Abstract
Background
Ginseng is a traditional herbal medicine for human health. Ginseng contains a bioactive ligand named gintonin. The active ingredient of gintonin is lysophosphatidic acid C18:2 (LPA C18:2). We previously developed a method for gintonin-enriched fraction (GEF) preparation to mass-produce gintonin from ginseng. However, previous studies did not show the presence of other bioactive lipids besides LPAs. The aim of this study was to quantify the fatty acids, lysophospholipids (LPLs), and phospholipids (PLs) besides LPAs in GEF.
Methods
We prepared GEF from white ginseng. We used gas chromatography-mass spectrometry for fatty acid analysis and liquid chromatography-tandem mass spectrometry for PL analysis, and quantified the fatty acids, LPLs, and PLs in GEF using respective standards. We examined the effect of GEF on insulin secretion in INS-1 cells.
Results
GEF contains about 7.5% linoleic (C18:2), 2.8% palmitic (C16:0), and 1.5% oleic acids (C18:1). GEF contains about 0.2% LPA C18:2, 0.06% LPA C16:0, and 0.02% LPA C18:1. GEF contains 0.08% lysophosphatidylcholine, 0.03% lysophosphatidylethanolamine, and 0.13% lysophosphatidylinositols. GEF also contains about 1% phosphatidic acid (PA) 16:0-18:2, 0.5% PA 18:2-18:2, and 0.2% PA 16:0-18:1. GEF-mediated insulin secretion was not blocked by LPA receptor antagonist.
Conclusion
We determined four characteristics of GEF through lipid analysis and insulin secretion. First, GEF contains a large amount of linoleic acid (C18:2), PA 16:0-18:2, and LPA C18:2 compared with other lipids. Second, the main fatty acid component of LPLs and PLs is linoleic acid (C18:2). Third, GEF stimulates insulin secretion not through LPA receptors. Finally, GEF contains bioactive lipids besides LPAs.
Keywords: Ginseng, Gintonin, Insulin, LPA, Phosphatidic acid
1. Introduction
Ginseng, the root of Panax ginseng Meyer, has been used as a tonic for human health [1]. Ginseng contains diverse bioactive medicinal components. Ginsenosides were the first ingredients of ginseng to be isolated. Ginsenosides are glycosides derived from triterpenoid dammaranes with 30 carbon atoms. Each ginsenoside has a common hydrophobic four-ring steroid-like backbone and has carbohydrate moieties at the carbon-3, carbon-6, or carbon-20 positions [2]. About 30 ginsenosides have been isolated from ginseng by changing the processing conditions such as pressure and temperature. Ginsenosides exhibit a variety of in vitro and in vivo pharmacological effects in biological systems [3], [4]. Acidic polysaccharides were identified next. They consist of a complex polymer chain of monosaccharides rich in l-arabinose, d-galactose, l-rhamnose, d-galacturonic acid, d-glucuronic acid, and d-galactosyl residue linked together through glycosidic bonds, resulting in complex macromolecular architectures [5]. Although the structures of acidic polysaccharides are not clearly understood, it is known that they regulate immune systems [5].
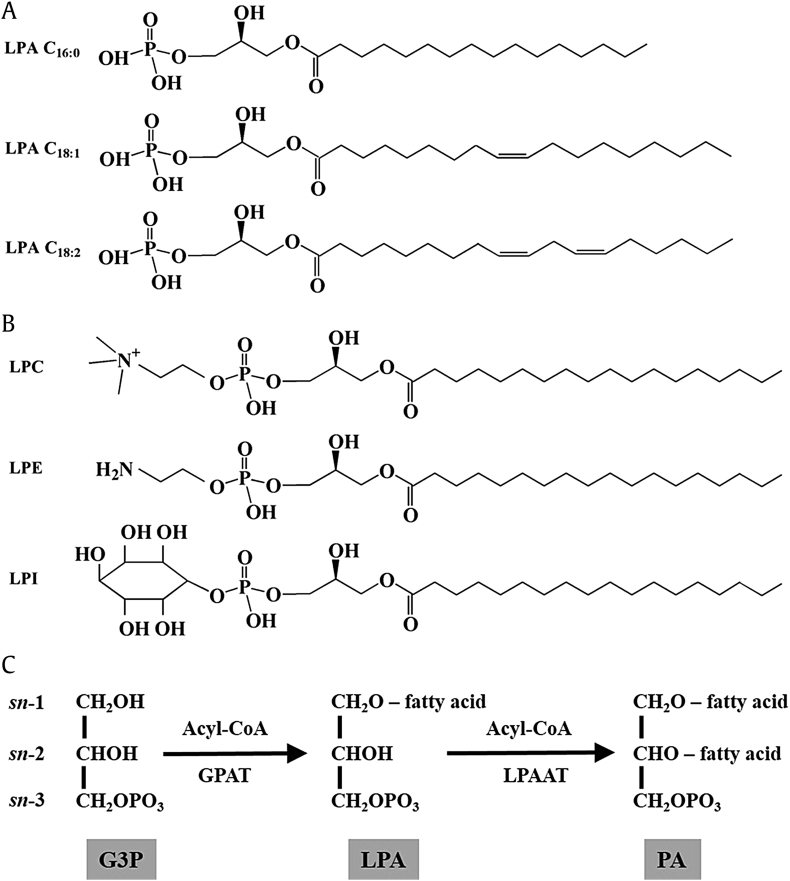
Recent studies found that ginseng also contains a novel phospholipid (PL) complex with ginseng proteins such as ginseng major latex-like protein 151, also called gintonin. Further study revealed that the functional component of gintonin is lysophosphatidic acid (LPA, 1-acyl-2-hydroxy-sn-glycero-3-phosphate) and that gintonin functions as an exogenous ginseng-derived G protein-coupled LPA receptor ligand [6], [7]. Thus, gintonin is distinguished from previously identified ginseng saponins and acidic polysaccharides, because the representative functional component is ginseng-derived lipid ligand [7]. LPA is a simple lysophospholipid (LPL; Fig. 1A) composed of glycerol 3-phosphate (G3P), fatty acids, and phosphates (Fig. 1) [8]. LPA is mainly synthesized in plants by glycerol 3-phosphate acyltransferase (GPAT) transferring fatty acids such as linoleic (C18:2), palmitic (C16:1), or oleic acid (C18:1) to the sn-1 or sn-2 positions of G3P [8] (Fig. 1C). In biological systems, LPA also plays an intermediate role in syntheses of phosphatidic acid (PA) and other PLs [8] (Fig. 1B, 1C). Interestingly, ginseng contains an extraordinarily high concentration of LPAs, especially LPA C18:2, about 10-fold greater than that in other herbal medicines and foodstuffs [9]. We previously developed a simple method for mass production of gintonin-enriched fraction (GEF) from ginseng using ethanol and water, and demonstrated that GEF exhibits various in vitro and in vivo physiological and pharmacological effects [10], [11], [12], [13], [14]. However, although we obtained qualitative evidence that GEF contains other lipid components along with LPAs [10], until now these bioactive lipids related to LPAs and other PL components of GEF have not been identified and quantified.
