Abstract
Aims
At present, the clinical burden of hypokalaemia and hyperkalaemia among European heart failure patients, and relationships between serum potassium and adverse clinical outcomes in this population, is not well characterized. The aim of this study was to investigate associations between mortality, major adverse cardiac events, and renin–angiotensin–aldosterone system inhibitor (RAASi) discontinuation across serum potassium levels, in a UK cohort of incident heart failure patients.
Methods and results
This was a retrospective observational cohort study of newly diagnosed heart failure patients listed in the Clinical Practice Research Datalink, with a first record of heart failure (index date) between 2006 and 2015. Hypokalaemia and hyperkalaemia episodes were defined as the number of serum potassium measurements exceeding each threshold (<3.5, ≥5.0, ≥5.5, and ≥6.0 mmol/L), without such a measurement in the preceding 7 days. Risk equations developed using Poisson generalized estimating equations were utilized to estimate adjusted incident rate ratios (IRRs) relating serum potassium and clinical outcomes (death, major adverse cardiac event, and RAASi discontinuation). Among 21,334 eligible heart failure patients, 1969 (9.2%), 7648 (35.9%), 2725 (12.8%), and 763 (3.6%) experienced episodes of serum potassium <3.5, ≥5.0, ≥5.5, and ≥6.0 mmol/L, respectively. The adjusted IRRs for mortality exhibited a U‐shaped association pattern with serum potassium. Relative to the reference category (4.5 to <5.0 mmol/L), adjusted IRRs for mortality were estimated as 1.98 (95% confidence interval: 1.69–2.33), 1.23 (1.12–1.36), 1.35 (1.14–1.60), and 3.02 (2.28–4.02), for patients with serum potassium <3.5, ≥5.0 to <5.5, ≥5.5 to <6.0, and ≥6.0 mmol/L, respectively. The adjusted IRRs for major adverse cardiac events demonstrated a non‐statistically significant relationship with serum potassium. Discontinuation of RAASi therapy exhibited a J‐shaped trend in association with serum potassium. Compared with the reference category (4.5 to <5.0 mmol/L), adjusted IRRs were estimated as 1.07 (0.89–1.28) in patients with serum potassium <3.5 mmol/L, increasing to 1.32 (1.14–1.53) and 2.19 (1.63–2.95) among those with serum potassium ≥5.5 to <6.0 and ≥6.0 mmol/L, respectively.
Conclusions
In UK patients with new onset heart failure, both hypokalaemia and hyperkalaemia were associated with increased mortality risk, and hyperkalaemia was associated with increased likelihood of RAASi discontinuation. Our results demonstrate the potential importance of serum potassium monitoring for heart failure outcomes and management.
Keywords: Hyperkalaemia, Serum potassium, Heart failure, Mortality, Renin–angiotensin–aldosterone system inhibitor therapy, Major adverse cardiac event
Introduction
Serum potassium concentrations below and exceeding the homeostatic range of 3.5–5.0 mmol/L are typically defined as hypokalaemia and hyperkalaemia, respectively.1 Both hypokalaemia and hyperkalaemia are considered burdensome electrolyte imbalances, associated with increased mortality and morbidity.2, 3, 4
As a consequence of renal insufficiency, patients with heart failure (HF) are at increased risk of hyperkalaemia, and medications routinely prescribed for management of HF are known to further affect serum potassium levels.5 In particular, combination therapy with renin–angiotensin–aldosterone system inhibitor (RAASi) and/or mineralocorticoid receptor antagonist (MRA) agents, while recommended to reduce the incidence of hospitalization,5 may further increase the risk of hyperkalaemia in this already‐vulnerable population.6, 7 Furthermore, the down‐titration or discontinuation of treatment to manage hyperkalaemia has been associated with worsening clinical outcomes and greater total costs in patients with HF and other co‐morbidities.8, 9, 10
At present, the clinical burden of hypokalaemia and hyperkalaemia among European HF patients, and relationships between serum potassium and adverse clinical outcomes in this population, is not well characterized. Existing real‐world studies have primarily focused on the chronic kidney disease (CKD) population11, 12, 13, 14 or utilized data derived from insurance‐based health care systems that may not be generalizable to other settings.2 Using primary care data obtained from the Clinical Practice Research Datalink (CPRD),15, 16 this study developed risk equations to describe associations between serum potassium and the incidence of death, major adverse cardiac events (MACE), and RAASi discontinuation, in a UK cohort of newly diagnosed HF patients.
Methods
Study data and patient population
Data were obtained from the CPRD, which contains primary care records for approximately 7% of the UK population and is broadly representative of the general population in terms of age, sex, and ethnicity.15, 16 Data from the CPRD were linked to the Hospital Episodes Statistics database,17 which contains information on all admissions, outpatient appointments, and emergency episodes recorded within National Health Service hospitals in England.
The study population composed of newly diagnosed HF patients (aged ≥18 years) listed on the CPRD between 1 January 2006 and 31 December 2015. The index date of the study was defined as the first record of HF after the study start date; consequently, patients with an HF diagnosis recorded prior to 1 January 2006 were excluded. To mitigate potential confounding, patients with CKD [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] or on dialysis prior to or at index date were excluded due to increased risk of hyperkalaemia5; however, eligible HF patients could develop CKD during follow‐up. Patients with HF and CKD were identified on the basis of Read codes (CPRD) and International Classification of Diseases‐10 codes (Hospital Episodes Statistics) provided in Supporting Information, Table S1 . Codes indicating HF type and severity, including left ventricular ejection fraction (LVEF), New York Heart Association functional classification, and N‐terminal pro‐B‐type natriuretic peptide, were not used to identify HF, due to a paucity of relevant CPRD data. Diagnoses of CKD were defined based on CKD stage (CKD 3a to CKD 5) and eGFR measurements, while Read and International Classification of Diseases‐10 codes were additionally used to define clinical outcomes and covariates. This study was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare Products Regulatory Agency database research on 15 December 2016 (study protocol 16_223R).
Study design and data structuring
The primary clinical outcomes were all‐cause mortality, MACE incidence (defined as a composite of arrhythmia, HF, myocardial infarction, and stroke), and RAASi discontinuation (defined as the first 90 day gap after the estimated end‐date of a RAASi prescription, using the medicines possession ratio). Patients receiving RAASi therapy, and dates of RAASi initiation/cessation during follow‐up, were identified using prescription data available on the CPRD; agents comprising RAASi included angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), MRAs, and renin inhibitors. Hypokalaemia was defined as a serum potassium concentration of <3.5 mmol/L, while hyperkalaemia was defined using serum potassium intervals of ≥5.0 to <5.5, ≥5.5 to <6.0, and ≥6.0 mmol/L.
