Abstract
Background
Amputations sustained owing to combat-related blast injuries are at high risk for deep infection and development of heterotopic ossification, which can necessitate reoperation and place immense strain on the patient. Surgeons at our institution began use of intrawound antibiotic powder at the time of closure in an effort to decrease the rate of these surgical complications after initial and revision amputations, supported by compelling clinical evidence and animal models of blast injuries. Antibiotic powder may be useful in reducing the risk of these infections, but human studies on this topic thus far have been inconclusive.
Purpose
We sought to determine whether administration of intrawound antibiotic powder at the time of closure would (1) decrease the risk of subsequent deep infections of major lower-extremity combat-related amputations, and (2) limit formation and decrease severity of heterotopic ossification common in the combat-related traumatic residual limb.
Methods
Between 2009 and 2015, 252 major lower extremity initial and revision amputations were performed by a single surgeon. Revision cases were excluded if performed specifically to address deep infection, leaving 223 amputations (88.5%) for this retrospective analysis. We reviewed medical records to collect patient information, returns to the operating room for subsequent infection, and microbiologic culture results. We also reviewed radiographs taken at least 3 months after surgery to determine the presence and severity of heterotopic ossification using the Walter Reed classification system. We grouped cases according to whether limbs underwent initial or revision amputations, and whether the limbs had a history of a prior infection. Apart from the use of antibiotic powder and duration of followup, the groups did not differ in terms of age, mechanism of injury, or sex. We then calculated the absolute risk reduction for infection and heterotopic ossification and the number needed to treat to prevent an infection.
Results
Overall, administration of antibiotic powder resulted in a 13% absolute risk reduction of deep infection (14 of 82 [17%] versus 42 of 141 [30%]; p = 0.03; 95% CI, 0.20%-24.72%). In revision amputation surgery, the absolute risk reduction of infection with antibiotic powder use was 16% overall (eight of 58 versus 17 of 57; 95% CI, 1.21%-30.86%), and 25% for previously infected limbs (eight of 46 versus 14 of 33; 95% CI, 4.93%-45.14%). The number needed to treat to prevent one additional deep infection in amputation surgery is eight in initial amputations, seven in revision amputations, and four for revision amputation surgery on previously infected limbs. With the numbers available, we observed no reduction in the risk of heterotopic ossification with antibiotic powder use, but severity was decreased in the treatment group in terms of the number of residual limbs with moderate or severe heterotopic ossification (three of 12 versus 19 of 34; p = 0.03).
Conclusions
Our findings show that administration of intrawound antibiotic powder reduces deep infection in residual limbs of combat amputees, particularly in the setting of revision amputation surgery in apparently aseptic residual limbs at the time of the surgery. Furthermore, administration of antibiotic powder for amputations at time of initial closure decreases the severity of heterotopic ossification formation, providing a low-cost adjunct to decrease the risk of two complications common to amputation surgery.
Level of Evidence Level III, therapeutic study
Introduction
As of December 1, 2016, more than 1705 United States service members have sustained traumatic extremity amputations related to combat operations in Iraq and Afghanistan. Primarily resulting from blast trauma, injuries resulting in immediate or subsequent limb amputation often are massive and subject to ubiquitous bacterial and foreign-body contamination. To preserve limb length and salvage functional joint levels, definitive amputation often is performed through the zone of injury after serial débridement of obviously devitalized tissue. This practice of amputation through the zone of injury, while independently associated with heterotopic ossification (HO) formation, has not been correlated with subsequent deep infection to date. Many combat extremity wounds are contaminated with virulent and drug-resistant organisms requiring multiple operations to adequately control [2, 14, 15]. Deep infection of residual limbs warrants deconstruction of the myoplasty and myodesis, frequently results in residual bone shortening, and may necessitate a prolonged period of nonweightbearing, prosthetic modification, and gait retraining when it occurs after initial rehabilitation. Twenty-seven percent of combat-related amputations require the patient be returned to the operating room for reexploration and débridement of wounds after initial closure, taxing health system resources, the patient, and the treatment team [26].
