Abstract
Mantle cell lymphoma (MCL) is an incurable type of B-cell lymphoma. It is typically composed of small-to-medium-sized cleaved lymphoid cells with cyclin D1 protein expression due to the chromosomal translocation t(11;14)(q13;q32). Even with the development of rituximab, an anti-CD20 antibody drug, the long-term outcome of patients with MCL has not improved. Recently, new agents have been used in clinical settings, and the outcome of patients with MCL is expected to improve. The treatment of MCL may be at a turning point from intensive chemotherapy to chemotherapy-free treatment. In this study, a recent progress in the diagnosis and treatment of MCL is reviewed.
Keywords: bendamustine, bortezomib, ibrutinib, lymphoma, mantle-cell
Mantle cell lymphoma (MCL) is a mature B-cell neoplasm accounting for 2%–6% of non-Hodgkin lymphomas.1, 2 Until 1991, the following names for lymphoma were used to refer to MCL: centrocytic lymphoma, intermediate lymphocytic lymphoma, mantle-zone lymphoma, lymphocytic lymphoma of intermediate differentiation, multiple lymphomatous polyposis, and mantle cell-derived lymphoma. Their association with a specific chromosomal abnormality (t(11;14)(q13;q32))3 and the identification of a novel cyclin gene at the breakpoint locus4 lead to the identification of MCL.5 In most patients with MCL, the cyclin D1 gene CCND1 on 11q13 is relocated adjacent to the immunoglobulin heavy chain gene IGH on 14q32 due to chromosomal translocation t(11;14)(q13;q32), and this results in the overexpression of cyclin D1 protein in the nucleus (Fig. 1).6
Fig. 1.
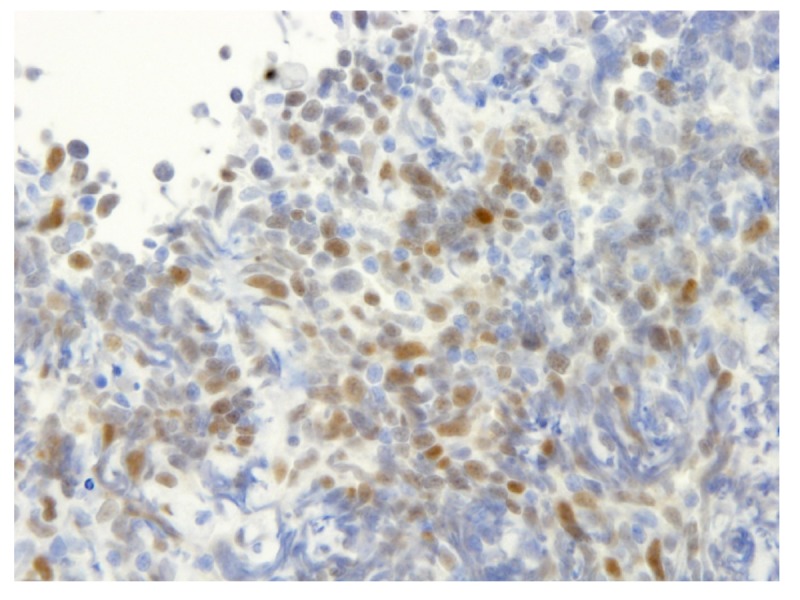
Immunohistochemistry of cyclin D1 protein. Cyclin D1 protein was positive in the nuclei of mantle cell lymphoma cells.
Despite its small-to-medium-sized cells and indolent clinical behavior, patients with MCL frequently experience relapse and have a poor prognosis even after intensive chemotherapy. With the development of novel agents, the outcomes of patients with MCL substantially improved even in actual clinical settings.7 This review discussed the recent progress in the diagnosis and treatment of MCL particularly focusing on the novel agents available in Japan, which include bendamustine, bortezomib, and ibrutinib.
