Ginsenosides are the major active ingredients of ginseng. In skin physiology, ginsenosides affect pigment regulation and exhibit antiaging activity. According to recent reports, ginsenoside Rg1 increases melanogenesis and tyrosinase activity in human melanocytes [1]. In contrast, ginsenoside F1 (GF1) (sFig. 1), a metabolite produced by the hydrolysis of Rg1, is reported to inhibit visible pigmentation. A clinical study by Han et al demonstrated a skin whitening effect of GF1 on artificially tanned human skin caused by interleukin 13 production from human epidermal γδ T cells [2]. Kim et al showed that GF1 reduces melanin secretion through dendrite retraction with no effects on the intracellular melanin content and tyrosinase expression in α-melanocyte stimulating hormone (MSH)-stimulated B16F10 murine melanoma cells [3]. To date, however, no direct findings have been obtained on the whitening effects of GF1 in the human epidermal environment consisting of melanocytes and keratinocytes in an in vitro experimental system.
Here, we investigated the antimelanogenic effects of GF1 on MNT-1/HaCaT cocultures cells and three-dimensional (3-D) human skin equivalent. In addition, we focused on the dendrite formation in melanocytes and melanosome transfer into keratinocytes after GF1 treatment. Collectively, our findings demonstrate for the first time that GF1 showed the whitening effects in the human epidermis coexisting with melanocytes and keratinocytes.
For this study, MNT-1 cells were cultured in minimum essential media (MEM) containing 20% Fetal Bovine Serum (FBS), 1% gentamicine, and 20 mM hydroxyethyl piperazineethanesulfonic acid (HEPES). HaCaT cells were cultured in dulbecco's modified eagle's medium (DMEM) containing 10% FBS, 1% gentamicine, and 20 mM HEPES. Normal human epidermal melanocytes (NHEM) were purchased from (Lonza, Basel, Switzerland) and were cultured in melanocyte growth medium-4 (MGM-4) media. Cells were incubated at 37°C under 5% CO2 atmosphere.
To measure the melanin contents, cells (2 × 105) were cultured in a 6-well plate for 24 h. Cells were treated with the indicated concentrations of GF1. Thereafter, cells were washed twice with phosphate-buffered saline (PBS) and then detached with trypsin/EDTA. Cells were lysed by incubating in 200 μL of 1 N NaOH at 80°C for 2 h. Cell lysates were transferred to a 96-well plate, and absorbance was measured at 405 nm.
To perform Western blotting, cells were washed twice with cold PBS and then lysed in ice-cold modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl) containing protease inhibitors and phosphatase inhibitor cocktail set (Calbiochem, San Diego, CA, USA). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies at 4°C for 24 h, then washed with tris-buffered saline including 0.1% Tween-20, exposed to peroxidase-conjugated secondary antibodies for 1 h at room temperature, washed, and then visualized using an Odyssey imaging system.
To analyze the melanosome transfer, MNT-1/HaCaT cells were cultured in a 12-well plate at the ratio of 1 to 10 for 24 h, and then, GF1 was treated with indicated concentration. Cells were washed twice with cold PBS and fixed with 3.5% paraformaldehyde for 10 min. Cells were washed and treated with 0.1% Triton X-100 for 10 min and then 0.5% bovine serum albumin (BSA) for 1 h. Antibodies against tyrosinase-related protein 1 (1:200, Red) and cytokeratin-18 (1:200, Green) were used for melanocytes and keratinocytes detection.
