 2 mm of acetabular cartilage erosion. Of these patients, two also demonstrated acetabular protrusio. In addition, three patients developed protrusio without acetabular erosion > 2 mm. There was no difference in the proportion of patients with acetabular migration > 2 mm based on age at the time of surgery (55 ± 15 years versus 56 ± 18 years, p = 0.89) or treatment with preoperative (four of 21 [19%] versus 26 of 127 [20%]; OR, 0.91; 95% confidence interval [CI], 0.28-2.94; p = 1.0) or postoperative (four of 21 [19%] versus 18 of 127 [14%]; OR, 1.42; 95% CI, 0.43-4.72; p = 0.74) radiotherapy or chemotherapy (10 of 21 [48%] versus 64 of 127 [50%]; OR, 0.89; 95% CI, 0.35-2.25; p = 0.99). However, we note that patients with acetabular erosion > 2 mm had a mean followup of 8 ± 5 years compared with 4 ± 3 years for those with less acetabular wear (p < 0.001).
Fig. 1A-B.
The images show magnification-corrected AP hip radiographs from a patient (A) immediately after surgery and (B) 7 years later. Measurement of the acetabular cartilage thickness postoperatively was 4.3 mm; however, this decreased to 0.7 mm at last followup, a 3.6-mm change.
Survivorship Analysis
The cumulative incidence of revision to THA for hip pain at the 2-, 5-, 10-, and 15-year postoperative marks was 0% (95% CI, 0%-0%), 2% (95% CI, -1% to 4%), 5% (95% CI, 0%-11%), and 9% (95% CI, 0%-19%), respectively (Table 2). During the same time period, the cumulative incidence of death at the 2-, 5-, 10- and 15-year postoperative marks was 46% (95% CI, 37%-54%), 57% (95% CI, 49%-66%), 70% (95% CI, 61%-79%), and 70% (95% CI, 61%-79%) (Fig. 2). Based on patient age (Table 3), patients < 50 years of age were at increased risk of death (p < 0.001) compared with patients ≥ 50 years of age at the time of surgery (Fig. 3). However, there was no difference (p = 0.43) in the cumulative incidence of conversion of a bipolar hemiarthroplasty to THA in patients < 50 years compared with those ≥ 50 years of age (Fig. 3). Of 148 patients, a total of six (4%) underwent revision of their endoprosthetic replacement to treat symptomatic hip pain and/or arthritis (range, 3–15 years). Six were the result of groin pain with or without arthritis. Revision was performed on three patients with a history of a primary bone sarcoma, two with metastatic disease, and one with a hematologic malignancy.
Table 2.
Cumulative risk of revision to THA as a result of symptomatic hip pain
Fig. 2.
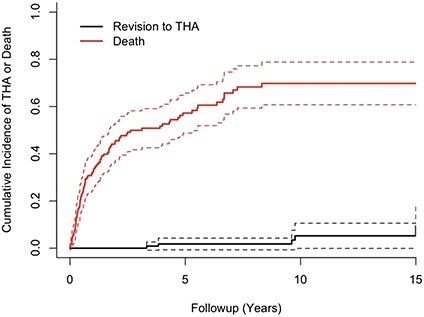
The graph shows the cumulative incidence of death and revision to THA after bipolar endoprosthetic replacement of the proximal femur with a 15-year incidence of 70% and 9%, respectively.
Table 3.
Cumulative risk of revision to THA resulting from symptomatic hip pain based on age
Fig. 3.
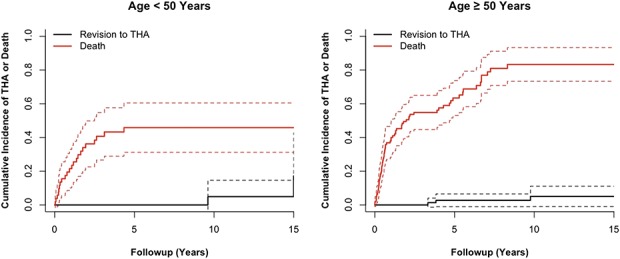
The graph shows the cumulative incidence of death and revision to THA after bipolar endoprosthetic replacement of the proximal femur based on patient age (≥ or < 50 years of age). For patients < 50 years, the 15-year incidence was 17% and 46%, whereas for patients ≥ 50 years, the 15-year incidence was 5% and 83%, respectively.
Association Between Erosions and MSTS Scores
There was no difference in MSTS scores between patients who developed > 2 mm of acetabular erosion (65% ± 25%) and those who did not (67% ± 20%; p = 0.77). At the most recent followup, the mean MSTS score was 58% ± 26% for the study group.
