Abstract
Background
Tanjin is an herbal medicine made from the root of salvia miltiorrhiza. It is predominantly given to arteriosclerotic patients as a supplement to ameliorate the clinical symptoms of cardiovascular diseases. In China, tanjin is used frequently in combination treatment for hypercholesterolemia. Thus, there is a high probability of combination of tanjin and statins in these arteriosclerotic patients. This study investigated the interaction between tanjin and rosuvastatin.
Methods
We performed a randomized single-blind, two-period crossover clinical trial on six healthy male volunteers. Volunteers were administered rosuvastatin with placebo or a tanjin-containing drug randomly. The blood samples were collected before drug administration, and at 0.5, 1, 1.5, 2, 4, 8, and 12 hours after administration. Lymphocytes were isolated from blood samples before and 12 hours after drug administration to measure mRNA. As an animal experiment, an in situ intestinal injection with portal vein sampling model was used to examine the interaction between tanjin and rosuvastatin during the absorption phase. Rosuvastatin or rosuvastatin combined with tanjin solution was injected into the intestine. After injection, blood from the portal vein was collected and the concentration of rosuvastatin was measured by LC/MS/MS analysis. A portion of the intestine and liver from the rats was collected and stored at –80°C for mRNA measurement.
Results
In the clinical trial, co-administration of tanjin decreased the maximum plasma concentration (Cmax) of rosuvastatin by 26.85% compared with rosuvastatin alone, and also decreased the area under the plasma concentration-time curve of rosuvastatin from 0 to 12 h (AUC0–12) by 19.43%. The relative expression of BCRP and OATP mRNA in human lymphocytes was increased by co-administration of tanjin. In the animal experiment, co-administration of tanjin extract reduced the concentration of rosuvastatin to 84.4, 64.4, and 50.0% at 15, 30, and 45 minutes, respectively. The tanjin-containing drug had a similar effect to tanjin extract. Furthermore, tanjin significantly reduced the absorption of rosuvastatin and the inhibitory effects lasted for at least 24 hours. Tanjin increased the relative expression of BCRP mRNA in the intestine, but it did not change the expression of OATP. Moreover, the concentration of rosuvastatin in the portal vein and systemic blood was reduced. In the liver, tanjin increased both BCRP and OATP mRNA expression, which was consistent with the results from human lymphocytes.
Conclusion
The clinical trial and animal experiment revealed that tanjin can significantly reduce the absorption of rosuvastatin. This interaction occurred, at least, at the absorption phase in the small intestine due to the enhanced efflux transport. Thus, as tanjin and rosuvastatin were found to interact, their combination needs to be paid attention to.
Keywords: absorption, drug interactions, herbal medicine, pharmacokinetics, rosuvastatin
Tanjin (TJ, the roots of Salvia miltiorrhiza) is a traditional herbal medicine that is widely used to treat cardiovascular diseases, such as angina pectoris, myocardial infarction, and stroke,1, 2 in China and the clinical symptoms of hypertension, such as headache, dizziness, and palpitations, in Japan. Some TJ-containing drugs are sold as class 2 OTC (over the counter) drugs in stores or online, and many TJ supplements are considered to be beneficial to prevent or ameliorate cardiovascular diseases for the healthy population and hypercholesterolemia patients. In recent years, TJ has been used to treat hypercholesterolemia.3–5 Statins, a kind of HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase inhibitor, are well-known lipid-lowering medications that have been found to reduce cardiovascular disease (CVD) and mortality in people with a high risk of cardiovascular disease.6 Statins have some rare but severe adverse effects, such as neuropathy, pancreatic, liver dysfunction, and muscle damage,7 especially in the case of excessive dosing. It is important to explore whether there are drug interactions between TJ and statins, and if there are, what role the absorption phase plays in this interaction.
A previous study reported the interaction between TJ and rosuvastatin (RST) in rats.8 However, the figure in this report showed a decreased plasma concentration of RST, whereas the text stated that the concentration was increased by co-administration of TJ. We were confused by this inconsistency and performed a preliminary study in rats to clarify whether the concentration of RST was increased or decreased by TJ. As a result, the concentration of RST was found to have decreased. We then investigated the drug interaction in vivo in humans.
In this study, we examined the influence of TJ on the pharmacokinetics of RST in six healthy volunteers. After the clinical study, we evaluated the drug interaction in rat to confirm the inhibitory effects of TJ on RST absorption using an in situ intestinal injection with portal vein sampling (IIPS) model.9
MATERIALS AND METHODS
Clinical trial
This clinical trial was registered in the UMIN Clinical Trials Registry under the Receipt No. R000029950, and was conducted in accordance with the ethical principles established in the Declaration of Helsinki and the Ethical Guidelines for Clinical Research. The final protocol was approved by the ethics committee of Tottori University, Faculty of Medicine in Japan (#1703B088).
