Abstract
Aims
There is debate on whether the beneficial effect of implantable cardioverter‐defibrillators (ICDs) is attenuated in patients with non‐ischaemic cardiomyopathy (NICM). We assess whether any ICD benefit differs between patients with NICM and those with ischaemic cardiomyopathy (ICM), using data from the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial.
Methods and results
We performed a post hoc analysis using WARCEF (N = 2293; ICM, n = 991 vs. NICM, n = 1302), where participants received optimal medical treatment. We developed stratified propensity scores for having an ICD at baseline using 41 demographic and clinical variables and created 1:2 propensity‐matched cohorts separately for ICM patients with ICD (N = 223 with ICD; N = 446 matched) and NICM patients (N = 195 with ICD; N = 390 matched). We constructed a Cox proportional hazards model to assess the effect of ICD status on mortality for patients with ICM and those with NICM and tested the interaction between ICD status and aetiology of heart failure. During mean follow‐up of 3.5 ± 1.8 years, 527 patients died. The presence of ICD was associated with a lower risk of all‐cause death among those with ICM (hazard ratio: 0.640; 95% confidence interval: 0.448 to 0.915; P = 0.015) but not among those with NICM (hazard ratio: 0.984; 95% confidence interval: 0.641 to 1.509; P = 0.941). There was weak evidence of interaction between ICD status and the aetiology of heart failure (P = 0.131).
Conclusions
The presence of ICD is associated with a survival benefit in patients with ICM but not in those with NICM.
Keywords: Heart failure with reduced ejection fraction, Implantable cardioverter‐defibrillator, Non‐ischaemic cardiomyopathy, Propensity score matching
Introduction
The 2013 American College of Cardiology/American Heart Association Guidelines for the Management of Heart Failure (HF) and the 2016 European Society of Cardiology Guidelines for the Diagnosis and Treatment of Acute and Chronic HF recommend implantable cardioverter‐defibrillator (ICD) implantation for prevention of death in patients with HF with reduced ejection fraction.1, 2 While there is considerable evidence that ICDs prevent death in patients with ischaemic cardiomyopathy (ICM),3, 4 the evidence for patients with non‐ischaemic cardiomyopathy (NICM) is less robust.
The Defibrillators in Non‐Ischaemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial5 did not show a significant survival benefit for ICD, though there was a trend towards mortality reduction. Similarly, although the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) demonstrated improved mortality with ICD therapy for patients with cardiomyopathy, the subgroup analysis for non‐ischaemic patients showed attenuated benefit.4 More recently, the Danish Study to Assess the Efficacy of ICDs in Patients with Non‐Ischaemic Systolic Heart Failure on Mortality (DANISH) trial6 failed to show a survival benefit for ICDs compared with optimal medical therapy. However, updated meta‐analyses7, 8, 9, 10, 11 continue to suggest a survival benefit for ICD implantation among patients with NICM, a recommendation that is reflected in current guidelines. For these reasons, there is considerable interest in additional analyses of whether the impact of having an ICD may differ depending on HF aetiology.12
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, a large randomized, clinical trial of patients with HF with reduced ejection fraction that tested the effect of warfarin vs. aspirin on death and stroke, provides an important opportunity to address this critical question. We conducted an analysis using WARCEF data to further elucidate the relation between ICD status and mortality for HF patients with ICM and NICM, using a propensity score‐based approach to limit confounding.
Methods
Study participants
The investigation conforms with the principles outlined in the Declaration of Helsinki. Details of the WARCEF trial have been published previously.13 A total of 2305 patients with left ventricular ejection fraction ≤ 35% in sinus rhythm were randomly assigned to warfarin (target international normalized ratio 2.75, with an acceptable target range of 2.0 to 3.5) or aspirin (325 mg/day). Left ventricular ejection fraction was measured by radionuclide ventriculography, left ventriculography, or quantitative echocardiography. Based on those findings and patients' past medical history, the aetiology of HF was determined by clinical judgement of each local site. Patients were enrolled at 168 centres in 11 countries between October 2002 and January 2010. The mean follow‐up time was 3.5 ± 1.8 years. Patients who had a clear indication for warfarin or aspirin were not eligible. Additional eligibility criteria were a modified Rankin score of 4 or less (on a scale of 0 to 6, with higher scores indicating more severe disability) and planned treatment with a beta‐blocker, an angiotensin‐converting enzyme (ACE)‐Inhibitor [or, if the side‐effect profile with ACE‐Inhibitors was unacceptable, with an angiotensin II receptor blocker (ARB)], or hydralazine and nitrates. Patients were ineligible if they had a condition that conferred a high risk of cardiac embolism, such as atrial fibrillation, a mechanical cardiac valve, endocarditis, or an intracardiac mobile or pedunculated thrombus.