MelanoDerm Skin Tissue Model (TM) is a viable reconstituted 3-D human skin equivalent containing normal melanocytes and keratinocytes (MatTek Corp., Ashland, MA). We used MelanoDerm TM (MEL-300-A) that is derived from Asian donors. They were maintained in the new maintenance medium-113 as recommended by the manufacturer. GF1 was applied to the MelanoDerm TM on days 0, 4, 6, 8, 11, 13, and 15. Before GF1 treatment, the tissues were washed with 1 mL of PBS to remove any residual compound. Tissues were fixed on day 18 for histological analysis. Histological evaluation was performed under light microscope (Bx-41; Olympus, Tokyo, Japan), and photomicrographs were taken using digital camera (DP72; Olympus). Melanoderm was fixed in 4% buffered formaldehyde solution for the staining. The samples were embedded in paraffin wax, cut to a thickness of 3 μm, stained using Hematoxylin and Eosin and Fontana and Mason staining solution. All data are expressed as means ± SD (standard deviation) values, and Student t test was used for statistical comparisons. A p value < 0.05 was considered statistically significant (individual p values are given in figure legends).
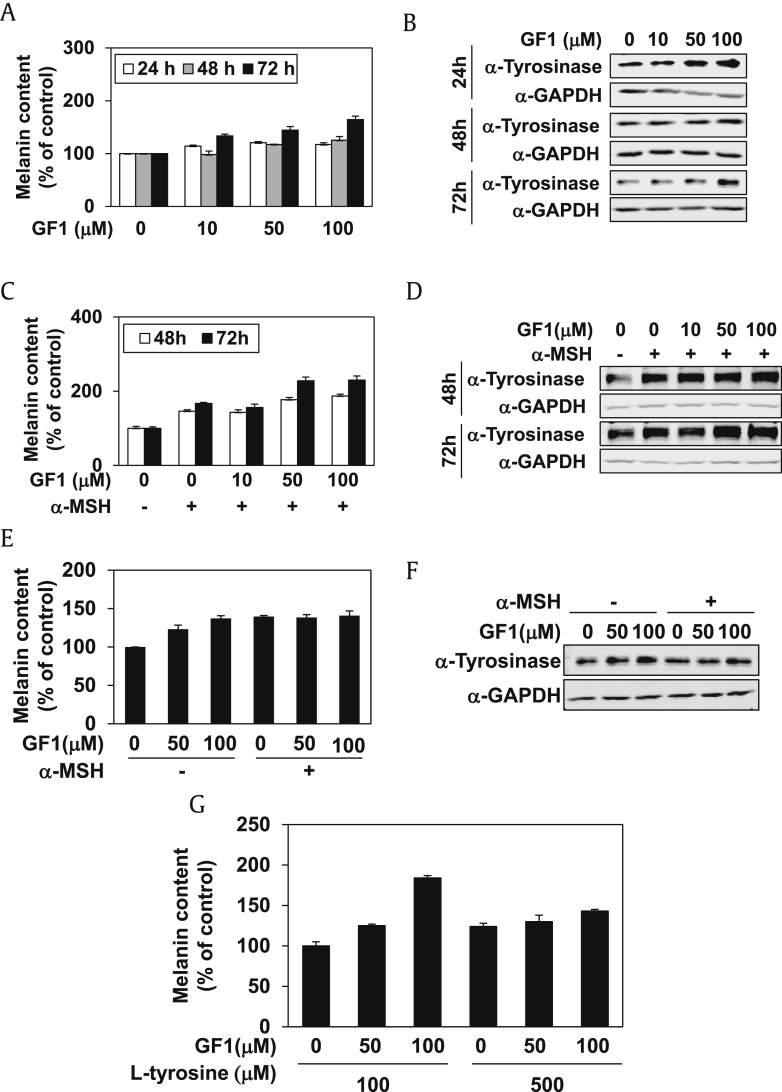
To establish the effect of GF1 on human melanocytes, we treated highly pigmented human melanoma cells (MNT-1) with GF1 for 24, 48, and 72 h, followed by analysis of the intracellular melanin content and expression of tyrosinase protein. As shown in Fig. 1A, GF1 had no inhibitory effect on melanin content within a concentration range of 10–100 μM. In addition, tyrosinase expression was not decreased by GF1 in MNT-1 cells (Fig. 1B, sFig. 2A). Treatment of α-MSH-stimulated MNT-1 cells with GF1 for 48 h and 72 h did not result in suppression of the melanin content (Fig. 1C) or tyrosinase expression (Fig. 1D, sFig. 2B). To further confirm the effect of GF1 on human melanocytes, we treated primary NHEM with GF1 in the presence or absence of α-MSH stimulation for 48 h. Similar to data obtained with MNT-1 cells, GF1 did not affect melanin content (Fig. 1E) or tyrosinase expression (Fig. 1F, sFig. 2C) in NHEM. Finally, we assessed whether the L-tyrosine level in the culture medium affects GF1 activity because L-tyrosine is the substrate of tyrosinase for melanin synthesis. Accordingly, MNT-1 cells were treated with GF1 for 48 h in the culture medium containing 100 μM or 500 μM L-tyrosine. As shown in Fig. 1G, GF1 did not inhibit the melanin content in MNT-1 cells, regardless of the concentration of L-tyrosine.