DIAGNOSIS
Typical MCL
MCL is commonly composed of small-to-medium-sized cleaved lymphoid cells, which are proliferating diffusely or vaguely in the nodules. Immunohistochemistry of lymphoma cells shows positivity for CD5, CD10, CD19, CD20, CD79a, and cyclin D1. Cyclin D1 positivity in the nucleus due to typical chromosomal translocation t(11;14)(q13;q32) supports the diagnosis, although cyclin D1 is also overexpressed in patients with multiple myeloma with the same chromosome aberration and hairy cell leukemia without any translocation, both of which are readily differentiated by the typical characteristics of their morphology and clinical presentation. MCL is usually in advanced stage when diagnosed, and extranodal involvement is common. The clinical course is indolent, and relapse frequently occurs after the standard chemotherapy for non-Hodgkin lymphoma. The MCL international prognostic index (MIPI) was elaborated with 455 patients with advanced stage MCL.8 The index was composed of age, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase level, and white blood cell count, and it was modified with the incorporation of Ki-67 positivity.9 Furthermore, tumor cells can be detected using polymerase chain reaction in the peripheral blood and/or bone marrow in 90% of patients with advanced MCL upon diagnosis, and minimal residual disease assessment at the level of 10-5 has an impact on predicting prognosis.10 In addition, cyclin D1-negative MCL exist with cyclin D2 or cyclin D3 overexpression, which may substitute cyclin D1.11 Cyclin D1 negativity is associated with better prognosis independent from other risk factors.12
Leukemic non-nodal MCL
t(11;14)(q13;q32) with complex cytogenetics expressing cyclin D1 mRNA was observed in a patient with prolymphocytic leukemia.13 Then, the leukemic variants of MCL were repeatedly reported.14 Among them, the indolent subtype of leukemic MCL was considered as leukemic non-nodal MCL, which developed through a pathway different from that of typical MCL.15, 16 This subtype is notably lacking in the expression of SOX11 protein, which is highly expressed in typical MCL.16
Blastoid and pleomorphic variant of MCL
The acquisition of TP53 mutations and other oncogenic abnormalities leads to the blastoid and pleomorphic variants of MCL that have poor prognosis.17, 18 These cases often have tetraploid DNA contents.19 The emergence of tetraploids can be recapitulated with mouse fibroblasts transduced with TP53 mutation that are selected under K condition.20 In evolutionary biology, K-selection was initially used to describe species evolution in isolated islands; K is the carrying capacity of the environment, i.e., the number of individuals in a population of a given species at the population equilibrium. Thus, the development of these variants may be associated with K selection during the evolution of MCL cells.
In-situ mantle cell neoplasia
Cells with cyclin D1 protein overexpression may be incidentally found in the mantle zone of lymphoid follicles in otherwise reactive lymph nodes. These cells may be the precursors of MCL; however, a low risk of developing overt MCL was observed.16
TREATMENT
Rituximab-based intensive chemotherapy
With the development of rituximab, anti-CD20 antibody drug, immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has substantially improved the outcome of patients with aggressive B-cell lymphomas. However, the long-term outcome of patients with MCL has not improved with the R-CHOP regimen. Intensive immunochemotherapy regimens, such as rituximab-based hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (R-HCVAD/MA), and MCL2 protocols conducted by the Nordic Lymphoma Group have significantly improved the outcomes of younger patients with MCL in general (median overall survival [OS]: 11-13 years), which impressed the efficacy of high-dose cytarabine as the frontline treatment of MCL (Table 1).21, 22 Furthermore, rituximab-based chemotherapy with high-dose cytarabine followed by autologous stem cell transplantation and rituximab maintenance was a better approach for younger patients with MCL in randomized studies.23, 24 Even after the introduction of intensive chemotherapy, MIPI can still discriminate risk groups and identify patients with poor prognosis.25 Recently, Eskelund et al. have evaluated the prognostic value of recurrent genetic aberrations in MCL and found that TP53 mutations but not deletions retained the prognostic impact for OS in multivariate analyses.26 TP53-mutated MCL cells may not be eradicated by intensive therapy, and patients with TP53-mutated MCL still have a dismal outcome with a median OS of 1.8 years.26 Thus, new treatment strategy using novel agents other than intensive chemotherapy should be explored for patients with TP53 mutations. Intriguingly, MIPI loses the potency of risk discrimination in patients without TP53 mutations, and this indicated the need for a new prognostic model other than MIPI.