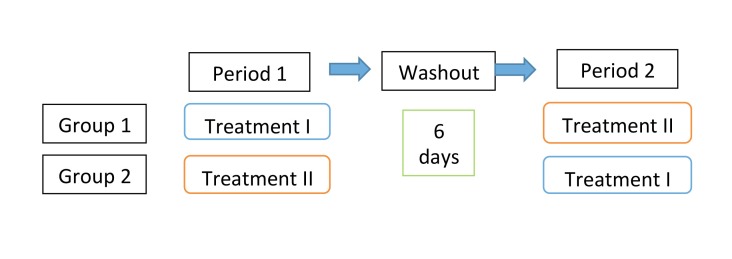
Physical examinations and laboratory tests for vital signs were conducted at screening. Volunteers with abnormalities in laboratory tests or drug hypersensitivity were excluded from this study. Six healthy volunteers (male, mean age of 21) were finally selected and written informed consent was obtained from all participants. The demographic characteristics of each group are listed in Table 1 and the experimental process is described in Fig. 1.
Table 1.
Group demographics and characteristics
| Group 1 (n = 3, Treatment I + II) | Group 2 (n = 3, Treatment II + I) | |
| Age (year) | 21.3 ± 1.5 [20, 23] | 20.7 ± 0.6 [20, 21] |
| Height (cm) | 173.3 ± 3.2 [171, 177] | 171.3 ± 2.1 [169, 173] |
| Weight (Kg) | 66.3 ± 2.5 [64, 69] | 64.3 ± 1.5 [63, 66] |
| BMI (body mass index) | 22.1 ± 0.2 | 21.9 ± 0.6 |
The data are shown as the mean ± standard deviation. The minimum and maximum of each characteristic are shown in square brackets. Treatment I: RST (single dose of 5 mg) with placebo for TJ (2.5 g). Treatment II: RST (single dose of 5mg) with TJ (single dose of 2.5 g). RST, rosuvastatin; TJ, tanjin.
Fig. 1.
Flow diagram of the clinical trial. Each group contained 3 volunteers, and between periods 1 and 2, there was a washout period of 7 days. Treatment I was administration of RST (single dose of 5 mg, p.o.) with placebo for TJ (2.5 g), whereas treatment II was administration of RST (single dose of 5 mg, p.o.) with TJ (2.5 g). RST, rosuvastatin; TJ, tanjin.
The six volunteers were divided into two equal groups (3 persons in each group). The study had a randomized, single-blind, two-period crossover design. One of the groups received a single dose of RST (CRESTOR Tablet 5 mg, rosuvastatin calcium salt, p.o.) with placebo (lactose, 2.5 g) as treatment I. The other group received a single dose of RST (5 mg, p.o.) with a TJ-containing drug (Kangenkaryu, 2.5 g, Iskra Industry, Tokyo, Japan) as treatment II. Kangenkaryu are granules that contain TJ as a main component. After a washout period of 6 days, the treatments were exchanged. Venous blood samples were collected at 0, 0.5, 1, 1.5, 2, 4, 8, and 12 hours after oral administration. The blood samples were centrifuged at 4000 rpm for 15 minutes to obtain the plasma and stored at –30°C for analysis. The concentration of RST was measured by LC/MS/MS. The blood samples at 0 and 12 hours after drug administration were collected, and lymphocytes were isolated using a Lymphoprep Tube (Alere Technologies AS, Oslo, Norway) according to the separation procedure.
Animal experiment
Animals and drugs
The experimental protocol was approved by the ethics committee on animal experiments at Tottori University (#15-Y-52 and #16-Y-12) and the experiments were carried out in accordance with the guidelines for animal experiments of the same facility. Twenty-one 8-week old rats were purchased from Shimizu Laboratory Supplies of Kyoto in Japan. RST calcium salt was purchased from Wako Pure Chemical Industries, Ltd (No.186-02731). The TJ-containing drug (Kangenkaryu) and TJ tablets were used in the animal experiment. TJ tablets made from TJ extract were purchased from Shanghai Leiyunshang Pharmaceutical (Shanghai, China).
RST was dissolved in distilled water to make a solution with a concentration of 0.083 mg/mL. Two TJ tablets were dissolved in 24.5 mL distilled water, and 2.05 mg of RST calcium was added to make mixed solution A. One package (2.5 g) of the TJ-containing drug was dissolved in 40.8 mL of distilled water and 3.41 mg of RST was added to make mixed solution B. These solutions were stored at 4°C.
