Abstract
Aims
The objective of the study was to evaluate whether the geriatric nutritional risk index (GNRI) at discharge may be helpful in predicting the long‐term prognosis of patients hospitalized with heart failure (HF) with preserved ejection fraction (HFpEF, left ventricular ejection fraction ≥50%), a common HF phenotype in the elderly.
Methods and results
Overall, 110 elderly HFpEF patients (≥65 years) from the Ibaraki Cardiovascular Assessment Study‐HF (n = 838) were enrolled. The mean age was 78.5 ± 7.2 years, and male patients accounted for 53.6% (n = 59). All‐cause mortality was compared between the low GNRI (<92) with moderate or severe nutritional risk group and the high GNRI (≥92) with no or low nutritional risk group. Cox proportional hazard regression models were constructed to evaluate the influence of the GNRI on all‐cause death with the following covariates using forward stepwise selection: age, sex, nutritional status based on the GNRI as a categorical variable, history of HF hospitalization, haemoglobin level, estimated glomerular filtration rate, log brain natriuretic peptide levels (logBNP), history of hypertension, log C‐reactive protein levels, left ventricular ejection fraction, left ventricular mass index, and the New York Heart Association functional classification (I/II or III class). The prognostic value of the GNRI was compared with that of serum albumin using C‐statistics. The GNRI was added to the logBNP, serum albumin or the body mass index was added to the logBNP, and the C‐statistic was compared using DeLong's test. Cox regression analysis revealed that age and a low GNRI were independent predictors of all‐cause death (P < 0.05, n = 103; hazard ratio = 1.095, 95% confidence interval = 1.031–1.163, for age, and hazard ratio = 3.075, 95% confidence interval = 1.244–7.600, for the GNRI). DeLong's test for the two correlated receiver operating characteristic curves [area under the receiver operating characteristic curve (AUROC) of serum albumin, 0.71; AUROC of the GNRI, 0.75] demonstrated significant differences between the groups (P = 0.038). Adding the GNRI to the logBNP increased the AUROC for all‐cause death significantly (0.71 and 0.80, respectively; P = 0.040, n = 105). The addition of serum albumin or the body mass index to the logBNP did not significantly increase the AUROC for all‐cause death (P = 0.082 and P = 0.29, respectively).
Conclusions
Nutritional screening using the GNRI at discharge is helpful to predict the long‐term prognosis of elderly HFpEF patients.
Keywords: Brain natriuretic peptide, Heart failure with preserved ejection fraction, Inflammation, Nutritional screening, Undernutrition
Introduction
The prevalence of cardiovascular disorders has increased markedly because of a rapidly ageing society and the westernization of lifestyle, both of which increase the risk of developing coronary artery disease and other cardiovascular disease. The growing prevalence of heart failure (HF) is also an important problem among the elderly because HF is observed predominantly in that population. According to the Acute Decompensated Heart Failure Syndromes registry in Japan, the mean age of patients with HF was 73.0 years, and 42.0% were women. Moreover, almost half of the patients presented a preserved ejection fraction (pEF), defined as a left ventricular ejection fraction (LVEF) >40%, and the endpoint of 1 year all‐cause mortality was achieved in 17.0% of patients.1 In clinical trial populations, the LVEF value used to define a ‘pEF’ ranged from 40 to 45%, and outcomes were better in patients with HF with preserved ejection fraction (HFpEF) than in those with a reduced ejection fraction (HFrEF).2 In previous studies, the LVEF value used to define the ‘pEF’ ranged from 40 to 55%, but current guidelines recommend a partition value of 50%.3, 4, 5 According to a recent analysis of a large national registry‐based cohort,6 cardiovascular and HF rehospitalizations rates are higher for patients with HFrEF (LVEF ≤ 40%) and those with HF with borderline ejection fraction (HFbEF) (LVEF 41–49%) than for those with HFpEF (LVEF ≥ 50%). However, patients with HFrEF, HFbEF, and HFpEF had very high rates of 5 year mortality (75–76%) and rehospitalization (82–86%) rates, which were similar. There are many effective treatment strategies for patients with HFrEF; unfortunately, effective treatment strategies for patients with HFpEF are lacking.
In HF patients, undernutrition is not uncommon7, 8, 9, 10, 11, 12, 13, 14, 15 and represents one of the most significant determinants of poor clinical outcomes.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 The geriatric nutritional risk index (GNRI) is a simple and well‐established nutritional screening tool for elderly HF patients.18, 19, 20 However, the predictive value of the assessment of nutritional status using GNRI in patients with HFpEF remains unclear.
In a multicentre registry setting, the present study evaluated whether determining the GNRI at discharge may be helpful to predict the long‐term prognosis of patients hospitalized with HFpEF (LVEF ≥ 50%), a common HF phenotype in the elderly population (≥65 years).