Fig. 1.
The effect of GF1 on melanin contents and tyrosinase expression in human melanocytes. GF1 (10 μM, 50 μM, 100 μM) was treated to MNT-1 cells for 24 h, 48 h, and 72 h. (A) Melanin contents were then analyzed. (B) Tyrosinase expression level was analyzed. In addition, GF1 (10, 50, 100 uM) was treated to α-MSH (500 nM)-stimulated MNT-1 cells for 48 h and 72 h. (C) Melanin contents were then analyzed. (D) Tyrosinase expression level was analyzed. We treated primary normal human epidermal melanocytes (NHEM) with GF1 (50 μM and 100 μM) in the presence or absence of α-MSH stimulation for 48 h. (E) Melanin contents were then analyzed. (F) Tyrosinase expression level was analyzed. MNT-1 cells were treated with GF1 (50 μM and 100 μM) for 48 h in culture medium containing 100 μM or 500 μM L-tyrosine. (G) Then, melanin contents were analyzed.
GADPH, glyceraldehyde 3-phosphate dehydrogenase; GF1, ginsenoside F1.
Following the finding that GF1 does not exert an antimelanogenic effect in mono-cultured human melanocytes, we further analyzed whether GF1 affects melanin synthesis in melanocytes in an experimental system containing both keratinocytes and melanocytes with the possibility of cell–cell communication. MNT-1 and HaCaT (an immortalized human normal keratinocyte cell line) cells were cocultured with 100 μM GF1 in the presence or absence of UV-B irradiation. After 48 h (sFig. 3A) or 72 h (sFig. 3B), we assessed the melanin contents from isolated MNT-1 and cocultured cells. Our data showed that GF1 does not inhibit melanin synthesis in both isolated MNT-1 and cocultured cell groups.
