Abstract
Background
For many cancer types, survival is improved when patients receive management at treatment centers that encounter high numbers of patients annually. This correlation may be more important with less common malignancies such as sarcoma. Existing evidence, however, is limited and inconclusive as to whether facility volume may be associated with survival in soft tissue sarcoma.
Questions/purposes
The purpose of this study was to examine the association between facility volume and overall survival in patients with soft tissue sarcoma of the extremities. In investigating this aim, we sought to (1) examine differences in the treatment characteristics of high- and low-volume facilities; (2) estimate the 5-year survival by facility volume; and (3) examine the association between facility volume and of traveling a further distance to a high-volume center and overall survival when controlling for confounding factors.
Methods
The largest sarcoma patient registry to date is contained within the National Cancer Database (NCDB) and captures > 70% of new cancer diagnoses annually. We retrospectively analyzed 25,406 patients with soft tissue sarcoma of the extremities in the NCDB from 1998 through 2012. Patients were stratified based on per-year facility sarcoma volume and we used univariate comparisons and multivariate proportional hazards analyses to correlate survival measures with facility volume and various other patient-, tumor-, and treatment-related factors. First, we evaluated long-term survival for all variables using the Kaplan-Meier method with statistical comparisons based on the log-rank test. Multiple patient, tumor, and treatment characteristics were compared between the two facility-volume groups and then included them in the multivariate proportional hazards model. Of the 25,406 patients analyzed, 3310 were treated at high-volume centers (≥ 20 patients annually) and 22,096 were treated at low-volume centers. Patient demographics were generally not different between both patient cohorts, although patients treated at high-volume centers were more likely to have larger and higher grade tumors (64% versus 56% size ≥ 5 cm, 28% versus 14% undifferentiated grade, p < 0.001).
Results
When controlling for patient, tumor, and treatment characteristics in a multivariate proportional hazards analysis, patients treated at high-volume facilities had an overall lower risk of mortality than those treated at low-volume centers (hazard ratio, 0.81 [0.75-0.88], p < 0.001). Patients treated at high-volume centers were also less likely to have positive margins (odds ratio [OR], 0.59 [0.52-0.68], p < 0.001) and in patients who received radiation, those treated at high-volume centers were more likely to have radiation before surgery (40.5% versus 21.7%, p < 0.001); there was no difference in the type of surgery performed (resection versus amputation) (OR, 1.01 [0.84-1.23], p = 0.883).
Conclusions
With the largest patient cohort to date, this database review suggests that certain patients with soft tissue sarcoma of the extremities, particularly those with large high-grade tumors, may benefit from treatment at high-volume centers. Further investigation is necessary to help improve the referral of appropriate patients to high-volume sarcoma centers and to increase the treatment capacity of and access to such centers.
Level of Evidence
Level III, therapeutic study.
Introduction
Given that soft tissue sarcomas (STS) are relatively rare [1, 33], and in light of the fact that surgical resection is a key element in their treatment, there is debate about whether conducting surgery at a high-volume center is associated with improved survival. A number of studies have suggested that this may be the case in sarcoma, including one study examining patients with STS of any location [9] and another specifically examining retroperitoneal STS [5]. Similar trends have also been observed with other cancer types [2, 10, 17, 18, 30], including pancreatic [3, 7, 14, 16, 26, 29], colorectal [11, 12, 24, 25], thoracic [11, 20, 22], and breast surgeries [23]. In addition, studies have demonstrated that patients undergoing resection and treatment of pancreatic and esophageal cancer had improved overall survival if they traveled further distances to high-volume centers than those who stayed close to home to receive their treatment at a low-volume center [15, 30].
However, evidence is limited and inconclusive as to whether facility volume may be associated with improved survival in soft tissue sarcoma of the extremities (STS-E). In a subanalysis, Gutierrez et al. [9] examined the role of facility volume on survival in 1965 STS-E; after controlling for confounding variables, that study failed to demonstrate a survival benefit with treatment of extremity tumors at a high-volume center. Although another study reached the opposite conclusion, that work did not investigate the specific impact on STS-E [5], and neither of these studies [5, 9] investigated the interplay between distance traveled and facility volume on survival measures. As such, whether facility volume is associated with survival in STS-E remains unclear; what is more, there remains the question of whether patients with STS-E should travel to a high-volume center to receive their treatment rather than stay closer to receive treatment at a lower volume center.