Fig. 1.
Structure of LPAs and LPLs and schematic diagrams of LPA and PA synthesis in ginseng. (A and B) Chemical structures of LPAs and representative LPLs (LPC, LPE, and LPI). (C) Synthesis of LPA and PA. GPAT catalyzes the esterification of a fatty acid moiety from acyl-CoA or acyl-ACP to the sn-1 position of G3P to form LPA. GPAT catalyzes the esterification of a fatty acid moiety from acyl-CoA to the sn-1 position of G3P to form LPA. LPAAT catalyzes the esterification of a fatty acid moiety from acyl-CoA to the sn-2 position of G3P to form LPA.
G3P, glycerol 3-phosphate; GPAT, glycerol 3-phosphate acyltransferase; LPA, lysophosphatidic acid; LPAAT, LPA acyltransferase; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; LPL, lysophospholipid; PA, phosphatidic acid.
We analyzed representative bioactive fatty acids, LPLs, and PLs in GEF using gas chromatography-mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). Here, we report that GEF contains bioactive lipid components closely related to LPAs. The main fatty acid compositions of GEF, in quantitative order, are linoleic (C18:2), palmitic (C16:0), and oleic (C18:1) acids. The main PL components of GEF, in quantitative order, are PAs, LPAs, lysophosphatidylinositol (LPI), lysophosphatidylcholine (LPC), and trace amounts of other LPLs. In experiments using non-neuronal cells, we also found that GEF contains other active ingredient for insulin secretion besides LPAs. The present report also discusses the possible contributions of fatty acids, PLs, and LPLs in GEF-mediated physiological and pharmacological roles in biological systems.
2. Materials and methods
2.1. Materials
Four-year-old Korean White ginseng was purchased from a local ginseng market. 1-Myristoyl-2-hydroxy-sn-glycero-3-phosphate (LPA C14:0), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphate (LPA C16:0), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphate (LPA C18:0), 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate (LPA C18:1), 1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC C14:0), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (lysophosphatidylethanolamine [LPE] C16:0), 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (LPE C18:1), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoinositol (LPI C16:0), 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1′-myo-inositol; LPI C18:1), 1,2-dimyristoyl-sn-glycero-3-phosphate (PA 14:0-14:0), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (PA 16:0-18:1), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphate (PA 16:0-18:2), 1,2-dilinoleoyl-sn-glycero-3-phosphate (PA 18:2-18:2), 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine (phosphatidylcholine [PC] 14:0-18:0), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PC 16:0-18:2), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (PC 18:2-18:2), and 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-myo-inositol; phosphatidylinositol [PI] 16:0-16:0) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). 1-Linoleoyl-2-hydroxy-sn-glycero-3-phosphate (LPA C18:2) was purchased from Echelon (Salt Lake City, UT, USA). 1-Linoleoyl-2-hydroxy-sn-glycero-3-phosphatidyicholine (LPC C18:2) was purchased from Larodan (Solna, Sweden). Fatty acids and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Insulin assay enzyme-linked immunosorbent assay (ELISA) kit was purchased from Shibayagi Co. Ltd. (Shibukawa, Gunma, Japan). RPMI 1640 Medium and all other materials for cell culture were purchased from ThermoFisher Scientific Korea (Gangnam-gu, Seoul, South Korea). Ki16425 were purchased from Cayman Chemicals (Ann Arbor, MI, USA). All other reagents used were purchased from Sigma-Aldrich.
2.2. Preparation of GEF
GEF was prepared according to a previously described method [10]. Briefly, 1 kg of 4-year-old ginseng was ground into small pieces (>3 mm) and refluxed with 70% fermentation ethanol eight times for 8 h at 80°C each. The ethanol extracts (350 g) were concentrated. Ethanol extract was dissolved in distilled cold water at a ratio of 1–10 and stored in a cold chamber at 4°C for 24 h. The supernatant and precipitate produced by water fractionation, after the ethanol extraction of ginseng, was separated by centrifuge (3000 rpm, 20 min). The precipitate was lyophilized after being centrifuged. This fraction was designated GEF and had a yield of 1.3%.
2.3. Quantitation of free fatty acids in GEF using GC-MS
Free fatty acid (FFA) analysis using GC-MS was performed at the Korea Basic Science Institute (Western Seoul Center). For GC-MS analysis of FFAs, samples were saponified then methylated. A 5-mg GEF sample in a screw-capped glass tube was hydrolyzed with 1 mL 1 M KOH in 70% ethanol at 90°C for 1 h then cooled down. Four milliliters of 10% boron triflouride (BF3) in methanol was added and derivatized at 60°C for 20 min for methylation. The reaction mixture was allowed to cool to room temperature, then fatty acid methyl esters (FAMEs) were extracted with 2 mL hexane. For phase separation, 1 mL water was added, the sample was shaken well, and the upper layer of hexane was collected through sodium sulfate filtration [15]. The FAMEs in the GEF samples were determined using a 7890B GC, equipped with a 5977A mass selective detector quadrupole mass spectrometer system (Agilent Technologies, Palo Alto, CA, USA). The DB-5 MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness, 5% diphenyl-95% dimethylsiloxane phase; J&W Scientific (Folsom, CA, USA) was used for separating the FAMEs using helium gas as a carrier at a flow rate of 1 mL/min. The GC oven temperature was maintained at 50°C for 1 min, and then ramped to 320°C at 5°C per min, then maintained for 10 min. Samples of 1 μL volume were injected with a 2:1 split ratio. The temperatures of the GC injection port and MS interface were set at 280°C and 300°C, respectively. The mass selective detector was run in electron impact mode, with an electron energy at 70 eV. The mass spectrometer was operated in full scan mode between 50 and 700 amu. Individual FAMEs were identified by the processes of peak separation and consultation with the Wiley 7n electron impact mass spectral library and comparing their retention times with authentic standards (Supelco, 37 Component FAME Mix certified reference material).