Table 1.
Selective studies of intensive immunochemotherapy for untreated MCL
| Phase | Treatment | n | Median age, years (range) | OR, % (CR) | Survival | TRM | Reference |
| II single-center | R-HCVAD/MA | 97 | 61 (41–80) | 97 (87) | median FFS: 4.8 years, OS: 10.7 years, 5-years FFS: 49%, OS: 67%, 10-year FFS: 26%, OS: 52% 15-year FFS: 22%, OS: 33% | 4% | Chihara et al. 201621 |
| II multicenter | R-Maxi-CHOP + HDA + ASCT (MCL2) | 159 | 56 (32–65) | 96 (82) | median PFS: 8.5 years, OS: 12.7 years | 5% | Eskelund et al. 201622 |
| III randomized | R-CHOP + dexa-BEAM/TC-ASCT vs R-CHOP/R-DHAP + TAM-ASCT | 234 vs 232 | 55 (IQR: 49–60) | 97 (76) vs 98 (83) | 5-year TTF: 40% vs 65%, OS: 68% vs 76% median TTF: 3.9 years vs 9.1 years, OS: NR vs 9.8 years | 3.4% vs 3.4% | Hermine et al. 201623 |
| III randomized | R-DHAP + ASCT + none vs Rm | 299 (120 vs 120)* | 57 (27–65) | 83 (77) | 4-year PFS: 68%, OS: 78% 4-year EFS: 61% vs 79%, OS: 80% vs 89%* | 4% | Le Gouill et al. 201724 |
| II multicenter | R-High-CHOP/CHASER + LEED-ASCT | 45 | 59 (35–65) | 96 (82) | median PFS 3.7 years, 2-year PFS 77% 5-year PFS: 52%, OS: 71% | 0% | Ogura et al. 201847 |
*after transplantation.
ASCT, autologous stem cell transplantation; CHASER, cyclophosphamide, high-dose cytarabine, dexamethasone, etoposide, and rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response rate; dexa-BEAM/TC, dexamethasone, carmustine, etoposide, cytarabine, and melphalan/total body irradiation and cyclophosphamide; DHAP, dexamethasone, high-dose cytarabine, and cisplatin; EFS, event-free survival; FFS, failure-free survival; HCVAD/MA, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose cytarabine and methotrexate; HDA, high-dose cytarabine; IQR, interquartile range; LEED, melphalan, cyclophosphamide, etoposide, and dexamethasone; MCL, mantle cell lymphoma; n, number of patients; NR, not reached; OR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab; Rm, rituximab maintenance; TAM, total body irradiation, high dose cytarabine, and melphalan; TRM, treatment-related mortality; TTF, time-to-treatment failure.
Bendamustine
Bendamustine is composed of two chemical structures. One is that of alkylating agents and the other is that of purine analogues. It was developed in East Germany in the Cold War era and found to be effective for non-Hodgkin lymphoma in the 21st century. Bendamustine plus rituximab is the first regimen that is non-inferior to the R-CHOP regimen for the treatment of untreated indolent lymphoma and MCL in the randomized clinical trial (Table 2).27, 28 Because of fewer toxic effects, bendamustine is a preferred substitute for all 4 drugs of the CHOP regimen. Compared with the R-HCVAD/MA regimen as a pre-transplant induction regimen for younger patients, bendamustine plus rituximab showed a similar efficacy, and it can achieve a deep remission with a better mobilization success.29 Furthermore, the phase 2 study of immunochemotherapy followed by autologous stem cell transplantation for transplant-eligible patients with MCL using bendamustine plus rituximab and then high-dose cytarabine plus rituximab showed a promising result and must be further assessed in comparative studies.30 In addition, low-dose cytarabine instead of high-dose one may have a role in the treatment of MCL and must be studied in randomized trials.31
Table 2.