In the acute experiment, rats were divided into three groups: the A group (n = 6), which received RST at 0.83 mg/kg and a TJ tablet (0.367 g/kg equivalent to crude TJ), the B group (n = 6), which received RST and the TJ-containing drug, and the C group (n = 6), which received RST alone at 0.83 mg/kg.
In the chronic experiment, 25 rats were divided into four groups. The A group (n = 7) and B group (n = 7) were pre-treated with TJ by gavage for 7 days, whereas the C group (n = 6) and D group (n = 5) were pre-treated with saline by gavage for 7 days. On the following day of the surgical experiment, A and C groups were injected with RST alone, and B and D groups were injected with RST and TJ. The experimental procedure of blood collection was the same as that for the acute experiment.
IIPS rat model analysis
The surgical procedures for the IIPS rat model were described previously.9 In brief, the rats were anesthetized by pentobarbital sodium (50 mg/kg) and 1 mL of heparin was intraperitoneally injected. Then, an incision was made along the linea alba of the abdomen to expose the intestines and portal vein. Next, 3 mL of the RST or RST + TJ solution was injected into the intestine by syringe, and a catheter was inserted into portal vein to collect 0.3–0.4-mL blood samples at 15, 30, 40, and 45 minutes after injection. After 2–3 mL of blood was collected from the heart, the rats were sacrificed by abdominal arterial bleeding. The blood samples were centrifuged at 4000 rpm for 15 minutes at 4°C and the plasma samples were stored at –80°C for analysis.
LC/MS/MS analysis
The detailed method for LC/MS/MS analysis was described in our previous study.9 RST was dissolved in methanol to make stock solution at concentrations of 1000, 500, 100, 50, 10, 5, and 1 ng/mL. Control plasma was diluted 10-times using water. Nine hundred µL of diluted plasma was added to 100 µL of 1000, 500, 100, 50, 10, 5, or 1 ng/mL RST in 100 µL of methanol.
Plasma with triple the volume of methanol was centrifuged at 15000 rpm for 5 min at 4°C, and 200 µL of the supernatant was diluted 10-times using water. One hundred and eighty µL of the diluted solution was added to 20 µL of methanol. These 200-µL samples were used to measure the concentration of RST by LC/MS/MS (AB SCIEX QTRAP 5500 LC/MS/MS System, SCIEX, Tokyo, Japan).
RT-PCR
RST is transported by organic-anion-transporting polypeptide (OATP) 1B110–12 and is mainly effluxed by breast cancer resistance protein (BCRP)13–15 in the intestine and liver. In rats, the homologous gene of OATP 1B1 is OATP 1b2.16 Thus, after measurement of the plasma concentration of RST, we also measured the relative expression of OATP and BCRP mRNA in human lymphocytes, and the rat intestine and liver.
Total RNA was extracted from lymphocyte, liver, and small intestine samples by TRIzol reagent (Promega, Carlsbad, CA) according to the instruction manual. Real-time fluorescence PCR (polymerase chain reaction) was carried out with LightCycler Nano (Roche, Basel, Switzerland) using KAPA SYBR FAST One-Step kit. The primers for BCRP, OATP1b2, and GAPDH were synthesized by Fasmac Company (Atsugi, Japan), and listed in Tables 2 and 3. The relative expression of mRNA was calculated by the double delta Ct analysis method. The expression of GAPDH mRNA was used as an internal standard reference.
Table 2.
PCR primer sequences for the clinical trial
| Gene | Primer |
| OATP1B1 | Forward 5’-AGTCCAGATTGCACCAAACC-3’ |
| Reverse 5’-TGACAAGCATACCTGGGACA-3’ | |
| BCRP | Forward 5’-AGCTGCAAGGAAAGATCCAA-3’ |
| Reverse 5’-TGCCCATCACAACATCATCT-3’ | |
| GAPDH | Forward 5’-CAATGACCCCTTCATTGACC-3’ |
| Reverse 5’-GACAAGCTTCCCGTTCTCAG -3’ |
BCRP, breast cancer resistance protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OATP, organic anion transporting polypeptide.
Table 3.
The PCR primer sequences for the animal experiment
| Gene | Primer |
| OATP1b2 | Forward 5’-CAGTGGCAGGCTTAACAACCT-3’ |
| Reverse 5’-GGATCCCATGTGTTCGTTGAG-3’ | |
| BCRP | Forward 5’-CCACTGGAATGCAAAATAGAG-3’ |
| Reverse 5’-CCTCATAGGTAGTAAGTCAGACACA-3’ | |
| GAPDH | Forward 5’-AGGGCTGCCTTCTCTCTTGAGT-3’ |
| Reverse 5’-AACTTGCCGTGGGTAGAGTCA-3’ |
BCRP, breast cancer resistance protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OATP, organic anion transporting polypeptide.