Methods
Study population
A total of 838 patients with HF symptoms were hospitalized between June 2012 and March 2015 and were enrolled in the Ibaraki Cardiovascular Assessment Study‐HF registry.7, 17, 21 Follow‐ups were conducted until 31 March 2016. The Ibaraki Cardiovascular Assessment Study is a multicentre registry study involving 11 hospitals in the Ibaraki Prefecture of Japan. The Ibaraki Cardiovascular Assessment Study registry inclusion criteria were patient age ≥20 years and the fulfilment of the Framingham criteria for HF.22 The registry exclusion criteria were age <20 years, not providing informed consent to the attending physician, limited life expectancy due to malignant neoplasms, patients in whom the 2 year observation was predicted to be impossible, and patients who were judged as medically inappropriate by the attending physician. Written informed consent was obtained from all patients, and data collection for this study was approved by the institutional review boards of the 11 participating hospitals. Additionally, the Ibaraki Cardiovascular Assessment Study registry study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Data from the Ibaraki Cardiovascular Assessment Study registry were retrospectively analysed. Two parameters are used to calculate the GNRI: serum albumin level and body mass index (BMI). We stratified the study patients into three groups: HF patients with in‐hospital death, HF patients who were discharged after alleviation of symptoms, and HF patients who were transferred elsewhere for continued medical care.7 Among the 838 patients enrolled in the registry, 590 patients were aged ≥65 years and were discharged after alleviation of symptoms. Seven patients on dialysis were excluded. Registry patients for whom GNRI could not be estimated were also excluded (n = 187). Ultimately, a total of 110 HFpEF patients with complete GNRI data were enrolled in this study (Figure 1 ).
Figure 1.

Study flow diagram. We included a total of 110 elderly heart failure (HF) with preserved ejection fraction (HFpEF) patients with geriatric nutritional risk index (GNRI) data. Low GNRI, group of HFpEF patients with moderate or severe nutritional risk; high GNRI, group of HFpEF patients with low or no nutritional risk.
Data collection
Baseline clinical data were collected for each patient. Patient‐related information collected at discharge included medical history, laboratory test results, echocardiographic findings, and prescriptions, and data were recorded in a computer database. Blood tests were performed to determine haemoglobin, sodium, serum creatinine, plasma brain natriuretic peptide (BNP), albumin, total cholesterol, and C‐reactive protein levels. The estimated glomerular filtration rate (eGFR) was calculated using the following formula: eGFR = 194 × serum creatinine−1.094 × age in years−0.287 for male patients. The adjusted eGFR value for female patients was calculated using the following formula: eGFR female = eGFR × 0.739.23 The BMI was calculated as body weight in kilogrammes divided by the square of the height in metres.
Assessment of nutritional status using geriatric nutritional risk index
The GNRI was developed by Bouillanne et al.24 as a screening tool for undernutrition in a hospital population. In the present study, the GNRI was calculated from serum albumin and BMI obtained at discharge. We adopted Kinugasa's measurement method18 as follows:
BMI/22 was set to 1 when the patient's BMI/22 was greater than 1.
The GNRI cut‐off values were also adopted from the study by Bouillanne et al.24 From these GNRI values, four grades of nutrition‐related risk were defined: major risk (GNRI < 82), moderate risk (GNRI 82 to <92), low risk (GNRI 92 to <98), and no risk (GNRI ≥ 98). In the present study, we defined the GNRI cut‐off value as 92. Clinical characteristics and mortality were compared between the low GNRI (<92) with moderate or severe nutritional risk group and the high GNRI (≥92) with low or no nutritional risk, according to previous reports.18, 19, 25
Correlation between brain natriuretic peptide levels and nutritional status
The correlation between the GNRI as a continuous variable and the logarithmically transformed plasma BNP (logBNP) level was evaluated. Blood was collected into tubes containing ethylenediaminetetraacetic acid, and plasma BNP concentrations were measured using a validated and commercially available immunoassay kit (Tosoh Co. Ltd., Tokyo, Japan). The upper limit of normal plasma BNP level was 18.4 pg/mL. The minimal and maximal detectable levels of BNP were 4 and 2000 pg/mL, respectively.
Assessment of prognosis using the geriatric nutritional risk index
We divided the study patients into two groups: (i) HFpEF patients with low or no nutritional risk (patients with a GNRI of ≥92) and (ii) HFpEF patients with moderate or severe nutritional risk (patients with GNRI of <92).
We investigated whether nutritional status assessed using the GNRI was associated with all‐cause death and cardiovascular death. Cardiovascular death was defined as death attributable to cardiovascular origin.
Assessment of heart failure with preserved ejection fraction
Heart failure with preserved ejection fraction was defined as follows: (i) presence of HF symptoms defined by the Framingham criteria22; (ii) preserved LVEF ≥ 50%, as previously described3, 4, 5; and (iii) absence of HF aetiologies, including severe valve disease, congenital disease, hypertrophic cardiomyopathy, acute myocarditis, cardiac amyloidosis, pericardial disease, primary pulmonary hypertension, or acute myocardial infarction.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation if normally distributed and as median [inter‐quartile range (IQR)] if non‐normally distributed. Differences between the two groups were compared using an unpaired Student's t‐test or a Mann–Whitney U test, as appropriate. The chi‐squared test was used to compare categorical variables. Pearson's correlation analysis was used to evaluate the correlation between the GNRI and the logBNP or log C‐reactive protein concentration. A partial correlation analysis was performed between the GNRI and the logBNP while controlling for the eGFR. Kaplan–Meier analysis with the log‐rank test was performed to determine whether nutritional screening using GNRI at discharge could be helpful in predicting long‐term prognosis in patients hospitalized with HFpEF. In addition, a Cox proportional hazards model analysis was performed to determine the significant predictors of prognosis. To evaluate the influence of the GNRI on all‐cause death, the following four Cox proportional hazard regression models were constructed: Model 1, unadjusted; Model 2, age and sex adjusted; and Model 3, age and logBNP adjusted. In Model 4, the following covariates were included using forward stepwise selection: age, sex, nutritional status based on the GNRI as a categorical variable, previous history of HF hospitalization, haemoglobin level, eGFR, logBNP, history of hypertension, log C‐reactive protein, LVEF, left ventricular mass index, and New York Heart Association (NYHA) functional classification (I/II or III class). The prognostic value of the GNRI was compared with that of serum albumin using the C‐statistic. We added the GNRI to the logBNP or the model of age and logBNP and compared the C‐statistics using DeLong's test. We also added the serum albumin or BMI to the logBNP and compared the C‐statistics using DeLong's test. As a severity assessment of HF, the BNP value is very useful; it is generally known that the BNP value at the post‐stability phase has a stronger prognostic ability than the value at the time of admission. A P‐value of <0.05 was considered statistically significant. All statistical analyses except the C‐statistics were performed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA), SPSS version 24 (IBM Corp., Armonk, NY, USA), or EZR version 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) for Windows. Statistical analysis using C‐statistics of the results was performed by R software package (version 3.3.3, R Development Core Team, https://www.r-project.org/).
