Abstract
Background
Nutritional status is strongly associated with prognosis in cancer patients. Controlling Nutritional Status (CONUT) score is a nutritional marker based on serum albumin, cholesterol, and total lymphocyte count. We investigated the prognostic significance of a combination of the tumor marker carcinoembryonic antigen (CEA) and CONUT score (T-CONUT) in colorectal cancer (CRC) patients.
Methods
A total of 522 patients who underwent surgery for CRC at our hospital were retrospectively enrolled in this study.
Results
Patients were divided into groups based on the results of receiver operating characteristic (ROC) curve analysis as follows: CONUThigh (CONUT score ≥ 3) and CONUTlow (CONUT score < 3), and CEAlow (< 5 ng/mL) and CEAhigh (≥ 5 ng/mL). The 5-year overall survival (OS) rates of patients in the CONUTlow and CONUThigh groups were 76.0% and 53.9%, respectively (P < 0.0001), and in the CEAlow and CEAhigh groups were 80.7% and 47.6%, respectively (P < 0.0001). Regarding T-CONUT, the 5-year OS rates of patients with CEAlow/CONUTlow, CEAlow/CONUThigh, CEAhigh/CONUTlow, and CEAhigh/CONUThigh were 84.7%, 69%, 55.3%, and 36.1%, respectively (P < 0.0001). Multivariate analysis identified T-CONUT score as an independent prognostic indicator in CRC patients.
Conclusion
T-CONUT may be a useful tool for predicting prognosis in CRC patients.
Keywords: carcinoembryonic antigen, colorectal cancer, Controlling Nutritional Status score, prognosis
Recent advances in surgical techniques, perioperative management, and chemotherapy, including the use of molecular targeting drugs, have improved the prognosis of colorectal cancer (CRC) patients; however, it still ranks fourth with respect to cancer-related deaths worldwide.1 Although colectomy with regional lymph node dissection is the main curative treatment for CRC, many patients experience recurrence even after complete removal of the tumor (R0 resection). Recent progress in chemotherapy has improved the prognosis of unresectable advanced and recurrent CRC,2 while early detection of recurrence has been shown to increase survival after curative colectomy for CRC.3–4 It is therefore important to determine the factors affecting postoperative prognosis in patients with CRC. To this end, serum tumor markers (TMs) are easy to measure and potentially useful for diagnosis, predicting survival rates, and monitoring recurrence following surgery. Carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 are the most commonly used TMs for diagnosis, treatment monitoring, and predicting the prognosis in patients with CRC.5
Recent studies demonstrated that the prognoses of various types of cancers were also affected by patient-related factors, including inflammation, immunocompetence, and nutrition, with the correlation between nutritional status and cancer prognosis being of particular interest. Nutritional status has been shown to be an important risk factor associated with postoperative morbidity and mortality,6–8 and various tools for assessing nutritional status have been reported.9–11 Furthermore, recent studies also demonstrated a close association between nutritional status and prognosis in cancer patients, including patients with CRC.12–14 The Controlling Nutritional Status (CONUT) score, which includes measures of serum albumin, total cholesterol, and peripheral lymphocyte count, was recently reported as a new tool for evaluating nutritional status.15 The CONUT score has been reported to be closely associated with prognosis in gastric cancer, esophageal cancer, and CRC.16–18 Serum TMs in CRC patients are mainly derived from the cancer itself, while the CONUT score reflects the patient’s nutritional status, and both approaches have been shown to be useful in predicting the prognosis of CRC patients. The different origins of TMs and CONUT score suggested that their combination might be superior to either TMs or CONUT score alone for predicting the prognosis of CRC patients. The current study thus aimed to evaluate the prognostic significance of the combination of serum TMs and CONUT score in patients with CRC.
MATERIALS AND METHODS
Patients
This retrospective study enrolled 522 stage I – IV CRC patients who underwent colorectal resection at our hospital between January 2007 and December 2015. The clinicopathologic findings were assessed according to the 8th edition of the Japanese Classification of Colorectal Carcinoma.19 Patients were checked periodically for early recurrence by diagnostic imaging (chest X-ray, colonoscopy, ultrasonography, and computed tomography). Causes of death and patterns of recurrence were determined by reviewing medical records, including laboratory data, ultrasonography, computed tomography, scintigrams, and laparotomies, or by direct inquiry with family members. We collected data on preoperative blood tests within one month before surgery, including serum albumin, total cholesterol, and total peripheral blood lymphocyte count (TLC) from the patients’ records. This study was approved by the Institutional Review Board of Tottori University (Approved number: 17A105).