In April 2009, to decrease the rate of deep surgical site infection after definitive or revision amputation we began application of antibiotic powder in amputation wounds at the time of closure. We progressively increased routine use until 2013 when antibiotic powder was used in all closures, as increasing evidence supported its use for infection prophylaxis. While there are few published orthopaedic trauma studies, to our knowledge, regarding the efficacy of antibiotic powder for decreasing deep wound infection [27], studies in spine surgery have shown a fairly consistent and promising decrease in the rate of deep surgical site infection with application of vancomycin powder [1, 4, 5, 10]. Additionally, recently established animal models of traumatic HO suggest that bacterial contamination of wounds can potentiate the severity of HO development, and that early intrawound antibiotic administration may mitigate chronic infection and HO formation [18, 23]. Seavey et al. [23] tested antibiotic use in traumatic amputations in a rodent model, and showed that vancomycin powder administration limits HO formation in infected and noninfected residual limbs when administered early.
We therefore asked the following: (1) Does routine administration of topical, intrawound antibiotic powder reduce deep surgical site infection at the time of initial closure or revision of combat-related lower extremity amputations? (2) Does antibiotic powder administration reduce the incidence and/or severity of HO in combat-related lower extremity amputations?
Materials and Methods
After receiving approval from our institutional review board, the surgical scheduling system at our institution was retrospectively reviewed to identify all patients who underwent planned definitive closure or revision of a lower extremity amputation by a single surgeon (BKP) between September 2008 and January 2015. We identified 252 major lower extremity initial and revision amputations meeting these criteria; we subsequently excluded all revision cases if performed specifically to address deep infection, leaving 223 amputations (88.5%) for final analysis. This surgeon’s patients were selected because he performs most of the primary and revision amputations at our institution, thus limiting the number of potential confounders in terms of differing thresholds for amputation closure or operative technique among surgeons, and because he had, with time, begun to administer antibiotic powder to all amputation wounds at the time of closure or revision (Fig. 1). He initiated the practice of intrawound antibiotic application in April 2009, primarily in difficult or previously infected cases, and gradually increased frequency as experiential evidence suggested positive results from routine use. By 2013, all initial and revision amputation closures received intrawound antibiotic powder, regardless of intraoperative concern.
Fig. 1.
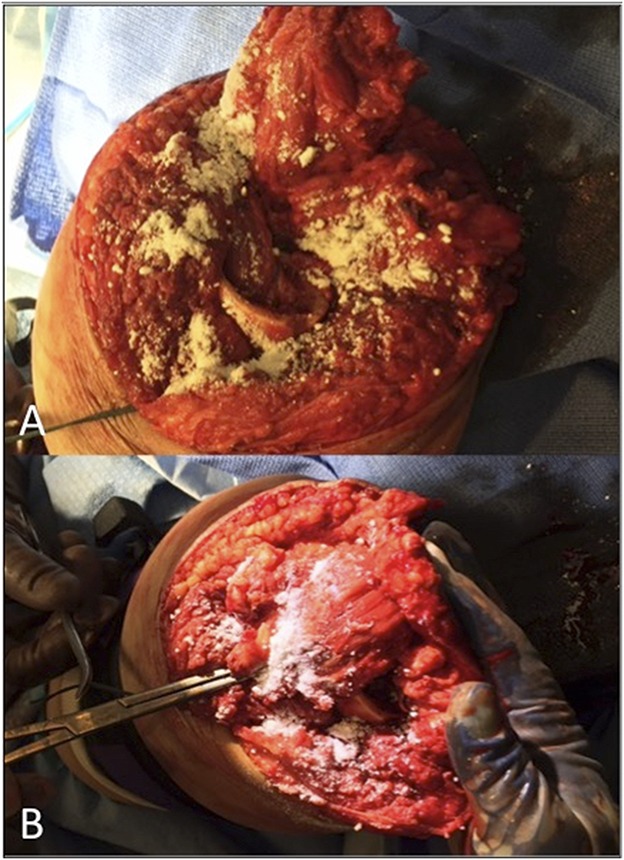
A-B Intraoperative photographs show placement of topical antibiotic powder (A) in the intramedullary canal and beneath the myodesis, and (B) above the myoplasty at the time of definitive closure of traumatic amputations sustained by combat-wounded service members.