We therefore sought to examine whether facility patient volume is associated with overall survival in patients with STS-E. In investigating this aim, we specifically sought to (1) examine differences in the treatment characteristics of high- and low-volume facilities; (2) estimate the 5-year survival by facility volume; and (3) examine the association between facility volume and of traveling a further distance to a high-volume center and overall survival when controlling for confounding factors.
Patients and Methods
The Duke University institutional review board approved this retrospective analysis of the National Cancer Database (NCDB) of patients diagnosed with STS-E from 1998 through 2012. The NCDB, a combined effort by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, includes > 70% of new cancer diagnoses every year from > 1500 accredited cancer programs in the United States [6]. This database is accessible by application and is available to any member of the Fellow of the American College of Surgeons; we accessed the NCDB Participant User File (PUF) for patients treated at NCDB-participating institutions and who had tumors in the extremities with International Classification of Diseases for Oncology, 3rd Edition topography codes C47.1 (peripheral nerves of the upper limb and shoulder), C47.2 (peripheral nerves of the lower limb and hip), C49.1 (soft tissues of the upper limb and shoulder), and C49.2 (soft tissues of the lower limb and hip).
Data from the NCDB were available to use from 1998 to 2012. All patients with primary, invasive STS-E were eligible for inclusion, including fibrosarcoma, liposarcoma, rhabdomyosarcoma, leiomyosarcoma, and synovial sarcoma. The volume of patients with STS-E at each facility was determined by the frequency of that facility within the NCDB data set. Patients were divided into two groups based on the average annual volume of patients treated at the facility from 1998 to 2012. Patient characteristics and overall survival were compared for those patients treated at facilities that recorded < 20 patients with STS-E annually relative to those patients treated at facilities recording ≥ 20 patients with STS-E annually. Exclusion criteria included benign tumors and patients < 18 years of age as well as entries that did not receive any treatment at the reporting facility (Class of case 00) or if endpoint time or status was not recorded before loss to followup. From the NCDB data set of 99,876 STS treated at a reporting facility between 1998 and 2012, our study criteria were met for 25,406 patients.
It should be noted that, although the NCDB does not report specific “followup,” for all qualifying patients who are alive and diagnosed within the last 5 years, the CoC accreditation standards require an annual followup rate of 90%. This patient followup is reported by the participating institutions to the NCDB annually [4, 6]. NCDB database records an endpoint as the time from diagnosis to either death or last known followup. Patients who were lost to followup before death were right-censored using the NCDB variable Vital Status, which documents whether the patient was alive or dead at last known followup. At 1 and 5 years, 924 and 6615 (4.2% and 30% of 22,096, respectively) low-volume facility patients had been lost to followup compared with 203 and 1066 (6.1% and 32% of 3310, respectively) high-volume facility patients.
We compared the following variables, as defined in the NCDB PUF data dictionary, between the groups: (1) patient characteristic variables: age, sex, race, Charlson Comorbidity Score, education level (based on the average education level in the zip code of the patient’s home), income (based on the average income level in the zip code of the patient’s home), crowfly distance (the distance from the zip code of the patient’s home to the facility), facility type (community cancer program, comprehensive community cancer program, or academic/research program), and insurance status (private insurance, Medicare, Medicaid, no insurance); (2) tumor characteristic variables: TNM stage, tumor size, histologic tumor type, and tumor grade; (3) treatment variables: surgery type (resection versus amputation), days from diagnosis to definitive surgery, whether the patient received radiotherapy, and surgical margins (negative or positive); and (4) endpoint/outcome variables: Kaplan-Meier survival and positive surgical margin status.
We compared distance traveled and facility volume with overall survival by comparing the patients in the lowest quartile of distance traveled (< 6 miles) to low-volume centers versus patients in the highest quartile of distance traveled (> 42 miles) to high-volume centers while controlling for confounding variables.