CONUT score
CONUT score was calculated based on serum albumin, total cholesterol, and TLC (Table 1). These factors were scored according to cut-off values, and the sum of the scores was used as the CONUT score. The patients were then divided into four categories according to CONUT score as follows: normal (score 0–1), light (score 2–4), moderate (score 5–8), and severe (score 9–12).
Table 1.
Controlling Nutritional Status (CONUT) scoring system
| Parameters | ||||
| Serum albumin (g/dL) | ≥ 3.5 | 3.0–3.49 | 2.5–2.9 | < 2.5 |
| Score | 1 | 2 | 4 | 6 |
| Total lymphocyte count (/mm3) | ≥ 1600 | 1200–1599 | 800–1199 | < 800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥ 180 | 140–180 | 100–139 | < 100 |
| Score | 0 | 1 | 2 | 3 |
| CONUT score | 0–1 | 2–4 | 5–8 | 9–12 |
| Assessment | Normal | Light | Moderate | Severe |
Statistical analysis
Individual variables were compared among groups using χ2 tests. Receiver operating characteristic (ROC) curves were generated and the area under the curve (AUC) was determined for survival analysis and to identify the optimal cut-off value for the CONUT score. Survival curves were calculated according to the Kaplan–Meier method. Differences between the curves were examined by log-rank tests. Possible prognostic factors for overall survival (OS) were subjected to multivariate analysis using a Cox’s proportional hazards model and stepwise analysis. The covariates included in the analysis were age, gender, tumor size, histology, tumor location, depth of invasion, lymph node metastasis, distant metastasis, lymphatic invasion, vascular invasion, and T-CONUT. The accepted level of significance was P < 0.05. All statistical analyses were performed using SPSS software (SPSS for Windows Version 24, SPSS Inc., Chicago, IL).
RESULTS
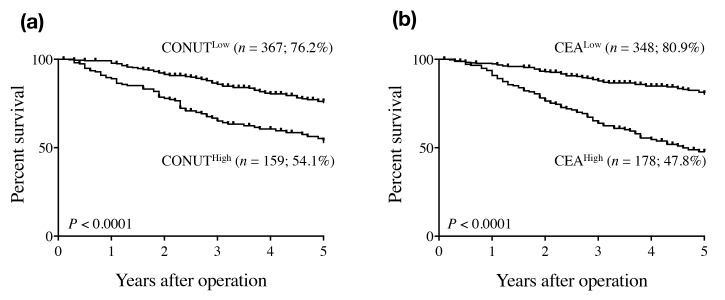
There were 271, 149, 68, and four patients with normal, light, moderate, and severe CONUT scores, respectively. According to ROC analysis, the optimal cut-off CONUT score for OS was 3 (AUC = 0.627, P < 0.0001). Based on these results, the patients were divided into two groups as follows: CONUThigh (CONUT score ≥ 3; n = 158) and CONUTlow (CONUT score < 3; n = 364). The relationships between CONUT score and clinicopathologic factors are shown in Table 2. CONUThigh was more common in elderly patients and in patients with large tumors, rectal cancer, and undifferentiated tumors, compared with non-elderly patients and patients with small tumors, colon cancer, and differentiated tumors, respectively. Furthermore, CONUThigh was also more frequent in patients with T3/T4 tumors, those with distant metastasis, those with Curability C compared with patients with T1/T2 tumors, those without distant metastasis, and those with Curability A and B. One hundred and fifty patients died during the follow up period, 80 patients died from CRC progression or recurrence, and 70 patients died from other causes, such as other cancers (n = 17), pneumonia (n = 4), stroke (n = 3), myocardial infarction (n = 1), and other remaining unknown causes. There were no surgically related deaths. The 5-year OS rate was significantly higher in patients in the CONUTlow (76%) compared with the CONUThigh (53.9%) group (P < 0.0001) (Fig. 1a).
Table 2.