Results
Clinical characteristics of study patients
Tables 1 and 2 show the clinical characteristics of the HFpEF patients with GNRI data according to the risk of undernutrition. At the time of admission, based on the NYHA functional classification, 10 patients were classified as Class II, 38 patients as Class III, and 62 patients as Class IV. Conversely, at the time of discharge, based on the NYHA functional classification, 52 patients were classified as Class I, 52 patients as Class II, and 6 patients as Class III. None of the patients were classified as Class IV. The median plasma BNP level of the overall study population was 206.9 (IQR 105.7–355.1) pg/mL, and as the distribution of BNP levels was highly skewed, we normalized the data through a logarithmic transformation. The median GNRI of the overall study population was 93.8 (IQR 84.9–98.3). Of the 110 enrolled HFpEF patients for whom GNRI could be calculated, 73 (66.4%) had low‐to‐major nutrition‐related risks (low, 21.8%; moderate, 23.6%; and major, 20.9%) at discharge.
Table 1.
Clinical characteristics of the patients by GNRI
| Overall (n = 110) | High GNRI (≥92) (n = 61) | Low GNRI (<92) (n = 49) | P‐value | |
|---|---|---|---|---|
| Age (years) | 78.5 ± 7.2 | 77.0 ± 6.5 | 80.4 ± 7.7 | 0.016 |
| Male, n (%) | 59 (53.6) | 30 (49.2) | 29 (59.2) | 0.34 |
| NYHA (2/3/4) on admission | 10/38/62 | 5/23/33 | 5/15/29 | 0.73 |
| NYHA (3 or 4) on admission, n (%) | 100 (90.9) | 56 (91.8) | 44 (89.8) | 0.75 |
| Clinical scenarios (1/2/3/4/5) on admission | 67/39/3/0/1 | 40/17/3/0/1 | 27/22/0/0/0 | — |
| NYHA (1/2/3) at discharge | 52/52/6 | 26/32/3 | 26/20/3 | — |
| NYHA (1 or 2) at discharge, n (%) | 104 (94.5) | 58 (95.1) | 46 (93.9) | 1 |
| Weight (kg) at discharge | 55.6 ± 11.1 | 59.8 ± 9.5 | 50.3 ± 10.7 | <0.001 |
| BMI (kg/m2) at discharge | 23.1 ± 4.1 | 25.0 ± 3.5 | 20.6 ± 3.5 | <0.001 |
| BMI (kg/m2) <18.5 at discharge, n (%) | 10 (9.1) | 0 (0) | 10 (20.4) | <0.001 |
| BMI (kg/m2) <22.0 at discharge, n (%) | 47 (42.7) | 12 (19.7) | 35 (71.4) | <0.001 |
| SBP (mmHg) at discharge | 120.0 [108.0–130.3] | 120.0 [108.0–126.8] | 121.5 [108.0–136.0] | 0.81 |
| Heart rate (b.p.m.) at discharge | 64.5 [58.0–71.0] | 64.0 [56.0–69.0] | 65.0 [58.8–75.3] | 0.182 |
| Medical history | ||||
| Current or past smoker, n (%) | 55 (50.0) | 30 (49.2) | 25 (51.0) | 1 |
| Readmission count for HF (0/1/2/≥3) | 81/12/7/10 | 45/6/5/5 | 36/6/2/5 | — |
| Previous history of HF hospitalization, n (%) | 29 (26.4) | 16 (26.2) | 13 (26.5) | 1 |
| HF aetiology, ischaemic, n (%) | 28 (25.5) | 13 (21.3) | 15 (30.6) | 0.28 |
| Atrial fibrillation, n (%) | 36 (32.7) | 22 (36.1) | 14 (28.6) | — |
| Hypertension, n (%) | 83 (75.5) | 51 (83.6) | 32 (65.3) | 0.044 |
| Dyslipidaemia, n (%) | 43 (39.1) | 28 (45.9) | 15 (30.6) | 0.119 |
| Diabetes mellitus, n (%) | 54 (49.1) | 31 (50.8) | 23 (46.9) | 0.71 |
| COPD, n (%) | 8 (7.3) | 4 (6.6) | 4 (8.2) | 1 |
| Cerebrovascular disease, n (%) | 12 (10.9) | 10 (16.4) | 2 (4.1) | 0.062 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; GNRI, geriatric nutritional risk index; HF, heart failure; n, number of patients; NYHA, New York Heart Association; SBP, systolic blood pressure.
Results are expressed as mean ± standard deviation or the median [inter‐quartile range]. Data were missing for the following characteristics: SBP, for six HF patients with high GNRI and three HF patients with low GNRI. ‘Atrial fibrillation’ demonstrates the rhythm at discharge.
