Abstract
Background
Streptococcus pneumoniae, more than 90 serotypes of which exist, is recognized as an etiologic agent of pneumonia, meningitis, and sepsis associated with significant morbidity and mortality worldwide. Immunization with a pneumococcal pep27 mutant (Δpep27) has been shown to confer comprehensive, long-term protection against even nontypeable strains. However, Δpep27 is effective as a vaccine only after at least three rounds of immunization. Therefore, treatments capable of enhancing the efficiency of Δpep27 immunization should be identified without delay. Panax ginseng Mayer has already been shown to have pharmacological and antioxidant effects. Here, the ability of Korean Red Ginseng (KRG) to enhance the efficacy of Δpep27 immunization was investigated.
Methods
Mice were treated with KRG and immunized with Δpep27 before infection with the pathogenic S. pneumoniae strain D39. Total reactive oxygen species production was measured using lung homogenates, and inducible nitric oxide (NO) synthase and antiapoptotic protein expression was determined by immunoblotting. The phagocytic activity of peritoneal macrophages was also tested after KRG treatment.
Results
Compared with the other treatments, KRG significantly increased survival rate after lethal challenge and resulted in faster bacterial clearance via increased phagocytosis. Moreover, KRG enhanced Δpep27 vaccine efficacy by inhibiting reactive oxygen species production, reducing extracellular signal–regulated kinase apoptosis signaling and inflammation.
Conclusion
Taken together, our results suggest that KRG reduces the time required for immunization with the Δpep27 vaccine by enhancing its efficacy.
Keywords: Korean Red Ginseng (KRG), Δpep27, Streptococcus pneumoniae
1. Introduction
Diseases caused by Streptococcus pneumoniae (pneumococcus) are public health problems of global importance. Pneumococcus is a commensal of the human respiratory tract and causes local infections (including otitis media) and several invasive diseases, such as pneumonia, sepsis, and meningitis, owing to its virulence factors [1]. According to a 2015 World Health Organization estimate, 0.9 million children under the age of 5 years die from pneumococcal disease, accounting for 16% of all deaths of children [2].
The well-known S. pneumoniae virulence factor pneumolysin has immunomodulatory effects, influencing cytokine production and complement activation, and increases intracellular reactive oxygen species (ROS) production [3]. ROS are important in inflammatory reactions, and their elevated production at sites of inflammation leads to endothelial disorder and tissue damage [4]. Inducible nitric oxide (NO) synthase (iNOS), a catalyst of NO production that induces the generation of ROS, is stimulated by inflammatory cytokines and interferons during bacterial infection [5], [6].
Reports concerning mitogen-activated protein kinases, originally called extracellular signal–regulated kinases (ERKs), are contradictory, with important roles in both cell survival and cell death have been described [7], [8]. In addition, induction of apoptosis by the activation of ERK has been associated to ROS [9], [10], [11]. ERK facilitates ROS-induced apoptosis by activating caspase-3 and inhibiting v-Akt Murine Thymoma Viral Oncogene signaling [12].
Korean Red Ginseng (KRG) is widely used in Korea to improve physical strength and treat fatigue [13]. The ginsenosides present in ginseng have antioxidant effects and protect against H2O2-mediated stress in astrocytes [14]. NO production during S. pneumoniae infection is lower in mice pretreated with KRG than in nonpretreated infected controls [15]. In addition, ginseng polysaccharide protects against influenza virus infection and enhances the efficacy of influenza vaccination [16]. To date, however, no study has been conducted to examine the effects of combining ginseng with pneumococcal vaccines.
Pneumococcal polysaccharide vaccines provide protection against only 23 of the more than 90 known S. pneumoniae serotypes. In addition, their utility is limited by their cost and weak immunogenicity [17], [18], [19]. Moreover, as the prevalent S. pneumoniae serotypes in developed countries (14, 6, 19, 18, 9, 23, 7, 4, 1, and 15) differ from those in underdeveloped countries (6, 14, 8, 5, 1, 19, 9, 23, 18, 15, and 7) [20], it is necessary to develop a vaccine that protects against a broader range of serotypes.
Mucosal immunization may be a more effective route than systemic immunization for individuals susceptible to pneumococcal infection [21], [22]. Several studies have shown that mucosal vaccination inhibits pneumococcal colonization and elicits antibody production, protecting against diseases such as sepsis [22], [23]. Mucosal vaccination also has the advantages of being needle free, yet simple and quick to deliver, contributing to safety, better compliance, lowered costs, and decreased vaccination-related pain [24]. However, conventional mucosal vaccines require adjuvants such as cholera toxin [22], [25], [26], which can cause Bell's palsy [27].
We previously confirmed that deletion of the pneumococcal pep27 gene completely abrogates virulence and that the resulting S. pneumoniae mutant, Δpep27, can be used as an attenuated mucosal vaccine [28]. In addition, Δpep27 confers protection against a greater number of serotypes than conventional pneumococcal vaccines by increasing sIgA levels and cytokine secretion [29] and has the notable advantage of not requiring an adjuvant. However, it has been found that two to three immunizations are required before Δpep27 becomes effective as a vaccine [28]. Therefore, the aim of this study was to investigate whether KRG enhances the efficacy of the Δpep27 vaccine and if so, how this effect is brought about.