Relationships between Controlling Nutritional Status (CONUT) score and clinicopathological variables in patients with colorectal cancer
| Variable | CONUTlow (n = 364) | CONUThigh (n = 158) | P value | |
| Age (years) | 0.013 | |||
| < 70 | 197 | 57 | ||
| ≥ 70 | 217 | 101 | ||
| Gender | 0.86 | |||
| Male | 202 | 89 | ||
| Female | 162 | 69 | ||
| Tumor size (cm) | < 0.0001 | |||
| < 4 | 215 | 58 | ||
| ≥ 4 | 149 | 100 | ||
| Tumor location | 0.024 | |||
| Colon | 218 | 111 | ||
| Rectum | 146 | 47 | ||
| Histology* | 0.019 | |||
| Differentiated | 337 | 136 | ||
| Undifferentiated | 27 | 22 | ||
| Depth of invasion† | < 0.0001 | |||
| T1 / 2 | 143 | 27 | ||
| T3 / 4 | 221 | 131 | ||
| Lymph node metastasis | 0.44 | |||
| Absent | 222 | 102 | ||
| Present | 142 | 56 | ||
| Distant metastasis | 0.0038 | |||
| Absent | 323 | 125 | ||
| Present | 41 | 33 | ||
| Lymphatic invasion‡ | 0.46 | |||
| Ly 0 / 1 | 204 | 83 | ||
| Ly 2 / 3 | 160 | 75 | ||
| Vascular invasion§ | 0.3 | |||
| V 0 / 1 | 245 | 99 | ||
| V 2 / 3 | 119 | 59 | ||
| Curability|| | 0.005 | |||
| A/B | 330 | 129 | ||
| C | 34 | 29 | ||
*Differentiated, papillary or tubular adenocarcinoma; undifferentiated, poorly differentiated or mucinous adenocarcinoma, or signet-ring cell carcinoma
†T1, tumor invasion of the lamina propria or submucosa; T2, tumor invasion of the muscularis propria; T3, tumor invasion of the subserosa or within adventitia; T4, tumor penetration of the serosa or tumor invasion of adjacent organs
‡Ly0–Ly3, grade of lymphatic invasion
§V0–V1, grade of vascular invasion
||Curability, A, R0 in Stage0, I, II, or III; B, R0 in Stage IV or R1 in any Stage; C, R2 in any Stage
Fig. 1.
Overall survival curves for colorectal patients according to CONUT score (a) and serum CEA level (b). CEA, carcinoembryonic antigen; CONUT, Controlling Nutritional Status.
The mean serum CEA level was 16.2 ng/mL (range: 0.1–1166 ng/mL). The patients were also divided into two groups according to serum CEA concentration as follows: CEAhigh (≥ 5 ng/mL; n = 177) and CEAlow (< 5 ng/mL; n = 345). The 5-year OS rate was significantly higher in patients in the CEAlow (80.7%) compared with the CEAhigh (47.6%) group (P < 0.0001) (Fig. 1b).
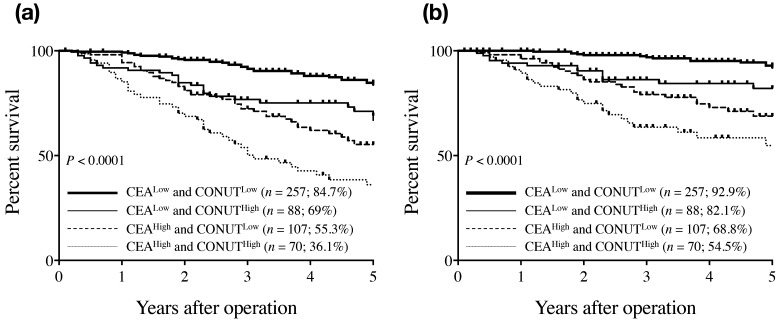
We determined the prognostic significance of the combination of CONUT score and CEA level (T-CONUT) by dividing the patients into four groups as follows: CEAlow/CONUTlow (n = 257); CEAlow/CONUThigh (n = 88); CEAhigh/CONUTlow (n = 107); and CEAhigh/CONUThigh (n = 70). Survival status was determined by ROC curves and the discriminatory abilities of CEA, CONUT, and T-CONUT were compared based on the AUCs. The AUCs of CEA, CONUT, and T-CONUT for OS were 0.66 (P < 0.0001), 0.627 (P < 0.0001), and 0.71 (P < 0.0001), respectively, indicating that T-CONUT was more useful than either indicator alone for predicting OS in CRC patients. The 5-year OS rates of patients with CEAlow/CONUTlow, CEAlow/CONUThigh, CEAhigh/CONUTlow, and CEAhigh/CONUThigh were 84.7%, 69%, 55.3%, and 36.1%, respectively (P < 0.0001) (Fig. 2a). Moreover, the equivalent 5-year disease-specific survival rates were 92.9%, 82.1%, 68.8%, and 54.5%, respectively (P < 0.0001) (Fig. 2b). We then examined the significance of T-CONUT for survival rate by Kaplan-Meier curve in stage I – IV. In stage I and IV CRC patients, there was no significant difference for survival rate among each T-CONUT group; however, in stage II and III patients, significant difference was observed for survival among each T-CONUT group similar to all stages (stage II: P = 0.001, and stage III: P = 0.006).
