Abstract
Aims
Each episode of acute decompensated heart failure (HF) incrementally adds to mortality. Peritoneal dialysis (PD) offers an alternative therapeutic option in refractory HF and reduces the incidence of decompensation episodes. The objective of this study was to determine the efficacy of PD, in terms of functional status, surrogate endpoints, rate of hospitalizations, and mortality.
Methods and results
This study is based on the registry of the German Society of Nephrology, involving 159 patients receiving PD treatment due to refractory HF between January 2010 and December 2014. Body weight was reduced by PD (82.2 ± 14.9 to 78.4 ± 14.8 kg, P < 0.001), and significant improvements in New York Heart Association functional class (3.38 ± 0.55 to 2.85 ± 0.49, P < 0.001) were found already after 3 months. Left ventricular ejection fraction did not change (31.5 ± 13.8 to 34.0 ± 15.7%, P = 0.175). C‐reactive protein improved with PD treatment (33.7 ± 52.6 to 17.1 ± 26.3 mg/L, P = 0.004). Blood urea nitrogen/creatinine ratio decreased significantly (148.7 ± 68.3 to 106.7 ± 44.8 mg/dL, P < 0.001). Hospitalization rates decreased significantly (total number 2.86 ± 1.88 to 1.90 ± 1.78, P = 0.001, and 39.2 ± 30.7 to 27.1 ± 25.2 days, P = 0.004). One year mortality was 39.6% in end‐stage HF patients treated with PD.
Conclusions
Peritoneal dialysis offers an additional therapeutic option in end‐stage HF and is associated with improved New York Heart Association classification and reduced hospitalization. Although PD treatment was associated with various benefits, further studies are necessary to identify which patients benefit the most from PD.
Keywords: Heart failure, Cardiorenal syndrome, Peritoneal dialysis, Ultrafiltration
Introduction
Congestive heart failure (CHF) is one of the fastest growing morbidities in industrial countries and the most common cause of hospital admission in elderly patients. In this patient cohort, CHF is accompanied by chronic kidney disease in up to 63% of patients1 and is associated with a very poor outcome.1, 2, 3, 4, 5, 6, 7, 8 Pathophysiological mechanisms of this cardiorenal syndrome are arterial underfilling and renal venous congestion, which hinder adequate volume control by ‘excretory renal insufficiency’, resulting in repeated hydropic decompensations.3, 5, 9, 10, 11, 12, 13, 14, 15, 16, 17 Although highly suggestive within this context, there is no evidence favouring ultrafiltration (UF) over conservative treatment with loop diuretics as first‐line therapy in patients with acute or chronic HF.18, 19 However, UF may be considered in patients with refractory congestion who failed to respond to diuretic‐based strategies.2 Whereas the UNLOAD‐HF20 and the AVOID‐HF trials21 showed some beneficial effects of UF, the CARESS‐HF trial not only failed to demonstrate the superiority of UF but was also associated with an increased number of adverse events. Peritoneal dialysis (PD), another therapeutic option that is frequently used in patients with refractory CHF,22 is associated with improved haemodynamic stability and lower costs and is able to drain ascites, which might at least theoretically improve outcome.
The aim of this study was therefore to provide more ‘real‐life’ information about outcome and treatment parameter patients (according to European Society of Cardiology 20122) treated with PD, employing the national registry of the German Society of Nephrology (DGfN).
Methods
For this prospective, multicentre, and national observational study, data from the registry of the DGfN were evaluated.
Between January 2010 and December 2014, a total of 159 patients with symptomatic end‐stage CHF were enrolled in ambulatory PD therapy after interdisciplinary assessment on the following conditions:
individually optimized pharmacological therapy according to the recommendation of the European Society of Cardiology2;
diuretic resistance defined as refractory hypervolaemia despite optimal sequential diuretic therapy [loop diuretics, thiazides, or, if possible, mineralocorticoid receptor antagonists (MRAs)] as recommended by national authorities2, 23;
device therapy as indicated by current guidelines2;
recurrent hospitalizations due to cardiac decompensation, at least two times within the last 6 months; and
patients not eligible for heart transplantation.
Before initiating PD, specific renal pathologies, for example, glomerulonephritis, were excluded, and conservative HF therapy was optimized. Echocardiography was performed before initiating PD. Exclusion criteria for this study were inotropic support and contraindication for PD. Renal function, as estimated by glomerular filtration rate, was calculated using the Modification of Diet in Renal Disease equation.
After implantation of a peritoneal dialysis catheter and careful instruction, patients performed PD by continuous ambulatory PD, automated PD, or intermittent PD. Scheduled study visits were recorded at initiation of PD, after 3 and 6 months, and every 6 months thereafter. All visits included assessment of patient history, physical examination, body weight, echocardiography, laboratory measurements, and medication. The main objective was hospitalizations.
All patients provided written informed consent, and the study was approved by the local ethics committee (vote number S‐106/2011).