Selective studies of bendamustine-based therapy for untreated MCL
| Phase | Treatment | n | Median age, years (range) | OR, % (CR) | Survival | TRM | Reference |
| III randomized | R-Bendamustine vs R-CHOP | 46 vs 48 | 70 (65–74) | 93 (40)* vs 91 (30)* | median PFS: 35.4 months vs 22.1 months | 0.4%* vs 2.0%* | Rummel et al. 201327 |
| III randomized | R-Bendamustine vs R-CHOP/R-CVP | 34 vs 33 | 60 (28–84)* vs 58 (25–86)* | 94 (50) vs 85 (27) | NA | 0.5%* vs 0.5%* | Flinn et al. 201428 |
| II two-center | R-Bendamustine + HDA + ASCT | 23 | 57 (42–69) | 96 (96) | 1-year PFS: 96%, OS: 96% | 0% | Armand et al. 201630 |
| II randomized, closed prematurely | R-HCVAD/MA + ASCT vs R-Bendamustine + ASCT | 17 vs 35 | 59 (44–66) vs 57 (33–64) | 94 (35) vs 83 (40) | 2-year PFS: 82% vs. 81%, OS: 88% vs. 87% | NA | Chen et al. 201729 |
| II multicenter | R-Bendamustine + low-dose cytarabine (RBAC500) | 57 | 71 (61–79) | 91 (91) | 2-year PFS: 81%, OS: 86% | 0% | Visco et al. 201731 |
*not restricted to MCL.
ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response rate; CVP, cyclophosphamide, vincristine, and prednisone; HDA, high-dose cytarabine; HCVAD/MA, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose cytarabine and methotrexate; MCL, mantle cell lymphoma; n, number of patients; NA, not available; OR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab; TRM, treatment-related mortality.
Bortezomib
Bortezomib is the first proteasome inhibitor used in clinical practice. The use of bortezomib rather than vincristine in the standard R-CHOP regimen (VR-CAP) had a better efficacy than the R-CHOP regimen for transplant-ineligible patients at the cost of increased hematologic toxicity (Table 3).32 The post-hoc analysis of this study showed the clear relationship between higher cumulative bortezomib dose and longer OS, and this result indicates the role of bortezomib in the treatment strategy for elderly patients with MCL in the condition of improved supportive care and optimization of bortezomib dose.33 Notably, bortezomib-based therapy is associated with a high incidence of painful peripheral neuropathy and herpes zoster, which requires dose adjustment and antiviral prophylaxis particularly in elderly patients.34–36
Table 3.
Selective studies of bortezomib-based therapy for untreated MCL
| Phase | Treatment | n | Median age, years (range) | OR, % (CR) | Survival | TRM | Reference |
| II multicenter | RiPAD + C | 39 | 72 (65–80) | 79 (59) | median PFS: 26 months, OS: NR | 5% | Houot et al. 201235 |
| III randomized | R-CHOP vs VR-CAP | 244 vs 243 | 66 (26–88) | 89 (42) vs 92 (53) | median PFS: 14.4 months vs 24.7 months. OS: 56.3 months vs NR 4-year OS: 54% vs 64% | 3% vs 2% | Robak et al. 201532 |
| II two-center | Bortezomib + R + HCVAD/MA | 95 | 61 (38–75) | 100 (82) | median TTF: 55 months | 4% | Romaguera et al. 201836 |
| II four-center | Bortezomib + R + modified HCVAD + Rm | 30 | 61 (48–74) | 90 (77) | median PFS: 8.1 years 6-year PFS: 53%, OS: 70% | 3%* | Chang et al. 201848 |
*following allogeneic stem cell transplant.
