Abstract
Mycosis fungoides, the most common subtype of cutaneous T-cell lymphoma, displays a broad spectrum of clinical, histological and phenotypic variants with different prognostic impacts. The classic immunophenotype is CD3+/CD4+/CD45RO+memory T cells. CD4/CD8 double-negative mycosis fungoides is rare. Here we describe the clinicopathological features of CD4/CD8 double-negative mycosis fungoides in a 55-year-old female with a review of the literature. Although the CD4/CD8 double-negative phenotype appears to be associated with an unusual clinical presentation, it does not appear to confer prognostic significance.
Keywords: CD4/CD8 double-negative, hypopigmented, mycosis fungoides
Although mycosis fungoides (MF) accounts for less than 1% of all non-Hodgkin lymphomas, MF is by far the most predominant cutaneous T-cell lymphoma subtype.1 Management of MF is based on the TNM classification categorized into 4 clinical stages.2 In addition, MF is classically divided according to its clinical presentation as patches in the early stage, plaques in the middle stage and tumors in the late stage.3 The clinical course and prognosis are depend on skin symptoms, and a mixture of those eruptions is usually seen during in its long clinical time course.1 Any area of skin may be affected in the early phases, and diagnosis is difficult. It is most often misdiagnosed as atopic dermatitis, psoriasis or chronic eczema, especially in the early stage.4 Since MF also resembles parapsoriasis en plaques, the definition and characterization of the early stage of MF is difficult.5 Occasionally, a plaque may ulcerate prior to the tumor stage.6 Patches and plaques may show poikiloderma including hypopigmentation, hyperpigmentation, atrophy and teleangiectasia.7 Other reported subtypes according to clinical, histological and immunological characteristics include erythrodermic, poikilodermic, purpura pigmentosa and papuloerythroderma types and a leukemic variant of MF.8–11
MF is a neoplasia of lymphocytes that generally coincides with cutaneous lymphoma originating in epidermotropic T cells, which express the T cell receptor with αβ+subunits and CD4+ immunophenotype, known as CD45RO+memory T-lymphocytes. Although a definitive diagnosis of MF may be made on the basis of clinical and histopathological features, determination of T-cell clonality and immunophenotypical analysis of T cells by immunohistochemistry or flowcytometry are important. MF is generally of the CD3+, CD4+, CD45RO+ and CD8- phenotype, which is a cytokine production pattern with a Th2 profile. However, a broad range of MF variants with differential diagnosis have been described, and some of them have different immunophenotypic profiles. Thus, understanding the immunophenotype based on the clinical features and staging is important for long-term follow-up.
PATIENT REPORT
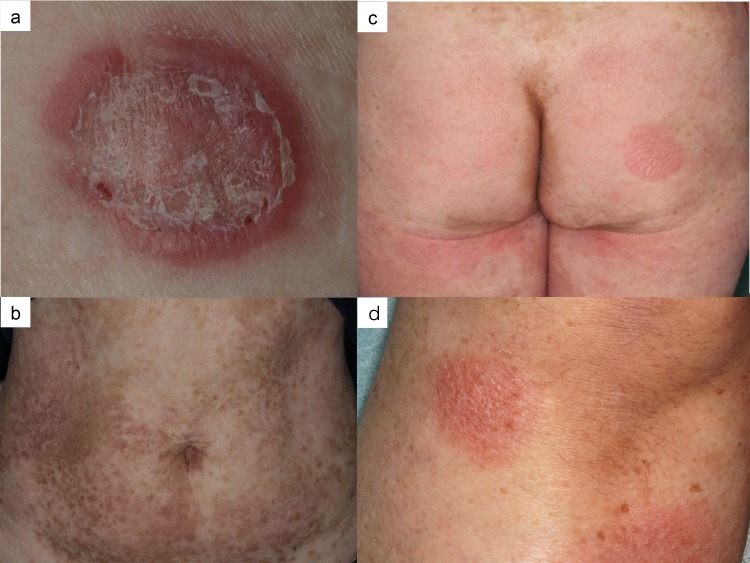
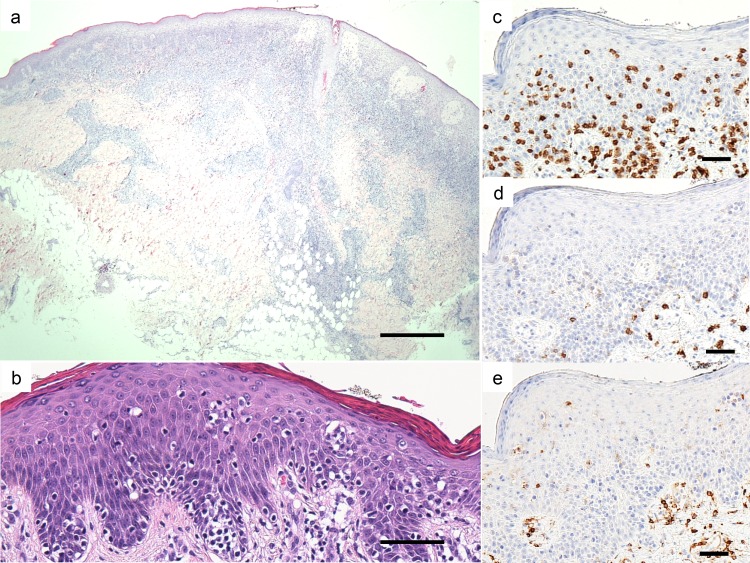