Statistical Analysis
Patients admitted to high- and low-volume facilities were assessed for differences in patient, tumor, and treatment characteristics using Pearson’s chi square test for categorical variables and two-tailed t-tests for continuous variables. Patient, tumor, and treatment variables to be included in the multivariate Cox proportional hazards model were first assessed in univariate Kaplan-Meier analysis, and those variables without evidence of nonproportionality were then considered for inclusion in the multivariate proportional hazards model. The functional form of continuous variables was also assessed for linearity and categorized if nonlinear. Variables that demonstrated evidence of nonproportionality and were not added to the multivariate proportional hazards model included: year of diagnosis, facility type, days from diagnosis to surgery, and use of radiation.
Multivariate proportional hazards analysis was used to identify patient, tumor, and treatment characteristics associated with increased mortality using backward selection at α = 0.1. No variables were removed in the selection process and hazard ratios were computed for all covariates with p values < 0.05 indicating statistical significance. Five-year survival estimates comparing low- and high-volume facilities were also obtained from Kaplan-Meier curves while stratifying across tumor grade, a significant predictor of mortality from the proportional hazards analysis that differed between facilities.
Another significant predictor of mortality, longer distance traveled to a treatment facility, is often associated with treatment at high-volume facilities. In another multivariate proportional hazards analysis including all the same patient, tumor, and treatment variables as possible confounders, patients in the lowest quartile of distance traveled for low-volume facilities (“close and low-volume”) were compared with those in the highest quartile of distance traveled for high-volume facilities (“far and high-volume”).
For comparing treatment characteristics, a Cochran-Mantel-Haenszel statistic was used to compute adjusted odds ratios and association statistics comparing the incidence of positive surgical margins, radiation use before or after surgery, and surgery types between low- and high-volume facilities. Positive surgical margin status was compared between high- and low-volume facilities while controlling for tumor grade, size, and surgery type. Radiation use was compared while controlling for tumor grade, size, and surgical margin status, and surgery type (resection versus amputation) was compared while controlling for tumor grade and size. All statistical analyses were performed using SAS/JMP (SAS Institute Inc, Cary, NC, USA).
Study Population
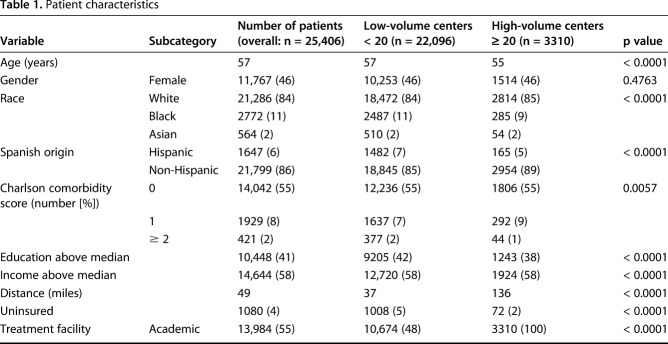
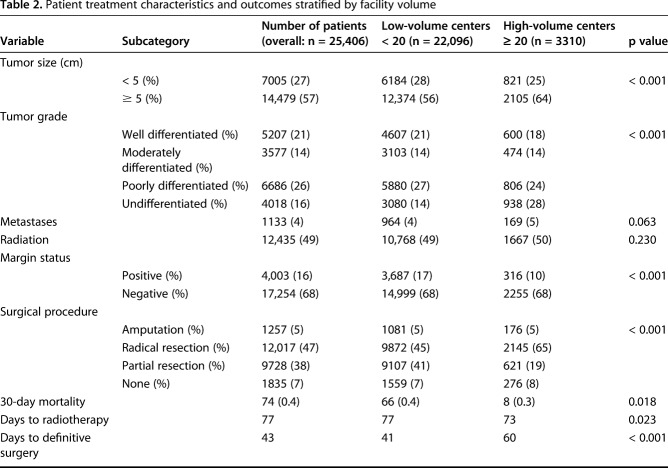
Facility volume over the study period ranged from one to 804 patients. Of the 25,406 patients analyzed, a total of 3310 patients were treated at nine high-volume centers (those that treated ≥ 20 STS-E patients annually), and 22,096 were treated at 1263 low-volume centers (those that treated < 20 patients per year) (Table 1). Patient demographics were generally similar within both patient cohorts, although more black and Hispanic patients received treatment at low-volume centers. Patients treated at high-volume centers tended to be from lower educational backgrounds and tended to travel farther for their treatment. Patients at low-volume centers were more likely to be uninsured. The tumor- and treatment-related characteristics differed between groups (Table 2). Patients treated at high-volume centers were more likely to have larger and higher grade tumors (64% versus 56% size ≥ 5 cm, 28% versus 14% undifferentiated grade, p < 0.001).