Statistical analysis included the Kolmogorov–Smirnov test, Wilcoxon signed‐rank test, or Student's t‐test for paired variables, Levene's test, Pearson's correlation, and Kaplan–Meier estimator and log‐rank test. Level of significance was α = 5%. Statistical analysis was performed using SPSS version 24 (IBM Corp., Armonk, NY, USA) and Excel version 2011 (Microsoft Copr., Redmond, WA, USA).
Results
Patient characteristics and treatment modalities are summarized in Table 1. Study population comprised a cohort of n = 159 patients with a mean follow‐up time of 13.3 ± 15.0 months (66 months at longest).
Table 1.
Baseline patient characteristics (medical and demographic data)
| n | (%) | |
|---|---|---|
| 159 | (100) | |
| Sex | ||
| Male | 133 | (83.7) |
| Female | 26 | (16.3) |
| Age (years) | 72.8 ± 12.1 | (100) |
| Aetiology of CHF | ||
| Ischaemic cardiomyopathy | 58 | (36.5) |
| Dilated cardiomyopathy | 50 | (31.5) |
| Pulmonary hypertension and right ventricular dysfunction | 7 | (4.40) |
| Hypertensive heart disease | 3 | (1.89) |
| Pericarditis constrictiva | 2 | (1.26) |
| Congenital heart defect | 2 | (1.26) |
| Not specified | 37 | (23.3) |
| Valvular heart disease | ||
| Tricuspid regurgitation | ||
| I | 12 | (7.55) |
| II | 27 | (17.0) |
| III | 17 | (10.7) |
| Mitral regurgitation | ||
| I | 18 | (11.3) |
| II | 33 | (20.8) |
| III | 10 | (6.29) |
| Medication | ||
| Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers | 82 | (51.6) |
| Beta‐blockers | 95 | (59.7) |
| Spironolacton | 57 | (35.8) |
| Erythropoietin | 24 | (15.1) |
| NYHA functional class | ||
| II | 7 | (4.40) |
| II–III | 5 | (3.14) |
| III | 41 | (25.8) |
| III–IV | 38 | (23.9) |
| IV | 41 | (25.8) |
| Not specified | 27 | (17.0) |
| PD regime at beginning | ||
| APD | 54 | (34.0) |
| CAPD | 79 | (49.7) |
| IPD | 5 | (3.14) |
| Not specified | 21 | (13.2) |
| Haemodialysis prior to PD | 18 | (11.3) |
APD, automatic peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; CHF, congestive heart failure; IPD, intermittent peritoneal dialysis; NYHA, New York Heart Association; PD, peritoneal dialysis.
Data are presented as mean ± standard deviation or n.
Seventy‐four patients underwent cardiac catheterization (46.5%), and 66 patients received an implantable cardioverter–defibrillator (41.6%) before PD was started. Eighteen patients (11.3%) needed precursory intermittent haemodialysis, primarily due to acute hypervolaemia or hyperkalaemia (mean duration of haemodialysis 12.4 ± 3.24 h/week or 3 times a week with average period of 4.15 ± 1.08 h, respectively; average blood flow 230.6 ± 67.1 mL/min).
Within the first 3 months, a slight increase of IPD was observed (Figure 1 ). Average Kt∕V was 2.2 ± 1.2.14. Patients required intermittent haemodialysis at different time points after beginning of PD.
Figure 1.
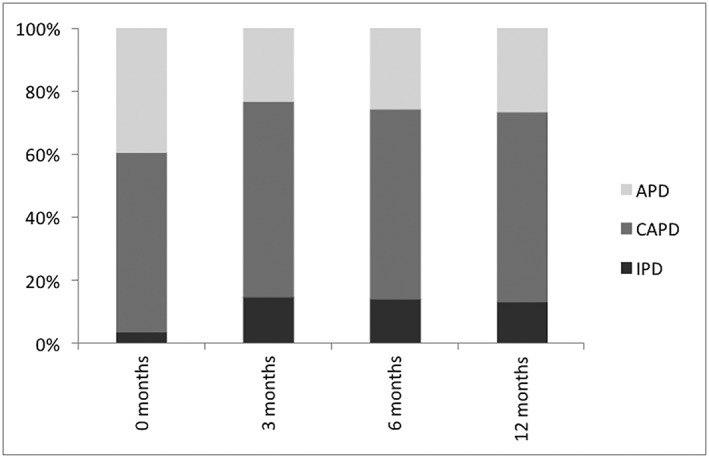
Peritoneal dialysis regime over the first year. APD, automatic peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; IPD, intermittent peritoneal dialysis.
Laboratory results are demonstrated in Table 2. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) revealed a negative absolute and relative personal change with PD treatment (−606 ng/L, relative change −3%).
Table 2.