2. Materials and methods
2.1. Bacterial strains
The type 2 S. pneumoniae strain D39 (NCTC 7466) [30] and an isogenic pep27 deletion mutant of this strain (THpep27) [31] were used. Bacteria were cultured at 37°C in Todd-Hewitt broth (Difco Laboratories Inc., Detroit, MI, USA) with yeast extract or on blood agar plates.
2.2. In vivo experiments
C57BL/6 mice (4-week-old females; Orient Bio Inc., Seongnam, South Korea) were used. The use of animals in this study was approved by the Animal Ethics Committee of Sungkyunkwan University, in accordance with the guidelines of the Korean Animal Protection Law. Euthanasia was carried out with CO2.
KRG extract, the composition of which has been described previously [32], was provided by the Korea Ginseng Corporation (Seoul, South Korea). It was dissolved in phosphate-buffered saline (PBS) and orally administered at 100 mg/kg twice a day for 15 days. On the 2nd and 9th day of KRG administration, mice were immunized once intranasally with 1 × 107–1 × 108 Colony-forming unit (CFU) of Δpep27. Seven days after the last immunization, they were challenged intranasally with 1 × 108 CFU of D39. All immunization and infection experiments were performed under anesthesia. The survival rate of the infected mice was recorded for 14 days.
To determine S. pneumoniae colonization profiles, the lungs were collected 24 h after challenge with D39 and homogenized in PBS with a homogenizer (Model 200; PRO Scientific Inc., Oxford, CT, USA) at the maximum rate. All samples were diluted in PBS and plated on blood agar plates containing 5–10 μg/ml gentamycin. After incubation of plates for 18 h at 37°C in an incubator containing 5% CO2, colonies were counted.
2.3. Peritoneal macrophage isolation and phagocytosis assay
Peritoneal macrophages were harvested by injecting PBS into the peritoneal cavities of mice and the subsequent isolation of peritoneal macrophages based on their adherence to the culture vessel. The peritoneal macrophages collected were seeded at 1 × 106 per well in a 96-well plate containing culture medium prepared by the addition of 10% fetal bovine serum (ATCC, Manassas, VA, USA) and 2% penicillin/streptomycin solution (Gibco, Waltham, MA, USA) to Dulbecco's modified Eagle's medium (DMEM; Corning Inc, Corning, NY, USA). After allowing the macrophages to adhere, the medium was replaced with antibiotic-free DMEM. The macrophages were then exposed to D39 at an Multiplicity Of Infection (MOI) of 1:50 for 4 h, transferred to antibiotic-containing media, and treated with cold distilled water. As a result, phagocytosed bacteria were released into the suspension. Serial dilutions of this bacterial suspension were then carried out using distilled water and plated on blood agar containing 5–10 μg/ml gentamycin. These plates were incubated overnight at 37°C in an incubator containing 5% CO2 before counting colonies.
2.4. Measurement of cytokine concentration
Concentrations of interleukin-1β, interferon-γ in bronchoalveolar lavage fluid were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences, San Diego, CA, USA) according to the manufacturer's instructions.
2.5. IgG antibody titer measurement
On the 9th day of KRG treatment, mice were immunized with 1 × 107–1 × 108 CFU Δpep27. Sera were then collected by retroorbital bleeding one week after immunization. Titers of antibodies against D39 bacteria and Pneumococcal surface protein A (PspA) protein were measured as previously described [22], [33].
2.6. ROS detection
The OxiSelect In Vitro ROS/RNS Assay (Cell Biolabs Inc., San Diego, CA, USA) was used to measure ROS production in the lungs, as described elsewhere [34]. In brief, the lungs were obtained from mice in each group and exposed to liquid nitrogen for 30 min. After homogenization, cells from these tissues were then lysed for ROS measurement.
2.7. Western blot analysis
Antibodies against iNOS (Novus Biologicals, Littleton, CO, USA), p-p44/42 mitogen-activated protein kinase (Cell Signaling Technology, Inc., Danvers, MA, USA), caspase-3 (GeneTex Inc., Irvine, CA, USA), B-cell lymphoma 2 (Bcl2) (GeneTex), p-AKT (Santa Cruz, Dallas, TX, USA), AKT (Santa Cruz), Bcl-xL (Santa Cruz), and β-actin (Santa Cruz) were used for the western blot analysis. Proteins were quantified using the Bradford method and electrophoresed on a 12.5% (w/v) sodium dodecyl sulfate–polyacrylamide gel before being transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was then incubated overnight with the desired primary antibody at 4°C. The following day, after three washes with Tris-buffered saline containing 0.5% Tween 20, the Horseradish Peroxidase (HRP)-conjugated anti-IgG secondary antibody (Bio-Rad Laboratories Inc., Hercules, CA, USA) was added at the appropriate dilution. Expression of each protein was detected by exposing the membrane to Enhanced chemiluminescence (ECL) solution (GenDEPOT, Katy, TX, USA). ImageJ 2.1.4.6 (https://imagej.net/Citing) was used to determine band densities.
2.8. Hematoxylin and eosin staining
Mice were sacrificed 24 h after infection, and their lungs were fixed in 10% neutral-buffered formalin (Sigma Aldrich, St. Louis, MO, USA) and embedded in paraffin. Five-micrometer sections were then cut and stained with hematoxylin and eosin (HE) [15].