Table 1.
Patient characteristics
Table 2.
Patient treatment characteristics and outcomes stratified by facility volume
Results
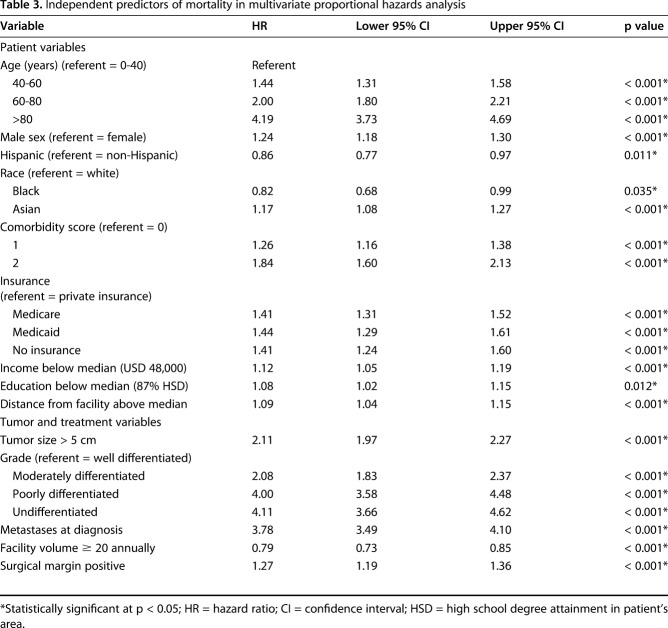
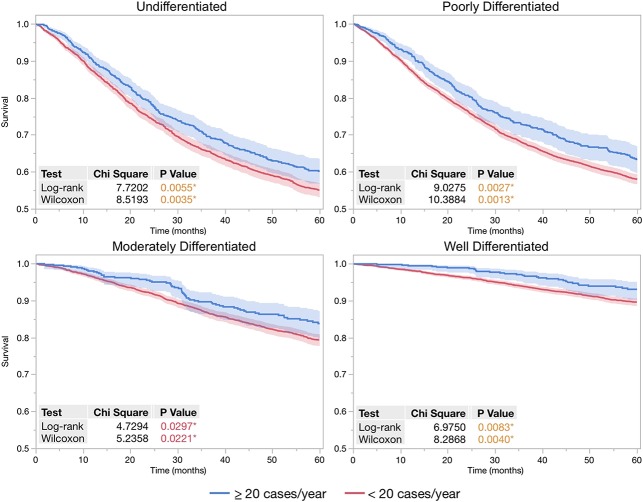
After controlling for potentially confounding variables such as tumor size, tumor grade, and treatment variables in multivariate proportional hazards analysis (Table 3), patients treated at high-volume centers had improved overall survival and a lower risk of mortality than those treated at low-volume centers (hazard ratio [HR], 0.79 [0.73-0.85], p < 0.001). Patients treated at high-volume facilities were more likely to have high-grade tumors, a strong predictor of mortality in the multivariate proportional hazards model. Adjusting for tumor grade, the Kaplan-Meier 5-year survival rates were higher for patients treated at high-volume facilities (Fig. 1). The relative survival benefit from treatment at a high-volume facility increased along with the increasing tumor grade, from a 3.5% survival difference (93.2% [90.4%-95.2%] versus 89.7% [88.6%-90.6%], log-rank p = 0.008) in patients with low-grade tumors to 4.6% (83.9% [79.8%-87.2%] versus 79.3 [77.7%-80.9%], log-rank p = 0.030) in those with intermediate-grade tumors and 5.5% (63.4% [59.7%-66.9%] versus 57.9% [56.6%-59.2%], log-rank p = 0.003) and 5.2% (60.1% [56.6%-63.5%] versus 54.9% [53.0-56.8%], log-rank p = 0.006) in those with poorly differentiated and undifferentiated high-grade tumors, respectively.