Fig. 2.
Overall survival curves (a) and disease-specific survival curves (b) for colorectal cancer patients according to the combination of CONUT score and serum CEA level. CEA, carcinoembryonic antigen; CONUT, Controlling Nutritional Status.
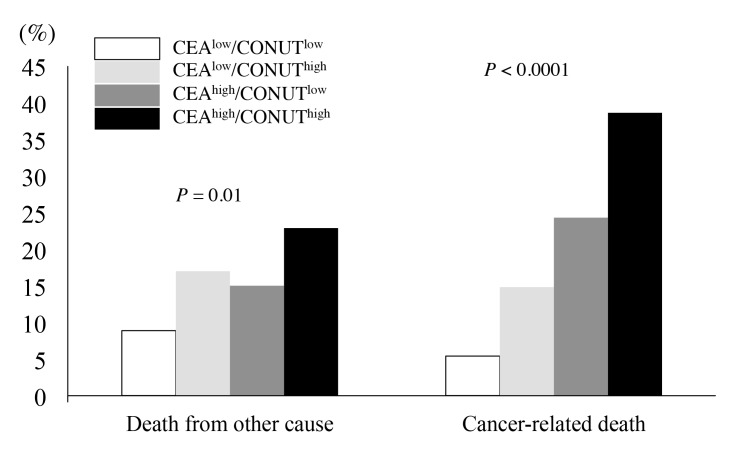
Regarding the cause of death, cancer-related deaths were observed in 14 (5.4%), 13 (14.8%), 26 (24.3%), and 27 patients (38.6%) in the CEAlow/CONUTlow, CEAlow/CONUThigh, CEAhigh/CONUTlow, and CEAhigh/CONUThigh groups, respectively (P < 0.0001) (Fig. 3), while deaths due to other causes were observed in 23 (8.9%), 15 (17%), 16 (15%), and 16 patients (22.9%), respectively (P = 0.01) (Fig. 3).
Fig. 3.
Cause of death of colorectal cancer patients according to the combination of CONUT score and serum CEA level. CEA, carcinoembryonic antigen; CONUT, Controlling Nutritional Status.
We then demonstrated ROC analysis of CEA, CONUT, and T-CONUT for cancer-related death and death from other cause, respectively. The AUCs of CEA, CONUT, and T-CONUT for cancer-related death were 0.762, 0.619, and 0.736, respectively, and while CEA had the highest AUC among these prognostic factors. Furthermore, the AUCs of CEA, CONUT, and T-CONUT for death from other causes were 0.589, 0.563, and 0.606, respectively, and T-CONUT was the highest AUC among them.
Multivariate analysis identified T-CONUT score as an independent prognostic indicator in CRC patients, together with age, lymph node metastasis, and distant metastasis (Table 3).
Table 3.
Multivariate analyses of prognostic factors for overall survival in patients with colorectal cancer
| P value | Hazard ratio | 95% CI | ||
| Age* | < 0.0001 | 1.049 | 1.030–1.069 | |
| Lymph node metastasis (N0–N3)† | 0.0007 | 1.385 | 1.148–1.672 | |
| Distant metastasis (absent or present) | < 0.001 | 3.273 | 2.189–4.893 | |
| T-CONUT | < 0.0001 | |||
| CEALow and CONUTLow vs. CEAHigh and CONUTHigh | < 0.0001 | 0.328 | 0.202–0.531 | |
| CEALow and CONUTHigh vs. CEAHigh and CONUTHigh | 0.15 | 0.693 | 0.42–1.144 | |
| CEAHigh and CONUTLow vs. CEAHigh and CONUTHigh | 0.21 | 0.752 | 0.482–1.174 | |
*Continuous variable
†N0, no regional lymph node metastasis; N1, metastasis in 1–3 pericolic, perirectal, or intermediate lymph nodes; N2, metastasis in ≥ 4 pericolic, perirectal, or intermediate lymph nodes; N3, metastasis in main lymph nodes or lateral lymph nodes
CEA, carcinoembryonic antigen; CI, confidence interval; CONUT, Controlling Nutritional Status.