Table 2.
Laboratory data, echocardiographic data, and medications at discharge by GNRI
| Overall (n = 110) | High GNRI (≥92) (n = 61) | Low GNRI (<92) (n = 49) | P‐value | |
|---|---|---|---|---|
| Laboratory measurement at discharge | ||||
| Haemoglobin (g/dL) | 11.5 ± 2.2 | 12.0 ± 2.3 | 10.8 ± 1.9 | 0.006 |
| Sodium (mEq/L) | 139.1 ± 3.7 | 139.2 ± 3.3 | 138.9 ± 4.1 | 0.64 |
| Estimated GFR (mL/min/1.73 m2) | 41.5 [31.8–56.0] | 43.4 [32.8–54.9] | 36.4 [25.6–56.6] | 0.36 |
| Estimated GFR <60 (mL/min/1.73 m2), n (%) | 91 (82.7) | 52 (85.2) | 39 (79.6) | 0.46 |
| BNP (pg/mL) | 206.9 [105.7–355.1] | 126.3 [76.0–264.9] | 297.0 [147.6–478.3] | <0.001 |
| logBNP | 2.26 ± 0.40 | 2.12 ± 0.42 | 2.42 ± 0.32 | 0.001 |
| Albumin (g/dL) | 3.60 [3.20–3.90] | 3.80 [3.60–4.20] | 3.10 [2.75–3.33] | <0.001 |
| Total cholesterol (mg/dL) | 167.1 ± 34.7 | 175.2 ± 34.3 | 155.9 ± 32.5 | 0.007 |
| C‐reactive protein (mg/dL) | 0.36 [0.16–0.92] | 0.29 [0.15–0.56] | 0.47 [0.19–1.77] | 0.015 |
| GNRI | 93.8 [84.9–98.3] | 98.3 [95.3–104.2] | 84.5 [77.3–88.4] | <0.001 |
| Echocardiography at discharge | ||||
| LVDd (mm) | 49.2 ± 5.9 | 48.6 ± 5.8 | 50.0 ± 6.0 | 0.22 |
| Left atrial volume index (mL/m2) | 48.5 [36.4–61.5] | 51.7 [38.1–61.8] | 44.3 [33.1–56.5] | 0.162 |
| Left atrial volume index >34 (mL/m2), n (%) | 86 (78.2) | 51 (83.6) | 35 (71.4) | 0.164 |
| LVMI (g/m2) | 119.4 ± 35.0 | 113.0 ± 34.4 | 127.3 ± 34.4 | 0.033 |
| E/mean E′ | 13.4 [11.0–17.7] | 13.5 [11.1–17.5] | 13.0 [10.3–18.4] | 0.79 |
| LVEF (%) | 60.0 [54.1–66.5] | 61.6 [55.9–68.7] | 58.3 [53.2–64.7] | 0.034 |
| Medication at discharge | ||||
| Diuretics, n (%) | 96 (87.3) | 54 (88.5) | 42 (85.7) | 0.78 |
| Loop diuretics, n (%) | 87 (79.1) | 49 (80.3) | 38 (77.6) | 0.81 |
| Thiazide diuretics, n (%) | 10 (9.1) | 6 (9.8) | 4 (8.2) | 1 |
| Tolvaptan, n (%) | 8 (7.3) | 5 (8.2) | 3 (6.1) | 0.73 |
| Aldosterone antagonist, n (%) | 59 (53.6) | 36 (59.0) | 23 (46.9) | 0.25 |
| ACEIs/ARBs, n (%) | 71 (64.5) | 42 (68.9) | 29 (59.2) | 0.32 |
| Beta‐blocker, n (%) | 78 (70.9) | 40 (65.6) | 38 (77.6) | 0.21 |
| Statin, n (%) | 44 (40) | 29 (47.5) | 15 (30.6) | 0.081 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BNP, brain natriuretic peptide; GFR, glomerular filtration rate; GNRI; geriatric nutritional risk index; LVDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; n, number of patients.
Results are expressed as mean ± standard deviation or the median [inter‐quartile range]. Data were missing for the following characteristics: BNP, for four heart failure patients with high GNRI and for one heart failure patient with low GNRI; total cholesterol, for six heart failure patients with high GNRI and nine heart failure patients with low GNRI; LVMI, for one heart failure patient with high GNRI and for one heart failure patient with low GNRI; and E/mean E′, for six heart failure patients with high GNRI.
The 110 HFpEF patients in the study population were categorized as follows: HFpEF patients with low GNRI (<92, n = 49) with moderate or major nutrition‐related risk and patients with high GNRI (≥92, n = 61) with low or no nutrition‐related risk. The clinical characteristics of the patients enrolled in the two groups are also shown in Tables 1 and 2. Patients' age, weight, BMI, hypertension history, haemoglobin level, plasma BNP level, serum albumin level, total cholesterol level, C‐reactive protein level, left ventricular mass index, and LVEF differed significantly between the two groups. However, factors such as sex, NYHA class, systolic blood pressure, heart rate, smoking status, HF‐related admission history, population of HF patients with ischaemic aetiology, dyslipidaemia, diabetes mellitus, chronic obstructive pulmonary disease, cerebrovascular disease, sodium level, eGFR, left ventricular end‐diastolic diameter, left atrial volume index, E/mean E′, and the use of diuretics, angiotensin‐converting enzyme inhibitors and/or angiotensin II receptor blockers, beta‐blockers, and statins did not differ significantly between the two groups. When the log C‐reactive protein level was plotted against the GNRI as a continuous variable for the overall patient population, there was a weak significant inverse correlation (r = −0.287, P = 0.002, n = 110), indicating that a greater increase in GNRI was associated with a greater decrease in C‐reactive protein levels.