2.4. Quantitation of LPLs and PLs in GEF using LC-MS/MS
Stock solutions of LPLs and PLs were prepared and diluted in high-pressure liquid chromatography (HPLC)–grade methanol. All solutions were stored at 4°C. LPLs and PLs were quantified by LC-MS/MS using an Agilent series 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA) instrument, which consisted of a G1311A quart pump, G1313A autosampler, G1322A degasser, G1316A column oven, and an API 2000 LC-MS/MS system (Applied Biosystems, Foster City, CA, USA) [16]. Tandem mass spectrometric (MS/MS) analysis was carried out using an electrospray ion source in positive (LPC, PC) and negative (LPA, LPE, LPI, PA, PI) modes. Data acquisition was performed in multiple reaction monitoring (MRM) mode and SCIEX Analyst 1.4.2 software (ABI Inc., Foster City, CA, USA) was used for data management and control. Chromatographic separations of LPA, LPC, LPE, and LPI were performed on a XBridge C18 analytical column (2.0 × 100 mm, 3.5 μm particle size; Waters Corporation, Milford, MA, USA; Fig. 2). The lipids for PA, PC, PI were separated on a XBridge Hydrophilic Interaction analytical column (2.1 × 100 mm, 3.5 μm particle size; Waters Corporation; Fig. 3). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid and 10 mM ammonium formate in water (B). The isocratic pump mode was run at a flow rate of 0.2–0.25 mL/min and 2-μL aliquots were injected into the column. The column temperature was maintained at 25°C. The ion spray voltage was set at −3.5 to −4.5 kV in negative ion mode and 5.5 kV in positive ion mode. The capillary temperature was set at 400–450°C. The operating conditions, optimized by a flow injection of all analytes, were as follows: nebulizing (GS1), auxiliary (GS2), and curtain gas flows of 30.0 to 50.0, 70.0, and 10 to 15 PSI, respectively. The MRM transitions of the analytes, the collision energy, and declustering potential for lipids are summarized in Table 1. Quantitation was performed via MRM of the precursor ions and the related product ions using an internal standard method with peak area ratios.
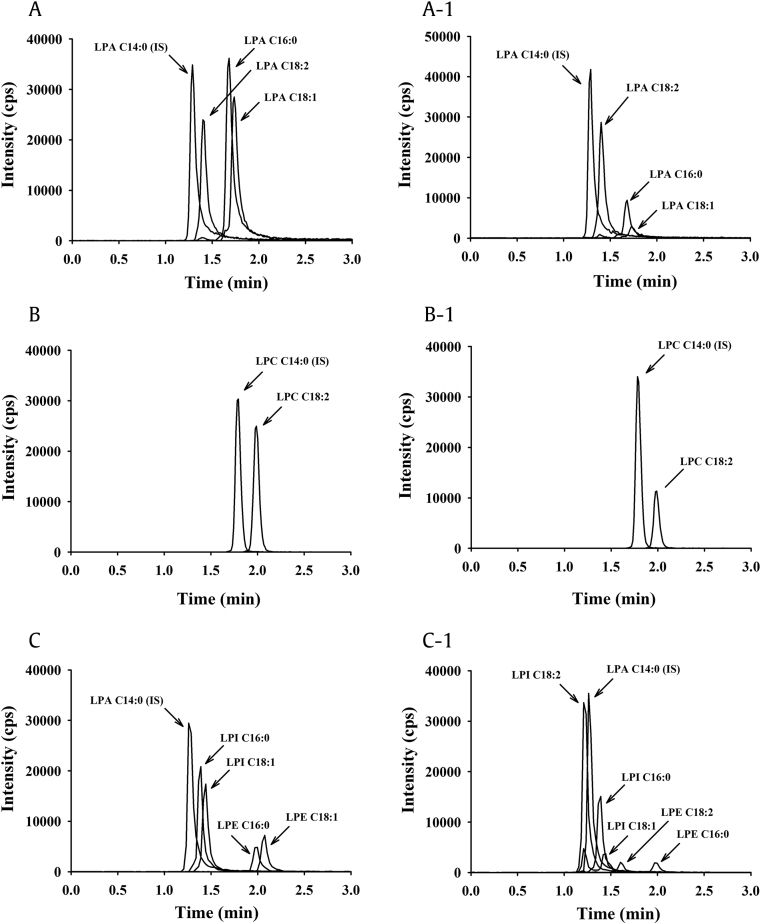
Fig. 2.
Representative chromatogram of LPLs in GEF using LC-MS/MS. (A) Simultaneous chromatograms of a standard mixture of LPAs and (A-1) the LPAs in GEF. (B) Simultaneous chromatograms of LPC standard and (B-1) the LPC in GEF. (C) Simultaneous chromatograms of standard mixture of LPEs and LPIs and (C-1) the LPEs and LPIs in GEF. Details of the methods of LPL analysis are given in the “Materials and Methods” section.
GEF, gintonin-enriched fraction; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; LPL, lysophospholipid.
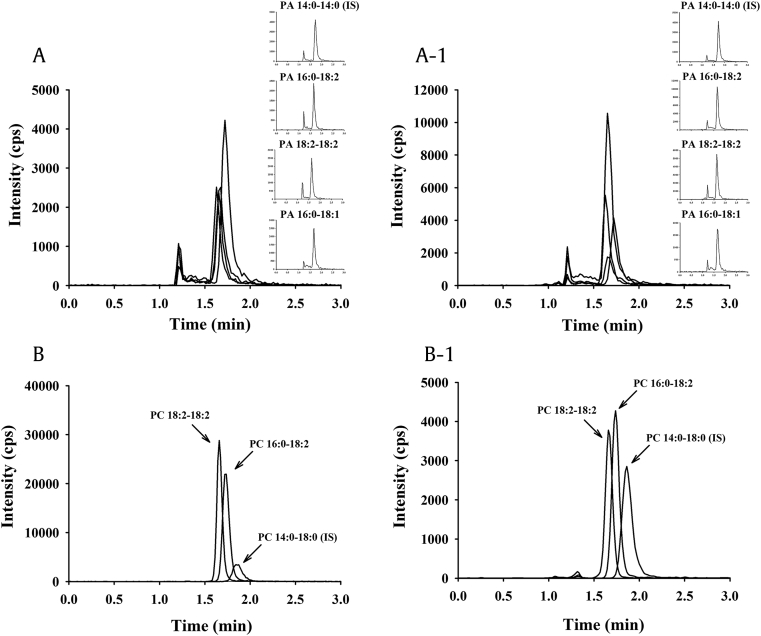
Fig. 3.
Representative chromatogram of PLs using LC-MS/MS in GEF. (A) Simultaneous chromatograms of standard mixture of PAs and (A-1) the PAs in GEF. (B) Simultaneous chromatograms of standard mixture of PCs and (B-1) the PCs in GEF. Details of the methods of PL analysis are given in the “Materials and Methods” section.
GEF, gintonin-enriched fraction; LC-MS/MS, liquid chromatography-tandem mass spectrometry; PA, phosphatidic acid; PC, phosphatidylcholine; PL, phospholipid.