DISCUSSION
The results of the current study demonstrated that CONUT score was useful for predicting the prognosis of CRC patients. Our results were similar to previous reports that demonstrated the significance of CONUT score for the patients after surgery with several cancers.16–18 Previous study also demonstrated that CONUT score was useful prognostic indicator in metastatic colorectal cancer patients receiving first line chemotherapy.20 CONUT includes measures of serum albumin, total cholesterol, and TLC. Albumin, which is the most abundant blood plasma protein, is produced in the liver and forms a large proportion of all plasma protein. Serum albumin is the standard factor used to assess a patient’s nutritional status and has been reported to be closely associated with the prognosis of various cancers, including CRC.21–23 TLC is also thought to reflect nutritional status, as well as being an indicator of immunocompetence. Lymphocytes include CD4+ and CD8+ T cells, natural killer (NK) cells, NK T cells, gamma-delta T cells, and B cells, which are reported to be closely associated with tumor immunity. Decreased numbers of these cells are thus likely to be associated with impaired tumor immunity, resulting in tumor progression. Several studies have demonstrated associations between the numbers of tumor-infiltrating lymphocytes, including CD4+ and CD8+ T cells, and cancer prognosis.24–26 Furthermore, decreased numbers of immune cells, including NK cells, B cells, and gamma-delta T cells, in peripheral blood and cancer tissue have been correlated with poor prognosis in various cancers.27–29 TLC might thus be a good indicator of cell-mediated immune status, including both acquired and adaptive immunities, as well as humoral immune status against CRC, indicating that the CONUT score reflects not only an individual’s nutritional status, but also their immune status.
Both serum albumin level and TLC are included in the prognostic nutritional index (PNI), which is one of the most frequently used indicators for evaluating nutritional status. PNI was recently shown to be closely associated with the prognosis of various types of cancer,13–14, 30 indicating the values of both nutritional and immune status as prognostic indicators in cancer patients. In addition to serum albumin and TLC, the CONUT score also includes a measure of serum cholesterol, which has been reported to correlate with tumor progression and patient survival in various cancers, including CRC.31–33
We also demonstrated that serum CEA level was closely associated with the prognosis of CRC patients in this study. Notably, however, CONUT score was useful for predicting the prognosis of CRC patients regardless of serum CEA level. Serum CEA mainly reflects the tumor status, whereas CONUT score reflects the patient’s overall condition, including their nutritional and immune statuses. These results suggest that the combination of these two factors (T-CONUT) might provide more accurate prognostic information for CRC patients than either factor alone, as demonstrated by the current ROC analysis. Furthermore, T-CONUT was a more useful prognostic factor in patients with stage II or III CRC; therefore, considering the indication of adjuvant chemotherapy, T-CONUT may be helpful in usual clinical practice. Moreover, T-CONUT was also identified as an independent prognostic indicator by multivariate analysis.
Regarding cause of death, T-CONUT was related to both cancer-related deaths and deaths due to other causes; however, it was more closely related to other causes of death. Migita et al. previously used PNI to evaluate the preoperative immunonutritional status of patients and found that low PNI scores were associated with a higher risk of non-cancer deaths.34 A similar study showed that low PNI scores increased the chance of respiratory failure due to pneumonia in older patients with gastric cancer, compared with patients with high PNI scores.35 Overall, these findings suggest that poor nutritional status increases the risk of death from non-cancer-related diseases after surgery. This indicates the benefit of using patient-related factors for predicting the prognosis of cancer patients.
Our study had some limitations. First it was a retrospective study and was therefore subject to bias. Second, we divided patients into two groups with high and low CONUT scores using a cut-off value of 3; however, cut-off values for CONUT scores have varied among reports, and the optimal cut-off value remains unclear. Third, the number of patients included in the current study was small, and further large-scale, prospective, randomized, controlled trials are needed to confirm the results.