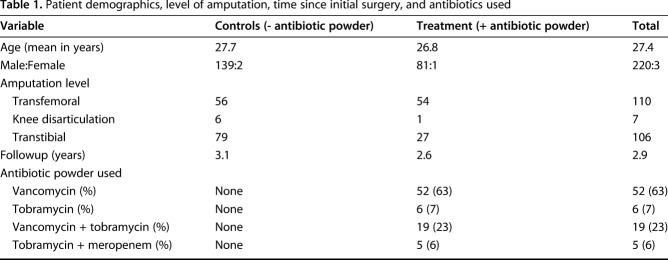
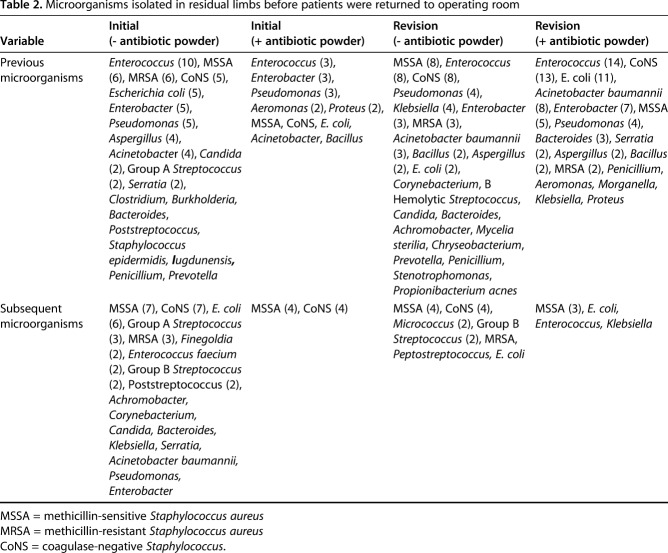
We distinguished patient cohorts based on whether the residual limb received antibiotic powder, and subclassified the treatment and control groups as initial amputation or revision closure and if the limb had a prior, culture-positive infection. Combat-related amputations were defined as initial amputations if no previous definitive closure was performed. Revision amputations consisted of a previously closed residual limb subsequently treated with reexploration and deconstruction of the myodesis or myoplasty. We collected patient demographics, level of amputation, time since initial surgery, and antibiotics used to describe the patient population (Table 1). Microbiologic culture data were collected from previous deep wound infections. This information was used to illustrate the frequency of infecting organisms before primary amputation closure, and in the setting of infection before revision (Table 2). Treating each limb as its own entity, all culture findings were reviewed when obtained in the zone of amputation before initial or revision closure, or when a subsequent débridement was performed in a clinically infected limb. One of the authors (GJP) reviewed all radiographs for evidence of subsequent HO formation after initial amputation closure, and graded severity according to the Walter Reed HO classification system [21].
Table 1.
Patient demographics, level of amputation, time since initial surgery, and antibiotics used
Table 2.