Table 3.
Independent predictors of mortality in multivariate proportional hazards analysis
Fig. 1.
When stratifying by tumor grades, 5-year Kaplan-Meier curves demonstrate patient survival was improved when treated at facilities with average patient volume ≥ 20 annually compared with lower volume facilities. Log-rank and Wilcoxon tests for survival difference were calculated for each set of Kaplan-Meier curves. Probability values < 0.05 were considered significant, indicated by an asterisk.
Despite traveling longer distances, patients who traveled to a high-volume center also had improved 5-year survival in a multivariate proportional hazards model in which facility volume and distance were combined, comparing the lowest quartile of distance traveled for low-volume facilities (“close and low-volume”) with the highest quartile of distance traveled for high-volume facilities (“far and high-volume”). While again controlling for all other patient, tumor, and treatment characteristics in a multivariate proportional hazards analysis, survivorship was greater in those patients who traveled the farthest distance to the high-volume facilities (HR, 0.80 [0.71-0.90], p < 0.001). In all, 6299 patients traveled < 6 miles to receive treatment at a low-volume center, whereas 1806 patients traveled > 42 miles to receive treatment at a high-volume center.
Among the treatment differences, those patients treated at high-volume centers were less likely to have positive margins (odds ratio [OR], 0.59 [0.52-0.68], p < 0.001) while controlling for tumor grade, size, and surgery type. Overall the use of radiation therapy was similar between high- and low-volume facilities (Table 1). In patients who received radiation, those treated at high-volume centers were more likely to have radiation before surgery (40.5% versus 21.7%, p < 0.001), even after controlling for tumor grade, size, and surgical margin status (OR, 1.62 [1.39-1.88], p < 0.001). There was no difference in the type of surgery performed (resection versus amputation) when controlling for tumor grade and size (OR, 1.01 [0.84-1.23], p = 0.883).
Discussion
For many cancer types, overall survival was improved when patients received management at treatment centers that encounter high numbers of patients annually [2, 3, 7, 10-12, 14, 16-18, 20, 22-26, 29, 30]; what is more, traveling to high-volume centers was associated with greater survivorship in some cancers [15, 30]. This correlation may be more important in less common malignancies such as sarcomas, in which proper identification and treatment are more challenging [19]. However, existing evidence is conflicted as to whether facility patient volume correlates with improved survival in STS [5, 9]. It has also been unclear previously whether there is a survival benefit to traveling to a high-volume center to receive treatment for sarcoma of the extremities. Using the largest sarcoma patient registry to date within the NCDB, our goal was to determine the association between facility patient volume and overall survival in patients with STS-E. We demonstrated that patients receiving treatment at higher volume centers (≥ 20 patients annually) had improved overall survival. When controlling for potentially confounding variables, patients treated at high-volume centers appeared to have lower rates of margin-positive surgery and higher utilization of neoadjuvant radiation therapy with no difference in type of surgery performed.