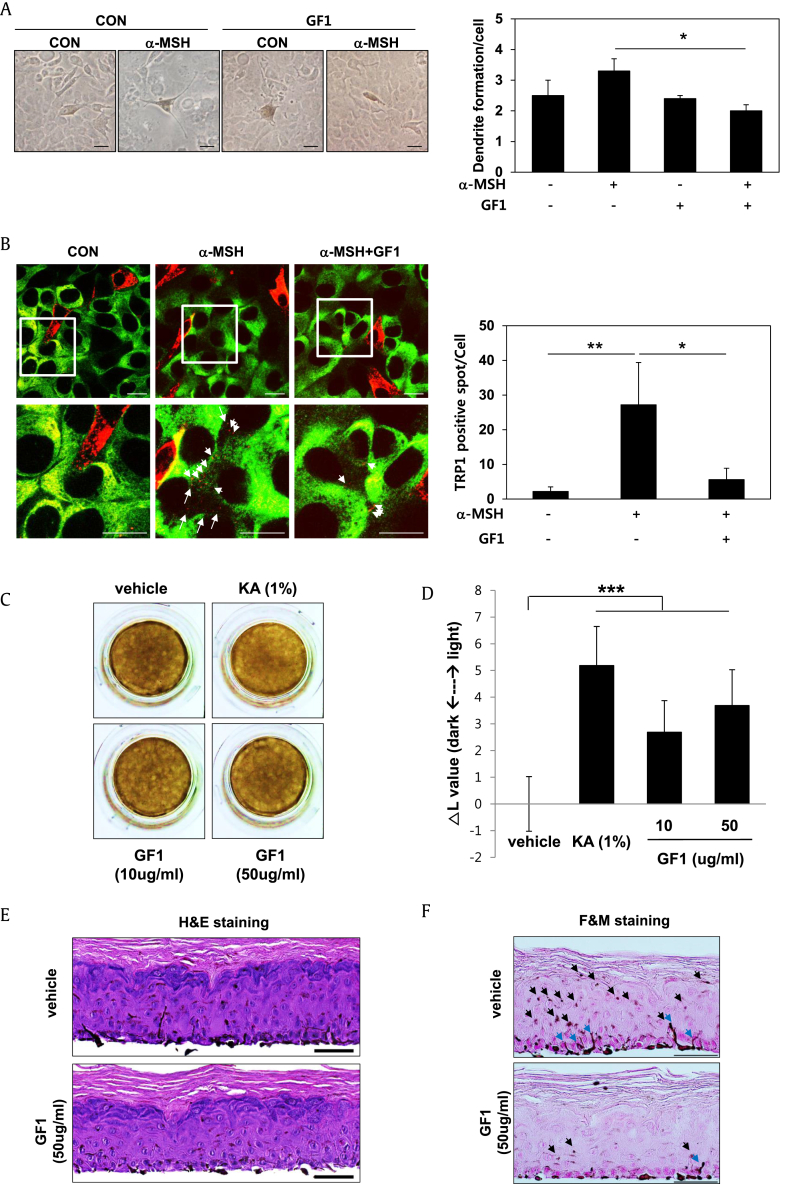
In addition to melanin synthesis, we focused on the inhibitory efficacy of GF1 on melanosome transfer from melanocytes to keratinocytes. Specifically, cocultured MNT-1/HaCaT cells were treated with 100 μM GF1 in the presence or absence of α-MSH stimulation (Fig. 2A), followed by examination of dendrite formation in melanocytes, which is required for melanosome transfer. Interestingly, α-MSH-induced stretched dendrites were retracted upon GF1 treatment. Then, we stained TRP-1-positive melanosomes (Red) for MNT-1 cells and cytokeratin-18 (Green) for HaCaT cells in cocultures. As shown in Fig. 2B, TRP-1-positive melanosomes in MNT-1 cells were transferred into HaCaT cells after α-MSH stimulation, which was significantly inhibited by GF1. These results clearly indicate that GF1 suppresses melanosome transfer, causing dendrite retraction of melanocytes. To extend this investigation to a more physiological system, we examined the whitening ability of GF1 in a 3-D human skin equivalent, which is an epidermal layer consisting of melanocytes and keratinocytes. In Fig. 2C, GF1-treated tissue appeared lighter than control tissue, and the L value (a lightness/darkness index) was altered upon GF1 treatment (Fig. 2D). In addition, hematoxylin and eosin staining of tissue sections revealed no tissue collapse or toxicity (Fig. 2E). Fontana and Mason staining experiments showed that GF1 inhibits dendrite formation of melanocytes in the basal layer and simultaneously suppresses melanosome transfer from melanocytes of the basal layer to keratinocytes of the differentiated upper layer (Fig. 2F). These findings collectively support the inhibitory efficacy of GF1 in melanosome transfer, leading to promotion of whitening in the human skin tissue model.
Fig. 2.