Microorganisms isolated in residual limbs before patients were returned to operating room
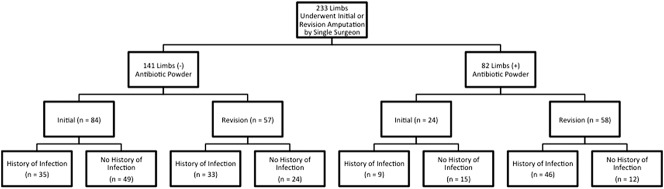
One hundred forty-one limbs were treated without topical antibiotic powder application. The antibiotic used was primarily vancomycin, unless prior cultures indicated resistance or the patient reported an allergy, in which case daptomycin was applied. This group included patients with 84 initial and 57 revision amputations. Eighty-two limbs were treated with intrawound antibiotic powder and included 24 initial and 58 revision closures (Fig. 2). Limbs in the control and the antibiotic powder treatment groups underwent revision amputation for various reasons including symptomatic HO (30 control, 36 treatment), myodesis failure (five control, four treatment), symptomatic neuromata refractory to nonsurgical methods (five control, one treatment), sterile dehiscence (two control, four treatment), soft tissue revision (eight control, four treatment), or a combination of these (eight control, eight treatment).
Fig. 2.
A demographic tree shows the cohort breakdown of residual limbs in which antibiotic powder was used. Cohorts are further subdivided into whether the treatment was done during the initial closure of an amputation or at the time of revision closure. These additionally were subclassified as having culture-positive infection in the limb before amputation closure.
Our primary study outcome was a culture-positive infection verified by tissue obtained during open débridement during an unplanned return to the operating room. Indications for unplanned return to the operating room included wound dehiscence, new fluid collection in the setting of inflammatory response, and other clinical indicators of deep residual limb infection. We compared the frequency of culture-positive infection in groups that received prophylactic antibiotic powder during the initial definitive or revision closure with control extremities which did not receive antibiotic powder before wound closure. We also performed a subgroup analysis comparing our primary study outcome on residual limbs that previously had positive cultures, and between limbs undergoing their initial definitive attempt at closure and those undergoing major revision procedures.
Our secondary outcome was presence and severity of HO after initial amputation. Where available, orthogonal residual limb radiographs were examined if obtained later than 3 months after amputation. Using these criteria, 72 limbs of the 108 initial amputations (52 control and 20 treatment) were available for radiographic analysis. Limb radiographs were measured at the most severe level of involvement on the orthogonal radiograph with the greatest radiographically apparent burden of HO. The measurements were performed by a single blinded reviewer (GJP) and graded according to the Walter Reed HO classification system as mild (0%-25%), moderate (25%-50%, or severe (> 50%) based on the cross-sectional percentage affected by HO at the level selected [26]. We compared the incidence of HO after treatment and the severity between the control and treatment groups for initial amputation closures only.
We obtained patient outcomes using the Armed Forces Health Longitudinal Technology Application, the outpatient medical records application of the Military Health System.
The average followup for the primary study outcome when last seen by a musculoskeletal specialty care provider for the operative limb was 1071 days (range, 383-2982 days). For the control group, the mean followup was 1139 days (range, 385-2596 days; SD, 520 days), which was significantly greater than that for the antibiotic powder treatment group at 957 days (range, 383-2982 days; SD, 445 days; p = 0.008). Return to the operating room for irrigation and débridement for the control group occurred at a median 77 days and for the antibiotic treatment group 52.5 days. No patients were lost to followup before 1 year. Followup was not calculated for radiographic outcomes as previous work in this patient population showed that no clinically meaningful development of HO or progression of radiographic severity beyond 3 months, if patients did not report symptoms [8, 20, 21].
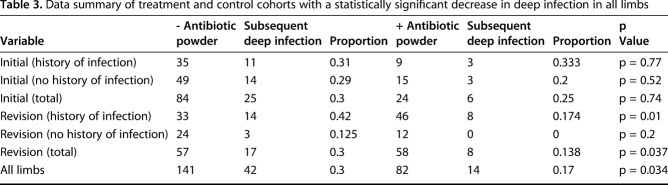
We performed data analysis using RStudio (Version 0.98.953; RStudio, Inc, Boston, MA, USA). Chi-square test was used to determine potential differences in comparing proportions between treatment and control groups among initial and revision limbs and with those that did and did not have prior infection (Table 3). We defined statistical significance as a two-tailed α less than 0.05. We identified the absolute risk reduction with the use of antibiotic powder and the number needed to treat to prevent one additional deep infection.