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response rate; HCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MA, high-dose cytarabine and methotrexate; MCL, mantle cell lymphoma; n, number of patients; NR, not reached; OR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab; RiPAD + C, rituximab, bortezomib, doxorubicin, dexamethasone, and chlorambucil; Rm, rituximab maintenance; TRM, treatment-related mortality; TTF, time-to-treatment failure; VR-CAP, bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
Ibrutinib
Ibrutinib is a first-in-class drug taken once daily via oral route, and it is a covalent inhibitor of Bruton tyrosine kinase, which is important for signaling via B-cell receptors and other B-cell surface receptors. Ibrutinib binds covalently to the kinase and inhibits B-cell receptor signaling leading to downstream mitigation of cell growth, proliferation, survival, adhesion, and migration. Bruton tyrosine kinase is commonly overexpressed in MCL, and its inhibition by ibrutinib induces apoptosis.37 A phase 2 study of ibrutinib involving patients with relapsed or refractory MCL had the best overall response rate (OR) of 68%, a complete response rate (CR) of 21%, and a median progression-free survival (PFS) of 13.9 months,38 and a phase 3 trial had an OR of 72%, a CR of 19%, and a median PFS of 14.6 months (Table 4).39 Interestingly, as observed in patients with chronic lymphoid leukemia, ibrutinib dose-dependently inhibits the adhesion and migration of MCL cells, resulting in transient lymphocytosis in patients with MCL who experience bone marrow involvement.40, 41 The common or serious side effects of ibrutinib include bleeding due to platelet dysfunction,42 diarrhea, rash, and atrial fibrillation.38, 39
Table 4.
Selective studies of chemotherapy-free treatment for MCL
| Phase | Treatment | n* | Median age, years (range) | OR, % (CR) | Survival | TRM | Reference |
| II multicenter | Ibrutinib | 111 | 68 (40-84) | 68 (21) | median PFS: 13.9 months 18-month OS: 58% | 1% | Wang et al. 201338 |
| II multicenter | Ibrutinib | 16 | 72 (55-83) | 88 (13) | 6-month PFS: 88% | 0% | Maruyama et al. 201649 |
| III randomized | Ibrutinib vs temsirolimus | 280 | 68 (IQR: 13) | 72 (19) vs 40 (1) | median PFS: 14.6 months vs 6.2 months 1-year OS: 68% vs 61% | 6% vs 8% | Dreyling et al. 201639 |
| II single-center | Ibrutinib and rituximab | 50 | 67 (45–86) | 88 (44) | 1-year PFS: 75%, OS: 86% | 2% | Wang et al. 201643 |
| II multicenter | Lenalidomide and rituximab | 38† | 65 (42–86) | 92 (64) | 3-year PFS: 80%, OS: 90%, 5-year PFS: 64%, OS: 77% | 0% | Ruan et al. 201845 |
| II two-center | Ibrutinib and venetoclax | 24‡ | 68 (47–81) | 71 (71) | 1-year PFS: 75%, OS: 79% | 8% | Tam et al. 201846 |
*relapsed or refractory MCL if not otherwise described. †untreated MCL. ‡not restricted to relapsed or refractory MCL.
CR, complete response rate; IQR, interquartile range; MCL, mantle cell lymphoma; n, number of patients; OR, overall response rate; OS, overall survival; PFS, progression-free survival; TRM, treatment-related mortality.
Moving towards chemotherapy-free treatment
Immunochemotherapy recommended for young fit patients with MCL, which is usually combined with autologous stem cell transplantation, is rarely performed in frail patients with comorbidities and elderly patients. If the risk among patients with MCL is low based on MIPI, the “watch and wait” strategy is an option particularly for elderly patients. However, novel agents without severe adverse effects are available and are given in outpatient clinics. Among them, the single use of bendamustine or in combination with rituximab is effective. Alternatively, chemotherapy-free regimens are preferred for elderly patients because it causes less severe hematologic toxicity. Ibrutinib can be administered orally, and the dose is adjusted for each patient. In addition, the combination of ibrutinib and rituximab showed favorable results for the treatment of relapsed MCL in the single-center phase 2 trial (Table 4).43 Although ibrutinib antagonized the rituximab anti-lymphoma effect in a mouse model partly via the off-target inhibition of interleukin-2-inducible tyrosine kinase in natural killer cells,44 a randomized trial of ibrutinib with rituximab against standard immunochemotherapy as first-line therapy is being conducted (EudraCT Number: 2015-000832-13).