Statistical analysis
The data are expressed as the mean ± standard deviation. Statistical comparisons were made using the two independent samples t-test with SPSS 11.0J. A value of P < 0.05 was considered significant.
RESULTS
TJ reduced the systemic concentration of RST in the clinical trial
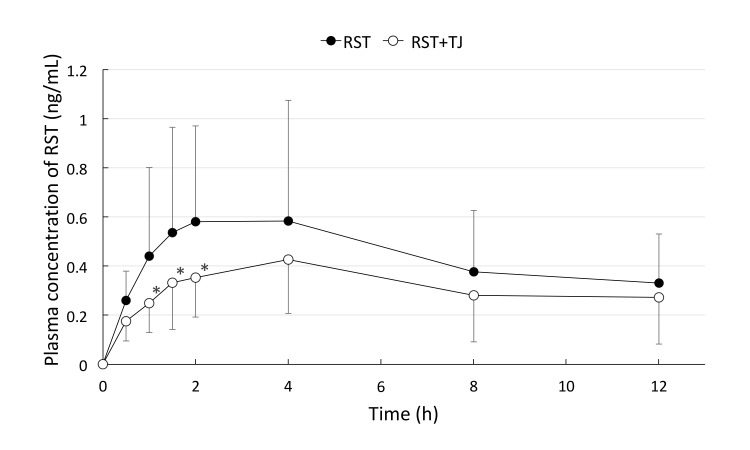
The plasma concentrations of RST after drug administration are shown in Fig. 2. TJ reduced the concentration of RST throughout the observation period.
Fig. 2.
The plasma concentration of RST in the volunteers. At 0, 0.5, 1, 1.5, 2, 4, 8, and 12 hours after RST administration (closed circle, n = 6) or RST + TJ administration (open circle, n = 6). The Tmax was at 4 hours after drug administration. TJ reduced the blood concentration of RST throughout the observation period. *P < 0.05 vs. group RST. Tmax, time of peak concentration; RST, rosuvastatin; TJ, tanjin.
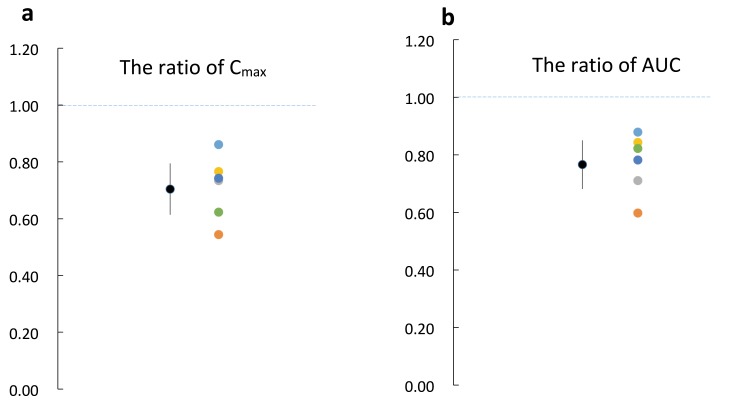
Ratios of the maximum concentration (Cmax) and the area under the curve (AUC) of RST in each subject between the two treatments (RST + TJ / RST) are shown in Figs. 3a and b.
Fig. 3.
The ratios of RST Cmax and AUC in combination with TJ to RST single administration. Color circles indicate ratios of AUC and Cmax in each subject, and black circles indicate the mean ratio and 95% confidence interval. (ratio is calculated by RST + TJ / RST alone). The geometric mean ratios of AUC and Cmax were 0.77 ± 0.08 and 0.7 ± 0.09, respectively. AUC, the area under the plasma RST concentration-time curve; Cmax, maximum plasma drug concentration; RST, rosuvastatin; TJ, tanjin.
The ratios of Cmax were all below 1-fold and the 95% confidence interval was 0.7 ± 0.09 (Fig. 3a). The ratios of AUC were also below 1-fold and the 95% confidence interval was 0.77 ± 0.08 (Fig. 3b). These results demonstrated that TJ can significantly reduce the Cmax and AUC of RST.