Table 3.
Data summary of treatment and control cohorts with a statistically significant decrease in deep infection in all limbs
Results
The risk of infection overall after amputation surgery was lower in patients who received antibiotic powder than those who did not (17%, 14 of 82, versus 30%, 42 of 141; absolute risk reduction [ARR], 12.7%; 95% CI, 1.6%-23.8%; p = 0.034). The number needed to treat to prevent one infection was eight (95% CI, 4.2-62.1). In cases of residual limbs after revision surgery, the overall risk of infection also was lower in patients who received antibiotic powder than those who did not (14%, eight of 58 versus 30%, 17 of 57; ARR, 16%; 95% CI, 1.2%-30.9%; p = 0.037). The number needed to treat to prevent one infection was seven (95% CI, 3.2-82.9). In cases of residual limbs after revision surgery with a previous history of infection, despite a higher infection incidence, those treated with antibiotic powder experienced fewer infections than those who did not (17%, eight of 46, versus 42%, 14 of 33; ARR, 25.03%; 95% CI, 4.9%-45.1%; p = 0.01). The number needed to treat to prevent one infection was four (95% CI, 2.0-28.1).
With the numbers available, there was no between-group difference in the proportion of patients who had HO develop after initial amputation, with HO developing in 65% (34 of 52) of control residual limbs compared with 60% (12 of 20) of limbs that received antibiotic powder (p = 0.68). However, fewer (25%) of the limbs that had HO develop in the treatment group were Grade 2 or 3 (moderate or severe), compared with 56% in the control group (p = 0.03) between cohorts.
Discussion
Deep infection in residual limbs of a patient with a combat-related amputation is a frequent complication that can have profound effects on rehabilitation of combat-wounded service members. A previous study showed a 27% rate of reoperation for combat-related lower extremity amputations for presumed infection and a 24% reoperation rate for symptomatic HO formation [24]. In our practice, reoperation for deep infection typically includes myodesis and bony revision with possible delayed closure, prolonged parenteral antibiotic therapy, at least 6 weeks nonweightbearing on the lower extremity, and often a costly replacement or modification of a previously used prosthesis and gait retraining. We sought to determine if use of inexpensive intraoperative antibiotic powder would decrease the risk of these costly deep infections. Secondarily, we attempted to answer whether its use would decrease the risk or severity of HO formation, which is also a frequent indication for amputation revision surgery in this patient population. Our study shows that the use of antibiotic powder, particularly in the setting of revision amputation surgery (including procedures performed for HO resection, myodesis failure, neuroma excision, or other major soft tissue revision), decreased subsequent clinical deep infections, and may reduce the severity of HO when used during initial amputation closure.
There are several limitations to the current study. First, it is a retrospective cohort analysis, which relies on the accuracy and completeness of the electronic medical record for acquisition of data. The mean followup of our patients was approximately 2.6 years for the treatment group and 3.1 years for the control group, which was found to represent a statistically significant difference, but not a clinically significant difference as these were well beyond the median time to reoperation for infection in the control and antibiotic powder treatment groups (median, 77 and 52.5 days, respectively). The times to followup represent the last visit of the patient to a musculoskeletal care provider who specifically commented on the health of the residual limb at the time of assessment. It is unlikely any patient with an infection treated elsewhere was missed, as our electronic medical record is universal among military treatment facilities across the Department of Defense. Loss to followup occurs only when patients retire from military service and seek treatment at either Veterans Affairs medical facilities or civilian treatment facilities. Furthermore, medical retirement in the military is a lengthy process that does not occur until at least a year after initial amputation closure owing to rehabilitation and administrative requirements.