Serum potassium was time updated during the study period, consistent with the approach of other database studies.14 An illustrative example of the last‐observation‐carried‐forward methodology adopted is provided in Supporting Information, Figure S1 . Time‐updated eGFR readings were taken to be the most recently available reading at the time of each serum potassium measurement. Patient intervals were defined as the period between serum potassium measurements, and clinical events were allocated to patient intervals based on the date on which they occurred. Additional analyses examined exposure time and events that occurred within a maximum of 30 days from each serum potassium measurement; after this period, time elapsed or events recorded were discarded and the remaining data reanalysed.
Time‐updated serum potassium was analysed as a categorical variable (<3.5, 3.5 to <4.0, 4.0 to <4.5, 4.5 to <5.0, 5.0 to <5.5, 5.5 to <6.0, and ≥6.0 mmol/L), and patient‐years spent in each serum potassium category were calculated. Patients were followed until the first occurrence of death, loss to follow‐up, or end of study. Loss to follow‐up was defined as the date a patient was transferred out of the practice, the date that the practice left the database, or the latest recorded event date.
Statistical analyses
Statistical analyses were performed using R version 3.3.2.18 Patient demographics were described using means, medians, counts and/or proportions, and associated measures of variability; summary statistics were calculated according to patient characteristics at their index date. Baseline clinical measurements were described using medians and interquartile ranges, based on the first measurement taken in the 3 months following the index date. Disease history was described by counts and proportions of patients who had history of disease during the 5 years prior to their index date. Medication usage at baseline was described using counts, and the proportion of patients prescribed a medication at least once during the 6 month period centred around the index date. Missing values were omitted from all calculations relating to baseline patient characteristics, disease history, and medication usage.
Hypokalaemia and hyperkalaemia episodes were defined as the number of serum potassium measurements exceeding each threshold (<3.5, ≥5.0, ≥5.5, and ≥6.0 mmol/L), without such a measurement in the preceding 7 days. Episodes were therefore assumed to persist for a maximum of 1 week and did not require a preceding measurement of normal serum potassium levels.
Risk equations predicting the incidence of each outcome (death, MACE, and RAASi discontinuation) were obtained by fitting a statistical model to the event count in each patient interval, using generalized estimating equations with an exchangeable working correlation structure. Events were assumed to be Poisson distributed, and the risk equations included a natural logarithm link function and an offset equal to the natural logarithm of patient‐years. Incident rate ratios (IRRs) were estimated using 4.5 to <5.0 mmol/L as the reference serum potassium group. To account for long intervals between serum potassium measurements and the occurrence of clinical events, IRRs were re‐estimated after restricting patient intervals to a maximum of 30 days post‐potassium measurement.
Five multiply imputed data sets were produced to inform missing baseline measurements, which were carried forward using the last‐observation‐carried‐forward approach. This included serum potassium, for which imputed data represented 17% of all patient time accumulated during the study. Model coefficients and their standard errors were pooled across imputed data sets using Rubin's Rules.19 Multiple imputation was performed on all clinical variables using the method of chained equations, as implemented in the R package ‘mice’,18 with all candidate covariates and outcome variables from the analysis models included in the imputation models.
To control for observed factors other than serum potassium, estimated IRRs were adjusted for confounding patient demographics, clinical histories and co‐morbidities, clinical measurements, and medication usage. Model assessment and final selection were performed based on the prediction mean squared error on a randomly selected validation subsample and the quasi‐likelihood information criterion.
Results
Baseline patient demographics and medication usage
A total of 21,334 incident HF patients were included in the study (Figure 1), with a mean follow‐up of 3.0 years. The mean age was 73 years, 12,092 patients (56.7%) were male, 3562 (22.6%) were current smokers, and 4539 (21.3%) had a history of arrhythmia (Table 1). Based on the earliest measurement recorded in the first 3 months post‐index, the median serum potassium concentration across the HF cohort was 4.4 mmol/L. At baseline, 9086 patients had serum potassium levels recorded, of which 372 patients were identified as having hypokalaemia (serum potassium <3.5 mmol/L) and 1223 had hyperkalaemia (serum potassium ≥5.0 mmol/L).
Figure 1.
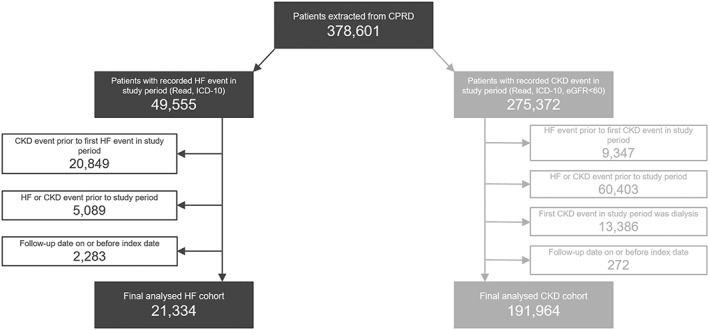
Study participation flow diagram.
CKD, chronic kidney disease; CPRD, Clinical Practice Research Datalink; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, International Classification of Diseases.
Table 1.
