Abstract
We report the case of a 6-year-old male who developed recurrent erythema nodosum (EN) at the age of 3 years. The patient exhibited hypertelorism, low-set ears, micrognathia, moderate intellectual disability, thin long fingers, loose anagen hair, and prominent palmoplantar wrinkles. A heterozygous single nucleotide variant in the SHOC2 gene (c.4 A > G, p.S2G) was identified. Patients with a SHOC2 mutation exhibit a unique combination of ectodermal abnormalities including darkly pigmented skin and loose anagen hair. This report is the first to describe EN in a patient with SHOC2 mutation, and to examine the patient’s hair using scanning electron microscopy. We hypothesize that the RAS/MAPK pathway is associated with the pathogenesis of cutaneous lesions in patients with SHOC2 mutations via autoinflammation and disturbance of epithelial stem cells.
Keywords: erythema nodosum, loose anagen hair, next-generation sequencing, Noonan syndrome, SHOC2
The SHOC2 gene on chromosome 10q25.2 encodes a product containing leucine-rich repeats and acts as a positive modulator of the RAS/MAPK pathway. Patients with a gain-of-function missense mutation c.4A > G (p.S2G) in SHOC2 exhibit Noonan syndrome-like symptoms with loose anagen hair.1
Previous studies have reported patients with a SHOC2 mutation manifesting multiple phenotypes including intellectual disability and dysmorphic facial features. Additionally, these patients display a unique combination of ectodermal abnormalities including darkly pigmented skin and loose anagen hair.1, 2 The present patient exhibited recurrent erythema nodosum (EN), which has not been reported previously in patients with a SHOC2 mutation. In this study, we describe recurrent EN using scanning electron microscopy (SEM) to examine the hair in a patient with a SHOC2 mutation. These findings may be useful in understanding the cutaneous symptoms of this syndrome.
PATIENT REPORT
A 6-year-old male was born to nonconsanguineous parents at 38 weeks with a birth weight of 3,190 g (standard deviation [SD], +0.4), height of 47 cm (SD, −0.9), and head circumference of 33.5 cm (SD, ±0). He was referred to our hospital at the age of 1 year 1 month due to exhibited global developmental delay. He was capable of walking at 2 years and 1 month of age and uttered the first meaningful word at 3 years and 5 months of age.
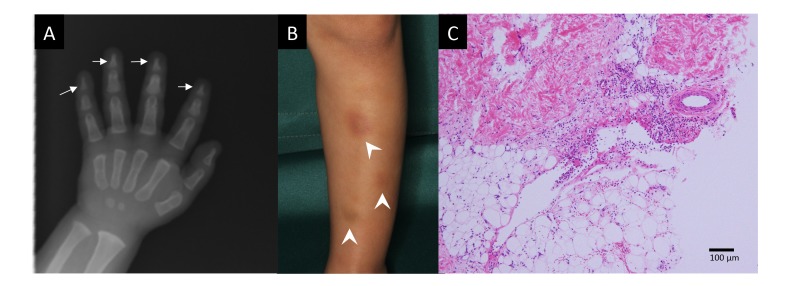
Physical examination at 1 year and 3 months of age revealed generalized hypotonia and facial dysmorphisms including hypertelorism, low-set ears, and micrognathia. He had thin long fingers and prominent palmoplantar wrinkles. Bone X-ray revealed tapered distal phalanges of the fingers (Fig. 1A).
Fig. 1.
A: Skeletal X-ray at 1 year of age showing tapered distal phalanges (arrows). B: Erythema appearing on the anterior surface of the lower leg at 3 years of age (arrow heads). C: Histopathology of an erythema showing perivascular and septal inflammatory infiltration in the subcutaneous adipose tissue (Hematoxylin–Eosin staining, bar = 100 μm).
He experienced the first emergence of multiple and painful erythema (2–3 cm in diameter) on the lower leg at 3 years and 9 months of age. The erythema recurred every 2–3 months without other symptoms or infectious episodes and spontaneously disappeared within 2 days (Fig. 1B). Skin biopsy revealed typical findings of septal panniculitis with lymphocytic, neutrophilic, and eosinophilic infiltration in the absence of vasculitis (Fig. 1C). The levels of autoantibodies, including antinuclear antibody and Sjögren syndrome-related antibodies, were not elevated in the serum.