Another limitation was patient selection. The treatment and control cohorts were not randomly selected, but rather the indications for treatment with intraoperative antibiotics, and therefore inclusion into the treatment group, evolved with time owing to increasing evidence from the spine literature and anecdotal evidence of efficacy in infection prophylaxis. Initially, antibiotic powder was used in cases that the primary surgeon felt to be at higher risk of infection typically owing to history of multiple or resistant prior infections. The indications expanded during the course of the study, and by the final year of the study, antibiotic powder was used in all residual limbs. While this lends toward a degree of selection bias, it would more likely favor the null hypothesis as the prior infection subgroup of the control cohort had a higher rate of infection than baseline, indicating that treatment was favored in higher-risk patients. There is also a possibility that as a single-surgeon study, infection outcomes may have been influenced by the orthopaedic learning curve and greater amputation experience acquired with time.
Next, our primary outcome measure, deep infection, was assessed by the findings of intraoperative cultures that were taken during débridement or revision surgery; therefore, subclinical or superficial infection did not receive consideration in our study. The decision to return for reoperation was made by the primary surgeon in nearly all cases, and was based on a reliable clinical picture concerning for infection taking into account pain, wound drainage or overt dehiscence, erythema, systemic inflammatory response, and elevated inflammatory markers [19]. The use of positive cultures as a component of the endpoint prevents this outcome measure from being unduly biased by false-positive indications for reoperation. While there is a possibility, albeit minor, that deep infections were missed, such a scenario is fairly unlikely given the aforementioned captive patient population, and that untreated deep infections of residual limbs are unlikely to remain subclinical. In addition, the Walter Reed classification for grading HO severity is not yet validated for intra- or interobserver reliability, although it was used effectively by one reviewer for this purpose [21].
Last, we do not know the utility of this treatment modality in other patient populations with amputations, and can only assume this study population is generalizable to other patients with trauma. The deep infection rates for major amputations attributable to civilian trauma are remarkably close to those sustained in combat [9]. However, the occurrence of HO in the civilian population with amputations is much lower than in patients with combat injuries, and does not appear to be affected by the indication for amputation, which may limit the generalizability of our findings [12].
The administration of intrawound vancomycin powder has been studied extensively in spine surgery and generally found to be safe and effective in decreasing postoperative surgical site infections [4, 5, 10, 11, 13, 17]. Procedures performed between 2001 and 2008, before systematic application of concentrated bacitracin powder, had a postoperative infection rate of 13%, whereas procedures performed with antibiotic powder between 2008 and 2013 had a postoperative infection rate of 1%. One of the largest studies in patients with trauma found no postoperative infections in 93 elbow contracture releases in which intrawound vancomycin powder was applied, compared with a 6.5% deep infection rate in patients who received perioperative parenteral antibiotics alone [27]. The introduction of intrawound vancomycin powder allows a high concentration of local antibiotic with little adverse clinical effects seen in prior studies, and has been shown to reach a minimum inhibitory concentration against methicillin-resistant Staphylococcus aureus (MRSA) and coagulase negative staphylococcus for several days [4, 25]. This is particularly important in combat-related wounds as a previous study showed MRSA colonization rates of 26% [2]. One potential drawback of intraoperative local antibiotic prophylaxis on a large scale is the risk of consequent selective pressure favoring the emergence of, or selection for, antibiotic-resistant strains [3]. This concern, however, largely focuses on the specter of potential biofilm formation, particularly with antibiotic-coated delivery systems used in the setting of treating active infection. Among the limited reports of selective antibiotic resistance is a case report of the presence of a gentamicin-resistant staphylococcal strain from the surface of gentamicin-impregnated antibiotic beads 5 years after being placed for periprosthetic infection [16]. In our culture data, we found no increased selection toward resistant organisms in patients who received intraoperative antibiotics (Table 2). In short, to the best of our knowledge there is no current evidence to make a compelling argument against prophylactic use of topical antibiotics.