Baseline patient demographics and clinical histories of UK heart failure patients, stratified by serum potassium category at baseline
| Variable | All (n = 21 334) | Serum potassium category at baseline (mmol/L)a | ||||||
|---|---|---|---|---|---|---|---|---|
| <3.5 (n = 372; 1.7%) | 3.5 to <4.0 (n = 1402; 6.6%) | 4.0 to <4.5 (n = 3323; 15.6%) | 4.5 to <5.0 (n = 2766; 13.0%) | 5.0 to <5.5 (n = 939; 4.4%) | 5.5 to <6.0 (n = 228; 1.1%) | ≥6.0 (n = 56; 0.3%) | ||
| Follow‐up years, mean (SD) | 3.0 (2.5) | 2.4 (2.2) | 3.1 (2.5) | 3.2 (2.4) | 3.2 (2.4) | 3.2 (2.5) | 2.9 (2.4) | 2.6 (2.4) |
| Patient demographics | ||||||||
| Age (years), mean (SD) | 73 (14) | 79 (11) | 75 (13) | 73 (13) | 73 (12) | 73 (12) | 74 (12) | 76 (12) |
| Male, n (%) | 12 092 (56.7%) | 152 (40.9%) | 714 (50.9%) | 2000 (60.2%) | 1749 (63.2%) | 573 (61.0%) | 139 (61.0%) | 35 (62.5%) |
| Current smoker, n (%) | 3562 (22.6%) | 64 (19.6%) | 233 (18.6%) | 626 (20.8%) | 589 (23.4%) | 208 (24.5%) | 49 (23.7%) | 13 (27.1%) |
| Clinical measurements at baseline, median (IQR)b | ||||||||
| Body mass index (kg/m2) | 27.4 (23.4, 32.1) | 26.2 (22.7, 30.7) | 27.4 (23.5, 31.7) | 27.6 (23.8, 32.3) | 27.7 (23.7, 32.5) | 28.4 (24.4, 33.8) | 25.0 (21.7, 31.1) | 24.8 (21.3, 28.2) |
| SBP (mmHg) | 129 (115, 141) | 127 (110, 140) | 130 (115, 143) | 129 (115, 140) | 129 (115, 141) | 128 (112, 142) | 125 (110, 140) | 119 (110, 135) |
| eGFR (mL/min/1.73 m2) | 69 (57, 79) | 65 (50, 77) | 70 (58, 79) | 70 (59.0, 79) | 69 (57, 80) | 67 (55, 77) | 59 (37, 70) | 49 (37, 77) |
| Serum potassium (mmol/L) | 4.4 (4.0, 4.7) | 3.3 (3.1, 3.4) | 3.8 (3.6, 3.9) | 4.2 (4.1, 4.3) | 4.7 (4.5, 4.8) | 5.1 (5.0, 5.3) | 5.6 (5.5, 5.7) | 6.1 (6.1, 6.4) |
| Serum phosphorus (mmol/L) | 1.2 (1.0, 1.3) | 1.1 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.3) | 1.2 (1.05, 1.30) | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.5) | 1.4 (1.3, 1.6) |
| Clinical history at baseline, n (%)c | ||||||||
| Diabetes | 3184 (15.0%) | 52 (14.0%) | 204 (14.6%) | 497 (15.0%) | 498 (18.0%) | 190 (20.2%) | 45 (19.7%) | 11 (19.6%) |
| Myocardial infarction | 2003 (9.4%) | 32 (8.6%) | 122 (8.7%) | 358 (10.8%) | 326 (11.8%) | 123 (13.10%) | 28 (12.3%) | 3 (5.4%) |
| Stroke | 1418 (6.7%) | 37 (10.0%) | 113 (8.1%) | 232 (7.0%) | 186 (6.8%) | 65 (6.9%) | 12 (5.3%) | 3 (5.4%) |
| Arrhythmia | 4539 (21.3%) | 109 (29.3%) | 371 (26.5%) | 809 (24.4%) | 685 (24.8%) | 231 (24.6%) | 68 (29.8%) | 11 (19.7%) |
| Peripheral vascular disease | 636 (3.0%) | 12 (3.2%) | 35 (2.5%) | 111 (3.3%) | 85 (3.1%) | 42 (4.5%) | 7 (3.1%) | 3 (5.4%) |
| Chronic pulmonary disease | 2901 (13.6%) | 58 (15.60%) | 224 (16.0%) | 574 (17.3%) | 478 (17.3%) | 164 (17.5%) | 42 (18.4%) | 5 (9.0%) |
| Malignancy | 2095 (9.8%) | 41 (11.0%) | 178 (12.7%) | 416 (12.5%) | 318 (11.5%) | 102 (10.9%) | 22 (9.7%) | 3 (5.4%) |
| Metastatic tumour | 355 (1.7%) | 11 (3.0%) | 36 (2.6%) | 67 (2.0%) | 55 (2.0%) | 12 (1.3%) | 5 (2.2%) | 1 (1.8%) |
| Rheumatic disease | 560 (2.6%) | 14 (3.8%) | 58 (4.1%) | 127 (3.8%) | 83 (3.0%) | 27 (2.9%) | 7 (3.1%) | 0 (0.0%) |
| Peptic ulcer | 225 (1.1%) | 2 (0.5%) | 18 (1.3%) | 37 (1.1%) | 30 (1.1%) | 9 (1.0%) | 1 (0.4%) | 0 (0.0%) |
| Dementia | 499 (2.3%) | 12 (3.2%) | 46 (3.3%) | 69 (2.1%) | 45 (1.6%) | 14 (1.5%) | 3 (1.3%) | 1 (1.9%) |
| Medication use at baseline, n (%)d | ||||||||
| ACE inhibitors | 10 957 (51.4%) | 223 (60.0%) | 964 (68.8%) | 2435 (73.3%) | 2072 (74.9%) | 732 (78.0%) | 174 (76.3%) | 41 (73.2%) |
| ARBs | 2547 (12.0%) | 46 (12.4%) | 240 (17.1%) | 519 (15.6%) | 508 (18.4%) | 140 (14.9%) | 37 (16.2%) | 12 (21.4%) |
| Renin inhibitors | 11 (0.1%) | 0 (0.0%) | 2 (0.1%) | 1 (0.0%) | 2 (0.1%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) |
| MRAs | 4017 (18.8%) | 124 (33.3%) | 317 (22.6%) | 857 (25.8%) | 873 (31.6%) | 339 (36.1%) | 100 (43.9%) | 28 (50.0%) |
| Any RAASi therapye | 13 376 (62.7%) | 298 (80.1%) | 1172 (83.6%) | 2890 (87.0%) | 2492 (90.1%) | 855 (91.1%) | 209 (91.7%) | 47 (83.9%) |
| CCBs (DHP) | 3151 (14.8%) | 84 (22.6%) | 309 (22.0%) | 635 (19.1%) | 476 (17.2%) | 167 (17.8%) | 38 (16.7%) | 15 (26.8%) |
| CCBs (non‐DHP) | 1142 (5.4%) | 36 (9.7%) | 115 (8.2%) | 228 (6.9%) | 189 (6.8%) | 54 (5.8%) | 10 (4.4%) | 3 (5.4%) |
| NSAIDs | 1611 (7.6%) | 38 (10.2%) | 148 (10.6%) | 333 (10.0%) | 255 (9.2%) | 99 (10.5%) | 21 (9.2%) | 5 (8.9%) |
| Diuretics | 13 107 (61.4%) | 360 (96.8%) | 1265 (90.2%) | 2750 (82.8%) | 2220 (80.3%) | 767 (81.7%) | 186 (81.6%) | 53 (94.6%) |
| Beta blockers | 9553 (44.8%) | 185 (49.7%) | 798 (56.9%) | 1984 (59.7%) | 1767 (63.9%) | 614 (65.4%) | 154 (67.5%) | 30 (53.6%) |
| Statins | 8859 (41.5%) | 178 (47.9%) | 739 (52.7%) | 1848 (55.6%) | 1619 (58.5%) | 548 (58.4%) | 129 (56.6%) | 21 (37.5%) |
| Bronchodilators | 4221 (19.8%) | 98 (26.3%) | 358 (25.5%) | 836 (25.2%) | 722 (26.1%) | 259 (27.6%) | 61 (26.8%) | 14 (25.0%) |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; DHP, dihydropyridine; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MRAs, mineralocorticoid receptor antagonists; NSAIDs, nonsteroidal anti‐inflammatory drugs; RAASi, renin–angiotensin–aldosterone system inhibitor; SBP, systolic blood pressure; SD, standard deviation.