For this analysis, we excluded 12 enrolled patients because of lack of data on either aetiology of HF or ICD status at baseline (Figure 1 ). Information regarding indications for ICD, and whether or not the patients received concurrent cardiac resynchronization therapy (CRT), was not recorded in WARCEF.
Figure 1.
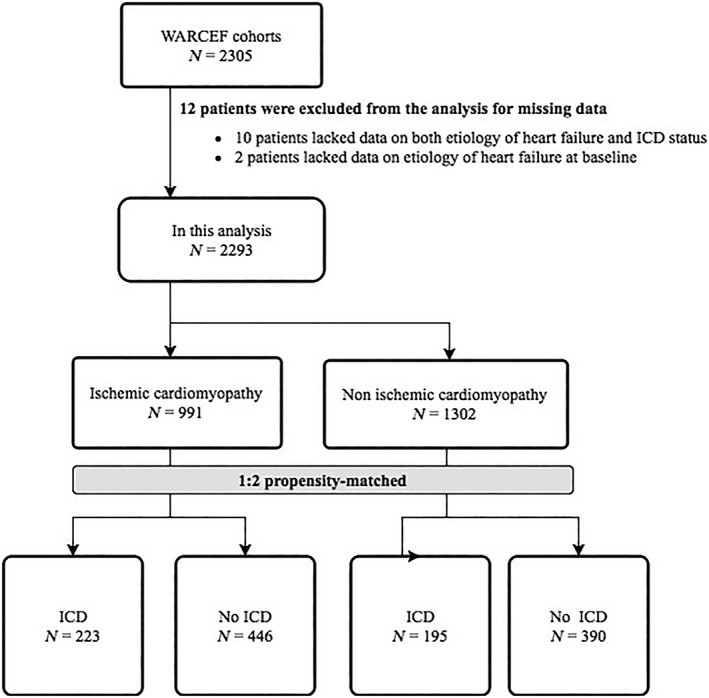
Overview of the study cohort. ICD, implantable cardioverter‐defibrillator.
Assessment of outcomes
In the WARCEF trial, an independent endpoint adjudication committee, whose members were unaware of the treatment assignment, adjudicated major clinical outcomes. The committee adjudicated cause of death based on the standardized narrative reports on patients' status preceding death. If applicable and available, discharge summary; detailed report by physician, nursing staff, or family; autopsy report; death certificate; and other materials were considered as materials for adjudication. Post‐mortem ICD interrogation was not always available but was considered in adjudication process if it was available. In the current analysis, the primary outcome was all‐cause death, and the secondary outcomes were cardiovascular death and sudden death. Deaths were adjudicated as having a cardiovascular or non‐cardiovascular cause, and cardiovascular deaths were then adjudicated as sudden or other types. The adjudication was not blinded to ICD status.
Statistical analysis
Baseline characteristics were assessed by ICD status and for ICM and NICM groups separately. Continuous variables are presented as mean ± SD and categorical variables as frequencies and percentages. They were considered balanced if the standardized bias was less than 0.25.14 This is the difference in means (for continuous variables) or proportions (for each category of a categorical variable) divided by the pooled standard deviation.
A propensity score matching approach was used to adjust for potential confounding in the comparison of patients who had or did not have implanted ICDs at randomization.15, 16 The goal is to assess whether any ICD benefit differs between NICM and ICM patients, rather than to measure ICD benefit in the overall population. We therefore match ICD to non‐ICD patients within the NICM stratum and, separately, ICD to non‐ICD patients within the ICM stratum.The propensity score model initially included 41 patient covariates, NICM vs. ICM status, and the interaction of this status with each of the covariates in a logistic regression model. To avoid overfitting, the final model included the subset of predictor variables that optimized the Akaike information criterion in a stepwise procedure.
We implemented the matching procedure with the R package MatchIt.17 We first selected matched controls using random selection within a calliper width of SD 0.1 on the logit scale. That is, for each case, we considered the set of controls whose logit propensity score was within 0.1 SDs of the logit propensity score of the case and randomly selected two from the set as the matched controls. If no matches were available within the calliper, we used the nearest neighbour control to match the case. In this way, we were able to match all cases to controls within each stratum.
To assess the association between ICD status and outcomes by aetiology of HF and to determine whether aetiology of HF modulated the association between ICD status and mortality, we constructed Cox proportional hazards models including ICD, ICM, and their interaction as covariates separately for all‐cause death, cardiovascular death, and sudden death. Time zero was defined as the time of randomization in the survival analyses. Robust variance estimators were used to adjust for any clustering caused by the matching. Two‐tailed P values < 0.05 were considered statistically significant. All analyses were performed using SAS (version 9.4; SAS Institute, Cary, North Carolina) and R.