Laboratory variables at baseline and during follow‐up after starting PD
| Pre‐PD | Post‐PD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 months | P | 6 months | P | 12 months | P | Last follow‐up | P | ||
| NT‐proBNP (pg/mL) | 3857 (IQR 2017–6303) | 2553 (IQR 1343–5001) | 0.931 | 2527 (IQR 1125–4874) | 0.904 | 1829 (IQR 958–3904) | 0.388 | 2747 (IQR 977–5743) | 0.770 |
| Albumin (g/L) | 38.5 ± 5.74 | 37.4 ± 6.18 | 0.048 | 37.8 ± 7.11 | 0.201 | 39.4 ± 4.94 | 0.988 | 37.2 ± 7.01 | 0.031 |
| Creatinine (mg/dL) | 3.06 ± 2.75 | 3.11 ± 2.43 | 0.939 | 3.46 ± 2.54 | 0.575 | 3.35 ± 2.64 | 0.020 | 3.89 ± 2.79 | 0.014> |
| MDRD eGFR (mL/min/1.73 m2) | 24.0 ± 11.3 | 28.5 ± 18.1 | 0.345 | 26.2 ± 15.5 | 0.669 | 25.9 ± 14.7 | 0.831 | 21.4 ± 14.0 | 0.019 |
| BUN (mg/dL) | 148.7 ± 68.3 | 105.9 ± 75.4 | <0.001 | 102.5 ± 41.9 | <0.001 | 96.8 ± 39.6 | <0.001 | 106.7 ± 44.8 | <0.001 |
| CRP (mg/L) | 33.7 ± 52.6 | 12.6 ± 25.1 | 0.001 | 13.2 ± 35.7 | 0.013 | 7.70 ± 7.65 | 0.002 | 17.1 ± 26.3 | 0.004 |
| Sodium (mmol/L) | 136.4 ± 4.77 | 137.9 ± 6.46 | 0.131 | 137.4 ± 4.96 | 0.111 | 137.9 ± 4.45 | 0.160 | 136.9 ± 5.55 | 0.635 |
| Potassium (mmol/L) | 4.31 ± 0.73 | 4.21 ± 0.66 | 0.013 | 4.27 ± 0.58 | 0.070 | 4.22 ± 0.55 | 0.106 | 4.37 ± 0.72 | 0.391 |
| Phosphate (mmol/L) | 1.57 ± 1.05 | 1.54 ± 0.81 | 0.299 | 1.57 ± 0.73 | 0.473 | 1.53 ± 0.85 | 0.834 | 1.70 ± 1.27 | 0.909 |
| Haemoglobin (mg/dL) | 11.2 ± 1.74 | 11.8 ± 1.82 | 0.004 | 11.7 ± 2.20 | 0.003 | 12.4 ± 1.85 | <0.001 | 11.5 ± 2.17 | 0.219 |
BUN, blood urea nitrogen; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; IQR, inter‐quartile range; MDRD, Modification of Diet in Renal Disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PD, peritoneal dialysis.
Median, standard deviation, and Student's t‐test for paired variables. Mean, IQR, and Wilcoxon signed‐rank text for not normally contributed paired variables.
Follow‐up results of clinical variables after beginning of PD are shown in Table 3. There were no significant differences regarding peritoneal UF, body weight, and urine volume (Figure 2 ).
Table 3.
Clinical variables at baseline and after starting PD
| Pre‐PD | Post‐PD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 months | P | 6 months | P | 12 months | P | Last follow‐up | P | ||
| NYHA | 3.38 ± 0.55 | 2.85 ± 0.49 | <0.001 | 2.76 ± 0.68 | <0.001 | 2.57 ± 0.73 | <0.001 | 2.73 ± 0.68 | <0.001 |
| Systolic BP (mmHg) | 110.9 ± 19.9 | 113.5 ± 18.9 | 0.605 | 111.8 ± 19.3 | 0.542 | 114.1 ± 22.2 | 0.524 | 110.6 ± 21.2 | 0.428 |
| Diastolic BP (mmHg) | 67.7 ± 12.1 | 65.5 ± 10.9 | 0.003 | 66.6 ± 12.6 | 0.112 | 67.7 ± 15.9 | 0.226 | 65.4 ± 13.5 | 0.009 |
| EF (%) | 31.5 ± 13.8 | 31.9 ± 12.6 | 0.409 | 32.6 ± 11.5 | 0.093 | 33.9 ± 14.9 | 0.486 | 34.0 ± 15.7 | 0.175 |
| Urine (mL) | 1283.0 ± 956.6 | 1380.5 ± 799.9 | 0.095 | 1333.6 ± 868.9 | 0.237 | 1258.4 ± 685.2 | 0.279 | 1077.7 ± 686.6 | 0.804 |
| pUF (mL) | NA | 1125.8 ± 1229.7 | NA | 1175.0 ± 1398.4 | 0.907 | 1025.3 ± 838.5 | 0.558 | 1078.3 ± 687.4 | 0.289 |
| Body Weight (kg) | 82.2 ± 14.9 | 78.4 ± 14.8 | <0.001 | 78.5 ± 13.8 | <0.001 | 79.0 ± 15.2 | 0.015 | 78.7 ± 15.8 | <0.001 |
BP, blood pressure; EF, ejection fraction; NA, not applicable; NYHA, New York Heart Association; PD, peritoneal dialysis; pUF, peritoneal ultrafiltration.