TJ reduced the absorption of RST in the animal experiment
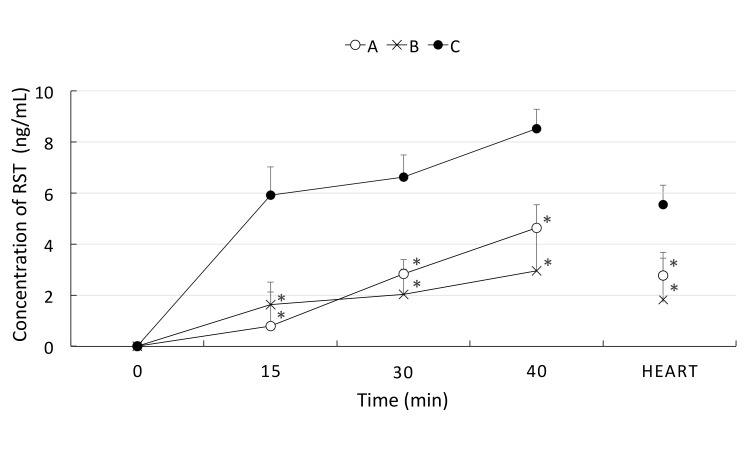
The interaction between TJ and RST in the absorption phase was also evaluated in the IIPS rat model. As shown in Fig. 4, both TJ tablets and the TJ-containing drug reduced the concentration of RST at 15, 30, and 40 minutes after oral administration compared with RST alone. Therefore, in the following experiment, we only used the TJ-containing drug as a representative of TJ. We measured the systemic plasma concentration of RST in the blood of the heart, and found that the concentration of RST was reduced by the combination of TJ early after administration. The differences in the systemic concentration of RST suggested similar inhibitory effects of TJ, consistent with the results of the clinical trial.
Fig. 4.
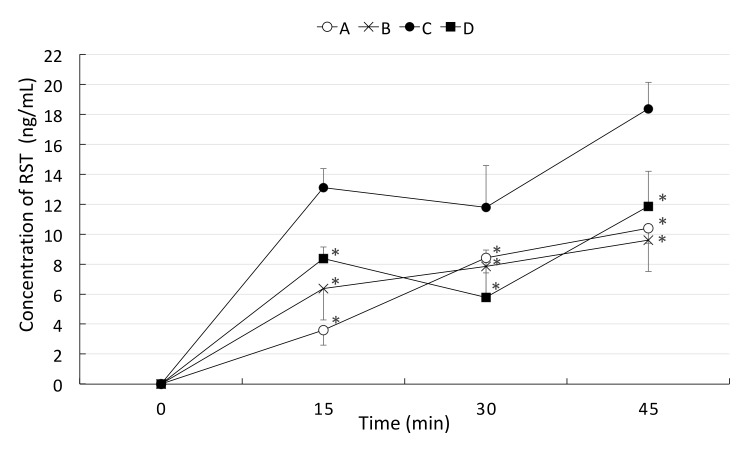
The concentration of RST in portal vein and systemic blood in the acute experiment. The concentrations of RST at 15, 30, and 40 minutes in portal vein blood and heart blood after drug injection were measured. A group (open circle, n = 6) received RST (0.83 mg/kg) and TJ tablets (0.367 g/kg equivalent to crude medicine), the B group (cross mark, n = 6) received RST and the TJ-containing drug, and the C group (closed circle, n = 6) were received RST alone at 0.83 mg/kg. Both TJ tablets and the TJ-containing drug significantly reduced the concentration of RST at 15, 30, and 40 min after injection. *P < 0.05 vs. C group. Heart: RST concentration in the systemic blood collected at 40 minutes after drug injection. RST, rosuvastatin; TJ, tanjin.
In the acute experiment, the concentration of RST was reduced significantly in both the TJ tablet group and TJ-containing drug group. There was no difference between TJ tablets and the TJ-containing drug in their influence on the absorption of RST. Thus, in the following chronic experiment, only the TJ-containing drug was used to estimate the interaction between TJ and RST.
As shown in Fig. 5, the A and B groups were pre-treated with TJ for 7 days, whereas the C and D groups were pre-treated with saline. The A and C groups were injected with RST alone, and B and D groups were injected with RST + TJ. On comparison of A with C and B with D, the concentration of RST was significantly reduced by TJ. By comparing A and B groups, TJ was demonstrated to inhibit the absorption of RST and the inhibitory effects of TJ lasted for at least one day.
Fig. 5.
The concentration of RST in the chronic experiment. The concentrations of RST at 15, 30, and 45 minutes after drug injection in the chronic experiment were measured. A group (open circle, n = 7) and B group (cross mark, n = 7) were pre-treated with TJ by gavage for 7 days, whereas the C group (closed circle, n = 6) and D group (closed square, n = 5) were pre-treated with saline by gavage for 7 days. On the experiment day, the A and C groups were injected with RST alone, and B and D groups were injected with RST and TJ. Pre-treatment with TJ for 7 days reduced the blood concentration of RST when comparing the A and C groups. *P < 0.05 vs. C group. RST, rosuvastatin; TJ, tanjin.