2.9. Macrophage 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
Peritoneal macrophages were harvested and seeded at a density of 1 × 104 per well in a 96-well plate, before being exposed to D39 for 2, 4, or 6 h. Macrophage 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide solution (Sigma Aldrich) was diluted to 2 mg/ml in DMEM and was added to the cells. The purple formazan formed as a consequence was dissolved in dimethyl sulfoxide (NobleBio B.V., Oldenzaal, The Netherlands), and absorbance at 540 nm was then measured using an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.10. Statistical analysis
Survival data were statistically analyzed using the log-rank test. For all other data, statistical analysis was carried out using one-way analysis of variance (with Bonferroni's multiple comparison test). GraphPad Prism software (version 5; GraphPad Software Inc., La Jolla, CA, USA) was used for all statistical analyses. p values less than 0.05 were defined as indicating statistically significant differences, and levels of significance are indicated as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
3. Results
3.1. KRG enhances Δpep27 vaccine efficacy
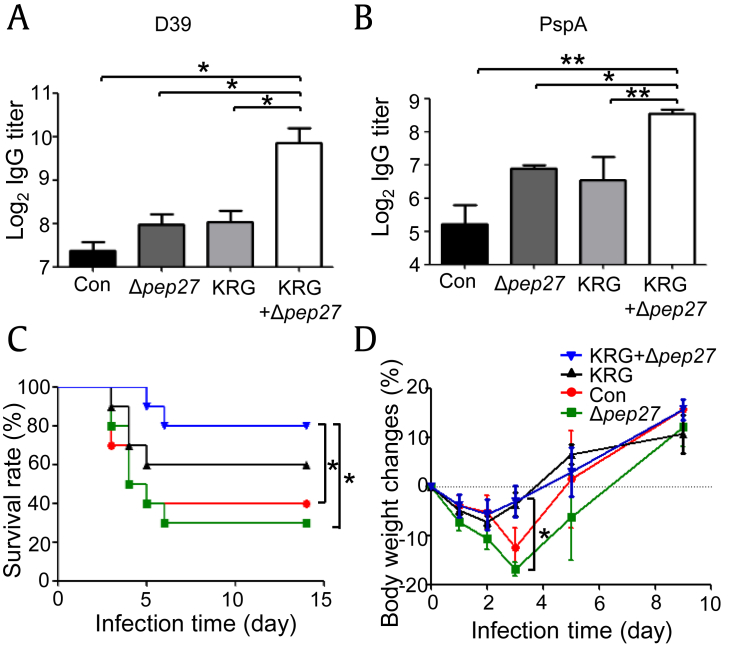
When vaccines are administered, the host immune system is activated, resulting in an increased antibody production by B cells [35]. Conjugate vaccines activate T cells, which leads to differentiation of B cells into the memory cells and plasma cells that generate IgG antibodies against the polysaccharide capsule [36]. Therefore, to determine vaccine efficacy in the present work, titers of IgG antibodies against the pathogenic pneumococcal D39 strain (serotype 2) and PspA were measured. Antibody titers in the KRG coadministered (KRG+Δpep27) group were significantly higher than those in all other treatment groups (Figs. 1A, 1B), suggesting that KRG resulted in an increased IgG antibody production, enhancing the efficacy of the Δpep27 vaccine.
Fig. 1.
KRG pretreatment enhances Δpep27 vaccine efficacy. Mice (n = 3) were treated with KRG and immunized once with Δpep27. (A) Seven days after immunization, levels of IgG antibodies against D39 were measured by ELISA. (B) Seven days after immunization, levels of IgG antibodies against PspA were measured by ELISA. Mice (n = 10) were treated with KRG for 15 days, immunized once with Δpep27, and subsequently challenged with D39. (C) Survival rate was monitored for 14 days. (D) Body weight was monitored for 9 days. Significant differences in survival rate and body weight were identified using the log-rank test and one-way ANOVA (with Bonferroni's multiple comparison test), respectively, (*p < 0.05).
ANOVA, analysis of variance; ELISA, enzyme-linked immuno sorbent assay; KRG, Korean Red Ginseng.
To confirm its effect on the efficacy of Δpep27, KRG was orally administered twice a day for 15 days to mice immunized once with Δpep27 during this period. One day after the completion of KRG treatment, the mice were infected with D39, and survival rate and body weight were monitored for 14 days and 9 days, respectively. Of the mice that received both KRG and Δpep27, 80% survived for 14 days, whereas survival rates among control mice and mice treated with Δpep27 only were 40% and 30%, respectively. Therefore, KRG-pretreated, Δpep27-immunized mice exhibited significantly higher survival than those in other treatment groups (Fig. 1C). In addition, a survival rate of 60% was recorded when mice were treated with KRG alone.
Body weight initially decreased in all groups after D39 challenge, most notably in the control and Δpep27 groups (by approximately 12% and 16%, respectively, by the 3rd day). However, body weight in the KRG coadministered group on the 3rd day was significantly higher than that in the Δpep27 group, although all groups subsequently exhibited weight gain (Fig. 1D). These findings confirmed that KRG coadministration increased the efficacy of the Δpep27 vaccine, resulting in a higher survival rate after S. pneumoniae challenge.