Median, standard deviation, and Student's t‐test for paired variables. Mean and Wilcoxon signed‐rank test for not normally contributed paired variables. Data are presented as mean ± standard deviation.
Figure 2.
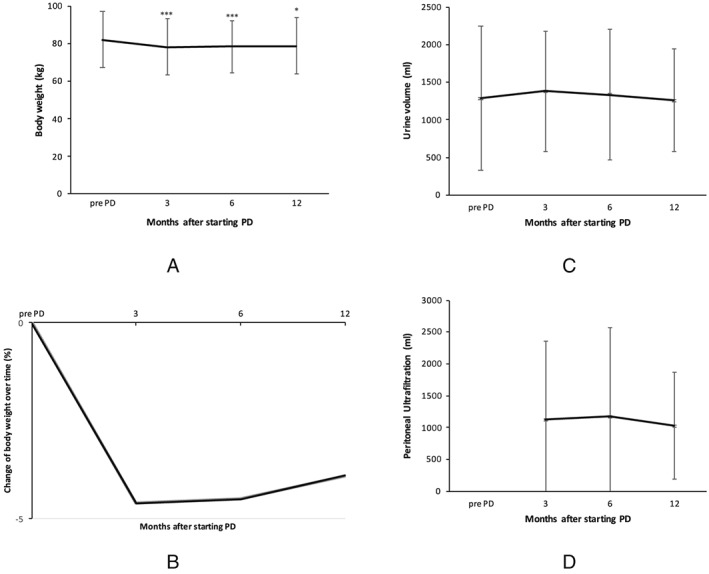
Comparison of body weight (A), relative change of body weight over time (B), urine volume (C), and peritoneal ultrafiltration (D) after starting peritoneal dialysis (PD). * P < 0.05; *** P < 0.001.
Regarding medication, use of MRA (35.8 vs. 35.7%) remained unchanged, while use of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) decreased (51.6 vs. 40.2%) during the first year after starting PD. Meanwhile, use of beta‐blockers increased during this period (59.7–73.1%).
Twenty‐four patients (15.1%) were treated with erythropoiesis stimulation agents (ESAs) pre‐PD. This number increased to 32.8% at 12 months (n = 19, patients at risk 58). In patients without ESAs or being on stable dosages of ESAs, we initially detected a significant increase of haemoglobin after 3 months (from 11.5 ± 1.89 to 12.0 ± 1.79 mg/dL, P = 0.024). But again, results were not of significance at the end of the observation period (11.6 ± 2.17 mg/dL, P = 0.724).
In total, number as well as days of hospitalization significantly decreased over the first year of PD from 2.86 ± 1.88 to 1.90 ± 1.78 (P = 0.001) and 39.2 ± 30.7 to 27.1 ± 25.2 days (P = 0.004), respectively (Figure 3 ). In a linear regression model, there was no significant correlation between left ventricular ejection fraction (LVEF) and number (R = −0.14, P = 0.319) as well as days of hospitalizations (R = 0.296, P = 0.155) after 1 year.
Figure 3.
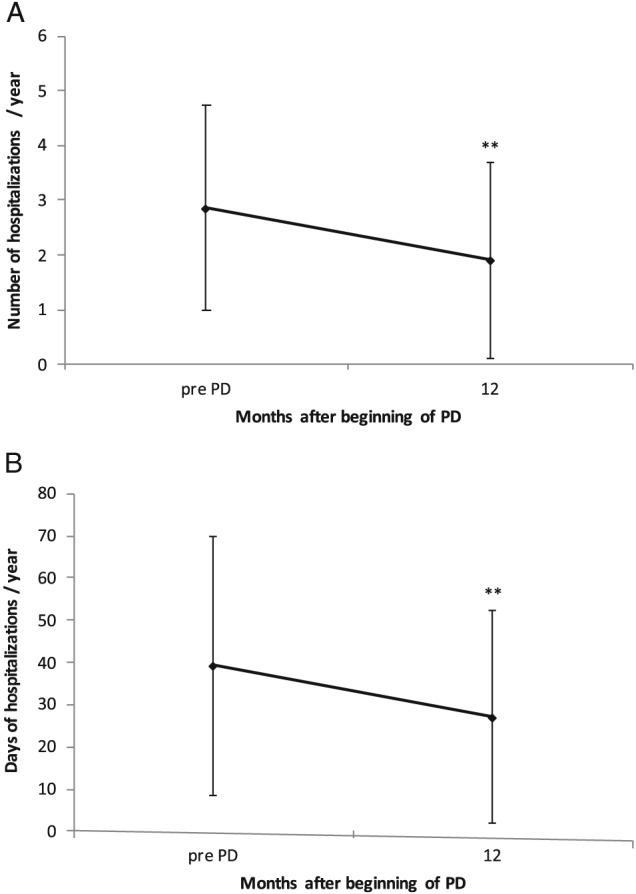
Number of hospitalizations per year after starting peritoneal dialysis (PD) (A) and days of hospitalizations per year after starting PD (B). ** P < 0.01.