Table 1.
LC-MS/MS operating conditions to analyze various LPLs and PLs
| Analyte | Mode | MS/MS |
LC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Precursor ion (m/z) | Product ion (m/z) | DP (v) | CE (v) | Mobile phase (A/B, v/v) |
Column | Flow rate (mL/min) | |||
| A | B | ||||||||
| LPA C18:2 | Neg | 433 | 79 | −46 | −106 | 85 | 15 | C18 | 0.25 |
| LPA C16:0 | Neg | 409 | 79 | −61 | −86 | ||||
| LPA C18:1 | Neg | 435 | 79 | −61 | −72 | ||||
| LPA C14:0 (IS) | Neg | 381 | 79 | −66 | −82 | ||||
| LPC C18:2 | Pos | 520 | 104 | 76 | 45 | 85 | 15 | C18 | 0.2 |
| LPC C14:0 (IS) | Pos | 468 | 104 | 81 | 37 | ||||
| LPE C18:2 | Neg | 476 | 79 | −61 | −112 | 85 | 15 | C18 | 0.25 |
| LPE C16:0 | Neg | 452 | 79 | −111 | −108 | ||||
| LPE C18:1 | Neg | 478 | 79 | −61 | −112 | ||||
| LPI C18:2 | Neg | 595 | 79 | −86 | −114 | ||||
| LPI C16:0 | Neg | 571 | 79 | −101 | −124 | ||||
| LPI C18:1 | Neg | 597 | 79 | −86 | −114 | ||||
| LPA C14:0 (IS) | Neg | 381 | 79 | −66 | −82 | ||||
| PA 16:0-18:2 | Neg | 671 | 79 | −121 | −130 | 90 | 10 | HILIC | 0.25 |
| PA 18:2-18:2 | Neg | 695 | 79 | −96 | −128 | ||||
| PA 16:0-18:1 | Neg | 674 | 79 | −76 | −128 | ||||
| PA 14:0-14:0 (IS) | Neg | 591 | 227 | −86 | −44 | ||||
| PC 18:2-18:2 | Pos | 782 | 184 | 116 | 49 | 80 | 20 | HILIC | 0.25 |
| PC 16:0-18:2 | Pos | 758 | 184 | 116 | 45 | ||||
| PC 14:0-18:0 (IS) | Pos | 735 | 184 | 136 | 37 | ||||
| PI 18:2-18:2 | Neg | 857 | 79 | −141 | −122 | 60 | 40 | HILIC | 0.25 |
| PI 16:0-16:0 | Neg | 810 | 79 | −141 | −122 | ||||
| PA 14:0-14:0 (IS) | Neg | 591 | 227 | −86 | −44 | ||||
CE, collision energy; DP, declustering potential; HILIC, Hydrophilic Interaction; IS: internal standard; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; LPL, lysophospholipid; PA, phosphatidic acid; PC, phosphatidylcholine; PI, phosphatidylinositol; PL, phospholipid.
A 100-mg GEF sample in a 50-mL centrifuge tube was dissolved with 10 mL water and vortex-mixed. One milliliter of the middle layer was transferred to a new centrifuge tube and diluted with methanol. The mixture was vortexed and then filtered through a 0.2-μm syringe filter (Millex-LG; Merck Millipore Corporation, Merck KGaA, Darmstadt, Germany). A volume of 2 μL was injected for LC-MS/MS analysis.
2.5. Cell culture
Rat beta cells INS-1 were kindly gifted from Prof. Yup Kang (Ajou University School of Medicine, Suwon, South Korea) and cultured in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 100 units/mL penicillin, and 100 μg/mL streptomycin.
2.6. Insulin secretion from INS-1 cells
Insulin secretion from INS-1 cells was determined using the ELISA. Briefly, INS-1 cells were seeded at 3 × 105 cells per well into 24-well plates. After 24 h, cells were washed with Krebs-Ringer bicarbonate (KRB) buffer (24 mM NaHCO3, 1.2 mM MgCl2, 1 mM HEPES, 129 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 2.5 mM CaCl2, 0.2 mM glucose, pH 7.4), and then preincubated in KRB buffer for 1 h [17]. The cells were incubated with gintonin, ginsenosides, or LPA at indicated concentrations for 2 h. In some experiments, Ki16425 was also added before gintonin treatment. Supernatants were collected and insulin concentration of each sample was determined using rat insulin ELISA kit according to the manufacturer's instruction (Shibayagi).
2.7. Data analysis
The calibration curves were evaluated via weighted linear (1/x) and quadratic (1/x2) regression with their correlation coefficients (R ≥ 0.95). The linearity of each calibration curve was determined by peak area analyte/peak area internal standard at six concentration levels of individual analytes. All values are presented as mean ± relative standard deviation (%) from three different samples of white ginseng.
3. Results
3.1. Quantitation of the representative fatty acids in GEF using GC-MS
In previous reports, we showed that GEF from ginseng contains about 20% lipids. We confirmed through qualitative analysis that gintonin and GEF contain three representative fatty acids: linoleic (C18:2), palmitic (C16:0), and oleic acids (C18:1), and other minors [6]. In the present study, we performed quantitative analysis to determine the concentrations of major FFAs in GEF. As shown in Table 2, we found that GEF contains a large amount (7.53%) of linoleic acid (C18:2) compared with two other fatty acids, palmitic (C16:0; 2.82%) and oleic acids (C18:1; 1.46%). This quantitative assay of fatty acids showed that GEF contains about 12% fatty acids and that the primary FFA in GEF is linoleic acid (C18:2).
Table 2.