3.2. KRG diminishes S. pneumoniae colonization by potentiating the Δpep27 vaccine
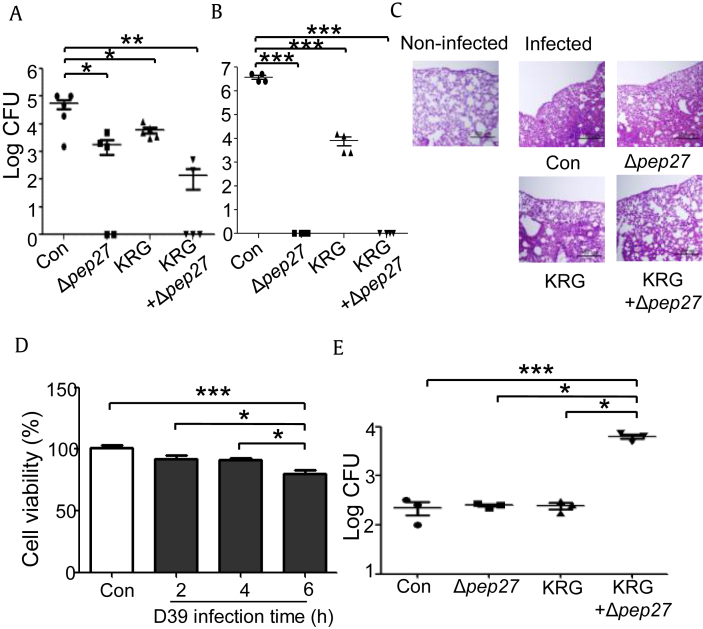
Previous reports have revealed that three rounds of immunization with Δpep27 inhibits pneumococcal colonization and confers protection against a wide range of S. pneumoniae serotypes [29]. To confirm that KRG increases survival in combination with the Δpep27 vaccine, S. pneumoniae colonization in KRG-pretreated, Δpep27-immunized mice was also determined. Compared with the control, KRG pretreatment or Δpep27 immunization alone led to significantly decreased bacterial load in the lungs 24 h after infection with 1 × 108 CFU D39 (p < 0.05). Moreover, KRG coadministration diminished bacterial burdens to a more significant extent, compared with the control group (p < 0.01) (Fig. 2A). In addition, when the mice were immunized two times and colony number was determined, no bacteria were detected in either the Δpep27 group or KRG+Δpep27 group (Fig. 2B), indicating that two times of immunization with Δpep27 showed similar colonization inhibition to three times of immunization [28]. Therefore, to determine the optimum KRG effect on Δpep27 vaccination, one time immunization experiment was performed further. Consistent with Fig. 2A, Hematoxylin and eosin staining indicated that inflammatory cell recruitment tended to be lower in the lungs of mice treated with the KRG+Δpep27 combination than in those of mice in the other groups (Fig. 2C).
Fig. 2.
KRG reduces pneumococcal colonization and inflammation and promotes phagocytosis by peritoneal macrophages during Δpep27 immunization. Mice (n = 5) were orally administered KRG (100 mg/kg) for 15 days and immunized once or twice with 1 × 107–1 × 108 CFU of Δpep27. Subsequently, they were infected with 1 × 108 CFU of D39, and the lungs were collected 24 h later. (A) After immunization with Δpep27 once, bacterial number in the lungs was determined. (B) After immunization with Δpep27 twice, bacterial number in the lungs was determined. (C) Lung sections were stained with HE. (D) Peritoneal macrophages from naive mice (1 × 104/well) were exposed to D39 (MOI 1:50) for the indicated time, and their viability was subsequently measured by MTT assay. (E) Mice (n = 3) were immunized once with Δpep27 during 15 days of KRG treatment. Macrophages (1 × 106/well) were exposed to D39 for 4 h, and their phagocytosis of bacterial cells was measured. Significant differences were identified using one-way ANOVA (with Bonferroni's multiple comparison test) (*p < 0.05, **p < 0.01, and ***p < 0.001). The experiments depicted were repeated three times.
ANOVA, analysis of variance; HE, hemolysin eosin; KRG, Korean Red Ginseng; MTT, Macrophage 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
Because macrophages play the most vital role in bacterial clearance, these cells were extracted from mice, and their survival was determined after infection with D39 for different time periods. The Macrophage 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay showed that the viability of macrophages from a naive mouse had not significantly decreased by 4 h after infection; however, a reduction was observed after 6 h of infection (Fig. 2D). Macrophages extracted from mice treated with KRG and Δpep27 were also infected with D39 for various periods and tested. The phagocytic potential of macrophages from the KRG coadministered group was significantly higher than that of macrophages from the other groups 4 h after infection (Fig. 2E). These results indicate that enhanced macrophage phagocytic activity and increased IgG titer were responsible for the KRG-induced inhibition of pneumococcal colonization.
3.3. KRG enhances Δpep27 vaccine potency by suppressing both ROS generation and ERK signaling–mediated cell death
KRG has been shown to reduce S. pneumoniae-induced ROS generation [15]. Therefore, we hypothesized that KRG diminishes ROS generation resulting from vaccination with Δpep27. To test this theory, mice were treated with KRG and Δpep27 vaccine and subsequently challenged with D39. Twenty-four hours after infection, lung lysates were subjected to western blotting and ROS assays. KRG coadministration was found to significantly decrease ROS production compared with the infected control and Δpep27 treatment alone (Fig. 3A). Furthermore, immunoblotting showed that in comparison with noninfected mice, those infected with D39 exhibited dramatically increased iNOS expression (Fig. 3B). Although iNOS levels were decreased by the administration of Δpep27 or KRG alone, these differences were not statistically significant; however, KRG coadministration reduced iNOS expression to a greater, and significant, extent (Fig. 3D, left panel). Coadministration also significantly decreased p-ERK levels compared with the control treatment (Fig. 3D, middle panel) and caspase-3 expression compared with Δpep27 alone (Fig. 3D, right panel).