Within 3 months, New York Heart Association (NYHA) significantly improved from 3.38 ± 0.55 to 2.85 ± 0.49 (P < 0.001) and remained at this level. In patients with worsening HF (n = 23), defined as reduction in ejection fraction (EF) ≥ 5%,24 a significant decrease in number of hospitalizations over 1 year was observed (3.00 ± 1.30 to 1.77 ± 1.93, P = 0.036) (Figure 4 ). There was a negative correlation between difference in NYHA classification and days of hospitalizations at 12 months (R = −0.281, P = 0.008). While overall LVEF did not change with PD treatment (31.5 ± 13.8% at baseline to 34.0 ± 15.7%, P = 0.175).
Figure 4.
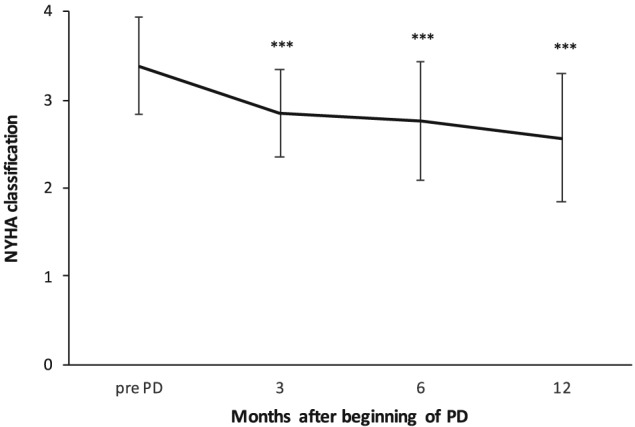
New York Heart Association (NYHA) classification after starting peritoneal dialysis (PD). ** P < 0.001.
Mortality during the first 2 years after starting PD is shown in Figure 5 . Average time until death was 437.6 ± 428.5 days. Seven patients recompensated and, therefore, intermittently stopped PD treatment. In addition, 13 patients changed medical centres for different reasons, and 36 were lost to follow‐up. One year mortality was 39.6% (n = 44), while 2 year mortality was found to be 59.1% (n = 65). In addition, mortality of ischaemic cardiomyopathy vs. dilated cardiomyopathy patients did not differ significantly in 1 and 2 year survival (log‐rank = 0.142 and 0.242, respectively).
Figure 5.
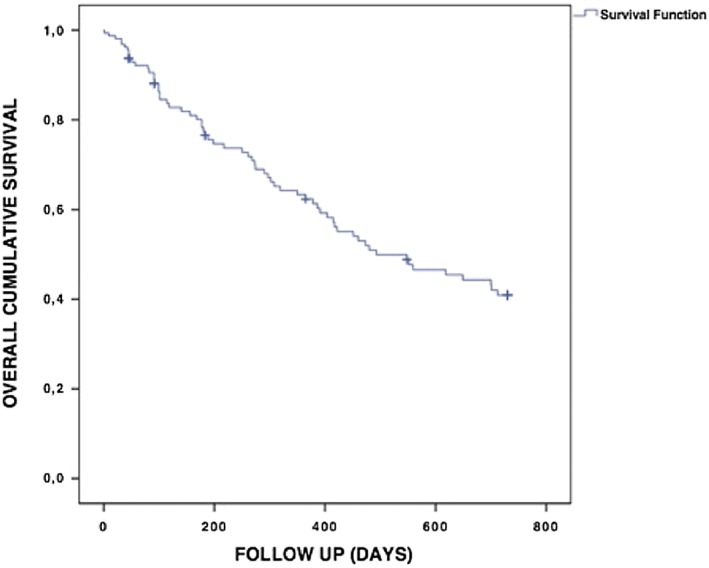
Kaplan–Meier survival curve. Cumulative survival 2 years after starting peritoneal dialysis.
Discussion
This is a prospective observational multicentre study, based on the national registry data of the DGfN, to evaluate the efficacy of PD in patients with refractory CHF.