Results
Baseline characteristics
The flowchart for the analysis procedure is presented in Figure 1 . Of the 2293 WARCEF participants, 418 had an ICD at time of enrolment (ICM, N = 223; NICM, N = 195) (Figure 1 ). Before matching, ICM patients with ICDs were more likely to be from high‐income countries (i.e. excluding Argentina and Ukraine); younger; have higher education, lower systolic blood pressure, alcohol consumption, ARB, lower sodium level; and less likely to have ACE‐Inhibitor, as compared with ICM patients without ICDs. NICM patients with ICDs were more likely to be from high‐income countries, have lower systolic blood pressure, and have a higher prevalence of past medical history of atrial fibrillation, have longer distance on 6 min walk test, and were more likely to take diuretics, as compared with NICM patients without ICDs (Table 1). We matched 205 ICM cases to controls and 189 NICM cases to controls using the calliper criterion and 18 ICM cases to controls and 6 NICM cases to controls using the nearest neighbour procedure. The standardized biases of all baseline covariates were less than 0.25 after matching (Table 2). Variables with high or moderate missingness include aldosterone blocker (32%), statin (21%), previous aspirin use (19%), 6 min walk (7%), and haemoglobin (6%). Missingness in other variables was negligible. Aldosterone blocker and 6 min walk entered the final propensity score model, with missing values imputed by mode and mean, respectively.
Table 1.
Patient characteristics before propensity score matching
| Characteristics | Ischaemic | Non‐ischaemic | ||||
|---|---|---|---|---|---|---|
| ICD (n = 223) | No ICD (n = 768) | Standardized bias | ICD (n = 195) | No ICD (n = 1107) | Standardized bias | |
| Treatment arm | ||||||
| Warfarin | 108/223 (48.4) | 380/768 (49.5) | 0.021 | 104/195 (53.3) | 546/1107 (49.3) | 0.08 |
| Demographics | ||||||
| Middle‐income countries (Argentina or Ukraine) | 3/223 (1.3) | 98/768 (12.8) | 0.377 | 3/195 (1.5) | 103/1107 (9.3) | 0.284 |
| Age, years | 61.1 ± 10.0 | 63.8 ± 10.2 | 0.271 | 58.1 ± 12.2 | 59.1 ± 11.8 | 0.09 |
| Age categories | ||||||
| <60 | 92/223 (41.3) | 265/768 (34.5) | 0.141 | 107/195 (54.9) | 558/1107 (50.4) | 0.089 |
| 60–74 | 114/223 (51.1) | 374/768 (48.7) | 0.048 | 72/195 (36.9) | 432/1107 (39.0) | 0.043 |
| >74 | 17/223 (7.6) | 129/768 (16.8) | 0.259 | 16/195 (8.2) | 117/1107 (10.6) | 0.078 |
| Male sex | 199/223 (89.2) | 655/768 (85.3) | 0.114 | 154/195 (79.0) | 829/1107 (74.9) | 0.095 |
| Race or ethnic group | ||||||
| Non‐Hispanic White | 177/223 (79.4) | 604/768 (78.6) | 0.018 | 139/195 (71.3) | 809/1107 (73.1) | 0.04 |
| Non‐Hispanic Black | 26/223 (11.7) | 65/768 (8.5) | 0.111 | 41/195 (21.0) | 198/1107 (17.9) | 0.081 |
| Hispanic | 13/223 (5.8) | 72/768 (9.4) | 0.127 | 9/195 (4.6) | 72/1107 (6.5) | 0.078 |
| Other | 7/223 (3.1) | 27/768 (3.5) | 0.021 | 6/195 (3.1) | 28/1107 (2.5) | 0.034 |
| Educational level | ||||||
| < High school | 73/221 (33.0) | 376/767 (49.0) | 0.321 | 72/195 (36.9) | 469/1106 (42.4) | 0.111 |