Discussion
Endoprosthetic replacement has become the primary form of reconstruction after resection of a proximal femoral malignancy because it allows for early return to weightbearing and improved functional status [4, 16, 21, 26, 30, 32, 36, 38]. This type of reconstruction is often performed using a bipolar hemiarthroplasty component to decrease the risk of instability. Although proximal femoral endoprostheses have been used for > 40 years, the long-term consequences, and particularly the rate of conversion to THA because of acetabular erosions, have not been well described. The results of this study demonstrated that the rate of conversion from bipolar hemiarthroplasty to THA was low (8%), especially when considering only acetabular erosion (5%).
The study has certain limitations. It was a retrospective study, which limited the data we were able to collect and the statistical analysis we were able to perform. However, our longitudinally maintained arthroplasty and tumor registries reduced the study’s recall and selection bias. Although the oncologic resections were based on the local extent of tumor involvement, we were unable to investigate the resultant size of the soft tissue defect and subsequent indications for prosthetic choice, and as such, we cannot comment on the influence of these variables on this study. Although this study combined patients from two large tertiary sarcoma centers, all procedures were performed by orthopaedic oncology subspecialty surgeons, so the indications and surgical techniques were likely very similar. Likewise, for a majority of the patients in the study, the followup was short because a substantial number of patients died of disease. As such, the proportion of patients who might develop erosions after this operation might be underestimated. This will not be a concern for most patients who receive these implants, because it appears that most will die before erosions develop; however, for those patients who survive for longer periods, it could well be a clinical concern. Another factor that might have caused us to underestimate the loss of acetabular cartilage was our use of nonweightbearing supine plain radiographs as the means of assessment; these may not be as sensitive as other modalities to detect small amounts of cartilage loss. However, radiographs are the most commonly used surveillance tool in practice, very small amounts of cartilage loss are unlikely to result in severe symptoms, and we also looked at conversion to THA and MSTS scores to ensure that we evaluated the clinical impact of acetabular erosions in these patients.
Although the incidence of protrusio was examined, the difference in the medial joint space over time was not nor was the incidence of condensation of the bony trabeculae of the acetabulum, which may herald acetabular erosion. In addition, a formal grading scale of the degenerative changes of the acetabulum was not assessed. The study examined the functional outcomes of the patients, but the impact of acetabular erosions on quality of life was not assessed. Finally, the assessment of the relationship between acetabular erosions on MSTS scores was relatively crude (it was assessed categorically, comparing patients with more than or less than 2 mm of cartilage loss); given the small patient numbers and the imprecision in measurement on plain radiographs, it did not seem reasonable to assess it using a correlation analysis. There is every reason to think that acetabular cartilage wear will, at some level, be associated with pain and functional limitations; however, for most patients in this population, this surgical approach seemed to meet the need. However, for patients who will survive for longer periods, erosions may become a larger problem. Longer term studies will need to evaluate this.
Acetabular erosion after endoprosthetic replacement of the proximal femur with a bipolar hemiarthroplasty has not been commonly reported. Drexler et al. [11] found degenerative changes occurred in 4.6% (three of 65) and protrusio acetabuli in 13.8% (nine of 65) in 65 patients after hemiarthroplasty endoprosthetic replacement with 4.6% (three of 65) patients converted to THA to treat groin pain. The revisions in the series by Drexler et al. occurred at 26, 34, and 47 months after surgery [11]. By contrast, we noted a higher proportion of patients with degenerative changes (13%) but a lower frequency of protrusion (3%) in a larger series of 148 patients; however, we observed a similar rate of conversion of the bipolar hemiarthroplasty to THA (4% [six of 148]) because of degenerative changes and groin pain. However, we noted that with longer followup, the risk of conversion of bipolar hemiarthroplasty to THA increased. In the nononcologic setting, the proportion of patients undergoing conversion to THA to treat symptoms of degenerative arthritis has been reported to be up to 18% after initial hemiarthroplasty for hip arthritis or fracture [2, 29, 39]. Although we noted a higher rate of degenerative changes than has been previously reported after reconstruction with a bipolar hemiarthroplasty for a proximal femoral malignancy, at the current followup, this did not seem to result in worse functional outcomes based on MSTS scores.
Of the 148 patients in this study, conversion of a bipolar hemiarthroplasty to THA was in six patients with symptomatic hip pain and degenerative joint disease with a 10-year cumulative risk of conversion to THA to treat symptomatic acetabular erosions of 5% (95% CI, 0%-11%). This finding is similar to other reports of proximal femoral endoprostheses [11, 14, 18, 27]. In randomized controlled trials of patients with femoral neck fractures, however, the rate of conversion from bipolar hemiarthroplasty to THA for symptomatic osteoarthritis is 4% to 10%, which seems similar to the results of this study [2, 39]. Because the overall rate of conversion to THA was so low, we do not advocate for the use of primary THA after proximal femoral resection for cancer.