Correlation between brain natriuretic peptide levels and nutritional status
When the logBNP was plotted against the GNRI as a continuous variable for the overall patient population, there was a weak significant inverse correlation (r = −0.30, P = 0.002, n = 105), indicating that a greater increase in GNRI was associated with a greater decrease in plasma BNP levels. After controlling for eGFR, the association between the GNRI and the logBNP persisted (r [partial] = −0.285, P = 0.003, n = 105).
Impact of nutritional screening using geriatric nutritional risk index for all‐cause death
During the follow‐up period (503.5 [IQR 328.0–790.0] days), 24 deaths occurred. Of these, 14 patients (58.3%) had a cardiovascular death: HF death (n = 7, 29.2%), sudden death (n = 4, 16.7%), and death due to other reasons (n = 3, 12.5%). Ten patients (41.7%) experienced non‐cardiovascular‐related (n = 8) or unknown (n = 2) deaths.
The Kaplan–Meier analysis revealed that all‐cause deaths occurred more frequently in HFpEF patients with a low GNRI (n = 17) compared with HFpEF patients with a high GNRI (n = 7) (log‐rank P < 0.001). Table 3 shows the impact of nutritional screening using GNRI on all‐cause death. The analysis revealed that HFpEF patients with a low GNRI had an increased risk of all‐cause death compared with patients in the high GNRI group (P < 0.05) but not in Model 3 (Table 3). In Model 4, the multivariate Cox regression analysis using forward stepwise selection revealed that age and a low GNRI as a categorical variable were independent predictors of all‐cause death [P < 0.05, n = 103; hazard ratio (HR) = 1.095, 95% confidence interval (CI) = 1.031–1.163, for age, and HR = 3.075, 95% CI = 1.244–7.600, for the GNRI]. After adjusting for age, all‐cause deaths occurred more frequently in HFpEF patients with a low GNRI compared with HFpEF patients with a high GNRI (P = 0.009, n = 110; HR = 3.334; 95% CI = 1.354–8.207) (Figure 2 ). The Cox proportional hazard analyses also revealed that each per point increase in the GNRI was associated with a decreased risk of all‐cause death (Table 3). In Model 3, using the GNRI as a continuous variable, Cox proportional hazard analyses also revealed that advanced age and higher logBNP were associated with an increased risk of all‐cause death (HR = 1.081, 95% CI = 1.018–1.147, for age; HR = 4.872, 95% CI = 1.358–17.477, for logBNP). In addition, the Kaplan–Meier analysis also revealed that cardiovascular deaths occurred more frequently in HFpEF patients with a low GNRI (n = 9) compared with HFpEF patients with a high GNRI (n = 5) (P = 0.025 by the log‐rank test).
Table 3.
Impact of nutritional screening using GNRI on all‐cause death
| No. of events (all‐cause deaths)/at risk (%) | Model 1: unadjusted | Model 2: adjusted for age and sex | No. of events (all‐cause deaths)/at risk (%) | Model 3: adjusted for age and logBNP | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
|
GNRI Low vs. high (high as per reference)a |
24/110 (21.8) | 4.311 (1.784–10.415) | 0.001 | 3.202 (1.295–7.918) | 0.012 | 24/105 (22.9) | 2.444 (0.953–6.267) | 0.063 |
| GNRI as a continuous variable | 0.912 (0.877–0.949) | <0.001 | 0.927 (0.889–0.967) | <0.001 | 0.921 (0.880–0.964) | <0.001 | ||
BNP, brain natriuretic peptide; CI, confidence interval; GNRI, geriatric nutritional risk index; HR, hazard ratio.
Data were missing for the following characteristics: logBNP for five patients.
Primary outcomes are presented as HR for the low GNRI (<92) using the high GNRI (≥92) as a reference.
Figure 2.

Survival curve adjusted for age. After adjusting for age, the analysis revealed that heart failure with preserved ejection fraction (HFpEF) patients with a low geriatric nutritional risk index (GNRI) had an increased risk of all‐cause death compared with patients in the high GNRI group (P = 0.009, n = 110; hazard ratio = 3.334; 95% confidence interval = 1.354–8.207). Low GNRI, group of HFpEF patients with moderate or severe nutritional risk; high GNRI, group of HFpEF patients with low or no nutritional risk.
Comparison with other nutritional indices
The C‐statistic of the GNRI was compared with that of serum albumin to assess its validity as a nutritional risk screening tool in elderly patients hospitalized with HFpEF (Figure 3 ). DeLong's test for two correlated receiver operating characteristic curves [area under the receiver operating characteristic curve (AUROC) of serum albumin, 0.71; AUROC of the GNRI, 0.75] demonstrated significant differences between the two groups (P = 0.038). We assessed whether the evaluation of the GNRI levels in addition to the other predictor improved the stratification of the risk of mortality in elderly patients hospitalized with HFpEF. Adding the GNRI to the logBNP increased the AUROC for all‐cause death significantly (0.71 and 0.80, respectively; P = 0.040, n = 105). However, adding the GNRI to the model of age and logBNP did not significantly increase the AUROC for all‐cause death (0.78 and 0.81, respectively; P = 0.197, n = 105). Conversely, adding serum albumin levels or BMI to the logBNP did not significantly increase the AUROC for all‐cause death (P = 0.082 and P = 0.29, respectively).
Figure 3.