A 55-year-old female presented with a 2-month history of an enlarged red nodule on her right thigh (Fig. 1a). She had a 30-year history of asymptomatic erythema on her trunk and extremities. Physical examination revealed multiple round to oval scaly erythematous patches and plaques of up to 15 cm in size on her whole body and mixed hypo-and hyperpigmented small patches disseminated on her trunk (Figs. 1b and c). A 3 cm subcutaneous indurated nodule was seen on her right thigh. A skin biopsy specimen from the right thigh showed dense infiltration of atypical lymphocytes in the subcutaneous tissue to the epidermis (Fig. 2a). The lesion disappeared after biopsy. Another biopsy specimen from a patch on her left leg showed mild acanthosis, dermal infiltration of small lymphocytes with prominent epidermotropism, and Pautrier’s microabscess (Figs.1d and 2b). Immunohistochemically, the tumor cells were positive for CD3, CD45RO and CD5 but were negative for CD7, CD4, CD8, CD30, CD20 and T-cell restricted intracellular antigen-1 (TIA-1) (Figs. 2c–e). Molecular biology investigations indicated a monoclonal rearrangement of the T-cell receptor beta gene. The peripheral blood cell count was normal, and there were no atypical cells. Neither internal organ involvement nor lymphadenopathy was detected by computed tomography. Based on these findings, we made a diagnosis of CD4/CD8 double-negative mycosis fungoides of stage ⅡB. The patient was treated with a topical corticosteroid and narrow-band ultraviolet B (NBUVB).
Fig. 1.
a: Red nodule on her right thigh. b: Disseminated hypopigmented and hyperpigmented small patches. c: Multiple round to oval erythematous patches and plaques. d: Erythematous patches on her left leg.
Fig. 2.
a: Skin biopsy specimen from a nodule on her right thigh. Bar = 500 µm. b: Skin biopsy specimen from a patch on left leg. (hematoxylin-eosin stain). Bar = 50 µm. c: Immunohistochemical staining for CD3. Bar = 50 µm. d: Immunohistochemical staining for CD4. Bar = 50 µm. e: Immunohistochemical staining for CD8. Bar = 50 µm.
DISCUSSION
Mycosis fungoides is a cutaneous T cell lymphoma with an indolent course and typically affects older adults (median age at diagnosis: 55–60 years; male-to-female ratio: 1.6–2.0:1).1 A total of 34 cases of CD4/CD8 double negative MF have been reported.12–16 Clinical features of the patients are summarized in Table 1. The patients included 14 males and 12 females, and the age at diagnosis ranged from 12 to 82 years (mean: 47.3 years). The duration of disease before diagnosis ranged from 6 months to 30 years (mean: 8.5 years). Eight patients had classic MF and 13 presented with unusual clinical variants: hypopigmented in 7 patients, localized in 3 patients, ichthyosiform in one patient, purpuric in one patient and erythema gyratum repens-like in one patient. Twenty-eight cases were early stage at diagnosis and 4 cases were advanced stage. Treatment was topical steroids, psoralen and ultraviolet A (PUVA), NBUVB or radiation. Four patients received interferon or chemotherapy in addition to skin-target therapy. The clinical course was indolent except for 3 cases: advanced stage at diagnosis due to lymph node and bone marrow metastasis in case 2, liver metastasis in case 4 and large cell transformation in case 5.