Fig. 3.
KRG inhibits ROS production and ERK signaling and increases cell survival during Δpep27 immunization. Mice (n = 3) were orally administered KRG for 15 days and immunized with 1 × 107–1 × 108 CFU Δpep27, before being infected with 1 × 108 CFU D39. (A) Twenty-four hours later, intracellular ROS levels were measured from lung lysate. (B) Twenty-four hours later, immunoblotting was performed to detect expression of iNOS, p-ERK, and casp-3 (caspase-3). (C) Expression of the cell survival factor AKT and the antiapoptotic proteins Bcl-xL and Bcl2 was detected by western blotting. (D) Relative immunoblot band densities were quantified using ImageJ. (E) Relative immunoblot band densities were quantified using ImageJ. All the experiments shown were conducted at least three times, and statistically significant differences were identified by one-way ANOVA (with Bonferroni's multiple comparison test) (*p < 0.05, **p < 0.01, and ***p < 0.001).
ANOVA, analysis of variance; ERK, extracellular signal–regulated kinase; iNOS, inducible nitric oxide synthase; KRG, Korean Red Ginseng; ROS, reactive oxygen species; DCF, 2′,7′-dichlorofluorescein.
To examine whether KRG coadministration increases cell survival, expression of p-AKT (involved in cell survival and growth) and that of Bcl-xL and Bcl2 (antiapoptotic proteins) were determined by western blotting. KRG coadministration significantly increased p-AKT and Bcl-xL levels compared with the administration of Δpep27 alone. It also raised Bcl2 expression, although this result was not statistically significant (Figs. 3C, 3E). These results indicate that KRG coadministration increased the expression of cell survival factors by inhibiting ROS generation.
3.4. KRG inhibits inflammatory cytokine and interferon secretion caused by Δpep27 vaccine administration
Because iNOS expression is induced by inflammatory cytokines and interferons [5], [6], we tested whether KRG coadministration downregulates the production of such molecules. After KRG coadministration, mice were challenged with D39, and cytokine levels in bronchoalveolar lavage fluid were determined by ELISA. In comparison with treatment with Δpep27 alone, KRG+Δpep27 significantly dampened the expression of inflammatory cytokines such as interleukin-1β and interferon-γ (Fig. 4), indicating that KRG coadministration did effectively restrict the production of proinflammatory cytokines.
Fig. 4.
KRG reduces secretion of inflammatory cytokines and interferons during Δpep27 immunization. Mice (n = 3) were orally administered KRG for 15 days, during which, they were immunized once with Δpep27. They were then challenged with 1 × 108 CFU D39. (A) Six hours after infection, IL-1β levels in BAL fluid were measured by ELISA. (B) Six hours after infection, IFN-γ levels in BAL fluid were measured by ELISA. Statistically significant differences were identified by one-way ANOVA (with Bonferroni's multiple comparison test) (*p < 0.05). Each experiment depicted was performed at least three times, with samples in duplicate.
ANOVA, analysis of variance; BAL, bronchoalveolar lavage; ELISA, enzyme-linked immuno sorbent assay; IL, interleukin; IFN, interferon; KRG, Korean Red Ginseng.
4. Discussion
S. pneumoniae causes diseases such as pneumonia and septicemia and is responsible for high rates of morbidity and mortality worldwide [37]. Although currently available pneumococcal vaccines are effective in preventing pneumococcal diseases, they are limited because they do not induce a T cell response [38] and can only protect against specific serotypes [39]. To overcome these limitations, a variety of attenuated pneumococci have been trialed as vaccines to date [23], [40]; however, the major disadvantage of these vaccines is that they are only effective when used in combination with adjuvants [23], [41]. The Δpep27 vaccine, which does not require adjuvants [28], [29], has the potential to overcome this problem but needs to be administered at least three times to be effective [28]. Therefore, reducing the number of Δpep27 immunizations required while retaining the same level of efficacy would be highly preferable.
The antimicrobial effects of KRG have been ascribed to reduced bacterial burden and tissue injury in the lungs [42]. KRG can also protect against pneumococcal sepsis by increasing cell survival [15]. Consistent with this, we confirmed that KRG coadministration conferred better protection against S. pneumoniae D39 infection than the control treatment and Δpep27 alone as it was associated with a higher survival rate than the latter two treatments (Fig. 1C). Conjugate vaccines activate T cells and induce differentiation of B cells, which ultimately increases the production of IgG antibodies that react specifically with S. pneumoniae [36]. In the present study, levels of IgG antibodies specific for D39 and PspA were significantly higher in the KRG coadministered group than in the other groups (Fig. 1A, B), suggesting that KRG pretreatment enhanced the efficacy of the Δpep27 vaccine.
Administration of KRG has been shown to inhibit pneumococcal colony formation in the lungs and reduce inflammation of this tissue [43]. In the present work, we found that S. pneumoniae colonization was inhibited by the coadministration of KRG with Δpep27 (Fig. 2A). Macrophage-mediated phagocytosis is the most important factor in bacterial clearance. The number of phagocytosed bacteria was significantly higher in the KRG coadministration group than in the other groups (Fig. 2E), indicating that the combined action of Δpep27 and KRG promoted macrophage-mediated bacterial clearance, thereby inhibiting colony formation in the lungs.