Research efforts in our institution have focused on the mitigation of traumatic HO formation in the extremities of patients with combat wounds. A previous study showed highly concentrated local antibiotic is inhibitory to osteoblast formation [6]. Furthermore, since HO potentiation has been shown to be associated with MRSA infection in rodent models of traumatic ectopic bone formation [18], vancomycin has been trialed as a potential prophylaxis in this model and shows promise as an adjunctive treatment for preventing HO formation in a recent study [23]. In the current investigation, while there was not a difference in the absolute rate of HO formation, we did note decreased HO severity, which may be clinically relevant in terms of the likelihood of symptoms resulting in surgical excision. While no published study, to our knowledge, specifically compares HO grade with severity of symptoms and incidence of surgical excision, our experience is strongly supportive of such an association, particularly when the ectopic bone burden is profound (Fig. 3). Whether the effect of intrawound antibiotics is the result of potent osteoblastic inhibition or secondary to reduction of bioburden-induced local inflammatory cytokine production requires further study; however, these effects were noted even in the absence of proven bacterial contamination and prior clinical infection.
Fig. 3.
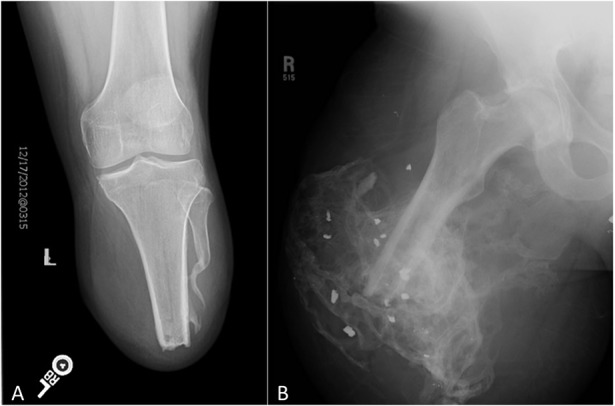
A-B AP radiographs are shown of the residual limb of a patient who (A) received intrawound antibiotic powder at the time of initial closure with development of less-severe heterotopic ossification formation, compared with (B) a limb that did not receive antibiotic powder at the time of initial closure.
Given the low cost of topical antibiotics in general, and vancomycin specifically, this intervention is likely to be highly cost-effective. Surgical site infections, particularly those involving deep tissues, strain hospital resources. One study that focused on change in hospital profit attributable to surgical site infections showed a USD 2,268,589 loss of potential profit during a 2-year period [24]. A study conducted in Veteran’s Affairs hospitals determined that patients with deep surgical site infections were associated with 1.93 times greater costs compared with patients without infection, with an average excess cost of USD 25,721 per patient with a deep surgical site infection [22]. In a spine study, Emohare et al. [7] noted that routine vancomycin powder application resulted in an overall cost reduction of USD 572,745. In our study, applying relatively inexpensive antibiotic powder prophylactically reduced the infection rate from 30% to 17%, potentially alleviating the cost of return to the operating room in addition to the substantial cost of amputee rehabilitation that is required for prosthesis revision, gait retraining after revision amputation surgery, government wages, and cost of nonmedical attendants and lodging during rehabilitation.
Combat-related major extremity amputations have a high risk of complications and revision surgery, the most frequent of which are deep infection and symptomatic HO. We found that routine use of antibiotic powder decreased the risk of deep infection, particularly after revision amputation surgery, and may help decrease the severity of HO after combat-related trauma. Given the potential for reducing these complications, future studies must be performed in this and other trauma populations. In the interim, given the low cost of topical antibiotics, there is little apparent downside to routine use of intrawound antibiotic powder during closure of combat-related amputations, and there may be a role for antibiotic powder application in other types of orthopaedic trauma surgery in which the risk of infection is high.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defined a U.S. Government work as a work prepared by a military service member or employees of the U.S. Government as part of that person’s official duties. The opinions or assertions contained in this paper are the private views of the authors and are not to be construed as reflecting the views, policy or positions of the Departments of the Navy, Department of Defense nor the U.S. Government.