Our study is not without limitations. The first major limitation is that this is a retrospective database study. Although this database captured over 25,000 patients with STS-E, this certainly is not the entire population of such patients in the United States. As such, it may not be representative of STS-E as a whole. Additionally, given the retrospective nature of this study, it is possible that there are potential confounding variables for which we are unable to account. Nonetheless, our study did include variables such as grade, tumor size, and treatment characteristics that have been demonstrated in prospective trials to be the most prognostic factors for survival [28]. Additionally, we used advanced statistical methodology to control for potential confounding variables in our data set; as such, we feel that this large retrospective study is still informative. Second, although we identified discrepancies in margin status between high-volume centers and low-volume centers, we were unable to calculate recurrence-free survival, which has been intimately related to margin status [21, 31]. This points to another limitation of the NCDB: the inability to evaluate local recurrence or disease-free survival. Although we acknowledge this limitation, these comparisons were not a specific aim of our study and did not affect our ability to examine overall survival. Third, our study lost nearly 30% of patients to followup at 5 years. To account for those patients who were lost to followup over the 5-year period, we appropriately censored those patients in our multivariate proportional hazards model based on last known status; as such, we do not feel that this greatly impacts our results or our overall message. Fourth, although we can identify general trends in patient characteristics and overall survival, we do not have specific patient or facility information. As such, we cannot identify the reasons why certain patients might seek out care at high-volume centers as opposed to low-volume centers. Similarly, we cannot say definitively that fellowship-trained orthopaedic oncologists were preferentially responsible for extremity resections at high-volume centers.
Another potential limitation was the decision to dichotomize volume (to < 20 versus ≥ 20 patients per year per center) rather than analyze it as a continuous variable. We did this for several reasons. First, facility volume cannot be used as a continuous variable in a proportional hazards model because of its nonlinear functional form. Although one option is to create a logarithmic or polynomial transformation of the facility volume variable, these can be difficult to interpret compared with discretization. Categorizing such a continuous variable is often used in such cases and is easier for clinicians to interpret. More importantly, we believe this approach has face validity; it seems reasonable to believe that there could be a threshold experience/volume to achieve improved survivorship. The cutoff of 20 annually is indeed somewhat arbitrary but reflects a few clinical and statistical factors, including what clinicians might view as a reasonably high cutoff to be considered a “high-volume” center but not too high that the number of entries in the database would be severely limited and the approximate inflection of the functional form of the data set (< 20 have a higher-than-predicted hazard rate, ≥ 20 hazard rate begins to decrease to the below-predicted hazard rate). Our findings presented are robust to changing of the high-volume threshold as well as increased categorization (ie, > 15 versus < 15 significant; > 20 annually was significant compared with < 10 and 10-20; however, the latter two categories were not significantly different, as we might expect, based on the functional form and whether there is a threshold effect of approximately 20). One might reasonably be concerned that by dichotomizing, unrealistic conclusions could be drawn such as whether there really is a difference between a center that took care of 19 or 21 patients per year; we believe that small differences in volume like that around the cutoff point probably in fact would be not very different in terms of survivorship or other endpoints. However, as a result of the skewed distribution, the < 20/year group was dominated by the low-volume facilities (median approximately 4/year) as expected.
Two studies have previously examined the role of facility volume in STS. Gutierrez et al. [9] and Bonvalot et al. [5] both demonstrated improved overall survival in patients diagnosed with STS who were treated at high-volume centers. However, these two studies did not focus on the specific role of facility volume in overall survival of patients with STS-E. Gutierrez et al. [9] retrospectively identified 4205 patients with STS in a large longitudinally maintained population database, the Florida Cancer Data System; only 1965 of these patients had a diagnosis of STS-E. In their study, 61.7% of patients treated at high-volume centers had STS-E as opposed to 39.6% of patients at low-volume centers; this was a possible confounding factor because it is well established that sarcoma body site impacts overall survival [8, 32]. Indeed, despite showing a survival difference in univariate analyses, the investigators did not find high-volume centers to be independently associated with an increase in survival after controlling for relevant confounding variables. The retrospective study by Bonvalot et al. [5] included 382 patients collected from participating institutions. Although this study did not specifically examine the extremities, it did demonstrate a survival benefit when patients were treated at high-volume centers. Although these studies offer novel contributions, our study has the advantage over both because we used the NCDB to specifically examine STS-E. This database collects contributions from nearly 1500 accredited CoC institutions, represents 70% of cancer diagnoses in the United States every year, and includes important details regarding patient-specific factors and tumor and characteristics.