Relative mRNA expression of transport proteins
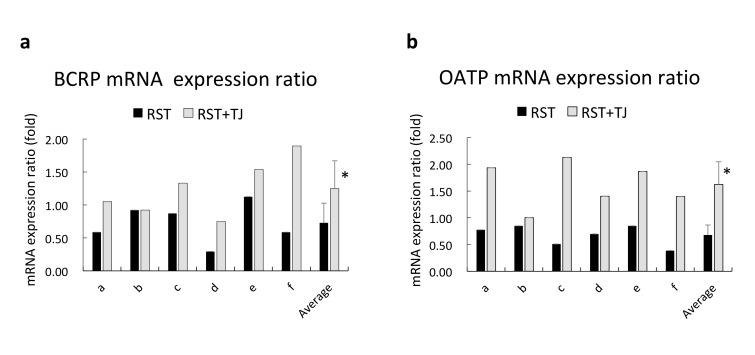
The mRNA expression ratio (after drug administration / before drug administration) in human lymphocytes of six subjects (a–f) and average data are shown in Fig. 6. The expression ratios of BCRP and OATP were reduced (below 1.00) in most subjects after administration of RST, while the expression ratios of BCRP and OATP were increased after administration of TJ + RST, and the average of expression ratio increased significantly after administration of RST + TJ.
Fig. 6.
The relative expression ratio of BCRP and OATP in human lymphocytes from each subject. The ratio is calculated by after drug administration / before drug administration. The 6 subjects are indicated as a to f. *P < 0.05 by t-test. Closed bar indicates RST alone, and open bar indicates RST + TJ. BCRP, breast cancer resistance protein; OATP, organic-anion-transporting polypeptide; RST, rosuvastatin; TJ, tanjin.
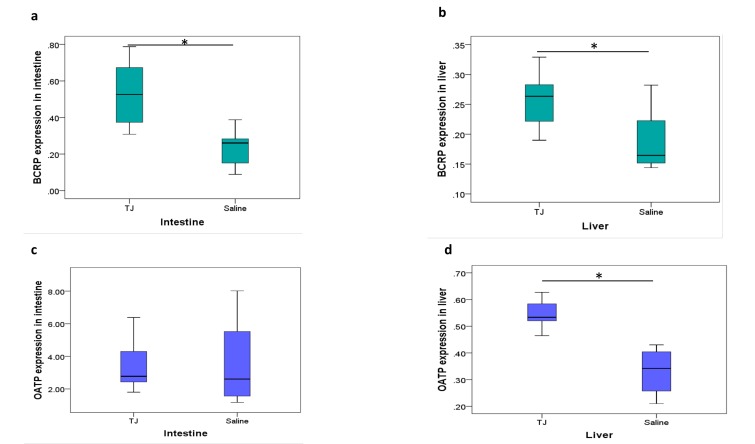
In the animal study, there was low BCRP expression in the intestine relative to the reference value for GAPDH mRNA; however, the expression of BCRP was increased significantly by TJ in both the intestine and liver (Figs. 7a and b). On the other hand, OATP expression was increased by TJ in the liver but not in the intestine (Figs. 7c and d).
Fig. 7.
The relative expression of BCRP and OATP mRNA in the intestine and liver. Treatment with TJ for 7 days increased the expression of BCRP mRNA in the intestine and liver, and increased the expression of OATP mRNA in the liver. The expression of OATP mRNA in intestine was not changed after TJ treatment. The relative expression of BCRP mRNA in the intestine (a) and liver (b). The relative expression of OATP mRNA in the intestine (c) and liver (d). *P < 0.05 by t-test. BCRP, breast cancer resistance protein; OATP, organic-anion-transporting polypeptide; RST, rosuvastatin; TJ, tanjin.
DISCUSSION
TJ was originally used to treat vascular disease according to ancient Chinese medical documents. As HMG-CoA reductase is the key enzyme in the production of cholesterol, statins (HMG-CoA reductase inhibitors) are widely used to treat hypercholesterolemia, which is associated with cardiovascular disease.17 However, statins cause some rare but severe adverse effects such as muscle damage,18 myopathy and rhabdomyolysis 19–22 and liver damage.23, 24 Therefore, the combination of TJ and statins has emerged in recent years for hyperlipidemia treatment in China.25 Several studies investigated this combination in animal models.26 Although the combination of drugs may be beneficial and reduce side effects, the risk of adverse drug interactions may increase.