Amounts of lipids in GEF
| Analyte | Calibration equation | R | Amount (mg/g) | Content (%) |
|---|---|---|---|---|
| Linoleic acid | y = 0.0156x − 0.4116 | 0.9929 (R2) | 75.28 ± 2.5 | 7.53 |
| Palmitic acid | y = 0.017x − 0.1685 | 0.9961 (R2) | 28.18 ± 0.8 | 2.82 |
| Oleic acid | y = 0.0163x − 0.4502 | 0.9910 (R2) | 14.64 ± 0.3 | 1.46 |
| LPA C18:2 | y = 1.38x + 0.00283 | 0.9995 | 2.01 ± 5.61 | 0.20 |
| LPA C16:0 | y = 2.25x − 0.00383 | 0.9999 | 0.57 ± 3.04 | 0.06 |
| LPA C18:1 | y = 1.83x − 0.000639 | 0.9994 | 0.19 ± 0.42 | 0.02 |
| LPC C18:2 | y = 1.81x − 0.0055 | 0.9999 | 0.82 ± 4.39 | 0.082 |
| LPE C18:21) | LPE 18:1 | — | 0.12 ± 8.56 | 0.012 |
| LPE C16:0 | y = 0.358x − 0.00058 | 0.9993 | 0.19 ± 2.22 | 0.019 |
| LPE C18:1 | y = 0.472x + 0.00063 | 0.9993 | BSL | BSL |
| LPI C18:21) | LPI 18:1 | — | 0.93 ± 3.01 | 0.093 |
| LPI C16:0 | y = 1.26x + 0.000244 | 0.9985 | 0.38 ± 4.17 | 0.038 |
| LPI C18:1 | y = 1.06x + 0.000765 | 0.9984 | 0.11 ± 6.25 | 0.011 |
| PA 16:0-18:2 | y = 0.00636x2 + 0.909x + 0.0159 | 0.9992 | 11.16 ± 0.95 | 1.12 |
| PA 18:2-18:2 | y = −0.00322x2 + 0.981x + 0.0258 | 0.9999 | 4.84 ± 5.16 | 0.48 |
| PA 16:0-18:1 | y = 0.000148x2 + 1.15x − 0.000798 | 0.9990 | 1.53 ± 4.96 | 0.15 |
| PC 18:2-18:2 | y = 1.79x2 + 8.04x − 0.0112 | 0.9992 | 0.23 ± 1.32 | 0.023 |
| PC 16:0-18:2 | y = 2.66x2 + 7.33x − 0.0026 | 1.0000 | 0.28 ± 1.47 | 0.028 |
| PI 18:2-18:21) | PI 16:0-16:0 | — | BSL | BSL |
| PI 16:0-16:0 | y = −0.00318x2 + 1.13x − 0.0317 | 0.9984 | BSL | BSL |
Amount (mg/g) = mean ± RSD (%) from three different samples of white ginseng.
BSL, below the sensitivity limit; GEF, gintonin-enriched fraction; LPA, lysophosphatidic acid; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; MRM, multiple reaction monitoring; PA, phosphatidic acid; PC, phosphatidylcholine; PI, phosphatidylinositol; RSD, relative standard deviation.
The amount of LPE C18:2, LPI C18:2, and PI 18:2-18:2 was determined without standards, and were identified and quantified using MRM transitions. The methods of LC-MS/MS conditions and calibration equations were obtained by LPE C18:1, LPI C18:1, and PI 16:0-16:0.
3.2. Quantitation of LPLs in GEF using LC-MS/MS
In previous reports, we showed that gintonin contains LPA C18:2, LPA C16:0, and LPA C18:1 as the representative functional components. LPAs in GEF are LPLs with simple structures (for review, see Ref. [18]). In biological systems, there are several additional bioactive LPLs such as LPC, LPE, lysophosphatidylglycerol (LPG), LPI, lysophosphatidylserine (LPS), and lysophosphatidylthreonine (LPT). Most of them also exhibit gintonin's effects through activation of G protein-coupled receptors [18]. Next, we determined the concentrations of free LPLs, including LPAs, in GEF. We found that GEF contains about 0.2% LPA C18:2 and lesser concentrations of LPA C16:0 and LPA C18:1 (Table 2). Next, we found that GEF contains a small amount, 0.082%, of LPC. Interestingly, we found that GEF contains 0.093% LPI C18:2, 0.038% LPI C16:0, and 0.011% LPI C18:1, which is known as a kind of endocannabinoid GPR55 receptor ligand [19]. We also found that GEF contains 0.012% LPE C18:2 and 0.019% LPE C16:0 (Table 2). GEF did not contain LPG, LPS, or LPT (data not shown). These results confirmed that LPAs are the major LPLs in GEF, along with lesser amounts of other LPL ligands, including LPC, LPE, and LPI. This quantitative assay of LPLs shows that GEF contains about 0.54% LPLs, including LPAs.
3.3. Quantitation of PLs in GEF using LC-MS/MS
Next, we examined the free PL contents of GEF and quantified PA, PC, phosphatidylethanolamine (PE), phosphatidylglycerol (PG), PI, phosphatidylserine (PS), and phosphatidylthreonine. Surprisingly, we found that GEF contains large amounts of PAs, in the order of 1.0% PA 16:0-18:2 > 0.5% PA 18:2-18:2 > 0.15% PA 16:0-18:1. In addition, GEF also contains 0.05% PC and a trace amount of PI. However, GEF does not contain PE, PG, PS, or phosphatidylthreonine. These results indicate that GEF contains about total 1.75% PLs and that PAs are the main PLs in GEF. The absence of PE, PG, and PS in GEF might be associated with the trace amounts of LPG, LPS, and LPT in GEF.
3.4. Effects of GEF on insulin secretion in INS-1 cell
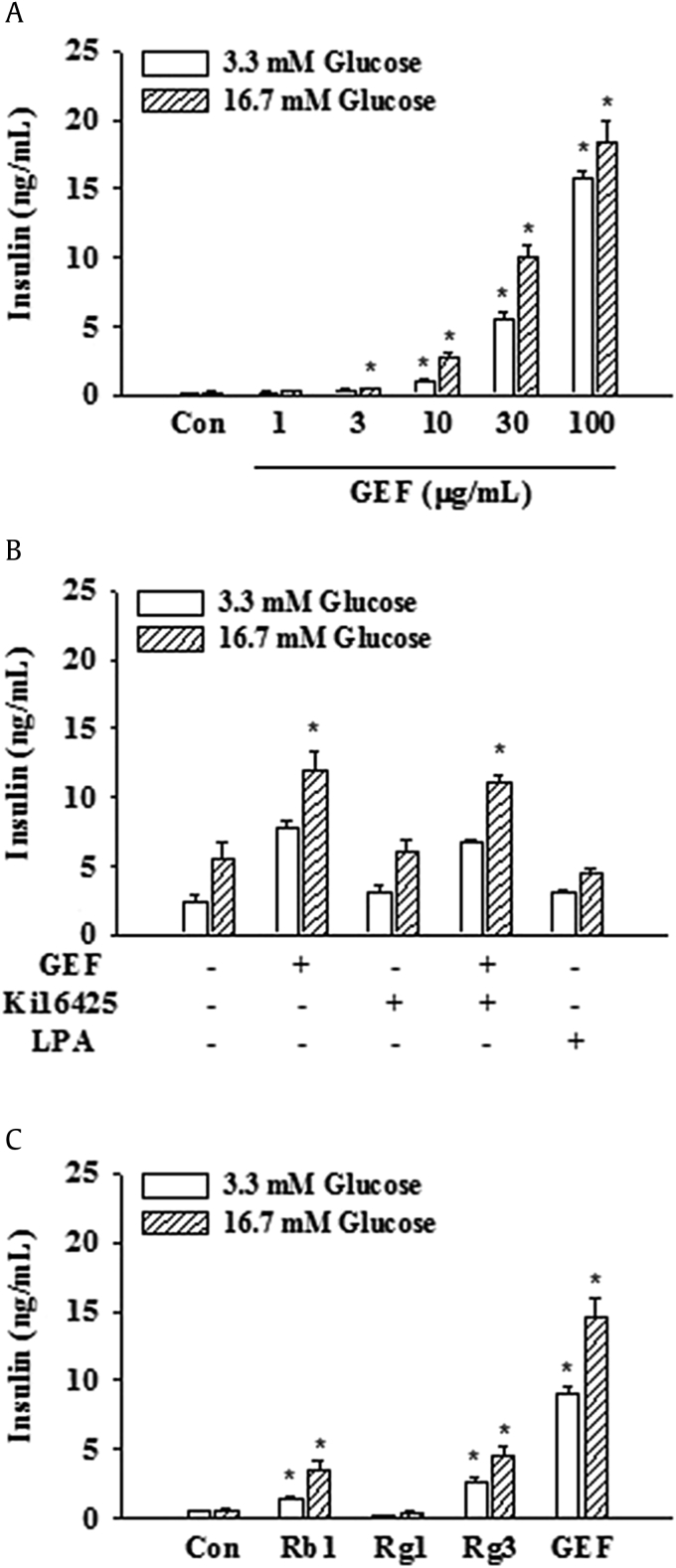