First of all, our study confirms previous observations that even in end‐stage CHF patients, PD significantly reduced both number and days of hospitalization for all causes. It has to be emphasized that hospitalizations were also reduced in patients with declining EF, which is of importance because as repeated decompensations are followed by an incremental worsening of prognosis.25
In general, conservative therapeutic options are rare for end‐stage CHF patients with refractory to pharmacological treatment alone. Therefore, in diuretic‐resistant CHF, current guidelines are directed at symptom relief and co‐morbidity conditions, with UF or haemofiltration recommended as beneficial options. Nevertheless, to date, no further specifications concerning patient selection, treatment modality, or outcome measures can be given.26 Moreover, evidence of extracorporeal UF in CHF is conflicting.18, 19, 27 Although extracorporeal UF might be an efficient method of treating decompensated HF on an acute basis, it may not be feasible to employ this treatment modality on a chronic basis for the growing number of patients with end‐stage HF. For this reason, in a recent recommendation, the German Societies of Cardiology and Nephrology suggested PD treatment of patients with chronic refractory cardiorenal syndrome.23
Against this background, PD as a treatment modality carries some potential advantages.23 PD offers, at least theoretically, the opportunity of a gentle and continuous UF that, in particular, relieves the increased renal venous28, 29 as well as intra‐abdominal pressure and elegantly drains ascites, thus potentially re‐establishing glomerular filtration and increasing diuresis. This was reflected by the relatively stable serum creatinine within the first months of PD treatment, which is in contrast to the study published by Bart et al., using an extracorporeal device.18, 19
In our patient cohort, an overall significant weight loss was observed. On the one hand, weight loss can be considered a surrogate for better volume management due to additional UF accompanied with remained urine output. On the other hand, it can reflect development of muscle loss and malnutrition, as PD patients lose several grammes of protein in the dialysate every day. Nevertheless, albumin levels remained within the normal range, which is of importance because albumin is regarded as a strong predictor of survival. Despite weight loss and improved dyspnoea, serial overall NT‐proBNP values remained unchanged throughout the study. However, the between‐person variation of NT‐proBNP is known to be large and markedly greater than the within‐person variation.30 Indeed, the relative NT‐proBNP levels decreased, indicating the positive effects of PD treatment in HF patients.
The finding of a weight loss accompanied by significantly lowered C‐reactive protein and blood urea nitrogen (BUN) with only slight glomerular filtration rate changes probably reflects a sustained loss of oedema, especially from intra‐abdominal compartments, that otherwise would trigger translocation of lipopolysaccharide and hinder the resorption of nutritional compounds, finally ending up with profound cachexia as described by Cicoira et al.31 A low BUN with a better nutritional status is associated with a better prognosis.32, 33, 34 PD treatment decreased the BUN/creatinine ratio, which is consistent with the described reduced mortality.
HF therapy in patients with chronic kidney disease is frequently limited by life‐threatening hyperkalaemia; therefore, patients were less likely to receive ACE inhibitors or ARBs.35 Of note, patients on PD therapy often display a mild hypokalaemia. Although well known, no data are available whether this is also true in patients with cardiorenal syndrome treated with PD and whether PD offers the chance for an increased dosage of renin–angiotensin–aldosterone system blockers as well as MRAs.23 In our patient cohort, use of ACE/ARBs decreased and use of MRAs remained stable. Whether higher MRA and ACE/ARB doses might influence prognosis in this special patient cohort has to be demonstrated in further studies.
Applying the Charlson Comorbidity Index, reflecting 1 year mortality of patients with co‐morbid states, we would have expected a mortality rate of 80% in our cohort, while historical collectives displayed mortality rates ranging from 45 to 75%36, 37 with conventional treatment. Various studies on PD reported 1 year mortality rates between 18 and 44%.38, 39, 40 As 43% of our patients were hospitalized, a maximum of two times in the previous year, and 25% of the annual in‐hospital days were less than 3 weeks, this might even strengthen the generalizability of the findings to a patient population frequently not assessed for PD. However, it limits the mortality comparisons with historical controls. Wang et al.41 confirmed an increased mortality in patients with HF with PD treatment, and furthermore, in a previous study, our group described similar mortality rates of 33% in HF patients undergoing PD and 23% in a propensity score‐matched HF cohort.42 Against this background and given that HF cohorts may vary considerably in co‐morbidity load, our finding of 1 year mortality of 39.6% in end‐stage HF patients treated with PD indeed insinuates a strong survival benefit.
Even from an economic point of view, PD may prove beneficial as it contributes to lower healthcare costs by reducing hospital days.43
Our observations accord with former results, as NYHA classification did significantly improve with PD,44 which is in line with previous results of Courivaud et al.40 and implies that the impact of PD might differ regarding the change/improvement in EF, depending on the baseline EF. Nevertheless, recent studies found that LVEF does not add significant prognostic information to relevant demographic and biochemical variables in patients with advanced chronic kidney disease. It seems that the determination of global longitudinal strain rates indeed allows a significantly better prognostication than EF with respect to cardiovascular death.45 Substantiating this thesis, the subgroup analysis of 1 and 2 year mortality did not reveal any difference between dilated cardiomyopathy and ischaemic cardiomyopathy patients.
There are a few limitations to be taken into consideration. Although, to the best of our knowledge, this study encompasses the largest PD patient population treated for HF, it is still a relatively small patient cohort to allow for any exclusion of a potential bias, considering the highly heterogeneous presentation of cardiorenal patients from 18 different centres. As a frequent problem of register data is incomplete data entry or follow‐up, some patients have to be excluded for statistics. There was no standardized quality of life assessment, so there might be concerns that the morbidity of hospital admission can be counterbalanced by the complexity of doing PD at home for these patients. However, our previous study can invalidate this objection by demonstrating an improved quality of life with PD.42 In addition, there could be a bias as patients that were not started on PD were excluded while patients who received PD were closely monitored. Finally, the majority of our patients were male and of White Caucasian origin, which may hamper the translation of our findings to other populations from different ethnic backgrounds.