Stratified baseline characteristics include only those patients with an observed serum potassium measurement recorded within +3 months of the index date.
Defined as first clinical measurement recorded within +3 months of the index date.
Defined as clinical history over 5 years (60 months) prior to the index date.
Defined as medication prescribed within ±3 months of the index date.
RAASi therapy was composed of ACE inhibitors, ARBs, renin inhibitors, and MRAs.
Despite the absence of LVEF data in our analyses, a substantial proportion of the overall HF cohort did not receive pharmacological therapies recommended for patients with reduced ejection fraction. Of note, beta blockers were received by 9553 (44.8%) patients at baseline, while RAASi agents and diuretics were received by 13,376 (62.7%) and 13,107 (61.4%) patients, respectively (Table 1). Compared with doses recommended in European Society of Cardiology 2016 Guidelines,5 the mean daily doses of individual ACE inhibitors, ARBs, and MRAs were consistently lower (Supporting Information, Table S2 ).
Serum potassium, hypokalaemia, and hyperkalaemia
A total of 3355 hypokalaemia episodes were experienced among 1969 (9.2%) patients, corresponding to a crude rate of 51.7 episodes per 1000 patient‐years (Table 2). The incidence of hyperkalaemia was highly sensitive to the serum potassium threshold used, with 7648 (35.9%), 2725 (12.8%), and 763 (3.6%) patients experiencing at least one episode of hyperkalaemia when defined as ≥5.0, ≥5.5, and ≥6.0 mmol/L, respectively. Corresponding crude rates of hyperkalaemia were 323.5, 79.9, and 16.1 episodes per 1000 patient‐years, respectively.
Table 2.
Hypokalaemia and hyperkalaemia episodes among UK heart failure patients during follow‐up
| Statistic | Hypokalaemiaa | Hyperkalaemiaa | ||
|---|---|---|---|---|
| <3.5 mmol/L | ≥5.0 mmol/L | ≥5.5 mmol/L | ≥6.0 mmol/L | |
| Incidence of hypokalaemia and hyperkalaemia at specified serum potassium threshold | ||||
| Total number of episodes | 3355 | 21 008 | 5190 | 1044 |
| Episodes per patient, mean (SD) | 0.16 (0.69) | 0.98 (2.30) | 0.24 (0.95) | 0.05 (0.31) |
| Crude rate of episodes per 1000 patient‐years (95% CI) | 51.7 (49.9, 53.4) | 323.5 (319.1, 327.9) | 79.9 (77.8, 82.1) | 16.1 (15.1, 17.1) |
| Patients with hypokalaemia and hyperkalaemia at specified serum potassium threshold, n (%) | ||||
| No hypokalaemia and/or hyperkalaemia episodes | 19 365 (90.8%) | 13 686 (64.2%) | 18 609 (87.2%) | 20 571 (96.4%) |
| Exactly one episode | 1342 (6.3%) | 3658 (17.1%) | 1733 (8.1%) | 600 (2.8%) |
| Exactly two episodes | 334 (1.6%) | 1487 (7.0%) | 474 (2.2%) | 107 (0.5%) |
| Exactly three episodes | 136 (0.6%) | 796 (3.7%) | 215 (1.0%) | 32 (0.1%) |
| Four or more episodes | 157 (0.7%) | 1707 (8.0%) | 303 (1.4%) | 24 (0.1%) |
| Time (years) to next episode at specified serum potassium threshold, median (IQR) | ||||
| To first episode (among patients with ≥1 episodes during follow‐up) | 0.82 (0.19, 2.35) | 0.94 (0.28, 2.34) | 1.42 (0.48, 2.95) | 1.70 (0.61, 3.44) |
| First to second episode (among patients with ≥2 episodes during follow‐up) | 0.19 (0.07, 0.80) | 0.50 (0.14, 1.18) | 0.46 (0.11, 1.19) | 0.28 (0.07, 1.04) |
| Second to third episode (among patients with ≥3 episodes during follow‐up) | 0.20 (0.06, 0.71) | 0.42 (0.12, 0.97) | 0.37 (0.11, 0.97) | 0.47 (0.20, 1.18) |
| Third to fourth episode (among patients with ≥4 episodes during follow‐up) | 0.25 (0.07, 0.65) | 0.38 (0.11, 0.90) | 0.31 (0.10, 0.79) | 0.39 (0.07, 0.67) |
CI, confidence interval; IQR, interquartile range; SD, standard deviation.
Hypokalaemia and hyperkalaemia episodes were quantified as the number of serum potassium measurements <3.5, ≥5.0, ≥5.5, or ≥6.0 mmol/L, without such a measurement in the preceding 7 days.
We observed a statistically significant association between the rate of the serum potassium testing and the proportion of measurements ≥5.0 mmol/L. Across the HF cohort, the median frequency of serum potassium measurements during follow‐up was 1.85 per year (interquartile range 1.06–3.27). When patients were stratified by serum potassium testing frequency, the proportion of measurements ≥5.0 mmol/L was greater among patients with ≥1.85 serum potassium measurements per year, compared with those with <1.85 measurements per year (17.8% vs. 13.8%; P < 0.001).