In this study, we investigated the interaction of TJ and RST in healthy volunteers. The plasma concentration of RST was reduced by co-administration of TJ compared with RST alone. The mean Cmax and AUC were decreased by 26.85% and 17.81%, respectively. RST is different from other statins in that almost all other statins are metabolized by CYP3A and have a marked first-pass effect.27, 28 However, RST is different and most RST is eliminated in feces without being metabolized 29; therefore, the impact of CYP3A on RST metabolism is negligible. The concentration of RST in the RST + TJ group at 1, 1.5, and 2 hours after drug administration was significantly reduced compared with that in the RST alone group, and the concentrations continuously decreased after peak concentrations and there is no significant decrease between the two groups. This suggests that the interaction between TJ and RST mainly occurs at the absorption phase, and there are almost no effects of the elimination phase on this drug interaction. Based on the results of the clinical trial, we speculated that absorption of RST plays a key role in the interaction between RST and TJ. To confirm this speculation, we used the IIPS rat model to examine whether this interaction occurred during intestinal absorption in rats. As described in our previous study, the IIPS model is used to investigate only the effects of absorption excluding other influencing elements such as the gastric emptying rate and intestinal motility. We confirmed that the concentration of RST in the portal vein was reduced by co-administration of TJ in both the acute and chronic experiments. In the chronic experiment, after pre-treatment with TJ for 7 days, the plasma concentration of RST was decreased even in the RST alone group. This demonstrated that the inhibitory effects of TJ last for at least 24 hours.
Next, we measured the relative mRNA expression levels of BCRP and OATP1b2 in the intestine of rats after TJ administration for 7 days, and found that the expression of BCRP was increased by TJ, whereas that of OATP, which functions in influx transport, was not changed. Increased BCRP expression in the intestine was able to reduce the concentration of RST in portal vein blood. This change in expression was consistent with the portal vein and systemic blood concentrations of RST. In the liver, TJ increased both OATP and BCRP expression levels. This may cause an increase in the biliary excretion of RST through hepatocytes. However, the acceleration of the excretion phase was not observed in the human trial. In this human study, only a single dose of RST and TJ was evaluated. And IIPS rat model evaluates only intestinal drug absorption. Therefore, further experiments are needed to determine the precise mechanism underlying the interaction between RST and TJ.
In conclusion, RST interacts with TJ in combination at least during the absorption phase in the intestine, and the final systemic concentration of RST is consequently reduced. As this will void the original intention of combination therapy, this combination should be clinically avoided.
Acknowledgments
Acknowledgments: We thank Dr. Katsumi Nagata at Research Center for Bioscience and Technology, Tottori University for LC/MS/MS analysis. We are grateful to the volunteers who participated in this clinical study.
The authors declare no conflict of interest.
REFERENCES
- 1. Fei YX, Wang SQ, Yang LJ, Qiu YY, Li YZ, Liu WY, et al. Salvia miltiorrhiza Bunge (Danshen) extract attenuates permanent cerebral ischemia through inhibiting platelet activation in rats. J Ethnopharmacol. 2017;207:57-66. [DOI] [PubMed] [Google Scholar]
- 2. Chang CC1, Chang YC2, Hu WL3, Hung YC4. Oxidative Stress and Salvia miltiorrhiza in Aging-Associated Cardiovascular Diseases. Oxid Med Cell Longev. 2016;2016:4797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim C, Lim S, Lee B, Kim B, Cho S. Effect of methanol extract of Salviae miltiorrhizae Radix in high-fat diet-induced hyperlipidemic mice. Chin Med. 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwok T, Leung PC, Lam C, Ho S, Wong CK, Cheng KF, et al. A randomized placebo controlled trial of an innovative herbal formula in the prevention of atherosclerosis in postmenopausal women with borderline hypercholesterolemia. Complement Ther Med. 2014;22:473-80. [DOI] [PubMed] [Google Scholar]
- 5. Wang P, Xu S, Li W, Wang F, Yang Z, Jiang L, et al. Salvianolic acid B inhibited PPARγ expression and attenuated weight gain in mice with high-fat diet-induced obesity. Cell Physiol Biochem. 2014;34:288-98. [DOI] [PubMed] [Google Scholar]