Table 1.
Clinical data
| Case | Age/Sex | Duration (Y) | Stage | Clinical subtype | Treatment | Prognosis and survival (Y) |
| 1 | 72/M | NA | Plaque | NA | PUVA,etretinate, and chemotherapy | CR 1.4 |
| 2 | 77/F | NA | Tumor | NA | PUVA, X-ray and chemotherapy | Death 6.8 |
| 3 | 52/F | NA | Tumor | NA | PUVA | PR 14.0 |
| 4 | 82/F | NA | Tumor | NA | PUVA, X-ray and chemotherapy | Death 3 |
| 5 | NA | NA | ⅡB | NA | Chemotherapy | Death 3.9 |
| 6 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 7 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 8 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 9 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 10 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 11 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 12 | NA | NA | ⅠA/ⅠB | NA | PUVA/X-ray /IFNα | Indolent course |
| 13 | 77/M | 3 | ⅠB | Classic | Skin target therapy | Indolent course |
| 14 | 12/M | 5 | ⅠB | Hypopigmented | Skin target therapy | Indolent course |
| 15 | 38/F | 4 | ⅠB | Hypopigmented | Skin target therapy | Indolent course |
| 16 | 11/M | 9 | ⅠB | Hypopigmented | Skin target therapy | Indolent course |
| 17 | 45/M | 4 | Patch | Localized/Unilesional | Skin target therapy | Indolent course |
| 18 | 72/M | 15 | ⅠA | Classic | Skin target therapy | Indolent course |
| 19 | 71/F | 0.5 | ⅠB | Classic | Skin target therapy | Indolent course |
| 20 | 61/F | 10 | ⅠA | Classic | Skin target therapy | Indolent course |
| 21 | 28/M | 2 | ⅠB | Classic | Skin target therapy | Indolent course |
| 22 | 55/M | 10 | ⅠB | Classic | Skin target therapy | Indolent course |
| 23 | 47/M | 20 | ⅠA | Classic | Skin target therapy | Indolent course |
| 24 | 73/F | 10 | Plaque | Localized/Unilesional | Skin target therapy | Indolent course |
| 25 | 14/F | 10 | ⅠA | Hypopigmented | Skin target therapy | Indolent course |
| 26 | 27/M | 9 | ⅠB | Classic | Skin target therapy | Indolent course |
| 27 | 14/M | 12 | ⅠB | Hypopigmented | Skin target therapy | Indolent course |
| 28 | 14/M | 8 | ⅠB | Ichthyosiform | Skin target therapy | Indolent course |
| 29 | 34/F | 1 | Plaque | Localized/Pagetoid reticulosis | Skin target therapy | Indolent course |
| 30 | 34/M | 2 | ⅠB | Classic and purpuric | Skin target therapy | Indolent course |
| 31 | 23/F | NA | NA | NA | NA | NA |
| 32 | 45/F | NA | NA | NA | NA | NA |
| 33 | 70/F | 5 | ⅠB | Hypopigmented | NBUVB | PR |
| 34 | 73/M | 10 | ⅠB | Erythema gyratum repens -like | PUVA | PR |
| Our case | 55/F | 30 | ⅡB | Hypopigmented | NBUVB | Indolent course |
CR, complete response; F, female; IFN, interferon; M, male; NA, not applicable; NBUVB, narrow-band ultraviolet B; PR, partial response; PUVA, psoralen ultraviolet A; Y, years.
The histopathology of classic MF in the patch/plaque stage shows superficial bandlike or lichenoid infiltration of lymphocytes and histiocytes. Atypical cells are small to medium-sized, highly indented (cerebriform) and mostly confined to the epidermis (epidermotropism). They are characteristically present in the basal layer of the epidermis either as single and often haloed cells or in a linear configuration. The presence of intraepidermal collections of atypical cells (Pautrier microabscesses) is a highly characteristic feature but is not always observed.17 In cases of CD4/CD8 double-negative MF, histopathology is almost the same as that of the conventional type.