Our analysis identified several important differences between patients treated at low-volume centers and high-volume centers. High-volume centers tended to treat patients with higher grade and larger tumors than patients treated at low-volume centers. Our findings are similar to those of Gutierrez et al. [9], who also demonstrated that, among patients with STS, high-volume center patients tended to have larger and higher grade tumors. Despite this, patients treated at high-volume centers were more likely to have surgery with a negative margin than those treated at low-volume centers. Additionally, patients treated at high-volume centers were more likely to receive neoadjuvant radiotherapy. Despite this, we found no difference in the overall rate of utilization of radiation therapy. This is in contrast to the cohort from Gutierrez et al. [9], who demonstrated double the proportion of radiotherapy use at high-volume centers. This is notable as we have previously identified inadequate use of radiation therapy in the United States when treating intermediate- and high-grade soft tissue sarcoma [13] and may be an area of further investigation moving forward. This discrepancy could be explained by the inclusion in their study of sarcomas of all locations. Importantly, our study demonstrated improved rates of margin-negative surgery at high-volume centers; Gutierrez et al. [9] included no specific analysis of margin status in their study. Another important difference between the two studies is that, in Gutierrez et al. [9], a high-volume center was defined as a facility averaging five to 24 patients per year, whereas low-volume centers averaged only one to four patients per year. We took a more conservative approach with our definition of a high-volume center, defining such institutions as averaging ≥ 20 patients annually, which may account for the differences seen in our results. It is our view that a high-volume facility accustomed to dealing with sarcoma would require more than five patients per year.
Closer distance of facility and higher sarcoma volume of facility both appeared to improve survival in multivariate analysis; however, in life, these qualities do not always coincide with many rural or local hospitals having a lower volume of patients with sarcomas as a result of either the lower population or number of specialists, but perhaps having improved followup and monitoring. We carried out an additional subanalysis, comparing the patients traveling shorter distances (< 5.6 miles, lowest quartile) to low-volume centers with those traveling farther distances (> 41.7 miles, highest quartile) to high-volume centers and stratified independent survival benefit by tumor grade. Across all grades, survival was improved by traveling to a high-volume center; this survival benefit became more pronounced for higher grade tumors. These findings correlate with results of prior studies in other rare cancers such as esophageal cancer and pancreatic cancer. Lidsky et al. demonstrated that patients traveling to high-volume centers for treatment of their pancreatic cancer had better short- and long-term survival overall [15]. In addition, Speicher et al. [30] found that patients traveling to high-volume centers for their esophagectomy for esophageal cancer had improved survival compared with patients who stayed close to home to receive treatment at low-volume centers. Our findings seem to corroborate those of other cancers; not only do high-volume centers have improved survival rates overall, but patients traveling to receive treatment at high-volume centers have improved survival as compared with those staying close to home to receive treatment.
It is important to note that one finding in our article was that there were only nine high-volume centers treating 3310 patients over the time period; in contrast, 22,000 patients were treated by low-volume centers. Even if all the patients were willing to travel, which is both unlikely and impractical, we acknowledge the difficulty of caring for so many patients at so few sites. Although we are not suggesting that all patients be treated at high-volume centers, our analysis can still help to inform decision-making regarding appropriate referral. Established risk factors for a soft tissue mass being a sarcoma include size > 5 cm, location deep to fascia, and a heterogeneous appearance on MRI [21, 27]. It is noteworthy that two of these red flags—size > 5 cm and location deep to fascia—are risk factors from death resulting from STS [21, 27]. In our analysis, when controlling for confounding factors, size was associated with worse survival. Although our data set did not specifically include data about tumor depth, it has been previously demonstrated that tumor depth is associated with a higher rate of margin-positive surgery [31]. Our analysis found that after controlling for confounding factors, margin status was associated with worse survival and that low-volume centers were more likely to have margin-positive surgery. In a similar manner, it also well established that grade is an important driver of survival in the treatment of STS [21, 27]. Our analysis corroborates this finding, because high-grade tumors had the largest association with worse survival. Given these findings, we suggest that all large, deep soft tissue masses, or all STS-E confirmed to be high grade, be referred to a high-volume center.