In conclusion, there are beneficial effects of PD as non‐classical palliative therapy in CHF patients. Our data underline the need for larger controlled studies to identify factors for patient selection, employing PD as an adjunct therapy to modern pharmacological and device therapies and to broaden the view in guidelines on the most important risk factor in patients with HF–renal failure.
Conflict of interest
C.B., L.F., L.G., L.P.K., M.M.K., K.M., A.R., V.S., and M.Z. declare that they have no conflicts of interest. B.S. reports a conference/travel grant from St Jude Medical—HeartWare and a travel grant as well as personal fees from Berlin Heart GmbH, outside the submitted work. R.W. has received personal fees from Baxter Germany and Fresenius Germany. H.A.K. reports personal fees from AstraZeneca, personal fees from Daiichi Sankyo, personal fees from NovoNordisk, personal fees from Novartis, personal fees from Roche Diagnostics, personal fees from Bayer Vital, outside the submitted work.
Funding
This work was supported by the Deutsche Gesellschaft für Nephrologie e.V. (DGfN).
Acknowledgements
Data collection
Germany:
B Bommersbach, Memmingen; C Brockmann, Bad Bevensen; J. Bunia, Iserlohn; U Dose, Siegburg; L Frankenstein, Heidelberg; C Hintzen‐Kruse, Chemnitz; MM Kreusser, Heidelberg; LP Kihm, Heidelberg; S. Ludwig, Eberswalde; H Martin, Zwickau; K Meyer, Bad Bevensen; A. Remppis, Bad Bevensen; E Schillinger‐Pokorny, Offenburg; J Tönges, Wittlich; G Tönne, G., Warendorf; M Toepfer, M., Garmisch; I Poludniak, Hilden; W Reinhard, Papenberg; V Schwenger, Stuttgart; R Wanninger, Braunschweig; L Wolf, Villingen‐Schwenningen; K Von Appen, Lohrügge; M Zeier, Heidelberg. Austria: M Mündle, Feldkirch.
Grossekettler L., Schmack B., Meyer K., Brockmann C., Wanninger R., Kreusser M. M., Frankenstein L., Kihm L. P., Zeier M., Katus H. A., Remppis A., and Schwenger V. (2019) Peritoneal dialysis as therapeutic option in heart failure patients, ESC Heart Failure, 6: 271–279. 10.1002/ehf2.12411.
References
- 1. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007; 13: 422–430. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ. Chronic kidney disease in patients with cardiac disease: a review of evidence‐based treatment. Kidney Int 2005; 68: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 4. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 5. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000; 102: 203–210. [DOI] [PubMed] [Google Scholar]
- 6. McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 7. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 8. Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta‐analysis. J Am Coll Cardiol 2006; 47: 1987–1996. [DOI] [PubMed] [Google Scholar]
- 9. Shamseddin MK, Parfrey PS. Mechanisms of the cardiorenal syndromes. Nat Rev Nephrol 2009; 5: 641–649. [DOI] [PubMed] [Google Scholar]
- 10. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121: 2592–2600. [DOI] [PubMed] [Google Scholar]
- 11. Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, Orsini E, Caravelli P, Marzilli M, Colombo PC. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 2012; 14: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doty JM, Saggi BH, Blocher CR, Fakhry I, Gehr T, Sica D, Sugerman HJ. Effects of increased renal parenchymal pressure on renal function. J Trauma 2000; 48: 874–877. [DOI] [PubMed] [Google Scholar]
- 13. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, Kirkpatrick JN. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol 2010; 105: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 16. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 17. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio‐renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010; 31: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers HL, Marshall J, Bock J, Dowling TC, Feller E, Robinson S, Gottlieb SS. A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail 2008; 14: 1–5. [DOI] [PubMed] [Google Scholar]
- 20. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA, UNLOAD Trial Investigators . Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 675–683. [DOI] [PubMed] [Google Scholar]
- 21. Costanzo MR, Negoianu D, Fonarow GC, Jaski BE, Bart BA, Heywood JT, Nabut JL, Schollmeyer MP. Rationale and design of the Aquapheresis versus Intravenous Diuretics and Hospitalization for Heart Failure (AVOID‐HF) trial. Am Heart J 2015; 170: 471–482. [DOI] [PubMed] [Google Scholar]