Incidence of adverse clinical events
Adjusted IRRs for mortality were found to exhibit a U‐shaped association pattern with serum potassium (Figure 2). Relative to the reference category (4.5 to <5.0 mmol/L), adjusted IRRs for mortality were estimated as 1.98 (95% confidence interval: 1.69–2.33), 1.23 (1.12–1.36), 1.35 (1.14–1.60), and 3.02 (2.28–4.02), for patients with serum potassium <3.5, ≥5.0 to <5.5, ≥5.5 to <6.0, and ≥6.0 mmol/L, respectively. Adjusted IRRs for MACE demonstrated a non‐significant relationship with serum potassium, which was similarly observed when the four components of MACE (arrhythmia, HF, myocardial infarction, and stroke) were considered individually in sensitivity analysis (data not shown).
Figure 2.
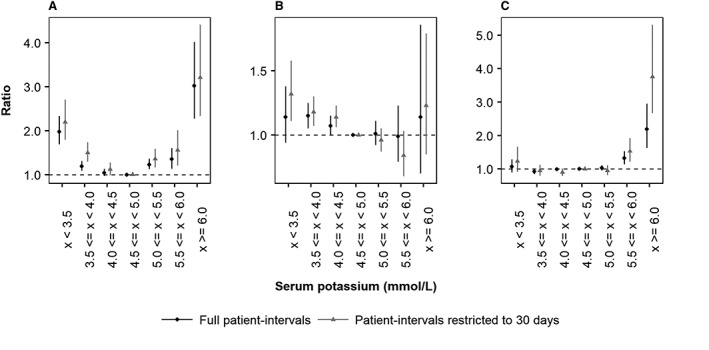
Adjusted incident rate ratios for (A) death, (B) major adverse cardiac event, and (C) renin–angiotensin–aldosterone system inhibitor discontinuation as a function of serum potassium level in UK heart failure patients. Black: full patient intervals; grey: patient intervals restricted to 30 days. Incident rate ratios were adjusted to account for confounding patient demographics, clinical histories and co‐morbidities, clinical measurements, and medication usage, as reported in Supporting Information, Table S3 .
The association between serum potassium concentration and mortality risk was more pronounced after restricting exposure time to a maximum of 30 days from each serum potassium measurement (Figure 2). Relative to the reference category, adjusted IRRs for mortality were estimated as 2.20 (1.80–2.70), 1.35 (1.17–1.58), 1.56 (1.21–2.01), and 3.21 (2.34–4.41), for patients with serum potassium <3.5, ≥5.0 to <5.5, ≥5.5 to <6.0, and ≥6.0 mmol/L, respectively. Restricting patient intervals had little impact on adjusted IRRs for MACE, which continued to exhibit a non‐significant association with serum potassium.
A J‐shaped trend in association between serum potassium and RAASi discontinuation was observed (Figure 2); however, statistically significant associations were only observed between high serum potassium levels and RAASi discontinuation. Compared with the reference category, adjusted IRRs were estimated as 1.07 (0.89–1.28) in patients with serum potassium <3.5 mmol/L, increasing to 1.32 (1.14–1.53) and 2.19 (1.63–2.95) among those with serum potassium ≥5.5 to <6.0 and ≥6.0 mmol/L, respectively. After restricting patient intervals to a maximum of 30 days from each serum potassium measurement, adjusted IRRs for RAASi discontinuation increased to 1.53 (1.22–1.92) and 3.76 (2.67–5.31) among patients with serum potassium levels ≥5.5 to <6.0 and ≥6.0, respectively.
The effect of serum potassium testing frequency on observed associations between serum potassium and adverse outcomes was explored in sensitivity analyses. After stratifying patients by the annual rate of serum potassium measurements, adjusted IRRs for mortality, MACE, and RAASi discontinuation demonstrated similar trends to the overall HF cohort; however, reduced sample sizes gave rise to wider confidence intervals and greater uncertainty (Supporting Information, Figures S2–S4 ).
Coefficient estimates and statistical inferences relating to the fitted risk equations are summarized in Supporting Information, Table S3 . To illustrate their predictive outputs, Figure 3 presents expected rates of death (per 1000 patient‐years), disaggregated by the four most important predictive factors: RAASi usage, age, diuretic usage, and years from index (Figure 3 A–D, respectively). Thus, in addition to hyperkalaemia and hypokalaemia, mortality risk was significantly elevated in patients not prescribed RAASi, older patients, those prescribed diuretics, and as time from index increased. Nevertheless, the observed U‐shaped mortality risk profile shown in Figure 2 remained evident. Association patterns between serum potassium and MACE incidence remained weak when event rates were further disaggregated by time with HF, diuretics use, age, and history of MACE (Supporting Information, Figure S5 ). When predicted RAASi discontinuation rates were disaggregated by diuretic usage, eGFR, insulin usage, and current smoking status, the J‐shaped association was preserved (Supporting Information, Figure S6 ).
Figure 3.
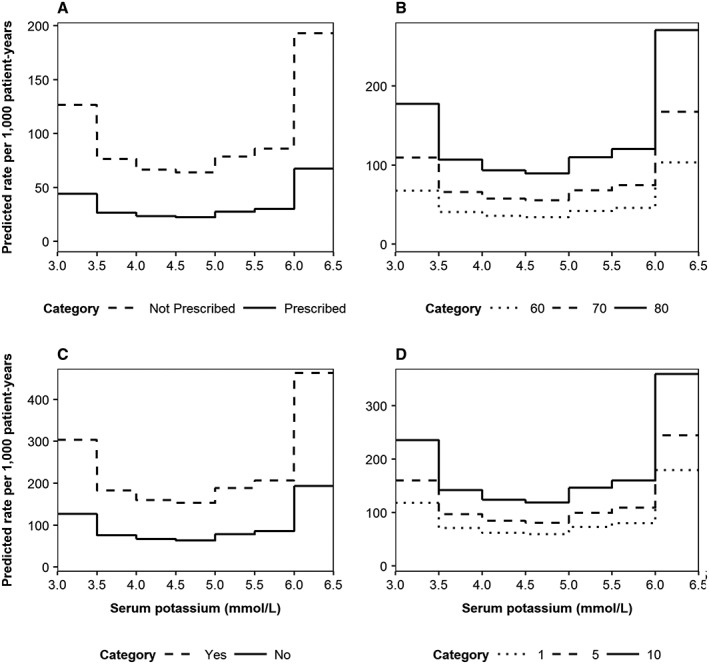
Predicted incidence rates of death, disaggregated by (A) renin–angiotensin–aldosterone system inhibitor usage (dashed line: not prescribed; solid line: prescribed), (B) age (dotted line: 60 years; dashed line: 70 years; solid line: 80 years), (C) diuretic usage (dashed line: yes; solid line: no), and (D) time from index date (dotted line: 1 year; dashed line: 5 years; solid line: 10 years). The top four most important variables (according to the absolute value of the t statistic) were varied, with all other baseline covariates reflective of the cohort average: male; aged 73 years; non‐smoker; no history of co‐morbidities; no medications prescribed; 710 days elapsed since initial heart failure event; estimated glomerular filtration rate 67 mL/min/1.73 m2; body mass index 28 kg/m2; haemoglobin 13.9 g/dL; phosphorous 1.17 mmol/L; and white blood cell count 7.89 × 109/L.