| High school graduate or some college | 109/221 (49.3) | 284/767 (37.0) | 0.251 | 95/195 (48.7) | 455/1106 (41.1) | 0.153 |
| College graduate or postgraduate | 39/221 (17.6) | 107/767 (14.0) | 0.104 | 28/195 (14.4) | 182/1106 (16.5) | 0.057 |
| Body measurement and vital statistics | ||||||
| Height, cm | 172.4 ± 8.9 | 171.5 ± 9.0 | 0.099 | 172.9 ± 9.5 | 171.4 ± 9.5 | 0.166 |
| Weight, kg | 86.5 ± 16.5 | 84.6 ± 17.6 | 0.111 | 89.0 ± 21.5 | 86.5 ± 20.8 | 0.118 |
| Body mass index mean | 29.1 ± 5.2 | 28.7 ± 5.4 | 0.07 | 29.7 ± 7.2 | 29.3 ± 6.3 | 0.067 |
| Body mass index distribution | ||||||
| <25 | 50/222 (22.5) | 186/766 (24.3) | 0.041 | 52/193 (26.9) | 271/1098 (24.7) | 0.052 |
| 25–30 | 88/222 (39.6) | 325/766 (42.4) | 0.057 | 57/193 (29.5) | 411/1098 (37.4) | 0.164 |
| >30 | 84/222 (37.8) | 255/766 (33.3) | 0.096 | 84/193 (43.5) | 416/1098 (37.9) | 0.116 |
| Systolic BP, mmHg | 119.1 ± 19.3 | 124.4 ± 17.6 | 0.29 | 119.9 ± 18.6 | 125.3 ± 19.4 | 0.278 |
| Pulse, beats/min | 69.6 ± 10.1 | 71.2 ± 11.6 | 0.141 | 71.9 ± 11.3 | 73.0 ± 12.5 | 0.119 |
| Past medical history/co‐morbidities | ||||||
| Hypertension | 141/216 (65.3) | 492/748 (65.8) | 0.01 | 107/187 (57.2) | 623/1076 (57.9) | 0.014 |
| Diabetes mellitus | 92/223 (41.3) | 283/768 (36.8) | 0.091 | 50/195 (25.6) | 297/1107 (26.8) | 0.027 |
| Atrial fibrillation | 13/223 (5.8) | 23/768 (3.0) | 0.152 | 17/195 (8.7) | 33/1107 (3.0) | 0.299 |
| Peripheral vascular disease | 42/223 (18.8) | 119/768 (15.5) | 0.091 | 14/195 (7.2) | 86/1107 (7.8) | 0.022 |
| Prior stroke or TIA | 29/223 (13.0) | 104/768 (13.5) | 0.016 | 19/195 (9.7) | 142/1106 (12.8) | 0.094 |
| Alcohol and smoking | ||||||
| Smoking status | ||||||
| Current smoker | 35/222 (15.8) | 138/767 (18.0) | 0.059 | 38/195 (19.5) | 196/1106 (17.7) | 0.046 |
| Former smoker | 148/222 (66.7) | 417/767 (54.4) | 0.249 | 92/195 (47.2) | 520/1106 (47.0) | 0.003 |
| Never smoked | 39/222 (17.6) | 212/767 (27.6) | 0.231 | 65/195 (33.3) | 390/1106 (35.3) | 0.04 |
| Alcohol consumption | ||||||
| Current consumption, >2 oz/day | 65/223 (29.1) | 179/768 (23.3) | 0.136 | 48/195 (24.6) | 279/1106 (25.2) | 0.014 |
| Previous consumption, >2 oz/day | 71/223 (31.8) | 149/768 (19.4) | 0.299 | 45/195 (23.1) | 241/1106 (21.8) | 0.031 |
| Never consumed alcohol | 87/223 (39.0) | 440/768 (57.3) | 0.366 | 102/195 (52.3) | 586/1106 (53.0) | 0.014 |
| Status of heart failure | ||||||
| NYHA classification | ||||||
| Class I | 26/223 (11.7) | 102/767 (13.3) | 0.049 | 27/193 (14.0) | 160/1102 (14.5) | 0.015 |
| Class II | 123/223 (55.2) | 402/767 (52.4) | 0.055 | 116/193 (60.1) | 621/1102 (56.4) | 0.076 |
| Class III | 73/223 (32.7) | 254/767 (33.1) | 0.008 | 48/193 (24.9) | 305/1102 (27.7) | 0.063 |
| Class IV | 1/223 (0.4) | 9/767 (1.2) | 0.073 | 2/193 (1.0) | 16/1102 (1.5) | 0.036 |
| Ejection fraction, % | 23.3 ± 6.8 | 25.0 ± 7.5 | 0.233 | 24.5 ± 7.3 | 24.7 ± 7.8 | 0.024 |
| LVEF ≤ 20% | 76/223 (34.1) | 195/768 (25.4) | 0.195 | 55/194 (28.4) | 324/1106 (29.3) | 0.021 |