The effect of GF1 on dendrite formation and melanosome transfer in MNT-1/HaCaT cocultured cells and a 3-D human skin equivalent. Cocultured MNT-1/HaCaT cells were treated with 100 μM GF1 in the presence or absence of α-MSH (500 nM) stimulation for 48 h. (A) After the fixation, we observed dendrite formation in melanocytes using optical microscopy (Scale bar; 50 μm). (B) For immunostaining, we stained melanosome with TRP-1 antibody (red color) and keratinocytes with cytokeratin-18 (green color). White arrows indicate transferred melanosome to HaCaT from MNT-1 cells (Scale bar; 20 μm). Human skin equivalent (MelanoDerm; n = 3) was treated with the 1% Kojic acid as a reference whitening compound and GF1 (10 and 50 ug/ml) for 18 d. (C) After treatment, skin equivalents were photographed. (D) ▵ L value (degree of lightness as compared to vehicle-treated control) for each test sample was calculated. (E) The Hematoxylin and Eosin (H&E) staining of tissue sections. (F) Fontana-Mason (F&M) staining of tissue sections. The slides were fixed in formaldehyde solution and embedded in paraffin wax for the staining (Scale bar; 50 μm). Black arrows indicate the transferred melanosome from melanocytes in the basal layer to keratinocytes in the upper layer. Blue arrows indicate melanocytes forming the extended dendrites. (*p < 0.05, **p < 0.01, *p < 0.001).
CON, control; GF1, ginsenoside F1; KA, Kojic acid.
In the present study, GF1 did not inhibit melanin synthesis and tyrosinase expression; rather, GF1 slightly increased intracellular melanin contents and tyrosinase expression in MNT-1 cells and cocultured cells (Fig. 1A and 1B and sFig. 3A). However, simultaneously, we found that GF1 inhibited melanosome transfer through the decreased dendrites formation of melanocytes. We suspect that increased intracellular melanin contents by GF1 could be caused by melanosome accumulation because of the blockade of melanosome transfer and induction of tyrosinase expression. Actually, visible skin pigmentation is determined by the amount of dispersion of melanosomes from the various areas of extended dendrites of melanocytes to surrounding keratinocytes [4]. Several compounds, such as centaureidin and methylophiopogonanone B, showed whitening efficacy, leading to melanocyte dendrite retraction without influencing melanogenic enzyme expression or melanin synthesis [5]. Therefore, the blockade of melanosome transfer into keratinocytes from melanocytes is considered an effective approach to induce whitening effect, although the melanin biosynthesis is not inhibited. Therefore, we considered that increased melanin contents by GF1 have little impact on the whitening efficacy of GF1. In addition, accumulated melanosome would be degraded by autophagy for skin homeostasis, although the fate of melanosomes is unclear [6].
We demonstrated for the first time that GF1 suppresses α-MSH-induced dendrite formation, resulting in inhibition of melanosome transfer into keratinocytes in human cell cocultures. In addition, GF1 disrupted melanin transfer from melanocytes in the basal layer to keratinocytes in the upper layer, exerting a whitening effect in the human skin equivalent. Recent coculture experiments indicate that melanocytes deliver pigment to keratinocytes via dendritic morphology changes, such as disorganization of β-tubulin microfilaments in the intracellular cytoskeleton [7], [8]. Cyclic adenosine monophosphate, a melanogenesis inducer, mediates dendrite formation through upregulation of Rac and downregulation of Rho activity [9], [10]. Therefore, future studies by our group will focus on comprehensive evaluation of the target proteins of GF1, such as molecules involved in the Cyclic adenosine monophosphate signaling pathway.