Discussion
In this national patient cohort of new onset HF patients in the UK, we demonstrate the potential importance of serum potassium for HF outcomes. Both hypokalaemia and hyperkalaemia were associated with increased mortality risk. In addition, hyperkalaemia was associated with increased likelihood of RAASi discontinuation. Although we could not establish whether potassium itself was the cause or marker of high risk, our results indicate that close monitoring of potassium values is needed in HF management.
We developed risk equations that can be used to predict the incidence of mortality, MACE, and RAASi discontinuation with respect to serum potassium levels and identify clinical factors that place newly diagnosed HF patients at greater risk of adverse outcomes. Our data showed that hypokalaemia (<4.0 nmol/L) and hyperkalaemia (≥5.0 mmol/L) were each associated with an increased risk of all‐cause mortality, while the incidence of MACE exhibited a non‐significant association with serum potassium. We observed a J‐shaped relationship between serum potassium and RAASi discontinuation with a small non‐significant increase in RAASi discontinuation associated with hypokalaemia (<3.5 mmol/L) and greater (and statistically significant) increases in RAASi discontinuation associated with hyperkalaemia (≥5.5 mmol/L). While our results are broadly consistent with relationships observed in previous studies,3, 4, 20, 21 our approach to restrict patient time to a maximum of 30 days from each serum potassium measurement resulted in a more robust estimation of associations between hypokalaemia and hyperkalaemia and adverse clinical outcomes. Given the publicly funded nature of the UK health care system, the CPRD represents a large, long‐term data set of patients with a broad range of demographic, social, and clinical characteristics. This may imply that our results are more generalizable to other countries and health care systems, compared with those founded on relatively short‐term, claims‐based data sets.2
Adjusted IRRs estimated in our study illustrated the association between serum potassium concentrations outside 4.0–5.0 mmol/L and an increased risk of mortality among incident HF patients. In contrast, the incidence of MACE exhibited a non‐significant association with serum potassium levels in this population. Although cause of death data were not captured in this study, our findings suggest that increased mortality observed among patients with hypokalaemia and hyperkalaemia may not be related to MACE. However, given our observation that patients with hypokalaemia at baseline were more likely to have a history of arrhythmia, which may predispose them to future events, we cannot rule out that some part of mortality may be related to sudden cardiac death.
Our results are generally consistent with available literature relating elevated serum potassium to increased mortality risk in populations with cardiovascular disease, including HF patients following myocardial infarction,22 cardiovascular disease patients receiving antihypertensive drugs,23 and HF patients enrolled in the EMPHASIS‐HF study (a randomized, double‐blind, placebo‐controlled, multi‐centre trial in which patients with mild HF symptoms and a low ejection fraction received eplerenone or placebo).24 More specifically, hypokalaemia and hyperkalaemia were each associated with increased risks of death and cardiovascular hospitalization in a large database study of 6073 chronic HF patients.20 Contrary to our findings, in which the optimal serum potassium range was 4.0–5.0 mmol/L, improved outcomes were reported in patients with high‐normal potassium (5.0–5.5 mmol/L).20 In a study of acute HF patients, serum potassium >4.6 mmol/L was associated with increased risk of 90 day mortality,21 while chronic HF patients had an elevated all‐cause mortality risk when serum potassium was >4.8 mmol/L.4 Moreover, serum potassium levels were independently associated with mortality in HF patients following discharge from acute HF‐related admissions.3 Similar to our findings, a U‐shaped relationship between mortality and serum potassium was reported, with higher mortality risk observed at low and high potassium values.3 Although comparisons between studies should consider differences in contextual settings, populations, and public health care systems, our findings illustrate the associations between serum potassium and mortality risk observed across multiple geographical locations and different co‐morbid populations.
We also report a J‐shaped association pattern between serum potassium and RAASi discontinuation risk. Adjusted IRRs presented in this study relate to RAASi discontinuation rates estimated among all patients and intervals observed during the study period, including those patients and intervals where RAASi therapy had not been prescribed. When alternative definitions of RAASi exposure were investigated, the relationship between elevated serum potassium level and increased likelihood of RAASi discontinuation persisted. The present study was restricted to an analysis of RAASi discontinuation; however, like recent literature relating serum potassium to RAASi dosing in HF patients,25 our future work will seek to investigate associations between serum potassium, RAASi down‐titration, and adverse outcomes among those in UK clinical practice.
Guideline‐recommended doses of ACE inhibitors, ARBs, and MRAs have been evidenced to reduce the risk of hospitalization and death in HF patients with reduced ejection fraction,5 while patients with preserved ejection fraction may be receiving these agents for other indications.26 However, previous trials have demonstrated an association between RAASi use and the incidence of hyperkalaemia events among HF patients.6, 7 Although in line with current clinical practice, where hyperkalaemia risk is managed through down‐titration or discontinuation of such agents,5, 27 suboptimal dosing has been associated with increased adverse event risks and health care costs.8, 9, 10 Such findings suggest that RAASi discontinuation is an intermediate event rather than an endpoint in its own right; thus, we included RAASi usage as a time‐varying covariate in our mortality risk equation to ensure that observed associations between serum potassium and the incidence of death were independent of RAASi use. Furthermore, although we explored potential interactions between RAASi usage and serum potassium when developing risk equations for adverse clinical outcomes, these results were not robust due to low event counts in each of the patient subdomains (e.g. 14 deaths were observed for patients on RAASi and with serum potassium ≥6.0 mmol/L).