S. pneumoniae releases pneumolysin at the time of infection resulting in the generation of ROS, which are highly reactive and can damage cells [4], [44], [45], [46]. During bacterial infection, inflammatory cytokines and interferons upregulate the production of iNOS [5], [47], which also leads to increased ROS levels [6]. ROS activate ERK signaling by inhibiting the dual-specificity phosphatase and ERK-specific phosphatases which negatively regulate ERK [48], [49]. ERK then downregulates the p-AKT pathway involved in cell survival and activates caspase-3 to induce apoptosis [12], [50]. KRG suppresses inflammation by inhibiting iNOS expression in Helicobacter pylori–infected gastric cells [42]. In addition, it restricts the production of NO during S. pneumoniae infection, reducing inflammation and preventing sepsis [15]. Here, inflammatory cytokine and interferon levels were significantly decreased in the KRG coadministration group compared with the Δpep27 group (Fig. 4). ROS production in infected mice administered KRG+Δpep27 was similar to that in noninfected mice and was significantly lower than that in infected control and Δpep27-treated mice (Fig. 3A). Expression of iNOS, p-ERK, and caspase-3 was also significantly decreased in the KRG coadministration group (Figs. 3B, 3D). In addition, as the expression of ERK was suppressed, the AKT pathway was activated, and the expression of antiapoptotic factors was significantly increased in mice coadministered KRG and Δpep27 (Figs. 3C, 3E). This suggests that such coadministration inhibited ROS production, which consequently suppressed ERK signaling, and ultimately restricted apoptosis. Furthermore, attenuated lung inflammation was noted in the KRG+Δpep27 group (Fig. 2C), suggesting that inhibition of ROS production had antiinflammatory effects.
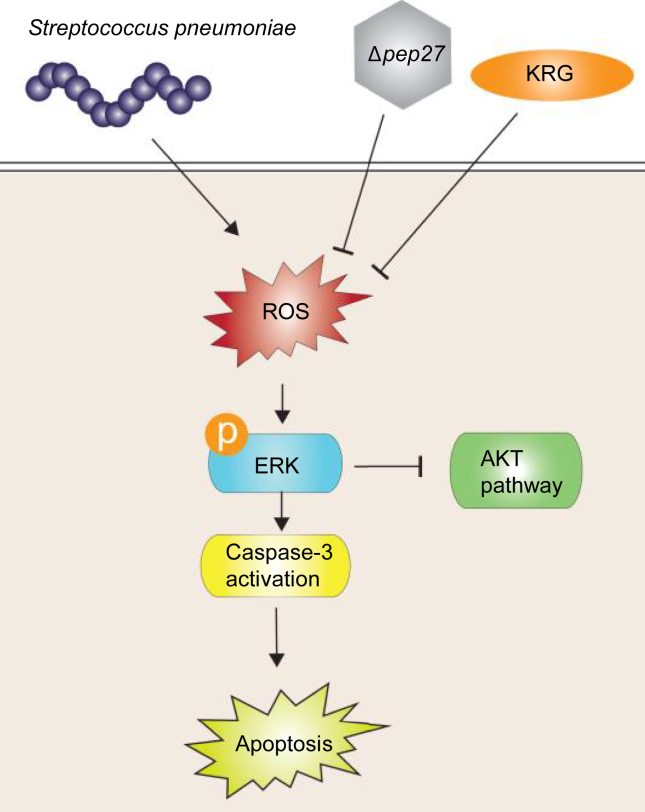
In conclusion, our results show that KRG enhances the efficacy of the Δpep27 vaccine as it was found to increase the survival of mice infected with pneumococcus and augment the phagocytic activity of macrophages, impeding pneumococcal colonization of the lungs. In addition, it hindered the pneumococcus-associated generation of ROS, thereby inhibiting ERK signaling–induced apoptosis and reducing inflammation (Fig. 5). Therefore, KRG coadministration may be an appropriate approach for increasing the efficacy of the Δpep27 vaccine while reducing the time required for immunization. However, whether KRG acts as an adjuvant has not yet been clarified and needs further study.
Fig. 5.
Schematic diagram illustrating suppression of the ROS/ERK pathway by KRG during Δpep27 immunization. KRG enhances the efficacy of the Δpep27 vaccine as it inhibits ROS generation triggered by S. pneumoniae, thereby resulting in a reduction in ERK signaling–induced apoptosis.