In previous reports, we showed that GEF exhibits cellular effects in neuronal cells via LPA receptor activation [20]. In the present study, we further examined whether GEF also stimulates insulin secretion through LPA receptors in non-neuronal INS-1 cell, which is known to secrete insulin and used for model cell for insulin secretion. As shown in Fig. 4A, GEF stimulated insulin secretion with concentration-dependent manner. Interestingly, GEF-induced insulin secretion was not blocked by LPA1/3 receptor antagonist Ki16425 and the effect of GEF on insulin secretion was much stronger than that of LPA (Fig. 4B). In addition, when we compared the effects of GEF on insulin secretion with ginsenosides such as Rb1, Rg1, and Rg3, we found that GEF was more effective than ginsenosides in insulin secretion. These results indicate that GEF-induced insulin secretion in INS-1 cell was not mediated via LPA receptors.
Fig. 4.
The effect of GT on insulin secretion from INS-1 cells. Cells were preincubated in KRB buffer for 1 h before incubation with GT or LPA. (A) Cells were incubated with GEF at the indicated concentrations in KRB buffer (3.3 or 16.7 mM glucose) for 2 h. (B) Cells were incubated either with GT (30 μg/mL) or LPA (30 μM) in KRB buffer (3.3 or 16.7 mM glucose) in the presence or absence of LPA1/3 receptor inhibitor, Ki16425 (10 μM) for 2 h. (C) Cells were incubated either with ginsenosides Rb1 (10 μM), Rg1 (10 μM), or Rg3 (10 μM), or GEF (100 μg/mL) for 2 h. Insulin concentration of supernatant was measured using an insulin ELISA kit. Data represent the means ± SD of four to six independent experiments; ∗p < 0.001, versus control (Con).
ELISA, enzyme-linked immunosorbent assay; GEF, gintonin-enriched fraction; GT, gintonin; KRB, Krebs-Ringer bicarbonate; LPA, lysophosphatidic acid.
4. Discussion
In previous reports, we showed that gintonin mainly consists of carbohydrates and lipid and ginseng protein complexes. We further demonstrated that one representative lipid component of gintonin, LPA C18:2, is the main functional ligand for the activation of G protein-coupled LPA receptors. We also showed gintonin-mediated effects from in vitro hormone or neurotransmitter releases to in vivo cognition-enhancing effects via LPA receptors [20]. As shown in Fig. 1, LPA is simply composed of G3P, fatty acids, and phosphate, but exhibits diverse biological effects. Previous reports also showed a possibility that GEF might contain other bioactive PLs related to LPAs and other bioactive lipids [6], [20]. In the present study, we focused on determining fatty acid, LPL, and PL contents in GEF, because previous reports showed that fatty acids, LPLs, and PL components could exhibit biological activities through membrane receptor signaling pathways (for review, see Ref. [18]). In this present study, we did not focus on simple glycolipids such as monoacylglycerol, diacylglycerol, and triacylglycerols, because they act as lipid storage for energy supply. We found three main characteristics of GEF through lipid content analysis. First, GEF contains about 12% FFAs, including linoleic acid (C18:2), followed by palmitic (C16:0) and oleic (C18:1). These results are also consistent with a previous report that Panax ginseng and American ginseng also contain high concentration of linoleic acid (C18:2) compared with other fatty acids [21]. Second, GEF contains a high concentration of LPA C18:2 compared with other LPLs such as LPC, LPE, and LPI, and its total LPL contents are 0.54%. Third, GEF also contains about 1.8% PLs such as PA 16:0-18:2, PA 18:2-18:2, PA 16:0-18:1, and PCs. Thus, the total lipid content of GEF was about 14.1%. These results indicate that linoleic acid (C18:2), LPA C18:2, and PA 16:0-18:2 might be the representative lipid components of GEF (Table 2).
Plants usually keep low concentrations of PAs, which are synthesized from LPAs by transferring one fatty acid to LPAs (Fig. 1C), because they are intermediate metabolites for larger molecules and are rapidly further processed into glycolipids and PLs for plasma membrane compositions or lipid storage [22]. Interestingly, GEF contains a relatively high concentration of PAs (Table 2). In addition, we observed a novel specificity of fatty acid components in PAs of GEF. The most abundant PA in GEF is PA 16:0-18:2 (Table 2). The concentration of PA 16:0-18:2 is about two-fold higher than that of PA 18:2-18:2 in GEF. It is noteworthy that most plant-derived PAs, including those in soy, consist of PA 18:2-18:2 [23], whereas most PAs derived from animals consist of PA 16:0-18:1, but GEF contains a small amount of the PA 16:0-18:1 found in animal systems [23]. On the other hand, the LPA C18:2 content in GEF is also much higher than that in other foodstuffs and herbal medicines [9]. Thus, ginseng contains unique LPA C18:2 and PA 16:0-18:2, different from those of animals and other plants.