The avoidance of hyperkalaemia requires monitoring of potassium intake, laboratory surveillance, and physician awareness of drug interactions. Guidelines recommend the combined use of RAASi and MRA agents to reduce mortality and HF hospitalizations, and emerging therapies may offer solutions to manage hyperkalaemia risk and facilitate such prescriptions in HF patients.28 Novel potassium‐binding agents that effectively manage serum potassium levels may enable continued RAASi and/or MRA therapy and provide significant value in the future.29, 30, 31, 32, 33
Comparing hyperkalaemia incidence and related outcomes across epidemiological studies is at present problematic, as there is no standard definition of hyperkalaemia, nor a consistent serum potassium threshold applied. The incidence of hyperkalaemia observed in this study was highly sensitive to the serum potassium threshold used, and estimated IRRs for death and RAASi discontinuation were higher with increasing serum potassium levels. Had the sample size allowed for such analysis, further stratification of patients with serum potassium ≥6.0 mmol/L may have strengthened the association pattern between serum potassium and the incidence of death and RAASi discontinuation.
Limitations of this study are generally attributed to observational nature of this study and the constraints of the data used to inform our analyses. Firstly, serum potassium data were obtained from measurements taken during routine primary care, rather than collected according to a clinical trial protocol. Our results suggest an association between frequency of testing and the percentage of serum potassium measurements ≥5.0 mmol/L; however, observed association patterns between serum potassium and adverse clinical outcomes were not significantly influenced by testing frequency in sensitivity analyses.
Secondly, this study was limited to the data available within the CPRD. Although causality cannot be inferred from our results, we accounted for potential confounding by excluding CKD and dialysis patients and adjusting IRRs for patient demographics, clinical histories and co‐morbidities, clinical measurements (including eGFR), and medication usage. Candidate covariates included those recorded within the CPRD; as such, potential confounders not available in this data set, particularly New York Heart Association classification, N‐terminal pro‐B‐type natriuretic peptide, and LVEF, were not explicitly considered. Consequently, we were unable to adjust for HF type and severity in our analyses, and a lack of patient‐level LVEF data precluded a robust analysis of prescribed RAASi doses with respect to maintenance doses recommended in European Society of Cardiology 2016 Guidelines.5 Moreover, we did not have access to serum creatinine levels and hence cannot ascertain whether RAASI discontinuation was due to increased creatinine levels, serum potassium change, or both. Additionally, we could not account for potential potassium supplement intake or potassium‐rich dietary intake of patients in the cohort.
Finally, our analyses may overestimate the incidence of hyperkalaemia, as genuine elevations in serum potassium were unable to be distinguished from pseudo‐hyperkalaemia resulting from haemolysis during venepuncture. Despite its limitations, one notable strength of the CPRD data set is its size, which allowed for statistically robust estimates of adverse clinical events risk that verified the association curves observed across different geographical locations and co‐morbid populations.
In conclusion, this study developed risk equations to describe the relationships between serum potassium and adverse clinical outcomes among incident HF patients in UK clinical practice. Serum potassium concentrations outside 4.0–5.0 mmol/L were associated with elevated mortality risk, while a J‐shaped association between categorical serum potassium and the incidence of RAASi discontinuation was observed. Our results demonstrate the potential importance of serum potassium in HF outcomes and management. Further work is needed to explore whether potassium itself causes adverse outcomes or is a marker of higher risk.
Conflict of interest
C.L. has received significant research grant funding from AstraZeneca (awarded to institution) and modest speaker honoraria from Biotronik, Medtronic, Abbot, Novartis, and Vifor. L.Q. is a full‐time employee of AstraZeneca, with modest ownership interests in AstraZeneca. A.B. has received modest advisory honoraria from AstraZeneca in relation to this study. H.F. has received modest research grant funding from AstraZeneca (principal investigator) and modest expert witness funding from Amgen (consultant). M.E. declares no conflict of interest. D.A., H.B., and P.M. have received significant research grant funding from AstraZeneca in relation to this study. E.P. is a full‐time employee of AstraZeneca.
Funding
This work was supported by a grant from AstraZeneca. The funding agreement ensured the authors' independence in designing the study, interpreting the data, and preparing the manuscript for publication.
Supporting information
Table S1. Read and International Classification of Diseases (ICD‐10) codes used to define heart failure and chronic kidney disease
Table S2. Renin‐angiotensin‐aldosterone system inhibitors (RAASi) use at baseline, compared to target maintenance doses recommended by European Society of Cardiology 2016 guidelines.1
Table S3. Model output for final risk equations
Figure S1. Illustrative example of time‐updated patient‐intervals based on timing of serum potassium measurements
Figure S2. Adjusted incident rate ratios for death as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S3. Adjusted incident rate ratios for major adverse cardiac events (MACE) as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S4. Adjusted incident rate ratios for renin‐angiotensin‐aldosterone system inhibitor (RAASi) discontinuation as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S5. Predicted incidence rates of MACE, disaggregated by (A) time with HF (dotted line: 1 year; dashed line: 5 years; solid line: 10 years), (B) diuretics usage (dashed line: yes; solid line: no), (C) age (dotted line: 60 years; dashed line: 70 years; solid line: 80 years), and (D) history of MACE (dashed line: yes; solid line: no)
Figure S6. Predicted incidence rates of RAASi discontinuation, disaggregated by (A) diuretics usage (dashed line: yes; solid line: no), (B) eGFR (dotted line: 15 mL/min/1.73m2; dashed line: 30 mL/min/1.73m2; solid line: 60 mL/min/1.73m2), (C) insulin usage (dashed line: yes; solid line: no), and (D) current smoking status (dashed line: yes; solid line: no)
Acknowledgements
Editorial assistance in the preparation of this manuscript was provided by Dr Karina Hamilton, Dr Daniel Sugrue, and Dr Angharad Morgan of Health Economics and Outcomes Research Ltd. The authors additionally thank Dr Susan Grandy and Dr Klas Bergenheim of AstraZeneca, for their support and contribution to this work.
Linde C., Qin L., Bakhai A., Furuland H., Evans M., Ayoubkhani D., Palaka E., Bennett H., and McEwan P. (2019) Serum potassium and clinical outcomes in heart failure patients: results of risk calculations in 21 334 patients in the UK, ESC Heart Failure, 6, 280–290. 10.1002/ehf2.12402.