| Distance covered on 6 min walk, m | 353.0 ± 126.6 | 341.4 ± 146.8 | 0.081 | 377.6 ± 124.4 | 352.8 ± 153.8 | 0.166 |
| Baseline MMSE score | 28.5 ± 2.0 | 28.4 ± 2.1 | 0.039 | 28.5 ± 2.0 | 28.6 ± 2.1 | 0.025 |
| Baseline MLWHF score | 37.5 ± 24.3 | 34.0 ± 22.5 | 0.153 | 32.9 ± 22.7 | 33.2 ± 24.4 | 0.011 |
| Medications | ||||||
| Aspirin or other antiplatelet agents | 136/190 (71.6) | 446/599 (74.5) | 0.065 | 104/141 (73.8) | 572/781 (73.2) | 0.012 |
| Warfarin or other oral anticoagulants | 24/223 (10.8) | 51/768 (6.6) | 0.156 | 14/195 (7.2) | 90/1107 (8.1) | 0.035 |
| ACE‐Inhibitor | 165/222 (74.3) | 656/768 (85.4) | 0.295 | 165/194 (85.1) | 945/1105 (85.5) | 0.013 |
| ARB | 57/221 (25.8) | 112/768 (14.6) | 0.298 | 32/193 (16.6) | 174/1104 (15.8) | 0.022 |
| Beta‐blocker | 209/223 (93.7) | 682/768 (88.8) | 0.163 | 181/194 (93.3) | 986/1105 (89.2) | 0.135 |
| Aldosterone blocker | 85/146 (58.2) | 283/472 (60.0) | 0.035 | 80/130 (61.5) | 362/594 (60.9) | 0.012 |
| Diuretic | 185/223 (83.0) | 614/768 (79.9) | 0.076 | 168/194 (86.6) | 884/1105 (80.0) | 0.168 |
| Statin | 185/196 (94.4) | 541/611 (88.5) | 0.194 | 118/150 (78.7) | 546/717 (76.2) | 0.059 |
| Lab data | ||||||
| Creatinine, mg/dL | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.184 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.113 |
| eGFR | 64.5 ± 19.6 | 66.5 ± 19.9 | 0.099 | 69.2 ± 20.7 | 70.4 ± 21.0 | 0.054 |
| Haemoglobin, g/dL | 14.0 ± 1.8 | 14.1 ± 1.5 | 0.047 | 14.1 ± 1.5 | 14.1 ± 1.5 | 0.007 |
| Sodium, mEq/L | 138.2 ± 9.5 | 139.8 ± 3.2 | 0.294 | 139.6 ± 3.4 | 139.8 ± 3.3 | 0.062 |
| WBC count, ×109/L | 7.7 ± 2.0 | 7.5 ± 2.0 | 0.097 | 7.5 ± 2.4 | 7.4 ± 2.0 | 0.084 |
ACE‐Inhibitor, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; ICD, implantable cardio‐defibrillator; LVEF, left ventricular ejection fraction; MLWHF, Minnesota Living with Heart Failure questionnaire; MMSE, Mini‐Mental Scale Examination; NYHA, New York Heart Association; TIA, transient ischaemic attack; WBC, white blood cell.
Table 2.
Patient characteristics after propensity matching
| Variable | Ischaemic | Non‐ischaemic | ||||
|---|---|---|---|---|---|---|
| ICD (n = 223) | No ICD (n = 446) | Standardized bias | ICD (n = 195) | No ICD (n = 390) | Standardized bias | |
| Treatment arm | ||||||
| Warfarin | 108/223 (48.4) | 223/446 (50.0) | 0.031 | 104/195 (53.3) | 184/390 (47.2) | 0.123 |
| Demographics | ||||||
| Middle‐income countries (Argentina or Ukraine) | 3/223 (1.3) | 9/446 (2.0) | 0.051 | 3/195 (1.5) | 6/390 (1.5) | 0 |
| Age, years | 61.1 ± 10.0 | 62.4 ± 9.6 | 0.135 | 58.1 ± 12.2 | 57.0 ± 11.7 | 0.091 |
| Age categories | ||||||
| <60 | 92/223 (41.3) | 171/446 (38.3) | 0.06 | 107/195 (54.9) | 228/390 (58.5) | 0.073 |
| 60–74 | 114/223 (51.1) | 224/446 (50.2) | 0.018 | 72/195 (36.9) | 137/390 (35.1) | 0.037 |
| >74 | 17/223 (7.6) | 51/446 (11.4) | 0.126 | 16/195 (8.2) | 25/390 (6.4) | 0.07 |
| Male sex | 199/223 (89.2) | 395/446 (88.6) | 0.021 | 154/195 (79.0) | 302/390 (77.4) | 0.037 |
| Race or ethnic group | ||||||