In addition, we found that polyunsaturated linoleic acid (C18:2) is the main fatty acid component of LPA, other LPLs, and PAs in GEF (Table 2), raising the possibility that linoleic acid (C18:2) is the primary fatty acid component of LPA, LPL, and PA synthesis in ginseng. Thus, Panax ginseng LPAs are synthesized from G3P by transferring an acyl group, which could be linoleic acid (C18:2), by GPAT in GEF. LPAs are further acylated to form PAs by LPA acyltransferase [24], of which the second acyl group could be linoleic (C18:2), palmitic (C16:0), or oleic acid (C18:1) in GEF (Fig. 1C). Thus, it is probable that in ginseng LPA and PA syntheses, the main acyl group will be in the order of linoleic (C18:2) >> palmitic (C16:0) > oleic acid (C18:1). As shown in Fig. 1A, LPA C18:2 can be transformed into PA 16:0-18:2, if C16:0 is transferred into LPA C18:2. LPA C18:2 can be transformed into PA 18:2-18:2, if C18:2 is transferred into LPA C18:2. LPA C18:1 can be transformed PA 16:0-18:1, if C16:0 is transferred into LPA C18:1. Those PAs could be further processed to form other PLs or glycerolipids. Thus, LPAs and PAs are intermediate metabolites for synthesis of diverse glycerolipids, LPLs, and PLs in ginseng. Interestingly, it was revealed that the amounts of LPAs and PAs in GEF were much higher than those in other foodstuffs or herbal medicines [25].
Considering physiological roles of fatty acids, linoleic acid (C18:2) is a polyunsaturated fatty acid. Linoleic acid (C18:2) acts as an endogenous ligand of GPR40, a fatty acid receptor found in various organs [26]. Linoleic acid (C18:2) is also an essential fatty acid, and, in animals, absorbed linoleic acid (C18:2) is usually further metabolized into arachidonic acid and further processed to other arachidonic acid-derived bioactive ingredients such as prostaglandins [27]. Thus, there is a possibility that the linoleic acid (C18:2) derived from GEF also could be further transformed into other physiologically active lipid-derived agents in animals. On the other hand, in plant systems, PAs are components of cellular membranes. PAs are also produced as a response to stresses, including osmotic stress, pathogens, and freezing/cold/wounding, and act as lipid-derived signaling molecules against stresses [28]. PAs also act as an intracellular second messenger signal molecule in animal systems [29]. A recent study showed that PAs are strong activators of mammalian target of rapamycin (mTOR), which functions as a nutrient/energy/redox sensor and controls protein synthesis and regulates cellular metabolism, growth, and proliferation [30]. Interestingly, it has been reported that the functions of PA in mTOR activation is dependent on the structure of its fatty acid components. For example, two saturated fatty acids promote storage, whereas one saturated and one unsaturated fatty acids promote signaling for the activation of mTOR [31]. Joy et al [32] further demonstrated that PA isolated from eggs with high saturated fatty acids exhibited less activation of mTOR than did PA from soy with unsaturated fatty acids. These previous reports suggest that GEF-derived PAs could be a candidate for mTOR activation.
Besides LPA and PAs, several LPLs act as plasma membrane signaling molecules. GEF contains small amounts of LPLs besides LPA, and one of them, LPI, may act as a G protein-coupled receptor ligand such as GPR55 [18]. Interestingly, we found that although GEF contains 0.26% LPC, LPE, and LPI, GEF does not contain LPG, LPS, or LPT (data not shown). In future studies, it will be interesting to examine the roles of linoleic acid (C18:2), PAs, and LPI in GPR40, mTOR, and GPR55 regulation, respectively, although their amounts in GEF are different from each other to some extent. If it is true for their individual functions, it will provide a chance to expand GEF application beyond LPA receptor-mediated biological effects.
Previous reports on whole ginseng root lipid analyses showed that Panax ginseng contains a total of about 1.13–1.24% free lipids [33]. Ginseng lipid content is composed of FFAs, mainly in the order of linoleic (C18:2) >> palmitic (C16:0) > oleic acid (C18:1), glycolipids in the order of sterol glucoside > monogalactosyl diglyceride > digalactosylglyceride, and PLs in the order of PE = PG > PC > PI > PA. Comparing total lipid contents of whole ginseng with that of GEF, GEF contains at least 10-fold higher lipid concentrations (Table 2), although we did not quantitate glycolipids in GEF. In other words, GEF could be enriched with lipids, especially with linoleic acid (C18:2), LPAs, and PAs, compared with other lipids. Currently, we cannot clearly explain why specific lipid components are enriched during GEF preparation from whole ginseng root. Further study will be required to explain this phenomenon. In addition, it should be noted that we observed some degree of variation in the concentrations of bioactive lipids in the GEF examined among white ginseng batches, although we kept 4-year old ginseng roots for the preparation of GEF. The variations in the contents of the analyzed bioactive lipids might be due to regional differences in ginseng cultivation areas, soil conditions, and ginseng harvest seasons.
It will be interesting to determine why GEF from ginseng contains such high concentrations of polyunsaturated linoleic acid (C18:2) and PAs compared with saturated fatty acids and other PLs. Ginseng is a representative perennial herbal medicine that grows in high mountains or high latitude areas with winter temperatures below 0°C. Therefore, it might be due to the cold growth environments of ginseng. It is known that linoleic acid (C18:2) and PA are required for tolerance to cold stress [34]. There is evidence that gene transcriptions related to fatty acid desaturation increase during cold stress and the increased unsaturated fatty acids and PA enhance membrane fluidization and protect against cell membrane destruction under freezing conditions [28], [35]. Thus, ginseng might produce polyunsaturated fatty acids, linoleic acid (C18:2), and PAs to keep itself alive under cold stress.
Interestingly, GEF-mediated increase of insulin secretion was not associated with LPA receptors (Fig. 4B), because LPA1/3 receptor antagonist did not block GEF action. In previous reports, we showed that the effects of gintonin and GEF on neuronal cells were attenuated or blocked by LPA1/3 receptor antagonist [20]. Considering the lipid compositions of GEF found in the present study, it seems that GEF-mediated insulin secretion achieved via other bioactive lipid(s) rather than LPAs. Further study will be required to identify novel candidate related with insulin secretion in INS-1 cells. Taken together, the present study shows that GEF might contain diverse bioactive components differentiating from LPAs.
In conclusion, we quantified the concentrations of the main lipid contents of GEF. We found that GEF has a unique composition of polyunsaturated linoleic acid (C18:2), LPA C18:2, and PA 16:0-18:2. GEF-induced insulin secretion in INS-1 cell was not mediated via LPA receptors, showing that GEF contains other bioactive component(s) besides LPAs.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Basic Science Research Program (NRF-2017R1D1A1A09000520) and the Brain Research Program (NRF2016M3C7A1913845) through the NRF of Korea funded by the Ministry of Science, ICT, and Future Planning to S. Y. Nah and (2017R1D1A1B03031395) to S. H. Hwang, the Ministry of Science, ICT, and Future Planning.