References
- 1. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician 2015; 92: 487–495. [PubMed] [Google Scholar]
- 2. Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA. Association of serum potassium with all‐cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017; 46: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Núñez J, Bayés‐Genís A, Zannad F, Rossignol P, Núñez E, Bodí V, Miñana G, Santas E, Chorro FJ, Mollar A, Carratalá A, Navarro J, Górriz JL, Lupón J, Husser O, Metra M, Sanchis J. Long‐term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018; 137: 1320–1330. [DOI] [PubMed] [Google Scholar]
- 4. Aldahl M, Jensen A‐SC, Davidsen L, Eriksen MA, Møller Hansen S, Nielsen BJ, Krogager ML, Køber L, Torp‐Pedersen C, Søgaard P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J 2017; 38: 2890–2896. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske P, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P , ESC Scientific Document Group. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R. Serum potassium and clinical outcomes in the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008; 118: 1643–1650. [DOI] [PubMed] [Google Scholar]
- 7. Desai AS, Swedberg K, McMurray JJ, Granger CB, Yusuf S, Young JB, Dunlap ME, Solomon SD, Hainer JW, Olofsson B, Michelson EL, Pfeffer MA, CHARM Program Investigators . Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50: 1959–1966. [DOI] [PubMed] [Google Scholar]
- 8. Epstein M. Hyperkalemia constitutes a constraint for implementing renin‐angiotensin‐aldosterone inhibition: the widening gap between mandated treatment guidelines and the real‐world clinical arena. Kidney International Supplements 2016; 6: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouwerkerk W, Voors A, Anker S, Cleland J, Dickstein K, Filippatos G, van der Harst P , Hillege HL, Lang CC, Ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ , Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J 2017; 38: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 10. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS. Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 11. Einhorn LM, Zhan M, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, Kotanko P, Pitt B, Saran R. Serum potassium and outcomes in CKD: insights from the RRI‐CKD cohort study. Clin J Am Soc Nephrol 2010; 5: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes J, Kalantar‐Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo‐and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract 2012; 120: c8–c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016; 11: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medicines and Healthcare Products Regulatory Agency . Clinical Practice Research Datalink 2017. https://www.cprd.com/home/ (1 October 2018).
- 16. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NHS Digital . Hospital episode statistics. http://content.digital.nhs.uk/hes (1 October 2018).
- 18. R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R‐project.org/ (1 October 2018).
- 19. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 20. Hoss S, Elizur Y, Luria D, Keren A, Lotan C, Gotsman I. Serum potassium levels and outcome in patients with chronic heart failure. Am J Cardiol 2016; 118: 1868–1874. [DOI] [PubMed] [Google Scholar]
- 21. Legrand M, Ludes P‐O, Massy Z, Rossignol P, Parenica J, Park J‐J, Ishihara S, AlHabib KF, Maggioni A, Miró O, Sato N, Cohen‐Solal A, Fairman E, Lassus J, Harjola VP, Mueller C, Peacock FW, Choi DJ, Plaisance P, Spinar J, Kosiborod M, Mebazaa A, Gayat E, GREAT (Global Research on Acute Conditions Team) Network and INI‐CRCT (Investigation Network Initiative-Cardiovascular and Renal Clinical Trialists) network. Association between hypo‐and hyperkalemia and outcome in acute heart failure patients: the role of medications. Clin Res Cardiol 2018: 107: 214–221. [DOI] [PubMed] [Google Scholar]
- 22. Krogager ML, Eggers‐Kaas L, Aasbjerg K, Mortensen RN, Køber L, Gislason G, Torp‐Pedersen C, Søgaard P. Short‐term mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother 2015; 1: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 2012; 109: 1510–1513. [DOI] [PubMed] [Google Scholar]
- 24. Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Circ Heart Fail 2014; 7: 51–58. [DOI] [PubMed] [Google Scholar]
- 25. Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin–angiotensin–aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT‐CHF. Eur J Heart Fail 2018; 20: 923–930. [DOI] [PubMed] [Google Scholar]
- 26. Lund LH, Benson L, Dahlström U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 27. Queen Mary University of London Clinical Effectiveness Group . Summary guidelines: heart failure update 2015. http://www.blizard.qmul.ac.uk/ceg‐resource‐library/clinical‐guidance/clinical‐guidelines/611‐heart-failure-guideline‐november‐2015/file.html (1 October 2018).
- 28. Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol 2017; 2: 79–85. [DOI] [PubMed] [Google Scholar]
- 29. Packham DK, Rasmussen HS, Lavin PT, El‐Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372: 222–231. [DOI] [PubMed] [Google Scholar]
- 30. Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, van der Meer P , Ponikowski P, Rasmussen HS, Lavin PT, Singh B, Yang A, Deedwania P. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS‐9) in heart failure patients: results from a phase 3 randomized, double‐blind, placebo‐controlled trial. Eur J Heart Fail 2015; 17: 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ‐Schmidt H, Berman L, Pitt B, OPAL‐HK Investigators . Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221. [DOI] [PubMed] [Google Scholar]
- 32. Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, Christ‐Schmidt H, Berman L, Weir MR. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail 2015; 17: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA, AMETHYST‐DN Investigators . Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST‐DN randomized clinical trial. JAMA 2015; 314: 151–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Read and International Classification of Diseases (ICD‐10) codes used to define heart failure and chronic kidney disease
Table S2. Renin‐angiotensin‐aldosterone system inhibitors (RAASi) use at baseline, compared to target maintenance doses recommended by European Society of Cardiology 2016 guidelines.1
Table S3. Model output for final risk equations
Figure S1. Illustrative example of time‐updated patient‐intervals based on timing of serum potassium measurements
Figure S2. Adjusted incident rate ratios for death as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S3. Adjusted incident rate ratios for major adverse cardiac events (MACE) as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S4. Adjusted incident rate ratios for renin‐angiotensin‐aldosterone system inhibitor (RAASi) discontinuation as a function of serum potassium level in UK heart failure patients, stratified by the annual frequency of serum potassium measurements: (A) <1.063; (B) 1.063 to <1.850; (C) 1.850 to <3.267; (D) ≥3.267. Grey: incident rate ratios for the overall cohort; black: incident rate ratios for patients stratified by serum potassium testing frequency.
Figure S5. Predicted incidence rates of MACE, disaggregated by (A) time with HF (dotted line: 1 year; dashed line: 5 years; solid line: 10 years), (B) diuretics usage (dashed line: yes; solid line: no), (C) age (dotted line: 60 years; dashed line: 70 years; solid line: 80 years), and (D) history of MACE (dashed line: yes; solid line: no)
Figure S6. Predicted incidence rates of RAASi discontinuation, disaggregated by (A) diuretics usage (dashed line: yes; solid line: no), (B) eGFR (dotted line: 15 mL/min/1.73m2; dashed line: 30 mL/min/1.73m2; solid line: 60 mL/min/1.73m2), (C) insulin usage (dashed line: yes; solid line: no), and (D) current smoking status (dashed line: yes; solid line: no)