| Non‐Hispanic White | 177/223 (79.4) | 358/446 (80.3) | 0.022 | 139/195 (71.3) | 277/390 (71.0) | 0.006 |
| Non‐Hispanic Black | 26/223 (11.7) | 49/446 (11.0) | 0.021 | 41/195 (21.0) | 88/390 (22.6) | 0.037 |
| Hispanic | 13/223 (5.8) | 19/446 (4.3) | 0.074 | 9/195 (4.6) | 16/390 (4.1) | 0.025 |
| Other | 7/223 (3.1) | 20/446 (4.5) | 0.068 | 6/195 (3.1) | 9/390 (2.3) | 0.049 |
| Educational level | ||||||
| < High school | 73/221 (33.0) | 186/445 (41.8) | 0.18 | 72/195 (36.9) | 134/390 (34.4) | 0.054 |
| High school graduate or some college | 109/221 (49.3) | 196/445 (44.0) | 0.106 | 95/195 (48.7) | 191/390 (49.0) | 0.005 |
| College graduate or postgraduate | 39/221 (17.6) | 63/445 (14.2) | 0.097 | 28/195 (14.4) | 65/390 (16.7) | 0.063 |
| Body measurement and vital statistics | ||||||
| Height, cm | 172.4 ± 8.9 | 172.2 ± 9.2 | 0.023 | 172.9 ± 9.5 | 172.9 ± 9.2 | 0.002 |
| Weight, kg | 86.5 ± 16.5 | 86.1 ± 18.7 | 0.022 | 89.0 ± 21.5 | 89.5 ± 23.0 | 0.022 |
| Body mass index mean | 29.1 ± 5.2 | 29.0 ± 5.7 | 0.016 | 29.7 ± 7.2 | 29.7 ± 6.8 | 0 |
| Body mass index distribution | ||||||
| <25 | 50/222 (22.5) | 111/445 (24.9) | 0.057 | 52/193 (26.9) | 103/387 (26.6) | 0.007 |
| 25–30 | 88/222 (39.6) | 176/445 (39.6) | 0.002 | 57/193 (29.5) | 120/387 (31.0) | 0.032 |
| >30 | 84/222 (37.8) | 158/445 (35.5) | 0.049 | 84/193 (43.5) | 164/387 (42.4) | 0.023 |
| Systolic BP, mmHg | 119.1 ± 19.3 | 120.9 ± 16.6 | 0.102 | 119.9 ± 18.6 | 121.7 ± 18.0 | 0.094 |
| Pulse, beats/min | 69.6 ± 10.1 | 70.3 ± 11.3 | 0.064 | 71.5 ± 11.3 | 71.4 ± 12.2 | 0.005 |
| Co‐morbidities | ||||||
| Hypertension | 141/216 (65.3) | 274/427 (64.2) | 0.023 | 107/187 (57.2) | 217/374 (58.0) | 0.016 |
| Diabetes mellitus | 92/223 (41.3) | 178/446 (39.9) | 0.027 | 50/195 (25.6) | 96/390 (24.6) | 0.024 |
| Atrial fibrillation | 13/223 (5.8) | 20/446 (4.5) | 0.062 | 17/195 (8.7) | 24/390 (6.2) | 0.1 |
| Peripheral vascular disease | 42/223 (18.8) | 70/446 (15.7) | 0.084 | 14/195 (7.2) | 33/390 (8.5) | 0.047 |
| Prior stroke or TIA | 29/223 (13.0) | 69/446 (15.5) | 0.07 | 19/195 (9.7) | 43/389 (11.1) | 0.043 |
| Alcohol and smoking | ||||||
| Smoking status | ||||||
| Current smoker | 35/222 (15.8) | 76/445 (17.1) | 0.035 | 38/195 (19.5) | 73/390 (18.7) | 0.02 |
| Former smoker | 148/222 (66.7) | 281/445 (63.1) | 0.073 | 92/195 (47.2) | 177/390 (45.4) | 0.036 |
| Never smoked | 39/222 (17.6) | 88/445 (19.8) | 0.056 | 65/195 (33.3) | 140/390 (35.9) | 0.054 |
| Alcohol consumption | ||||||
| Current consumption, >2 oz/day | 65/223 (29.1) | 130/446 (29.1) | 0 | 48/195 (24.6) | 107/390 (27.4) | 0.064 |
| Previous consumption, >2 oz/day | 71/223 (31.8) | 118/446 (26.5) | 0.12 | 45/195 (23.1) | 89/390 (22.8) | 0.006 |
| Never consumed alcohol | 87/223 (39.0) | 198/446 (44.4) | 0.109 | 102/195 (52.3) | 194/390 (49.7) | 0.051 |
| Status of heart failure | ||||||
| NYHA classification | ||||||
| Class I | 26/223 (11.7) | 64/445 (14.4) | 0.08 | 27/193 (14.0) | 56/389 (14.4) | 0.012 |
| Class II | 123/223 (55.2) | 229/445 (51.5) | 0.074 | 116/193 (60.1) | 235/389 (60.4) | 0.006 |