Collectively, our results provide preliminary evidence that GF1 has potential for development as an effective whitening reagent for the treatment of pigmentary disorders and lightening of darkened skin color as a cosmetic ingredient.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported and funded by the Amorepacific R&D Center.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2017.12.005
Appendix A. Supplementary data
The following is the supplementary data related to this article:
sFig. 1. The structure of ginsenoside F1 (GF1).
sFig. 2. Densitometry for tyrosinase expression by GF1 in MNT-1 cells. (A) MNT-1 cells were treated with GF1 for 24, 48, and 72 h, followed by analysis of expression of tyrosinase protein. (B) Treatment of α-MSH-stimulated MNT-1 cells with GF1 for 48 and 72 h, followed by analysis of expression of tyrosinase protein. (C) Primary normal human epidermal melanocytes (NHEM) were treated with GF1 in the presence or absence of α-MSH stimulation for 48 h, followed by analysis of expression of tyrosinase protein. GADPH, glyceraldehyde 3-phosphate dehydrogenase.
sFig. 3. The effect of GF1 on melanin contents in MNT-1/HaCaT cocultured cells. MNT-1 and HaCaT cells were cocultured with 100 μM GF1 in the presence or absence of uv-b irradiation. (A) After 48 h, we assessed the melanin contents from isolated MNT-1 and cocultured cells. (B) After 72 h, we assessed the melanin contents from isolated MNT-1 and cocultured cells.
References
- 1.Lin M., Zhang B.X., Zhang C., Shen N., Zhang Y.Y., Wang A.X., Tu C.X. Ginsenosides Rb1 and Rg1 stimulate melanogenesis in human epidermal melanocytes via PKA/CREB/MITF signaling. Evid. Based. Complement. Alternat Med. 2014;2014:892073. doi: 10.1155/2014/892073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han J., Lee E., Kim E., Yeom M.H., Kwon O., Yoon T.H., Lee T.R., Kim K. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of Ginsenoside F1. Exp Dermatol. 2014;23:860–862. doi: 10.1111/exd.12531. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.H., Baek E.J., Lee E.J., Yeom M.H., Park J.S., Lee K.W., Kang N.J. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp Dermatol. 2015;24:150–152. doi: 10.1111/exd.12586. [DOI] [PubMed] [Google Scholar]
- 4.Ando H., Niki Y., Ito M., Akiyama K., Matsui M.S., Yarosh D.B., Ichihashi M.J. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. Invest Dermatol. 2012;132:1222–1229. doi: 10.1038/jid.2011.413. [DOI] [PubMed] [Google Scholar]
- 5.Ebanks J.P., Wickett R.R., Boissy R.E. Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Int J Mol Sci. 2009;10:4066–4087. doi: 10.3390/ijms10094066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsuyama Y., Taira N., Yoshioka M., Okano Y., Masaki H. Disruption of melanosome transport in melanocytes treated with theophylline causes their degradation by autophagy. Biochem Biophys Res Commun. 2017;485:126–130. doi: 10.1016/j.bbrc.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Ni J., Wang N., Gao L., Li L., Zheng S., Liu Y., Ozukum M., Nikiforova A., Zhao G., Song Z. The effect of the NMDA receptor-dependent signaling pathway on cell morphology and melanosome transfer in melanocytes. J Dermatol Sci. 2016;84:296–304. doi: 10.1016/j.jdermsci.2016.08.534. [DOI] [PubMed] [Google Scholar]
- 8.Scott G. Rac and rho: the story behind melanocyte dendrite formation. Pigment Cell Res. 2002;15:322–330. doi: 10.1034/j.1600-0749.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- 9.Scott G., Leopardi S. The cAMP signaling pathway has opposing effects on Rac and Rho in B16F10 cells: implications for dendrite formation in melanocytic cells. Pigment Cell Res. 2003;16:139–148. doi: 10.1034/j.1600-0749.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 10.Ma H.J., Ma H.Y., Yang Y., Li P.C., Zi S.X., Jia C.Y., Chen R. α-Melanocyte stimulating hormone (MSH) and prostaglandin E2 (PGE2) drive melanosome transfer by promoting filopodia delivery and shedding spheroid granules: evidences from atomic force microscopy observation. J Dermatol Sci. 2014;76:222–230. doi: 10.1016/j.jdermsci.2014.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.