ERK, extracellular signal–regulated kinase; KRG, Korean Red Ginseng; ROS, reactive oxygen species.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Acknowledgments
The authors thank the Korea Ginseng Corporation for supplying the KRG extract, which was produced by a standardized process. This work was supported by the National Research Foundation (NRF-2015R1 A2 A1 A10052511 and NRF-2018R1A2A1A05078102). The funding body played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2017.11.007.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bogaert D., De Groot R., Hermans P.W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Organization, W.H.O . 2015. Pneumonia. [Google Scholar]
- 3.Martner A., Dahlgren C., Paton J.C., Wold A.E. Pneumolysin released during Streptococcus pneumoniae autolysis is a potent activator of intracellular oxygen radical production in neutrophils. Infect Immun. 2008;76(9):4079–4087. doi: 10.1128/IAI.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saura M., Zaragoza C., Bao C., McMillan A., Lowenstein C.J. Interaction of interferon regulatory factor-1 and nuclear factor kappaB during activation of inducible nitric oxide synthase transcription. J Mol Biol. 1999;289(3):459–471. doi: 10.1006/jmbi.1999.2752. [DOI] [PubMed] [Google Scholar]
- 6.Teng X., Zhang H., Snead C., Catravas J.D. Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am J Physiol Cell Physiol. 2002;282(1):C144–C152. doi: 10.1152/ajpcell.2002.282.1.C144. [DOI] [PubMed] [Google Scholar]
- 7.Bacus S.S., Gudkov A.V., Lowe M., Lyass L., Yung Y., Komarov A.P., Keyomarsi K., Yarden Y., Seger R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20(2):147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- 8.Tang D., Wu D., Hirao A., Lahti J.M., Liu L., Mazza B., Kidd V.J., Mak T.W., Ingram A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277(15):12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 9.Dong J., Ramachandiran S., Tikoo K., Jia Z., Lau S.S., Monks T.J. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol. 2004;287(5):F1049–F1058. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandiran S., Huang Q., Dong J., Lau S.S., Monks T.J. Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol. 2002;15(12):1635–1642. doi: 10.1021/tx0200663. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Martindale J.L., Holbrook N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275(50):39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang S., Yan Y., Daubert R.A., Han J., Schnellmann R.G. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292(1):F440–F447. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]
- 13.Nam Ki Y. Clinical applications and efficacy of Korean ginseng. Journal of Ginseng Research. 2002;26(3):111–131. [Google Scholar]
- 14.Lopez M.V., Cuadrado M.P., Ruiz-Poveda O.M., Del Fresno A.M., Accame M.E. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim Biophys Acta. 2007;1770(9):1308–1316. doi: 10.1016/j.bbagen.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen C.T., Luong T.T., Lee S.Y., Kim G.L., Kwon H., Lee H.G., Park C.K., Rhee D.K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine. 2015;22(11):1055–1061. doi: 10.1016/j.phymed.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Yoo D.G., Kim M.C., Park M.K., Park K.M., Quan F.S., Song J.M., Wee J.J., Wang B.Z., Cho Y.K., Compans R.W. Protective effect of ginseng polysaccharides on influenza viral infection. PloS One. 2012;7(3):e33678. doi: 10.1371/journal.pone.0033678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogaert D., Hermans P.W., Adrian P.V., Rumke H.C., de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22(17-18):2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Oosterhuis-Kafeja F., Beutels P., Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998-2006) Vaccine. 2007;25(12):2194–2212. doi: 10.1016/j.vaccine.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Koul P.A. Vaccination in the prevention of community-acquired pneumonia in adults. Journal of The Association of Physicians of India. 2016;64(12):22–27. [Google Scholar]
- 20.Sniadack D.H., Schwartz B., Lipman H., Bogaerts J., Butler J.C., Dagan R., Echaniz-Aviles G., Lloyd-Evans N., Fenoll A., Girgis N.I. Potential interventions for the prevention of childhood pneumonia: geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children–implications for vaccine strategies. Pediatr Infect Dis J. 1995;14(6):503–510. [PubMed] [Google Scholar]
- 21.Briles D.E., Hollingshead S.K., Nabors G.S., Paton J.C., Brooks-Walter A. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine. 2000;19(Suppl 1):S87–95. doi: 10.1016/s0264-410x(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 22.Roche A.M., King S.J., Weiser J.N. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun. 2007;75(5):2469–2475. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K., Yao R., Wang H., Pang D., Liu Y., Xu H., Zhang S., Zhang X., Yin Y. Mucosal and systemic immunization with a novel attenuated pneumococcal vaccine candidate confer serotype independent protection against Streptococcus pneumoniae in mice. Vaccine. 2014;32(33):4179–4188. doi: 10.1016/j.vaccine.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Giudice E.L., Campbell J.D. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58(1):68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Hvalbye B.K., Aaberge I.S., Lovik M., Haneberg B. Intranasal immunization with heat-inactivated Streptococcus pneumoniae protects mice against systemic pneumococcal infection. Infect Immun. 1999;67(9):4320–4325. doi: 10.1128/iai.67.9.4320-4325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malley R., Anderson P.W. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc Natl Acad Sci U S A. 2012;109(10):3623–3627. doi: 10.1073/pnas.1121383109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., Spyr C., Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 28.Kim E.H., Choi S.Y., Kwon M.K., Tran T.D., Park S.S., Lee K.J., Bae S.M., Briles D.E., Rhee D.K. Streptococcus pneumoniae pep27 mutant as a live vaccine for serotype-independent protection in mice. Vaccine. 2012;30(11):2008–2019. doi: 10.1016/j.vaccine.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 29.Kim G.L., Choi S.Y., Seon S.H., Lee S., Park S.S., Song J.Y., Briles D.E., Rhee D.K. Pneumococcal pep27 mutant immunization stimulates cytokine secretion and confers long-term immunity with a wide range of protection, including against non-typeable strains. Vaccine. 2016;34(51):6481–6492. doi: 10.1016/j.vaccine.2016.10.071. [DOI] [PubMed] [Google Scholar]