With the largest patient cohort to date, this database review suggests that patients with STS-E, particularly those with large, high-grade tumors, may benefit from treatment at high-volume centers. Patients receiving treatment at high-volume centers had lower rates of margin-positive surgery and improved survival. Further investigation is necessary to help improve the referral of appropriate patients to high-volume sarcoma centers and to increase the treatment capacity of and access to such centers.
Acknowledgments
For their assistance with the preparation of the manuscript, we thank Sadie Hawkins and Virgil Leonard. The data used in this study are derived from a deidentified NCDB file. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Footnotes
Each author certifies that neither he nor any member of his immediate family has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Duke University Medical Center, Durham, NC, USA.
References
- 1.Adigun IA, Rahman GA. A review of soft tissue sarcoma. Niger J Med. 2007;16:94–101. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 4.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP. Using the National Cancer Database for outcomes research: A Review. JAMA Oncol. 2017;3:1722–1728. [DOI] [PubMed] [Google Scholar]
- 5.Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, Laplanche A. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. [DOI] [PubMed] [Google Scholar]
- 6.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. [DOI] [PubMed] [Google Scholar]
- 7.Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med. 1996;165:294–300. [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez JC, Perez EA, Franceschi D, Moffat FL, Jr, Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–114. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 11.Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6–15. [DOI] [PubMed] [Google Scholar]
- 12.Harmon JW, Tang DG, Gordon TA, Bowman HM, Choti MA, Kaufman HS, Bender JS, Duncan MD, Magnuson TH, Lillemoe KD, Cameron JL. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou CH, Lazarides AL, Speicher PJ, Nussbaum DP, Blazer DG, 3rd, Kirsch DG, Brigman BE, Eward WC. The use of radiation therapy in localized high-grade soft tissue sarcoma and potential impact on survival. Ann Surg Oncol. 2015;22:2831–2838. [DOI] [PubMed] [Google Scholar]
- 14.Imperato PJ, Nenner RP, Starr HA, Will TO, Rosenberg CR, Dearie MB. The effects of regionalization on clinical outcomes for a high risk surgical procedure: a study of the Whipple procedure in New York State. Am J Med Qual. 1996;11:193–197. [DOI] [PubMed] [Google Scholar]
- 15.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG., 3rd Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. 2017;266:333–338. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luft HS. The relation between surgical volume and mortality: an exploration of causal factors and alternative models. Med Care. 1980;18:940–959. [DOI] [PubMed] [Google Scholar]
- 18.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. [DOI] [PubMed] [Google Scholar]
- 19.Noria S, Davis A, Kandel R, Levesque J, O'Sullivan B, Wunder J, Bell R. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–655. [DOI] [PubMed] [Google Scholar]
- 20.Patti MG, Corvera CU, Glasgow RE, Way LW. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–192. [DOI] [PubMed] [Google Scholar]
- 21.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 22.Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest. 1992;101:1332–1337. [DOI] [PubMed] [Google Scholar]
- 23.Roohan PJ, Bickell NA, Baptiste MS, Therriault GD, Ferrara EP, Siu AL. Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 25.Simons AJ, Ker R, Groshen S, Gee C, Anthone GJ, Ortega AE, Vukasin P, Ross RK, Beart RW., Jr Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum. 1997;40:641–646. [DOI] [PubMed] [Google Scholar]
- 26.Simunovic M, To T, Theriault M, Langer B. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ. 1999;160:643–648. [PMC free article] [PubMed] [Google Scholar]
- 27.Singer S, Corson JM, Gonin R, Labow B, Eberlein TJ. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skubitz KM, D'Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82:1409–1432. [DOI] [PubMed] [Google Scholar]
- 29.Sosa JA, Bowman HM, Gordon TA, Bass EB, Yeo CJ, Lillemoe KD, Pitt HA, Tielsch JM, Cameron JL. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speicher PJ, Englum BR, Ganapathi AM, Wang X, Hartwig MG, D'Amico TA, Berry MF. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer. Ann Surg. 2017;265:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–2543. [DOI] [PubMed] [Google Scholar]
- 33.Zahm SH, Fraumeni JF., Jr The epidemiology of soft tissue sarcoma. Semin Oncol. 1997;24:504–514. [PubMed] [Google Scholar]