- 6. Horita N, Miyazawa N, Kojima R, Inoue M, Ishigatsubo Y, Ueda A, et al. Statins reduce all-cause mortality in chronic obstructive pulmonary disease: a systematic review and meta-analysis of observational studies. Respir Res. 2014;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golomb BA, Evans MA. Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen J, Hu J, Cai J, Lai Y, Zheng X, Zou D, et al. Effect of danshen on pharmacokinetics of rosuvastatin in rats. Acad J Second Military Med Univ. 2012;3:320. [Google Scholar]
- 9. Qian W, Hasegawa J, Yang J, Endo Y, Miake J. Establishment of a novel in situ rat model for direct measuring of intestinal drug absorption: confirmation of inhibitory effects of daijokito on the absorption of ranitidine. Yonago Acta Med. 2018;61:192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25:2822-35. [DOI] [PubMed] [Google Scholar]
- 11. Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71:329-40. [DOI] [PubMed] [Google Scholar]
- 12. Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330-41. [DOI] [PubMed] [Google Scholar]
- 13. Karibe T, Hagihara-Nakagomi R, Abe K, Imaoka T, Mikkaichi T, Yasuda S, et al. Evaluation of the usefulness of breast cancer resistance protein (BCRP) knockout mice and BCRP inhibitor-treated monkeys to estimate the clinical impact of BCRP modulation on the pharmacokinetics of BCRP substrates. Pharm Res. 2015;32:1634-47. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi K, Sugiura T, Matsubara K, Sato R, Shimizu T, Masuo Y, et al. Interaction of novel platelet-increasing agent eltrombopag with rosuvastatin via breast cancer resistance protein in humans. Drug Metab Dispos. 2014;42:726-34. [DOI] [PubMed] [Google Scholar]
- 15. Kunze A, Poller B, Huwyler J, Camenisch G. Application of the extended clearance concept classification system (ECCCS) to predict the victim drug-drug interaction potential of statins. Drug Metab Pers Ther. 2015;30:175-88. [DOI] [PubMed] [Google Scholar]
- 16. Iusuf D, van Esch A, Hobbs M, Taylor M, Kenworthy KE, van de Steeg E, et al. Murine Oatp1a/1b uptake transporters control rosuvastatin systemic exposure without affecting its apparent liver exposure. Mol Pharmacol. 2013;83:919-29. [DOI] [PubMed] [Google Scholar]
- 17. Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829-39. [DOI] [PubMed] [Google Scholar]
- 18. Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373-87. [DOI] [PubMed] [Google Scholar]
- 19. Vrablik M, Zlatohlavek L, Stulc T, Adamkova V, Prusikova M, Schwarzova L, et al. Statin-associated myopathy: from genetic predisposition to clinical management. Physiol Res. 2014;63 Suppl 3:S327-34. [DOI] [PubMed] [Google Scholar]
- 20. Keen HI, Krishnarajah J, Bates TR, Watts GF. Statin myopathy: the fly in the ointment for the prevention of cardiovascular disease in the 21st century. Expert Opin Drug Saf. 2014;13:1227-39. [DOI] [PubMed] [Google Scholar]
- 21. Sakamoto K, Kimura J. Mechanism of statin-induced rhabdomyolysis. J Pharmacol Sci. 2013;123:289-94. [DOI] [PubMed] [Google Scholar]
- 22. Hilton-Jones D. Statin-related myopathies. Pract Neurol. 2018;18:97-105. [DOI] [PubMed] [Google Scholar]
- 23. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-9. [DOI] [PubMed] [Google Scholar]
- 24. Bellosta S, Corsini A. Statin drug interactions and related adverse reactions: an update. Expert Opin Drug Saf. 2018;17:25-37. [DOI] [PubMed] [Google Scholar]
- 25. Chu S, Shih W, Yang Y, Chen P, Chu Y. Use of traditional Chinese medicine in patients with hyperlipidemia: A population-based study in Taiwan. J Ethnopharmacol. 2015;168:129-35. [DOI] [PubMed] [Google Scholar]
- 26. Cheung D, Koon C, Wong P, Yau K, Wat E, Hung A, et al. Evaluating Efficacy and Safety of Combination Medication of Atorvastatin and a Herbal Formula Containing Salvia miltiorrhiza and Pueraria lobata on Hyperlipidemia. Phytother Res. 2017;31:1579-89. [DOI] [PubMed] [Google Scholar]
- 27. Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117-25. [DOI] [PubMed] [Google Scholar]
- 28. Gelissen IC, McLachlan AJ. The pharmacogenomics of statins. Pharmacol Res. 2014;88:99-106. [DOI] [PubMed] [Google Scholar]
- 29. Hu M, Tomlinson B. Evaluation of the pharmacokinetics and drug interactions of the two recently developed statins, rosuvastatin and pitavastatin. Expert Opin Drug Metab Toxicol. 2014;10:51-65. [DOI] [PubMed] [Google Scholar]