| Class III | 73/223 (32.7) | 149/445 (33.5) | 0.016 | 48/193 (24.9) | 93/389 (23.9) | 0.022 |
| Class IV | 1/223 (0.4) | 3/445 (0.7) | 0.029 | 2/193 (1.0) | 5/389 (1.3) | 0.023 |
| LVEF, % | 23.3 ± 6.8 | 24.2 ± 7.6 | 0.117 | 24.5 ± 7.3 | 24.3 ± 7.2 | 0.031 |
| LVEF ≤ 20% | 76/223 (34.1) | 139/446 (31.2) | 0.062 | 55/194 (28.4) | 111/390 (28.5) | 0.002 |
| Distance covered on a 6 min walk, m | 353.0 ± 126.6 | 359.3 ± 151.4 | 0.044 | 377.6 ± 124.4 | 377.7 ± 155.4 | 0.001 |
| Baseline MMSE score | 28.5 ± 2.0 | 28.4 ± 2.0 | 0.019 | 28.5 ± 2.0 | 28.6 ± 2.1 | 0.029 |
| Baseline MLWHF score | 37.5 ± 24.3 | 34.2 ± 22.7 | 0.144 | 32.9 ± 22.7 | 32.1 ± 24.7 | 0.033 |
| Medications | ||||||
| Aspirin or other antiplatelet agents | 136/190 (71.6) | 248/344 (72.1) | 0.011 | 104/141 (73.8) | 196/273 (71.8) | 0.044 |
| Warfarin or other oral anticoagulants | 24/223 (10.8) | 37/446 (8.3) | 0.086 | 14/195 (7.2) | 34/390 (8.7) | 0.056 |
| ACE‐Inhibitor | 165/222 (74.3) | 354/446 (79.4) | 0.121 | 165/194 (85.1) | 319/390 (81.8) | 0.086 |
| ARB | 57/221 (25.8) | 91/446 (20.4) | 0.13 | 32/193 (16.6) | 71/390 (18.2) | 0.043 |
| Beta‐blocker | 209/223 (93.7) | 410/446 (91.9) | 0.068 | 181/194 (93.3) | 369/390 (94.6) | 0.056 |
| Aldosterone blocker | 85/146 (58.2) | 157/276 (56.9) | 0.027 | 80/130 (61.5) | 129/229 (56.3) | 0.106 |
| Diuretic | 185/223 (83.0) | 370/446 (83.0) | 0 | 168/194 (86.6) | 339/390 (86.9) | 0.01 |
| Statin | 185/196 (94.4) | 319/352 (90.6) | 0.138 | 118/150 (78.7) | 187/256 (73.0) | 0.13 |
| Lab data | ||||||
| Creatinine, mg/dL | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.124 | 1.2 ± 0.3 | 1.2 ± 0.4 | 0.003 |
| eGFR | 64.5 ± 19.6 | 66.4 ± 19.9 | 0.094 | 69.2 ± 20.7 | 69.7 ± 20.4 | 0.023 |
| Haemoglobin, g/dL | 14.0 ± 1.8 | 14.0 ± 1.6 | 0.024 | 14.1 ± 1.5 | 14.1 ± 1.5 | 0.041 |
| Sodium, mEq/L | 138.2 ± 9.5 | 139.2 ± 3.2 | 0.161 | 139.6 ± 3.4 | 139.5 ± 3.3 | 0.014 |
| WBC count, ×109/L | 7.7 ± 2.0 | 7.7 ± 2.1 | 0.033 | 7.5 ± 2.4 | 7.5 ± 2.2 | 0.019 |
ACE‐Inhibitor, angiotensin‐converting enzyme inhibitor; ARB, Angiotensin II receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; ICD, implantable cardio‐defibrillator; LVEF, left ventricular ejection fraction; MLWHF, Minnesota Living with Heart Failure questionnaire; MMSE, Mini‐Mental Scale Examination; NYHA, New York Heart Association; TIA, transient ischaemic attack; WBC, white blood cell.
Values are mean ± SD or n (%).
Primary outcome: All‐cause death
Kaplan–Meier curves for all‐cause death are presented in Figure 2 . Results from the Cox models are presented in Table 3. There were 279 deaths in the current analysis (205 deaths among those without ICD and 74 deaths among those with ICD). For the 585 patients with NICM in the matched cohort, a total of 102 (17.4%) died during the follow‐up. These include 31 (15.9%) of the 195 patients with ICDs at baseline and 71 (18.2%) of the 390 matched patients who did not have ICDs. ICD status did not predict mortality [hazard ratio (HR): 0.984; 95% confidence interval (CI): 0.641 to 1.509; P = 0.941].
Figure 2.