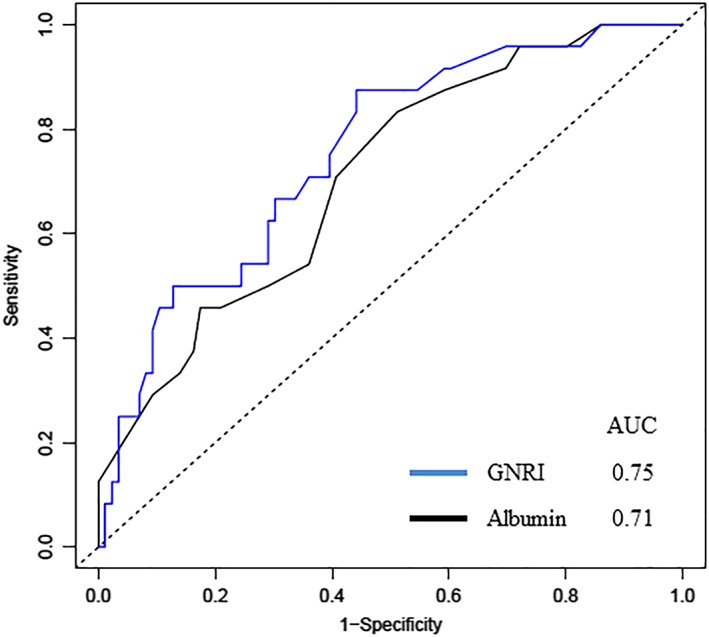
Predictive performance of serum albumin and the geriatric nutritional risk index (GNRI) for all‐cause death. AUC, area under the curve.
Discussion
In the present study, patient nutritional status, assessed using the GNRI at discharge, was examined to determine its usefulness in predicting the long‐term prognosis of patients hospitalized with HF in a multicentre registry setting. Our results showed that all‐cause death occurred more frequently in HFpEF patients with moderate or major nutrition‐related risk than in those with low or no nutrition‐related risk (Table 3 and Model 4). Evidence in support of a lower GNRI at discharge as a significant predictor of the occurrence of all‐cause death in patients hospitalized with HFpEF includes the following: a per point increase in the GNRI was associated with a lower risk of all‐cause death (Table 3). The results of the present study indicate that screening nutritional status using a GNRI at discharge further refines risk assessment in patients hospitalized with HFpEF.
Undernutrition is known as one of the most critical determinants of poor clinical outcomes in HF patients.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 However, to our knowledge, all but one18 of the previous studies reported on so‐called HF patients, and therefore, our specific findings for ‘HFpEF patients’ are novel. Kinugasa et al.18 reported that malnutrition assessed by the GNRI on admission was an independent determinant of long‐term death in acute HF with pEF. This finding18 supports those of the present study, which demonstrated that a lower GNRI is a significant predictor of the occurrence of all‐cause death in patients hospitalized with HFpEF.
Fluid status, in particular, has been shown to influence serum albumin levels and the BMI. Increased extracellular fluid volume decreases serum albumin, whereas it increases BMI. Considering such a counteracting effect, the GNRI, which is a combined index of albumin and BMI, may lead to a minimization of the effect of fluid status. However, the influence of serum albumin and BMI may influence the calculation of GNRI, as GNRI might prefer the stable state. In addition, HFpEF has been variably classified as LVEF > 40, >45, >50, and ≥55%.4 Currently, HF patients with an ejection fraction in the range of 41–49% are allocated to HFbEF.4 HFbEF patients present a mild systolic dysfunction, but in the clinical setting, there are many cases with a similar pathophysiology as HFpEF.5 Therefore, HFbEF patients are often treated with guideline‐directed medical therapy that is similar to that used in patients with HFpEF,4 whereas efficient therapeutic agents for HFpEF are poorly established. However, unlike for HFpEF, sufficient evidence‐based therapy for HFrEF patients may also be effective for HFbEF patients.5 For example, in patients with HFbEF, data suggest that HFrEF therapeutic agents such as beta‐blockers are also effective.26 According to Tsuji et al., the prognostic impacts of these agents in HFbEF differ from those in HFpEF but are almost comparable with those in HFrEF. Thus, the use of beta‐blockers has been positively associated, and the use of diuretics has been negatively associated with improved mortality in HFbEF and HFrEF but not in HFpEF patients.26 The HFpEF criterion used in the study by Kinugasa et al.18 was LVEF ≥ 40% and included HFbEF. Conversely, in our study, the HFpEF criterion was LVEF ≥ 50% and did not include HFbEF. Unfortunately, there are a lack of effective treatment strategies for patients with HFpEF, and the investigation of the potential predictive value of nutritional status assessment using the GNRI in patients with HFpEF is important.