- 22. Lu R, Mucino‐Bermejo MJ, Ribeiro LC, Tonini E, Estremadoyro C, Samoni S, Sharma A, Zaragoza Galvan Jde J, Crepaldi C, Brendolan A, Ni Z, Rosner MH, Ronco C. Peritoneal dialysis in patients with refractory congestive heart failure: a systematic review. Cardiorenal Med 2015; 5: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwenger V, Remppis BA, Westenfeld R, Weinreich T, Brunkhorst R, Schieren G, Krumme B, Haller H, Schmieder R, Schlieper G, Frye B, Hoppe UC, Hoyer J, Keller T, Blumenstein M, Schunkert H, Mahfoud F, Rump LC. Dialysis and ultrafiltration therapy in patients with cardio‐renal syndrome: recommendations of the working group “heart‐kidney” of the German Cardiac Society and the German Society of Nephrology. Dtsch Med Wochenschr 2014; 139: 1–8. [DOI] [PubMed] [Google Scholar]
- 24. Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013; 61: 391–403. [DOI] [PubMed] [Google Scholar]
- 26. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009; 53: 1–90. [DOI] [PubMed] [Google Scholar]
- 27. Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, Mackedanz S, Sobotka PA, Schollmeyer M, Goldsmith SR. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid‐Overloaded Patients With Decompensated Congestive Heart Failure (RAPID‐CHF) trial. J Am Coll Cardiol 2005; 46: 2043–2046. [DOI] [PubMed] [Google Scholar]
- 28. Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, Young JB, Rouleau JL, Granger CB, McMurray JJ. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM‐study programme. Eur J Heart Fail 2016; 18: 1508–1517. [DOI] [PubMed] [Google Scholar]
- 29. Mullens W, Nijst P. Cardiac output and renal dysfunction: definitely more than impaired flow. J Am Coll Cardiol 2016; 67: 2209–2212. [DOI] [PubMed] [Google Scholar]
- 30. Fahim MA, Hayen A, Horvath AR, Dimeski G, Coburn A, Johnson DW, Hawley CM, Campbell SB, Craig JC. N‐terminal pro‐B‐type natriuretic peptide variability in stable dialysis patients. Clin J Am Soc Nephrol 2015; 10: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cicoira M, Anker SD, Ronco C. Cardio‐renal cachexia syndromes (CRCS): pathophysiological foundations of a vicious pathological circle. J Cachexia Sarcopenia Muscle 2012; 2: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heywood JT, Elatre W, Pai RG, Fabbri S, Huiskes B. Simple clinical criteria to determine the prognosis of heart failure. J Cardiovasc Pharmacol Ther 2005; 10: 173–180. [DOI] [PubMed] [Google Scholar]
- 34. Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic‐associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol 2011; 58: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berger AK, Duval S, Manske C, Vazquez G, Barber C, Miller L, Luepker RV. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J 2007; 153: 1064–1073. [DOI] [PubMed] [Google Scholar]
- 36. Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348: 2007–2018. [DOI] [PubMed] [Google Scholar]
- 37. Khand A, Gemmel I, Clark AL, Cleland JG. Is the prognosis of heart failure improving? J Am Coll Cardiol 2000; 36: 2284–2286. [DOI] [PubMed] [Google Scholar]
- 38. Sanchez JE, Ortega T, Rodriguez C, Diaz‐Molina B, Martin M, Garcia‐Cueto C, Vidau P, Gago E, Ortega F. Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant 2010; 25: 605–610. [DOI] [PubMed] [Google Scholar]
- 39. Koch M, Haastert B, Kohnle M, Rump LC, Kelm M, Trapp R, Aker S. Peritoneal dialysis relieves clinical symptoms and is well tolerated in patients with refractory heart failure and chronic kidney disease. Eur J Heart Fail 2012; 14: 530–539. [DOI] [PubMed] [Google Scholar]
- 40. Courivaud C, Kazory A, Crepin T, Azar R, Bresson‐Vautrin C, Chalopin JM, Ducloux D. Peritoneal dialysis reduces the number of hospitalization days in heart failure patients refractory to diuretics. Perit Dial Int 2014; 34: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure with preserved or reduced ejection fraction in patients treated with peritoneal dialysis. Am J Kidney Dis 2013; 61: 975–83:10. [DOI] [PubMed] [Google Scholar]
- 42. Frohlich H, Katus HA, Tager T, Lossnitzer N, Grossekettler L, Kihm L, Zeier M, Remppis A, Frankenstein L, Schwenger V. Peritoneal ultrafiltration in end‐stage chronic heart failure. Clin Kidney J 2015; 8: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sens F, Schott‐Pethelaz AM, Labeeuw M, Colin C, Villar E. Survival advantage of hemodialysis relative to peritoneal dialysis in patients with end‐stage renal disease and congestive heart failure. Kidney Int 2010; 80: 970–977. [DOI] [PubMed] [Google Scholar]
- 44. Cnossen TT, Kooman JP, Krepel HP, Konings CJ, Uszko‐Lencer NH, Leunissen KM, van der Sande FM. Prospective study on clinical effects of renal replacement therapy in treatment‐resistant congestive heart failure. Nephrol Dial Transplant 2012; 27: 2794–2799. [DOI] [PubMed] [Google Scholar]
- 45. Stabton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain. Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009; 2: 356–364. [DOI] [PubMed] [Google Scholar]