Table 2 summarizes the immunophenotypes of neoplastic cells. Deletion of CD7 was observed in 23 of the 29 cases tested. CD45RO, a memory phenotype marker, was expressed in 18 (69%) of 26 cases and TIA-1, a cytotoxic phenotype marker, was expressed in 11 (65%) of 17 cases. CD56 was positive in 2 (7%) of 28 cases, and CD30 was positive in 6 (20%) of 30 cases. T-cell clonality was evaluated in 21 cases by detection of a monoclonal rearrangement of T cell receptor gamma or beta gene using a polymerase chain reaction technique. Monoclonality was detected in 15 (71%) of 21 cases.
Table 2.
Immunophenotype of intraepidermal atypical lymphocytes
| Case | CD3 | CD4 | CD8 | CD7 | CD45RO | TIA-1 | CD56 | CD30 | TCR-β | TCR-δ | Clonality |
| 1 | + | – | – | NA | NA | NA | NA | + | + | – | NA |
| 2 | + | – | – | + | NA | NA | NA | – | + | – | NA |
| 3 | + | – | – | – | NA | NA | NA | – | + | – | NA |
| 4 | + | – | – | – | NA | NA | NA | – | – | + | NA |
| 5 | 3+ | – | – | 2+ | – | NA | 2+ | – | 3+ | – | NA |
| 6 | 3+ | – | – | – | 2+ | NA | – | – | 3+ | – | NA |
| 7 | 3+ | – | – | – | – | NA | – | 2+ | – | 3+ | NA |
| 8 | - | – | – | – | 2+ | NA | – | 2+ | 3+ | – | NA |
| 9 | 3+ | – | – | 2+ | – | NA | 2+ | 2+ | – | – | NA |
| 10 | 3+ | – | – | – | – | NA | – | – | – | 3+ | NA |
| 11 | 3+ | – | – | 2+ | – | NA | – | 2+ | – | 3+ | NA |
| 12 | 3+ | – | – | 2+ | – | NA | – | – | 3+ | – | NA |
| 13 | 3+ | – | – | – | 3+ | 3+ | – | – | 2+ | – | + |
| 14 | 3+ | – | – | – | 3+ | 3+ | 3+ | – | 2+ | – | NA |
| 15 | 3+ | – | – | – | 3+ | – | – | – | – | – | + |
| 16 | 3+ | – | – | – | – | 3+ | – | – | NA | NA | + |
| 17 | 3+ | – | – | – | 3+ | NA | – | – | – | – | + |
| 18 | 3+ | – | – | – | 3+ | – | – | – | – | 1+ | + |
| 19 | 3+ | – | – | – | 3+ | – | – | – | 3+ | – | – |
| 20 | 3+ | – | – | – | 3+ | 3+ | – | – | – | – | – |
| 21 | 3+ | – | – | – | 3+ | 3+ | – | – | 2+ | – | + |
| 22 | 3+ | – | – | – | 3+ | 3+ | – | – | – | – | + |
| 23 | 3+ | – | – | – | 3+ | NA | – | – | 2+ | 1+ | + |
| 24 | 3+ | – | – | – | 3+ | 3+ | – | 1+ | – | 3+ | – |
| 25 | 3+ | – | – | – | 3+ | 3+ | – | – | NA | NA | NA |
| 26 | 3+ | – | – | – | 3+ | 3+ | – | 2+ | – | – | – |
| 27 | 3+ | – | – | – | 3+ | 3+ | – | – | – | 1+ | – |
| 28 | 3+ | – | – | NA | NA | NA | NA | NA | 1+ | – | + |
| 29 | 3+ | – | – | – | NA | NA | NA | NA | 3+ | – | – |
| 30 | 3+ | – | – | – | 3+ | 1+ | – | – | 3+ | – | + |
| 31 | NA | – | – | NA | NA | – | – | NA | + | NA | + |
| 32 | NA | – | – | NA | NA | – | – | NA | + | NA | + |
| 33 | 3+ | – | – | 3+ | – | – | – | – | – | – | + |
| 34 | + | – | – | NA | + | NA | NA | NA | NA | NA | + |
| Our case | 3+ | – | – | – | 3+ | – | – | – | + | NA | + |
–; < 10%, 1+; < 25%, 2+; < 50%, 3+; > 50%. NA, not applicable; TCR, T cell receptor; TIA-1, T cell intracellular antigen 1.