In conclusion, T-CONUT may be a useful prognostic indicator in patients with CRC. Given that serum markers can be measured quickly, easily, and non-invasively, T-CONUT may represent a useful biological marker in routine clinical settings.
Acknowledgments
Acknowledgments: We thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
The authors declare no conflict of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 2. Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal Cancer Survival Gains and Novel Treatment Regimens: A Systematic Review and Analysis. JAMA Oncol. 2015;1:787-95. [DOI] [PubMed] [Google Scholar]
- 3. Fora A, Patta A, Attwood K, Wilding G, Fakih M. Intensive radiographic and biomarker surveillance in stage II and III colorectal cancer. Oncology. 2012;82:41-7. [DOI] [PubMed] [Google Scholar]
- 4. Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, Lopez-Calvino B, Seoane-Pillado T, Pertega-Diaz S, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644-56. [DOI] [PubMed] [Google Scholar]
- 5. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-94. [DOI] [PubMed] [Google Scholar]
- 6. Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23:227-32. [DOI] [PubMed] [Google Scholar]
- 7. Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996;12:23-9. [DOI] [PubMed] [Google Scholar]
- 8. Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut. 2000;46:813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779-85. [DOI] [PubMed] [Google Scholar]
- 10. Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Muhlebach S, Stanga Z, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92-7. [DOI] [PubMed] [Google Scholar]
- 11. Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. The Am J Clin Nutr. 2004;80:1634-8. [DOI] [PubMed] [Google Scholar]
- 12. Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cance. Ann Surg Oncol. 2016;23:525-33. [DOI] [PubMed] [Google Scholar]
- 13. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-74. [DOI] [PubMed] [Google Scholar]
- 14. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688-92. [DOI] [PubMed] [Google Scholar]
- 15. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38-45. [PubMed] [Google Scholar]
- 16. Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204-12. [DOI] [PubMed] [Google Scholar]
- 17. Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tokunaga R, Sakamoto Y, Nakagawa S, Ohuchi M, Izumi D, Kosumi K, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32:99-106. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. Tokyo: Kanehara; 2013. [Google Scholar]
- 20. Daitoku N, Miyamoto Y, Tokunaga R, Sakamoto Y, Hiyoshi Y, Iwatsuki M, et al. Controlling Nutritional Status (CONUT) Score Is a Prognostic Marker in Metastatic Colorectal Cancer Patients Receiving First-line Chemotherapy. Anticancer Res. 2018;38:4883-8. [DOI] [PubMed] [Google Scholar]
- 21. Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381-9. [DOI] [PubMed] [Google Scholar]
- 22. Siddiqui A, Heinzerling J, Livingston EH, Huerta S. Predictors of early mortality in veteran patients with pancreatic cancer. Am J Surg. 2007;194:362-6. [DOI] [PubMed] [Google Scholar]
- 23. Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40:592-5. [DOI] [PubMed] [Google Scholar]
- 24. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-4. [DOI] [PubMed] [Google Scholar]
- 26. Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PloS One. 2014;9:e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu H, Xiao-Jun W, Zhi-Wei Z, Gong C, Guo-Qiang W, Li-Yi Z, et al. The prognostic significance of peripheral T-lymphocyte subsets and natural killer cells in patients with colorectal cancer. Hepatogastroenterology. 2009;56:1310-5. [PubMed] [Google Scholar]
- 28. Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322-7. [DOI] [PubMed] [Google Scholar]
- 29. Berntsson J, Nodin B, Eberhard J, Micke P, Jirstrom K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139:1129-39. [DOI] [PubMed] [Google Scholar]
- 30. Sun KY, Xu B, Chen SL, Yuan YJ, Wu H, Peng JJ, et al. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21:5961-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Martino M, Leitner C V, Seemann C, Hofbauer S L, Lucca I, Haitel A, et al. Preoperative serum cholesterol is an independent prognostic factor for patients with renal cell carcinoma (RCC). BJU Int. 2015;115:397-404. [DOI] [PubMed] [Google Scholar]
- 32. Cengiz O, Kocer B, Surmeli S, Santicky M J, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma?. Med Sci Monit. 2006;12:Cr240-7. [PubMed] [Google Scholar]
- 33. Lee YL, Li WC, Tsai TH, Chiang HY, Ting CT. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan - a cohort study. Oncotarget. 2016;7:22948-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647-54. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H, et al. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg. 2012;36:1632-9. [DOI] [PubMed] [Google Scholar]