Inflammation may play an important role in the pathogenesis of HFpEF owing to its significant contribution to myocardial fibrosis.27 Koller et al.27 reported that C‐reactive protein was a strong prognostic marker for risk stratification in patients with HFpEF. Recently, in emerging pathophysiological models of HFpEF, systemic microvascular endothelial inflammation related to coexisting conditions has been proposed as an additional mechanism leading to myocardial inflammation and fibrosis, increased oxidative stress, and alterations in cardiomyocyte signalling pathways.28 These alterations promote cardiomyocyte remodelling and dysfunction, in addition to microvascular dysfunction and rarefaction in cardiac and skeletal muscle.28 Conversely, the GNRI was developed as a screening tool to assess not only the nutritional but also the inflammatory status of older inpatients.29 The GNRI has been shown to correlate well with indicators of inflammation and length of hospital stay.29 Indeed, in the present study, the C‐reactive protein level was significantly higher in the moderate or major nutrition‐related risk group (low GNRI, 0.47 [IQR 0.19–1.77]) than in the low or no nutrition‐related risk group (high GNRI, 0.29 [IQR 0.15–0.56], P < 0.05). In addition, when the log C‐reactive protein was plotted against the GNRI as a continuous variable in the overall patient population, there was a weak but significant inverse correlation (r = −0.287, P = 0.002, n = 110), indicating that a greater increase in GNRI would be associated with a greater decrease in C‐reactive protein levels. Intestinal oedema or anorexia‐induced low nutritional intake, liver dysfunction, cytokine‐induced hypercatabolism, insulin resistance, and other mechanisms may all lead to HF‐related undernutrition.12 HF patients with undernutrition enter a vicious cycle of inflammation, catabolic drive, and undernutrition, which further exacerbates HF.30 Unfortunately, because effective treatment options are currently unavailable for HFpEF, this might be considered a valuable target for intervention in the future.
Several limitations of the present study should be mentioned. The total number of HFpEF patients enrolled in the Ibaraki Cardiovascular Assessment Study registry and the number of all‐cause deaths and cardiovascular death events were not large. Therefore, the number of indices that could be incorporated into the Cox proportional hazard regression models was small. In addition, we did not exclude co‐morbid diseases such as nephrotic syndrome, liver cirrhosis, cancer, collagen disease, the presence of infectious diseases, and blood disorders, all of which may affect albumin levels. In general, female HFpEF patients have occupied two thirds of the entire HFpEF study population.31 Conversely, in some cohort studies, female HFpEF patients represented only 48–50% of the entire HFpEF patient population.32, 33 Similarly, in our sample, there was a lower number of women (46%) enrolled, which is in contrast with the epidemiology of HFpEF in the real‐world setting. Because our study differs from a complete case registration study, the proportion of female patients with HFpEF was likely lower.
Conclusions
This multicentre registry study suggests that nutritional screening using GNRI at discharge from hospital is helpful in predicting the long‐term prognosis of elderly patients hospitalized with HFpEF.
Conflict of interest
None declared.
Funding
None.
Acknowledgements
We would like to thank Koichi Hashimoto and the staff at the Tsukuba Clinical Research and Development Organization (T‐CReDO) for supporting data management. List of participating hospitals and investigators: Akihiro Suzuki and Haruhiko Higuchi (Hitachi General Hospital), Takayoshi Yamanouchi and Ryo Kawamura (Hitachinaka General Hospital), Rihito Yamada (Mito Medical Center), Noriyuki Takeyasu and Daisuke Abe (Ibaraki Prefectural Central Hospital), Yuichi Noguchi and Hidetaka Nishina (Tsukuba Medical Center Hospital), Tsuyoshi Enomoto and Masayuki Igawa (Tsukuba Memorial Hospital), Kimito Ishikawa (Ryugasaki Saiseikai General Hospital), Hiroshi Maeda (Ibaraki Seinan Medical Center Hospital), and Masae Endo and Ikuo Yoshida (Moriya Daiichi General Hospital).
Nishi I., Seo Y., Hamada‐Harimura Y., Yamamoto M., Ishizu T., Sugano A., Sato K., Sai S., Obara K., Suzuki S., Koike A., Aonuma K., Ieda M., and Ibaraki Cardiovascular Assessment Study‐Heart Failure Investigators (2019) Geriatric nutritional risk index predicts all‐cause deaths in heart failure with preserved ejection fraction, ESC Heart Failure, 6, 396–405. 10.1002/ehf2.12405.
Investigators in the Ibaraki Cardiovascular Assessment Study‐Heart Failure registry are listed in the Acknowledgements.
References
- 1. Kajimoto K, Sato N, Takano T, Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry . Association of age and baseline systolic blood pressure with outcomes in patients hospitalized for acute heart failure syndromes. Int J Cardiol 2015; 191: 100–106. [DOI] [PubMed] [Google Scholar]
- 2. Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from DIG‐PEF, CHARM‐preserved, and I‐PRESERVE? J Am Coll Cardiol 2012; 60: 2349–2356. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 5. Tsutsui H. Guidelines for diagnosis and treatment of acute and chronic heart failure (JCS 2017JHFS 2017) http://www.j‐circ.or.jp/guideline/pdf/JCS2017tsutsuih.pdfJapanese.