A physical examination at 6 years and 4 months of age revealed generalized hypotonia; however, muscle power and deep tendon reflexes were normal. His weight was 14.7 kg (SD: −1.2), height was 100.8 cm (SD: −1.7), and head circumference was 50.8 cm (SD: −0.1). At 4 years, the patient’s intellectual quotient was determined to be 52 according to the Tanaka–Binet scale of intelligence.
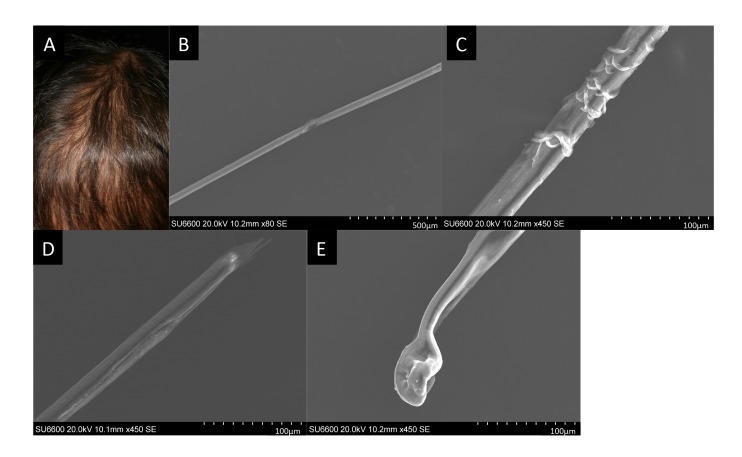
Easily pluckable, sparse hair had been prominent since infancy (Fig. 2A). SEM revealed rough-surfaced hair shafts (Figs. 2B and C), longitudinal splitting of distal hair shafts (Fig. 2D), and distorted hair bulbs (Fig. 2E).
Fig. 2.
A: Sparse scalp hair at 5 years of age. The surface of the scalp is partially exposed because of hair loss. B–E: Scanning electron microscopy images of the patient’s hair at 5 years of age. (B) Trichorrhexis represented as a focal longitudinal vulnerable lesion of the hair shaft. (C) The hair shaft showing a sagged or peeled surface. (D) Trichoptilosis represented as a longitudinal splitting and cracking lesion at the distal end of the hair shaft. (E) Misshapen and depressed hair bulb.
Next-generation sequencing using an Illumina TruSight One Sequencing Panel on an Illumina MiSeq platform (Illumina, San Diego, CA) was performed, and a heterozygous single nucleotide variant in SHOC2 (c.4 A > G, p.S2G) was found, which was validated by Sanger sequencing. The parents of the patient did not consent to a genetic analysis of their SHOC2 genes. However, neither parent displayed phenotypic features of SHOC2. Informed consent for his genetic analysis was obtained from his parents following the Helsinki Declaration. This study was approved by the ethics committee at the Tottori University (dated September 22, 2014, approval number G152).
DISCUSSION
The histopathological findings of the biopsy using a specimen from the leg were compatible with EN.3 Erythema has been identified in patients diagnosed with RASopathies including SHOC2 mutations.1 However, the recurrent or single event was not specified, and the term “erythema nodosum” was not used. Generally, EN occurs as an isolated condition or in association with drug administration, infection, and the presence of connective tissue diseases.3 Notably, systemic lupus erythematosus has been established as a complication in patients with PTPN11, KRAS, and SHOC2 mutations.4 Remarkably, activation of the H-Ras protein in the RAS/MAPK pathway is reported to result in the pronounced accumulation of immune cells in mouse epidermis.5 The causative factors for the differential manifestation of either erythema or EN among patients with SHOC2 mutations is still unknown, but genetically driven cutaneous autoinflammation may be linked to the dysregulation of the RAS/MAPK pathway.