Furthermore, lenalidomide, a second-generation immunomodulatory compound, is an emerging new player in the treatment of lymphoma, and it was approved for the treatment of patients with relapsed or refractory MCL by the Food and Drug Administration in the Unites States in 2013. It has been approved in Japan for adult T-cell leukemia/lymphoma. A long-term follow-up in the phase 2 study of the R-squared regimen, i.e., lenalidomide and rituximab, for untreated MCL had 5-year PFS of 64% and OS of 77%; The OR was 92% and CR was 64%.45 A randomized phase 3 trial on lenalidomide compared with placebo in combination with rituximab is being conducted for the treatment of relapsed or refractory indolent lymphoma (ClinicalTrials Number: NCT01938001), and favorable results were observed. Another emerging compound is venetoclax, a BCL-2 inhibitor, which has been approved by the Food and Drug Administration for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma. The combination of venetoclax and ibrutinib improved the outcome of patients with MCL who had been predicted to have poor outcomes with current therapy.46
CONCLUSIONS
For young fit patients with MCL, intensive immunochemotherapy with autologous stem cell transplantation is still recommended. However, novel approaches should be examined for a high-risk subgroup of patients with TP53 mutations. In addition, less intensive regimens using new agents should be compared with intensive approaches, and the establishment of frontline chemotherapy-free treatment is eagerly awaited. We should focus on the progress of clinical trials on new agents since we are at a turning point from intensive immunochemotherapy to less toxic chemotherapy-free treatment. In the near future, the outcome of patients with MCL is expected to be further improved.
Acknowledgments
Acknowledgments: I would like to thank Editage (www.editage.jp) for English language editing. This study is partially supported by a research fund from Chugai Pharmaceutical Co., Ltd.
The author declares no conflict of interest.
REFERENCES
- 1. The Non-Hodgkin’s Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909-18. [PubMed] [Google Scholar]
- 2. Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536-45. doi: 10.1111/bjh.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78:259-63. [PubMed] [Google Scholar]
- 4. Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, et al. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512-5. doi: 10.1038/350512a0 [DOI] [PubMed] [Google Scholar]
- 5. Banks PM, Chan J, Cleary ML, Delsol G, De Wolf-Peeters C, Gatter K, et al. Mantle cell lymphoma, a proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992;16:637-40. [DOI] [PubMed] [Google Scholar]
- 6. Yang WI, Zukerberg LR, Motokura T, Arnold A, Harris NL. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994;145:86-96. [PMC free article] [PubMed] [Google Scholar]
- 7. Smith A, Roman E, Appleton S, Howell D, Johnson R, Burton C, et al. Impact of novel therapies for mantle cell lymphoma in the real world setting: a report from the UK’s Haematological Malignancy Research Network (HMRN). Br J Haematol. 2018;181:215-28. doi: 10.1111/bjh.15170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558-65. [DOI] [PubMed] [Google Scholar]
- 9. Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, et al. Prognostic value of Ki-67 Index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-94. doi: 10.1200/JCO.2015.63.8387 [DOI] [PubMed] [Google Scholar]
- 10. Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Bottcher S, Asnafi V, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115:3215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315-21. doi: 10.1182/blood-2005-04-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, et al. Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253-61. [PubMed] [Google Scholar]