- 6. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017; 70: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 7. Nishi I, Seo Y, Hamada‐Harimura Y, Sato K, Sai S, Yamamoto M, Ishizu T, Sugano A, Obara K, Wu L, Suzuki S, Koike A, Aonuma K, Ibaraki Cardiovascular Assessment Study‐Heart Failure Investigators . Utility of nutritional screening in predicting short‐term prognosis of heart failure patients. Int Heart J 2018; 59: 354–360. [DOI] [PubMed] [Google Scholar]
- 8. Bonilla‐Palomas JL, Gámez‐López AL, Anguita‐Sánchez MP, Castillo‐Domínguez JC, García‐Fuertes D, Crespin‐Crespin M, López‐Granados A, Suárez de Lezo J. Impact of malnutrition on long‐term mortality in hospitalized patients with heart failure. Rev Esp Cardiol (Engl Ed) 2011; 64: 752–758. [DOI] [PubMed] [Google Scholar]
- 9. Aziz EF, Javed F, Pratap B, Musat D, Nader A, Pulimi S, Alivar CL, Herzog E, Kukin ML. Malnutrition as assessed by nutritional risk index is associated with worse outcome in patients admitted with acute decompensated heart failure: an ACAP‐HF data analysis. Heart Int 2011; 6: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki N, Kida K, Akashi Y, Musha H, Miyake F. Usefulness of nutritional assessment using CONUT at admission and short‐term prognosis in patients with acute heart failure. Jomyaku Keicho Eiyo 2013; 28: 1083–1090 Japanese. [Google Scholar]
- 11. Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Watanabe T, Kubota I. The prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol 2013; 62: 307–313. [DOI] [PubMed] [Google Scholar]
- 12. Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev 2016; 21: 549–565. [DOI] [PubMed] [Google Scholar]
- 13. La Rovere MT, Maestri R, Olmetti F, Paganini V, Riccardi G, Riccardi R, Pinna GD, Traversi E. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis 2017; 27: 274–280. [DOI] [PubMed] [Google Scholar]
- 14. Agra Bermejo RM, González Ferreiro R, Varela Román A, Gómez Otero I, Kreidieh O, Conde Sabarís P, Rodríguez‐Mañero M, Moure González M, Seoane Blanco A, Virgós Lamela A, García Castelo A, González Juanatey JR. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol 2017; 230: 108–114. [DOI] [PubMed] [Google Scholar]
- 15. Iwakami N, Nagai T, Furukawa TA, Sugano Y, Honda S, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T, NaDEF Investigators . Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long‐term mortality in patients with acute heart failure. Int J Cardiol 2017; 230: 529–536. [DOI] [PubMed] [Google Scholar]
- 16. Adejumo OL, Koelling TM, Hummel SL. Nutritional risk index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant 2015; 34: 1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishi I, Seo Y, Hamada‐Harimura Y, Sato K, Sai S, Yamamoto M, Ishizu T, Sugano A, Obara K, Wu L, Suzuki S, Koike A, Aonuma K, Ibaraki Cardiovascular Assessment Study‐Heart Failure Investigators . Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long‐term prognostic prediction in patients with heart failure requiring hospitalization. Heart Vessels 2017; 32: 1337–1349. [DOI] [PubMed] [Google Scholar]
- 18. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 19. Honda Y, Nagai T, Iwakami N, Sugano Y, Honda S, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T, NaDEF Investigators . Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged ≥65 years with acute heart failure. Am J Cardiol 2016; 118: 550–555. [DOI] [PubMed] [Google Scholar]
- 20. Sargento L, Vicente Simões A, Rodrigues J, Longo S, Lousada N, Palma Dos Reis R. Geriatric nutritional risk index as a nutritional and survival risk assessment tool in stable outpatients with systolic heart failure. Nutr Metab Cardiovasc Dis 2017; 27: 430–437. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto M, Seo Y, Ishizu T, Nishi I, Hamada‐Harimura Y, Machino‐Ohtsuka T, Sato K, Sai S, Sugano A, Obara K, Aonuma K. Effect of dipeptidyl peptidase‐4 inhibitors on cardiovascular outcome and cardiac function in patients with diabetes and heart failure—insights from the Ibaraki Cardiac Assessment Study‐Heart Failure (ICAS‐HF) registry. Circ J 2017; 81: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 22. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators Developing the Japanese Equation for Estimated GFR . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 24. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 25. Kaneko H, Suzuki S, Goto M, Yuzawa Y, Arita T, Yagi N, Murata N, Kato Y, Kano H, Matsuno S, Otsuka T, Uejima T, Takai H, Oikawa Y, Kunihara T, Nagashima K, Kirigaya H, Sagara K, Sawada H, Aizawa T, Yajima J, Yamashita T. Geriatric nutritional risk index in hospitalized heart failure patients. Int J Cardiol 2015; 181: 213–215. [DOI] [PubMed] [Google Scholar]
- 26. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, CHART‐2 Investigators . Characterization of heart failure patients with mid‐range left ventricular ejection fraction—a report from the CHART‐2 Study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 27. Koller L, Kleber M, Goliasch G, Sulzgruber P, Scharnagl H, Silbernagel G, Grammer T, Delgado G, Tomaschitz A, Pilz S, März W, Niessner A. C‐reactive protein predicts mortality in patients referred for coronary angiography and symptoms of heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 758–766. [DOI] [PubMed] [Google Scholar]
- 28. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2016; 375: 1868–1877. [DOI] [PubMed] [Google Scholar]
- 29. Gärtner S, Kraft M, Krüger J, Vogt LJ, Fiene M, Mayerle J, Aghdassi AA, Steveling A, Völzke H, Baumeister SE, Lerch MM, Simon P. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr 2017; 36: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 30. Nakaya Y. Nutritional assessment in patients with heart failure. Jpn J Clin Nutr 2015; 127: 296–302 Japanese. [Google Scholar]
- 31. Goyal P, Paul T, Almarzooq ZI, Peterson JC, Krishnan U, Swaminathan RV, Feldman DN, Wells MT, Karas MG, Sobol I, Maurer MS, Horn EM, Kim LK. Sex‐ and race‐related differences in characteristics and outcomes of hospitalizations for heart failure with preserved ejection fraction. J Am Heart Assoc 2017; 6z pii: e003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Cameron VA, Poppe K, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J 2018; 39: 1770–1780. [DOI] [PubMed] [Google Scholar]
- 33. Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, Anzai T, JASPER Investigators . Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction—a report from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) registry. Circ J 2018; 82: 1534–1545. [DOI] [PubMed] [Google Scholar]