This work was done at the Uniformed Services University – Walter Reed National Military Medical Center Department of Surgery, Bethesda, MD, USA
References
- 1.Beckman JM, Amankwah EK, Tetreault LL, Tuite GF. Reduction in CSF shunt infection over a 10-year period associated with the application of concentrated topical antibiotic powder directly to surgical wounds prior to closure. J Neurosurg Pediatr. 2015;16:648–661. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campoccia D, Montanaro L, Speziale P, Arciola CR. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials. 2010;31:6363–6377. [DOI] [PubMed] [Google Scholar]
- 4.Caroom C, Tullar JM, Benton EG, Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976). 2013;38:1183–1187. [DOI] [PubMed] [Google Scholar]
- 5.Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014;14:397–407. [DOI] [PubMed] [Google Scholar]
- 6.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;333:245–251. [PubMed] [Google Scholar]
- 7.Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW, Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014;14:2710–2715. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. [DOI] [PubMed] [Google Scholar]
- 9.Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R; Lower Extremity Assessment Project (LEAP) Study Group. Complications following limb-threatening lower extremity trauma. J Orthop Trauma. 2009;23:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Kang DG, Holekamp TF, Wagner SC, Lehman RA., Jr Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015;15:762–770. [DOI] [PubMed] [Google Scholar]
- 11.Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, Camillo FX, Klimo P., Jr A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21:974–983. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto ME, Khan M, Jayabalan P, Ziebarth J, Munin MC. Heterotopic ossification in civilians with lower limb amputations. Arch Phys Med Rehabil. 2014;95:1710–1713. [DOI] [PubMed] [Google Scholar]
- 13.Molinari RW, Khera OA, Molinari WJ., 3rd Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21(suppl 4):S476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma. 2008;64(3 suppl):S221–231. [DOI] [PubMed] [Google Scholar]
- 15.Murray CK, Roop SA, Hospenthal DR, Dooley DP, Wenner K, Hammock J, Taufen N, Gourdine E. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171:826–829. [DOI] [PubMed] [Google Scholar]
- 16.Neut D, van de Belt H, van Horn JR, van der Mei HC, Busscher HJ. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24:1829–1831. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, Devin CJ. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011;11:641–646. [DOI] [PubMed] [Google Scholar]
- 18.Pavey GJ, Qureshi AT, Hope DN, Pavlicek RL, Potter BK, Forsberg JA, Davis TA. Bioburden increases heterotopic ossification formation in an established rat model. Clin Orthop Relat Res. 2015:473:2840–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polfer EM, Hoyt BW, Senchak LT, Murphey MD, Forsberg JA, Potter BK. Fluid collections in amputations are not indicative or predictive of infection. Clin Orthop Relat Res. 2014;472:2978–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14(10 Spec No):S191–197. [DOI] [PubMed] [Google Scholar]
- 21.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. [DOI] [PubMed] [Google Scholar]
- 22.Schweizer ML, Cullen JJ, Perencevich EN, Vaughan Sarrazin MS. Costs associated with surgical site infections in Veterans Affairs hospitals. JAMA Surg. 2014;149:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seavey JG, Wheatley BM, Pavey GJ, Tomasino AM, Hanson MA, Sanders EM, Dey D, Moss KL, Potter BK, Forsberg JA, Qureshi AT, Davis TA. Early local delivery of vancomycin suppresses ectopic bone formation in a rat model of trauma-induced heterotopic ossification. J Orthop Res. 2017;35:2397–2406. [DOI] [PubMed] [Google Scholar]
- 24.Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E, Perl T. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148:907–914. [DOI] [PubMed] [Google Scholar]
- 25.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011;36:2084–2088. [DOI] [PubMed] [Google Scholar]
- 26.Tintle SM, Shawen SB, Forsberg JA, Gajewski DA, Keeling JJ, Andersen RC, Potter BK. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232–237. [DOI] [PubMed] [Google Scholar]
- 27.Yan H, He J, Chen S, Yu S, Fan C. Intrawound application of vancomycin reduces wound infection after open release of post-traumatic stiff elbows: a retrospective comparative study. J Shoulder Elbow Surg. 2014;23:686–692. [DOI] [PubMed] [Google Scholar]