Endoprosthetic replacement of the proximal femur is a functionally successful procedure for most patients [13, 14, 18, 26, 30, 40] and in the current series, radiographic changes in acetabular erosion were not indicative of the patients’ functional outcome based on the MSTS score. After hemiarthroplasty, functional improvement based on the Harris hip score has been found to peak 1 to 2 years after the index procedure [2, 7, 22, 31]. In prospective randomized studies comparing a bipolar hemiarthroplasty with a THA for patients with displaced femoral neck fractures, it has been found that erosion can be a problem, necessitating revision to THA [2, 3]. Likewise, Pellegrini et al. [29] noted that although radiographic evidence of cephalad or medial migration of a bipolar hemiarthroplasty occurred, it did not correspond with pain, satisfaction, or clinical outcome measures, similar to the outcome of the current series.
Overall, the rates of acetabular erosion and conversion to THA were low after endoprosthetic reconstruction of the proximal femur using bipolar hemiarthroplasty. Although some patients may demonstrate radiographic evidence of acetabular cartilage wear, with the current followup, it does not typically affect their functional outcome. Studies with longer clinical followup are needed to determine if the expected increased incidence of continued acetabular erosion translates into clinical impairment.
Acknowledgments
We thank Omar S. Dahduli MBBS, for his assistance in the review of the radiographs.
Footnotes
Each author certifies that neither he nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University Musculoskeletal Oncology Unit, Department of Orthopedic Surgery, Mount Sinai Hospital, University of Toronto, Toronto, Canada.
References
- 1.Armbuster TG, Guerra J, Jr, Resnick D, Goergen TG, Feingold ML, Niwayama G, Danzig LA. The adult hip: an anatomic study. Part I: the bony landmarks. Radiology. 1978;128:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Avery PP, Baker RP, Walton MJ, Rooker JC, Squires B, Gargan MF, Bannister GC. Total hip replacement and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck: a seven- to ten-year follow-up report of a prospective randomised controlled trial. J Bone Joint Surg Br. 2011;93:1045–1048. [DOI] [PubMed] [Google Scholar]
- 3.Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:2583–2589. [DOI] [PubMed] [Google Scholar]
- 4.Bernthal NM, Schwartz AJ, Oakes DA, Kabo JM, Eckardt JJ. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin Orthop Relat Res. 2010;468:2867–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickels J, Meller I, Henshaw RM, Malawer MM. Reconstruction of hip stability after proximal and total femur resections. Clin Orthop Relat Res. 2000;375:218–230. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 7.Cadossi M, Chiarello E, Savarino L, Tedesco G, Baldini N, Faldini C, Giannini S. A comparison of hemiarthroplasty with a novel polycarbonate-urethane acetabular component for displaced intracapsular fractures of the femoral neck: a randomised controlled trial in elderly patients. Bone Joint J. 2013;95:609–615. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abudu A, Buckley L. Modular endoprosthetic replacement for tumours of the proximal femur. J Bone Joint Surg Br. 2009;91:108–112. [DOI] [PubMed] [Google Scholar]
- 9.Damron TA, Sim FH. Surgical treatment for metastatic disease of the pelvis and the proximal end of the femur. Instr Course Lect. 2000;49:461–470. [PubMed] [Google Scholar]
- 10.Dobbs HS, Scales JT, Wilson JN, Kemp HB, Burrows HJ, Sneath RS. Endoprosthetic replacement of the proximal femur and acetabulum. A survival analysis. J Bone Joint Surg Br. 1981;63:219–224. [DOI] [PubMed] [Google Scholar]
- 11.Drexler M, Gortzak Y, Sternheim A, Kollender Y, Amar E, Bickels J. The radiological evaluation of the hip joint after prosthetic arthroplasty of the proximal femur in patients with a tumour using a bipolar femoral head. Bone Joint J. 2015;97:1704–1709. [DOI] [PubMed] [Google Scholar]
- 12.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 13.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. [DOI] [PubMed] [Google Scholar]
- 14.Finstein JL, King JJ, Fox EJ, Ogilvie CM, Lackman RD. Bipolar proximal femoral replacement prostheses for musculoskeletal neoplasms. Clin Orthop Relat Res. 2007;459:66–75. [DOI] [PubMed] [Google Scholar]
- 15.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. [DOI] [PubMed] [Google Scholar]