Our review of CD4/CD8 double-negative MF cases revealed that the age at diagnosis was younger and the male-to-female ratio was smaller than those in patients with conventional MF. Hodak et al. reported that most of the CD4/CD8 double-negative MF cases were clinically characterized by an unusual clinical presentation.13 We also found the same results in our review. Especially, the hypopigmented type was most commonly seen and occurred at younger ages than the other types. This was consistent with the characteristics of the hypopigmented MF already reported as having a good prognosis in young age.18
Clonal T cell receptor gene rearrangement was detected in 57–71% of cases of early lesions of MF and was not related to prognosis.15 In some cases of CD4/CD8 double-negative MF, the cells expressed cytotoxic markers such as TIA-1 and CD56. Although a cytotoxic phenotype was occasionally seen in MF, clinical behavior did not differ from conventional MF.19–21
The prognosis of patients with MF depends on the stage and particularly on the type and extent of skin lesions and the presence of extracutaneous disease.22, 23 The 5-year disease-specific survival rates were reported to be 73%-96% for patients in the patch/plaque stage and 44% for patients in the tumor stage.23 Patients with effaced lymph nodes, visceral involvement and transformation into a large T-cell lymphoma have an aggressive clinical course.1 The clinical course of CD4/CD8 double-negative MF was indolent except for 3 patients with an advanced stage at diagnosis and it was the same as that of conventional MF.
As long as the disease is confined to the skin, skin-targeted therapies are preferred. In patients with early stage MF (1A1B2A), topical corticosteroids, UVB, PUVA, localized radiotherapy or interferon gamma can be used.24, 25 Systemic therapy such as treatment with retinoids, interferon alpha, low-dose MTX or chemotherapy should be mainly considered for patients with advanced stages or for patients with refractory cutaneous disease.26, 27 Biologicals such as interferon alpha and other cytokines, traditional and new retinoids such as bexarotene, and receptor-targeted cytotoxic fusion proteins, are being increasingly used in the treatment of MF.28
Our patient had a hypopigmented type and indolent course, which was typical of case in a review of CD4/CD8 double-negative MF cases.
In conclusion, CD4/CD8 double-negative MF is a rare immunophenotype of MF. Unusual phenotypes and clinical presentations sometime make diagnosis of MF difficult. It is important to consider variants such as CD4/CD8 double-negative MF, and clinicopathologic correlation is always required.
The authors declare no conflict of interest.
REFERENCES
- 1. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-85. [DOI] [PubMed] [Google Scholar]
- 2. Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-22. [DOI] [PubMed] [Google Scholar]
- 3. Wilcox RA. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:151-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nashan D, Faulhaber D, Stander S, Luger TA, Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;156:1-10. [DOI] [PubMed] [Google Scholar]
- 5. Vakeva L, Sarna S, Vaalasti A, Pukkala E, Kariniemi AL, Ranki A. A retrospective study of the probability of the evolution of parapsoriasis en plaques into mycosis fungoides. Acta Derm Venereol. 2005;85:318-23. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita T, Abbade LP, Marques ME, Marques SA. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-28; quiz 829-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, Stevens S, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-63. [DOI] [PubMed] [Google Scholar]
- 8. Haththotuwa R, Zilinskiene L, Oliff J, Vydianath B, Amel-Kashipaz R, Stevens A, et al. Biopsy correlation of surface area vs. single-axis measurements on computed tomography scan of lymph nodes in patients with erythrodermic mycosis fungoides and Sezary syndrome. Br J Dermatol. 2017;177:877-8. [DOI] [PubMed] [Google Scholar]
- 9. Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004;18:397-415. [DOI] [PubMed] [Google Scholar]