Hair abnormality is a common feature in RASopathies, and loose anagen hair is a distinctive feature of patients with a SHOC2 mutation. Two previous reports described the hair characteristics of patients with this mutation using light microscopy.1, 6 Similar to the findings observed using SEM in our patient, deformity of the hair shafts was described as trichorrhexis nodosa and trichoptilosis, and the hair bulbs were distorted and misshapen. Whereas, loose anagen hair (LAH) is also shown in cases without SHOC2 mutation.7 Similar to our findings, previously report of SEM in LAH reveals distorted hair bulbs and cuticle scales.8 We observed the first to confirm these hair abnormality using SEM in a patient with SHOC2 mutation. KRAS-knockout mice have been reported to show defective hair cycling.9 The exact pathogenesis of loose anagen hair in patients with a SHOC2 mutation remains unknown; however, the RAS/MAPK pathway, which is required for the development and regeneration of hair follicles, may be involved in this process.10 The mutation in the SHOC2 gene is believed to affect the proliferation, survival, or differentiation of epithelial stem cell-derived cells residing in hair follicles.11
We described EN in a patient with SHOC2 mutation and observation of the abnormal hair using SEM. Other RAS/MAPK pathway related disease; tuberous sclerosis complex (TSC) is a genetic disorder caused by mutation in either of the two genes TSC1 or TSC2. Recently, mTOR inhibitor everolimus is approved by the Food and Drug Administration for TSC associated partial onset seizure. Cortical tuber in TSC patients is important etiology for epileptogenesis, and an increase of inflammatory cells in cortical tubers has been previously described.12 Certain etiology of skin lesions and hair abnormalities in patients with SHOC2 mutation are still unknown, but genetically pathologies including inflammation may be linked to the symptoms. Further research are needed for development of treatment for these symptoms in patients with SHOC2 mutation.
The authors declare no conflict of interest.
REFERENCES
- 1. Komatsuzaki S, Aoki Y, Niihori T, Okamoto N, Hennekam RC, Hopman S, et al. Mutation analysis of the SHOC2 gene in Noonan-like syndrome and in hematologic malignancies. J Hum Genet. 2010;55:801-9. [DOI] [PubMed] [Google Scholar]
- 2. Baldassarre G, Mussa A, Banaudi E, Rossi C, Tartaglia M, Silengo M, et al. Phenotypic variability associated with the invariant SHOC2 c.4A>G (p.Ser2Gly) missense mutation. Am J Med Genet A. 2014;164A:3120-5. [DOI] [PubMed] [Google Scholar]
- 3. Chowaniec M, Starba A, Wiland P. Erythema nodosum-review of the literature. Reumatologia. 2016;54:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uehara T, Hosogaya N, Matsuo N, Kosaki K. Systemic lupus erythematosus in a patient with Noonan syndrome-like disorder with loose anagen hair 1: More than a chance association. Am J Med Genet A. 2018;176:1662-6. [DOI] [PubMed] [Google Scholar]
- 5. Golomb L, Sagiv A, Pateras IS, Maly A, Krizhanovsky V, Gorgoulis VG, et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 2015;22:1764-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kane J, Berrebi K, McLean R, Petkiewicz S, Hay B, Martin M, et al. Noonan syndrome with loose anagen hair associated with trichorrhexis nodosa and trichoptilosis. Clin Case Rep. 2017;5:1152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhurat RP, Deshpande DJ. Loose anagen hair syndrome. Int J Trichology. 2010;2:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dicle O, Velipasaoglu S, Ozenci CC, Akkoyunlu G, Demir N. Report of a new case with loose anagen hair syndrome and scanning electron microscopy findings. Int J Dermatol. 2008;47:936-8. [DOI] [PubMed] [Google Scholar]
- 9. Mukhopadhyay A, Krishnaswami SR, Yu BD. Activated Kras alters epidermal homeostasis of mouse skin, resulting in redundant skin and defective hair cycling. J Invest Dermatol. 2011;131:311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doma E, Rupp C, Baccarini M. EGFR-ras-raf signaling in epidermal stem cells: roles in hair follicle development, regeneration, tissue remodeling and epidermal cancers. Int J Mol Sci. 2013;14:19361-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mühlebner A, van Scheppingen J, Hulshof HM, Scholl T, Iyer AM, Anink JJ, et al. Novel Histopathological Patterns in Cortical Tubers of Epilepsy Surgery Patients with Tuberous Sclerosis Complex. PLoS One. 2016;11:e0157396. DOI: 10.1371/journal.pone.0157396 [DOI] [PMC free article] [PubMed] [Google Scholar]