- 16.Grimer RJ, Carter SR, Pynsent PB. The cost-effectiveness of limb salvage for bone tumours. J Bone Joint Surg Br. 1997;79:558–561. [DOI] [PubMed] [Google Scholar]
- 17.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. [DOI] [PubMed] [Google Scholar]
- 18.Houdek MT, Watts CD, Wyles CC, Rose PS, Taunton MJ, Sim FH. Functional and oncologic outcome of cemented endoprosthesis for malignant proximal femoral tumors. J Surg Oncol. 2016;114:501–506. [DOI] [PubMed] [Google Scholar]
- 19.Houdek MT, Wyles CC, Labott JR, Rose PS, Taunton MJ, Sim FH. Durability of hemiarthroplasty for pathologic proximal femur fractures. J Arthroplasty. 2017;32:3607–3610. [DOI] [PubMed] [Google Scholar]
- 20.Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14:2887–2895. [DOI] [PubMed] [Google Scholar]
- 21.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271. [DOI] [PubMed] [Google Scholar]
- 22.Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88:249–260. [DOI] [PubMed] [Google Scholar]
- 23.Kiekens G, Somville J, Taminiau A. Clinical relevance of acetabular erosion in young patients with a bipolar hip prosthesis. Acta Orthop Belg. 2000;66:455–460. [PubMed] [Google Scholar]
- 24.LaBelle LW, Colwill JC, Swanson AB. Bateman bipolar hip arthroplasty for femoral neck fractures. A five- to ten-year follow-up study. Clin Orthop Relat Res. 1990;251:20–25. [PubMed] [Google Scholar]
- 25.Masterson EL, Ferracini R, Griffin AM, Wunder JS, Bell RS. Capsular replacement with synthetic mesh: effectiveness in preventing postoperative dislocation after wide resection of proximal femoral tumors and prosthetic reconstruction. J Arthroplasty. 1998;13:860–866. [DOI] [PubMed] [Google Scholar]
- 26.Menendez LR, Ahlmann ER, Kermani C, Gotha H. Endoprosthetic reconstruction for neoplasms of the proximal femur. Clin Orthop Relat Res. 2006;450:46–51. [DOI] [PubMed] [Google Scholar]
- 27.Ogilvie CM, Wunder JS, Ferguson PC, Griffin AM, Bell RS. Functional outcome of endoprosthetic proximal femoral replacement. Clin Orthop Relat Res. 2004;426:44–48. [DOI] [PubMed] [Google Scholar]
- 28.Park DH, Jaiswal PK, Al-Hakim W, Aston WJ, Pollock RC, Skinner JA, Cannon SR, Briggs TW. The use of massive endoprostheses for the treatment of bone metastases. Sarcoma. 2007;2007:62151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrini VD, Jr, Heiges BA, Bixler B, Lehman EB, Davis CM., 3rd Minimum ten-year results of primary bipolar hip arthroplasty for degenerative arthritis of the hip. J Bone Joint Surg Am. 2006;88:1817–1825. [DOI] [PubMed] [Google Scholar]
- 30.Potter BK, Chow VE, Adams SC, Letson GD, Temple HT. Endoprosthetic proximal femur replacement: metastatic versus primary tumors. Surg Oncol. 2009;18:343–349. [DOI] [PubMed] [Google Scholar]
- 31.Ravikumar KJ, Marsh G. Internal fixation versus hemiarthroplasty versus total hip arthroplasty for displaced subcapital fractures of femur–13 year results of a prospective randomised study. Injury. 2000;31:793–797. [DOI] [PubMed] [Google Scholar]
- 32.Refaat Y, Gunnoe J, Hornicek FJ, Mankin HJ. Comparison of quality of life after amputation or limb salvage. Clin Orthop Relat Res. 2002;397:298–305. [DOI] [PubMed] [Google Scholar]
- 33.Schneiderbauer MM, von Knoch M, Schleck CD, Harmsen WS, Sim FH, Scully SP. Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am. 2004;86:1684–1689. [DOI] [PubMed] [Google Scholar]
- 34.Selek H, Basarir K, Yildiz Y, Saglik Y. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty. 2008;23:112–117. [DOI] [PubMed] [Google Scholar]
- 35.Sim FH, Chao EY. Hip salvage by proximal femoral replacement. J Bone Joint Surg Am. 1981;63:1228–1239. [PubMed] [Google Scholar]
- 36.Sim FH, Pritchard DJ, Ivins JC, Shives TC. Total joint arthroplasty. Applications in the management of bone tumors. Mayo Clin Proc. 1979;54:583–589. [PubMed] [Google Scholar]
- 37.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 38.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 39.van den Bekerom MP, Hilverdink EF, Sierevelt IN, Reuling EM, Schnater JM, Bonke H, Goslings JC, van Dijk CN, Raaymakers EL. A comparison of hemiarthroplasty with total hip replacement for displaced intracapsular fracture of the femoral neck: a randomised controlled multicentre trial in patients aged 70 years and over. J Bone Joint Surg Br. 2010;92:1422–1428. [DOI] [PubMed] [Google Scholar]
- 40.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. [PubMed] [Google Scholar]