Contributor Information
Sung-Hee Hwang, Email: Sunghhwang@sangji.ac.kr.
Seung-Yeol Nah, Email: synah@konkuk.ac.kr.
References
- 1.Brekhman, Dardymov New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–430. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 2.Nah S.Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014;5:98. doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loh S.H., Park J.Y., Cho E.H., Nah S.Y., Kang Y.S. Animal lectins: potential receptors for ginseng polysaccharides. J Ginseng Res. 2017;41:1–9. doi: 10.1016/j.jgr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M., Lim Y.H., Kim D.H., Nah S.Y. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35:92–103. [Google Scholar]
- 7.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.-H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Snyder C.L., Truksa M., Shah S., Weselake R.J. sn-Glycerol-3-phosphate acyltransferases in plants. Plant Signal Behav. 2011;6:1695–1699. doi: 10.4161/psb.6.11.17777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B.H., Choi S.H., Kim H.J., Jung S.W., Kim H.K., Nah S.Y. Plant lysophosphatidic acids: a rich source for bioactive lysophosphatidic acids and their pharmacological applications. Biol Pharm Bull. 2016;39:156–162. doi: 10.1248/bpb.b15-00575. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.H., Jung S.W., Kim H.S., Kim H.J., Lee B.H., Kim J.Y., Kim J.H., Hwang S.H., Rhim H., Kim H.C. A brief method for preparation of gintonin-enriched fraction from ginseng. J Ginseng Res. 2015;39:398–405. doi: 10.1016/j.jgr.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.J., Shin E.J., Lee B.H., Choi S.H., Jung S.W., Cho I.H., Hwang S.H., Kim J.Y., Han J.S., Chung C. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Mol Cells. 2015;38:796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.J., Kim D.J., Shin E.J., Lee B.H., Choi S.H., Hwang S.H., Rhim H., Cho I.H., Kim H.C., Nah S.Y. Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer's disease. Neurochem Int. 2016;101:56–65. doi: 10.1016/j.neuint.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.J., Park S.D., Lee R.M., Lee B.H., Choi S.H., Hwang S.H., Rhim H., Kim H.C., Nah S.Y. Gintonin attenuates depressive-like behaviors associated with alcohol withdrawal in mice. J Affect Disord. 2017;215:23–29. doi: 10.1016/j.jad.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Lee B.H., Kim H.K., Jang M., Kim H.J., Choi S.H., Hwang S.H., Kim H.C., Rhim H., Cho I.H., Nah S.Y. Effects of gintonin-enriched fraction in an atopic dermatitis animal model: involvement of autotaxin regulation. Biol Pharm Bull. 2017;40:1063–1070. doi: 10.1248/bpb.b17-00124. [DOI] [PubMed] [Google Scholar]
- 15.Chung S.W.C., Tong S.K., Xiao Y., Ho Y.Y. Methylmercury and long-chain n-3 fatty acids of 88 fish species commonly consumed in Hong Kong. J Anal Sci and Technol. 2015:1–9. [Google Scholar]
- 16.Yoon H.R., Kim H., Cho S.H. Quantitative analysis of acyl-lysophosphatidic acid in plasma using negative ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788:85–92. doi: 10.1016/s1570-0232(02)01031-0. [DOI] [PubMed] [Google Scholar]
- 17.Choi S.E., Shin H.C., Kim H.E., Lee S.J., Jang H.J., Lee K.W., Kang Y. Involvement of Ca2+, CaMK II and PKA in EGb 761-induced insulin secretion in INS-1 cells. J Ethnopharmacol. 2007;110:49–55. doi: 10.1016/j.jep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Makide K., Uwamizu A., Shinjo Y., Ishiguro J., Okutani M., Inoue A., Aoki J. Novel lysophospholipid receptors: their structure and function. J Lipid Res. 2014;55:1986–1995. doi: 10.1194/jlr.R046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laprairie R.B., Bagher A.M., Denovan-Wright E.M. Cannabinoid receptor ligand bias: implications in the central nervous system. Curr Opin Pharmacol. 2017;32:32–43. doi: 10.1016/j.coph.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X.J., Huang L.L., Cai X.J., Li P., Wang Y.T., Wan J.B. Fatty acid variability in three medicinal herbs of Panax species. Chem Cent J. 2013;7:12. doi: 10.1186/1752-153X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raho N., Ramirez L., Lanteri M.L., Gonorazky G., Lamattina L., ten Have A., Laxalt A.M. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J Plant Physiol. 2011;168:534–539. doi: 10.1016/j.jplph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Foster D.A. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.U., Huang A.H. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004;134:1206–1216. doi: 10.1104/pp.103.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T., Kassai A., Ohmoto M., Morito K., Kashiwada Y., Takaishi Y., Urikura M., Morishige J., Satouchi K., Tokumura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem. 2012;60:4156–4161. doi: 10.1021/jf300147y. [DOI] [PubMed] [Google Scholar]
- 26.Yamashima T. Dual effects of the non-esterified fatty acid receptor ‘GPR40’ for human health. Prog Lipid Res. 2015;58:40–50. doi: 10.1016/j.plipres.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Cunnane S.C. Problems with essential fatty acids: time for a new paradigm? Progr Lipid Res. 2003;42:544–568. doi: 10.1016/s0163-7827(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 28.Testerink C., Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot. 2011;62:2349–2361. doi: 10.1093/jxb/err079. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 30.Menon D., Salloum D., Bernfeld E., Gorodetsky E., Akselrod A., Frias M.A., Sudderth J., Chen P.H., DeBerardinis R., Foster D.A. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J Biol Chem. 2017;292:6303–6311. doi: 10.1074/jbc.M116.772988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster D.A. Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol Metab. 2013;24:272–278. doi: 10.1016/j.tem.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joy J.M., Gundermann D.M., Lowery R.P., Jäger R., McCleary S.A., Purpura M., Roberts M.D., Wilson S.M., Hornberger T.A., Wilson J.M. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab. 2014;11:29. doi: 10.1186/1743-7075-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi K.J., Kim M.W., Kim D.H. Studies on the lipid components of various ginsengs I. Lipid and fatty acid compositions of the free lipids. Korean J Ginseng Sci. 1985;9:193–203. [Google Scholar]
- 34.D'Angeli S., Altamura M.M. Unsaturated lipids change in olive tree drupe and seed during fruit development and in response to cold-stress and acclimation. Int J Mol Sci. 2016;12(17):1889. doi: 10.3390/ijms17111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arisz S.A., Munnik T. Distinguishing phosphatidic acid pools from de novo synthesis, PLD, and DGK. Methods Mol Biol. 2013;1009:55–62. doi: 10.1007/978-1-62703-401-2_6. [DOI] [PubMed] [Google Scholar]