- 13. Kobayashi H, Kitano K, Saito H, Aoki K, Narita A, Terada N, et al. Overexpression of the PRAD1 oncogene in a patient with prolymphocytic leukemia with t(11;14)(q13;q32). Cancer Genet Cytogenet. 1995;84:69-72. [DOI] [PubMed] [Google Scholar]
- 14. Ruchlemer R, Parry-Jones N, Brito-Babapulle V, Attolico I, Wotherspoon AC, Matutes E, et al. B-prolymphocytic leukaemia with t(11;14) revisited: a splenomegalic form of mantle cell lymphoma evolving with leukaemia. Br J Haematol. 2004;125:330-6. doi: 10.1111/j.1365-2141.2004.04913.x [DOI] [PubMed] [Google Scholar]
- 15. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-90. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122:3416-23. doi: 10.1172/JCI61272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greiner TC, Moynihan MJ, Chan WC, Lytle DM, Pedersen A, Anderson JR, et al. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood. 1996;87:4302-10. [PubMed] [Google Scholar]
- 18. Zoldan MC, Inghirami G, Masuda Y, Vandekerckhove F, Raphael B, Amorosi E, et al. Large-cell variants of mantle cell lymphoma: cytologic characteristics and p53 anomalies may predict poor outcome. Br J Haematol. 1996;93:475-86. [DOI] [PubMed] [Google Scholar]
- 19. Ott G, Kalla J, Ott MM, Schryen B, Katzenberger T, Muller JG, et al. Blastoid variants of mantle cell lymphoma: frequent bcl-1 rearrangements at the major translocation cluster region and tetraploid chromosome clones. Blood. 1997;89:1421-9. [PubMed] [Google Scholar]
- 20. Chikatsu N, Nakamura Y, Sato H, Fujita T, Asano S, Motokura T, et al. p53 mutations and tetraploids under r- and K-selection. Oncogene. 2002;21:3043-9. doi 10.1038/sj.onc.1205413 [DOI] [PubMed] [Google Scholar]
- 21. Chihara D, Cheah CY, Westin JR, Fayad LE, Rodriguez MA, Hagemeister FB, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol. 2016;172:80-8. doi: 10.1111/bjh.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eskelund CW, Kolstad A, Jerkeman M, Raty R, Laurell A, Eloranta S, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175:410-8. doi: 10.1111/bjh.14241 [DOI] [PubMed] [Google Scholar]
- 23. Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565-75. doi: 10.1016/S0140-6736(16)00739-X [DOI] [PubMed] [Google Scholar]
- 24. Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-60. doi: 10.1056/NEJMoa1701769 [DOI] [PubMed] [Google Scholar]
- 25. Geisler CH, Kolstad A, Laurell A, Raty R, Jerkeman M, Eriksson M, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010;115:1530-3. doi: 10.1182/blood-2009-08-236570 [DOI] [PubMed] [Google Scholar]
- 26. Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-10. doi: 10.1182/blood-2017-04-779736 [DOI] [PubMed] [Google Scholar]
- 27. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203-10. doi: 10.1016/S0140-6736(12)61763-2 [DOI] [PubMed] [Google Scholar]
- 28. Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944-52. doi: 10.1182/blood-2013-11-531327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen RW, Li H, Bernstein SH, Kahwash S, Rimsza LM, Forman SJ, et al. RB but not R-HCVAD is a feasible induction regimen prior to auto-HCT in frontline MCL: results of SWOG Study S1106. Br J Haematol. 2017;176:759-69. doi: 10.1111/bjh.14480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armand P, Redd R, Bsat J, Mayuram S, Giardino A, Fisher DC, et al. A phase 2 study of rituximab-bendamustine and rituximab-cytarabine for transplant-eligible patients with mantle cell lymphoma. Br J Haematol. 2016;173:89-95. doi: 10.1111/bjh.13929 [DOI] [PubMed] [Google Scholar]
- 31. Visco C, Chiappella A, Nassi L, Patti C, Ferrero S, Barbero D, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4:e15-e23. doi: 10.1016/S2352-3026(16)30185-5 [DOI] [PubMed] [Google Scholar]
- 32. Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944-53. doi: 10.1056/NEJMoa1412096 [DOI] [PubMed] [Google Scholar]