- 10. Shimauchi T, Nishio D, Isoda H, Sugita K, Kabashima K, Tokura Y. Leukaemic mycosis fungoides in an atomic bomb survivor with lung and renal cancers. Clin Exp Dermatol. 2009;34:e322-4. [DOI] [PubMed] [Google Scholar]
- 11. Sugita K, Kabashima K, Nakamura M, Tokura Y. Drug-induced papuloerythroderma: analysis of T-cell populations and a literature review. Acta Derm Venereol. 2009;89:618-22. [DOI] [PubMed] [Google Scholar]
- 12. Fierro MT, Novelli M, Savoia P, Cambieri I, Quaglino P, Osella-Abate S, et al. CD45RA+ immunophenotype in mycosis fungoides: clinical, histological and immunophenotypical features in 22 patients. J Cutan Pathol. 2001;28:356-62. [DOI] [PubMed] [Google Scholar]
- 13. Hodak E, David M, Maron L, Aviram A, Kaganovsky E, Feinmesser M. CD4/CD8 double-negative epidermotropic cutaneous T-cell lymphoma: an immunohistochemical variant of mycosis fungoides. J Am Acad Dermatol. 2006;55:276-84. [DOI] [PubMed] [Google Scholar]
- 14. Kempf W, Kazakov DV, Cipolat C, Kutzner H, Roncador G, Tomasini D. CD4/CD8 double negative mycosis fungoides with PD-1 (CD279) expression--a disease of follicular helper T-cells?. Am J Dermatopathol. 2012;34:757-61. [DOI] [PubMed] [Google Scholar]
- 15. Massone C, Crisman G, Kerl H, Cerroni L. The prognosis of early mycosis fungoides is not influenced by phenotype and T-cell clonality. Br J Dermatol. 2008;159:881-6. [DOI] [PubMed] [Google Scholar]
- 16. Nagase K, Shirai R, Okawa T, Inoue T, Misago N, Narisawa Y, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90. [DOI] [PubMed] [Google Scholar]
- 17. Nickoloff BJ. Light-microscopic assessment of 100 patients with patch/plaque-stage mycosis fungoides. Am J Dermatopathol. 1988;10:469-77. [DOI] [PubMed] [Google Scholar]
- 18. Rodney IJ, Kindred C, Angra K, Qutub ON, Villanueva AR, Halder RM, et al. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808-14. [DOI] [PubMed] [Google Scholar]
- 19. Wain EM, Orchard GE, Mayou S, Atherton DJ, Misch KJ, Russell-Jones R. Mycosis fungoides with a CD56+ immunophenotype. J Am Acad Dermatol. 2005;53:158-63. [DOI] [PubMed] [Google Scholar]
- 20. Santucci M, Pimpinelli N, Massi D, Kadin ME, Meijer CJ, Muller-Hermelink HK, et al. Cytotoxic/natural killer cell cutaneous lymphomas. Report of EORTC Cutaneous Lymphoma Task Force Workshop. Cancer. 2003;97:610-27. [DOI] [PubMed] [Google Scholar]
- 21. Vermeer MH, Geelen FA, Kummer JA, Meijer CJ, Willemze R. Expression of cytotoxic proteins by neoplastic T cells in mycosis fungoides increases with progression from plaque stage to tumor stage disease. Am J Pathol. 1999;154:1203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zackheim HS, Amin S, Kashani-Sabet M, McMillan A. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J Am Acad Dermatol. 1999;40:418-25. [DOI] [PubMed] [Google Scholar]
- 23. Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-66. [DOI] [PubMed] [Google Scholar]
- 24. Shimauchi T, Sugita K, Nishio D, Isoda H, Abe S, Yamada Y, et al. Alterations of serum Th1 and Th2 chemokines by combination therapy of interferon-gamma and narrowband UVB in patients with mycosis fungoides. J Dermatol Sci. 2008;50:217-25. [DOI] [PubMed] [Google Scholar]
- 25. Haruyama S, Sugita K, Kawakami C, Nakamura M, Tokura Y. Development of a prominent granulomatous eruption after interferon-gamma therapy in a patient with mycosis fungoides. Acta Derm Venereol. 2010;90:190-1. [DOI] [PubMed] [Google Scholar]
- 26. Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2017. Eur J Cancer. 2017;77:57-74. [DOI] [PubMed] [Google Scholar]
- 27. Hamada T, Sugaya M, Tokura Y, Ohtsuka M, Tsuboi R, Nagatani T, et al. Phase I/II study of the oral retinoid X receptor agonist bexarotene in Japanese patients with cutaneous T-cell lymphomas. J Dermatol. 2017;44:135-42. [DOI] [PubMed] [Google Scholar]
- 28. Leuchte K, Schlaak M, Stadler R, Theurich S, von Bergwelt-Baildon M. Innovative Treatment Concepts for Cutaneous T-Cell Lymphoma Based on Microenvironment Modulation. Oncol Res Treat. 2017;40:262-9. [DOI] [PubMed] [Google Scholar]