- 30.Avery O.T., Macleod C.M., McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types : induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type Iii. J Exp Med. 1944;79(2):137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi S.Y., Tran T.D., Briles D.E., Rhee D.K. Inactivated pep27 mutant as an effective mucosal vaccine against a secondary lethal pneumococcal challenge in mice. Clin Exp Vaccine Res. 2013;2(1):58–65. doi: 10.7774/cevr.2013.2.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E.H., Kim I.H., Lee M.J., Thach Nguyen C., Ha J.A., Lee S.C., Choi S., Choi K.T., Pyo S., Rhee D.K. Anti-oxidative stress effect of red ginseng in the brain is mediated by peptidyl arginine deiminase type IV (PADI4) repression via estrogen receptor (ER) beta up-regulation. J Ethnopharmacol. 2013;148(2):474–485. doi: 10.1016/j.jep.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Miyaji E.N., Dias W.O., Gamberini M., Gebara V.C., Schenkman R.P., Wild J., Riedl P., Reimann J., Schirmbeck R., Leite L.C. PsaA (pneumococcal surface adhesin A) and PspA (pneumococcal surface protein A) DNA vaccines induce humoral and cellular immune responses against Streptococcus pneumoniae. Vaccine. 2001;20(5-6):805–812. doi: 10.1016/s0264-410x(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.N., Gil C.H., Kim Y.R., Shin H.K., Choi B.T. Anti-photoaging properties of the phosphodiesterase 3 inhibitor cilostazol in ultraviolet B-irradiated hairless mice. Sci Rep. 2016;6:31169. doi: 10.1038/srep31169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper N.R., Nemerow G.R. The role of antibody and complement in the control of viral infections. J Invest Dermatol. 1984;83(1 Suppl):121s–127s. doi: 10.1111/1523-1747.ep12281847. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard-Rohner G., Pollard A.J. Long-term protection after immunization with protein-polysaccharide conjugate vaccines in infancy. Expert Rev Vaccines. 2011;10(5):673–684. doi: 10.1586/erv.11.14. [DOI] [PubMed] [Google Scholar]
- 37.Fedson D.S., Scott J.A. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine. 1999;17(Suppl 1):S11–S18. doi: 10.1016/s0264-410x(99)00122-x. [DOI] [PubMed] [Google Scholar]
- 38.Song J.Y., Nahm M.H., Moseley M.A. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci. 2013;28(1):4–15. doi: 10.3346/jkms.2013.28.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cutts F.T., Zaman S.M., Enwere G., Jaffar S., Levine O.S., Okoko J.B., Oluwalana C., Vaughan A., Obaro S.K., Leach A. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 40.Moffitt K.L., Yadav P., Weinberger D.M., Anderson P.W., Malley R. Broad antibody and T cell reactivity induced by a pneumococcal whole-cell vaccine. Vaccine. 2012;30(29):4316–4322. doi: 10.1016/j.vaccine.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogenesch H., Dunham A., Hansen B., Anderson K., Maisonneuve J.F., Hem S.L. Formulation of a killed whole cell pneumococcus vaccine - effect of aluminum adjuvants on the antibody and IL-17 response. J Immune Based Ther Vaccines. 2011;9:5. doi: 10.1186/1476-8518-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150(2):761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Song Z., Johansen H.K., Faber V., Moser C., Kharazmi A., Rygaard J., Hoiby N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother. 1997;41(5):961–964. doi: 10.1128/aac.41.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.O., Ryu S.H., Lee Y.S., Kim J.I., Moon G.S. Protective effect of dietary buchu (Allium tuberosum Rottler) on oxidative stress and lipofuscin formation in streptozotocin – induced diabetic rats. J Korean Soc Food Sci Nutr. 2003;32(8):1337–1343. [Google Scholar]
- 45.Taylor N.L., Millar A.H. Oxidative stress and plant mitochondria. Methods Mol Biol. 2007;372:389–403. doi: 10.1007/978-1-59745-365-3_28. [DOI] [PubMed] [Google Scholar]
- 46.Wolfmeier H., Radecke J., Schoenauer R., Koeffel R., Babiychuk V.S., Drucker P., Hathaway L.J., Mitchell T.J., Zuber B., Draeger A. Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack. Biochim Biophys Acta. 2016;1860(11 Pt A):2498–2509. doi: 10.1016/j.bbagen.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto T., Khan S., Oyama K., Fujii S., Sawa T., Akaike T. A new paradigm for antimicrobial host defense mediated by a nitrated cyclic nucleotide. J Clin Biochem Nutr. 2010;46(1):14–19. doi: 10.3164/jcbn.SR09-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M., Bao W., Aizman R., Huang P., Aspevall O., Gustafsson L.E., Ceccatelli S., Celsi G. Activation of extracellular signal-regulated kinase mediates apoptosis induced by uropathogenic Escherichia coli toxins via nitric oxide synthase: protective role of heme oxygenase-1. J Infect Dis. 2004;190(1):127–135. doi: 10.1086/421243. [DOI] [PubMed] [Google Scholar]
- 49.Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 50.Tan B.J., Chiu G.N. Role of oxidative stress, endoplasmic reticulum stress and ERK activation in triptolide-induced apoptosis. Int J Oncol. 2013;42(5):1605–1612. doi: 10.3892/ijo.2013.1843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.