- 33. Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, et al. Association between bortezomib dose intensity and overall survival in mantle cell lymphoma patients on frontline VR-CAP in the phase 3 LYM-3002 study. Leuk Lymphoma. 2017;1-8. doi: 10.1080/10428194.2017.1321750 [DOI] [PubMed] [Google Scholar]
- 34. Solh M, Fisher RI, Goy A, de Vos S, Bernstein SH, Esseltine DL, et al. Herpes zoster complicating bortezomib therapy of relapsed/refractory indolent B-cell and mantle cell lymphoma: an analysis of two phase II trials. Leuk Lymphoma. 2013;54:2185-9. doi: 10.3109/10428194.2013.772294 [DOI] [PubMed] [Google Scholar]
- 35. Houot R, Le Gouill S, Ojeda Uribe M, Mounier C, Courby S, Dartigeas C, et al. Combination of rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil (RiPAD+C) as first-line therapy for elderly mantle cell lymphoma patients: results of a phase II trial from the GOELAMS. Ann Oncol. 2012;23:1555-61. doi: 10.1093/annonc/mdr450 [DOI] [PubMed] [Google Scholar]
- 36. Romaguera JE, Wang M, Feng L, Fayad LE, Hagemeister F, McLaughlin P, et al. Phase 2 trial of bortezomib in combination with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with bortezomib, rituximab, methotrexate, and cytarabine for untreated mantle cell lymphoma. Cancer. 2018;124:2561-9. doi: 10.1002/cncr.31361 [DOI] [PubMed] [Google Scholar]
- 37. Cinar M, Hamedani F, Mo Z, Cinar B, Amin HM, Alkan S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by ibrutinib induces apoptosis. Leuk Res. 2013;37:1271-7. doi: 10.1016/j.leukres.2013.07.028 [DOI] [PubMed] [Google Scholar]
- 38. Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507-16. doi: 10.1056/NEJMoa1306220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770-8. doi: 10.1016/S0140-6736(15)00667-4 [DOI] [PubMed] [Google Scholar]
- 40. Chang BY, Francesco M, De Rooij MF, Magadala P, Steggerda SM, Huang MM, et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122:2412-24. doi: 10.1182/blood-2013-02-482125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furtado M, Wang ML, Munneke B, McGreivy J, Beaupre DM, Rule S. Ibrutinib-associated lymphocytosis corresponds to bone marrow involvement in mantle cell lymphoma. Br J Haematol. 2015;170:131-4. doi: 10.1111/bjh.13275 [DOI] [PubMed] [Google Scholar]
- 42. Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783-7. doi: 10.1038/leu.2014.247 [DOI] [PubMed] [Google Scholar]
- 43. Wang ML, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17:48-56. doi: 10.1016/S1470-2045(15)00438-6 [DOI] [PubMed] [Google Scholar]
- 44. Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123:1957-60. doi: 10.1182/blood-2014-01-547869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruan J, Martin P, Christos P, Cerchietti L, Tam W, Shah B, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132:2016-25. doi: 10.1182/blood-2018-07-859769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-23. doi: 10.1056/NEJMoa1715519 [DOI] [PubMed] [Google Scholar]
- 47. Ogura M, Yamamoto K, Morishima Y, Wakabayashi M, Tobinai K, Ando K, et al. R-High-CHOP/CHASER/LEED with autologous stem cell transplantation in newly diagnosed mantle cell lymphoma: JCOG0406 STUDY. Cancer Sci. 2018;109:2830-40. doi: 10.1111/cas.13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang JE, Carmichael LL, Kim K, Peterson C, Yang DT, Traynor AM, et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab produces durable remissions in mantle cell lymphoma: A Wisconsin Oncology Network Study. Clin Lymphoma Myeloma Leuk. 2018;18:e61-e7. doi: 10.1016/j.clml.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maruyama D, Nagai H, Fukuhara N, Kitano T, Ishikawa T, Shibayama H, et al. Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma. Cancer Sci. 2016;107:1785-90. doi: 10.1111/cas.13076 [DOI] [PMC free article] [PubMed] [Google Scholar]