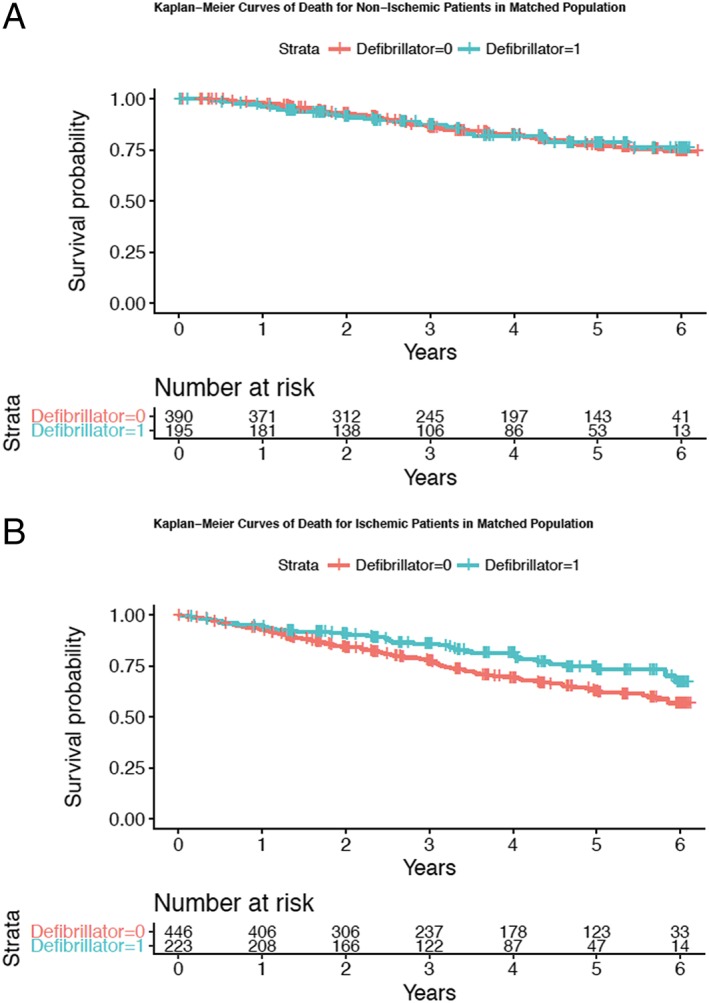
Kaplan–Meier curves showing the survival of those with and without an implantable cardioverter‐defibrillator: (A) patients with non‐ischaemic heart failure and (B) patients with ischaemic heart failure.
Table 3.
Hazard ratio to assess the effect of ICD status on mortality for those with ICM and with NICM based on the matched sample
| Patients with ICM | Patients with NICM | Interaction P value | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| All‐cause death | 0.640 (0.448–0.915) | 0.015 | 0.984 (0.641–1.509) | 0.941 | 0.131 |
| Cardiovascular death | 0.713 (0.473–1.075) | 0.107 | 0.967 (0.578–1.618) | 0.898 | 0.365 |
| Sudden death | 0.673 (0.385–1.176) | 0.164 | 0.246 (0.077–0.781) | 0.017 | 0.124 |
CI, confidence intervals; ICD, implantable cardioverter‐defibrillator; ICM, ischaemic cardiomyopathy; NICM, non‐ischaemic cardiomyopathy.
Of the 669 patients with ICM in the matched cohort, 177 (26.5%) died during follow‐up. These included 43 (19.3%) of the 223 patients with ICDs at baseline and 134 (30.0%) of the 446 matched patients who did not have ICDs. Having an ICD at baseline was significantly associated with lower mortality (HR: 0.640; 95% CI: 0.448 to 0.915; P = 0.015). There was a weak evidence of interaction between ICD status and the aetiology of HF (P = 0.131).
Secondary outcomes: Cardiovascular and sudden death
Of 279 deaths, a total of 184 (65.9%) were classified as cardiovascular (133 deaths among patients without ICD and 51 deaths among those with ICD). The relation between ICD status and cardiovascular mortality was similar to its relation with all‐cause mortality, though it did not achieve statistical significance in either subgroups (for ICM, HR: 0.713, 95% CI: 0.473 to 1.075, P = 0.107; for NICM, HR: 0.967, 95% CI: 0.578 to 1.618; P = 0.898; interaction P = 0.365).
A total of 97 deaths were classified as sudden (77 deaths among those without ICD and 20 deaths among those with ICD). The association between having ICD and sudden death was not significant among patients with ICM but was significant among patients with NICM (for ICM, HR: 0.673, 95% CI: 0.385 to 1.176, P = 0.164; patients with NICM, HR: 0.246, 95% CI: 0.077 to 0.781; P = 0.017; interaction P = 0.124).
Discussion
In this retrospective, propensity‐matched analysis of the WARCEF trial, we found that the presence of ICD at baseline was associated with a lower risk of all‐cause death among those with ICM but not among those with NICM. There was weak evidence of an interaction effect for ICD status and whether a patient had NICM or ICM. These findings are consistent with prior studies showing that the benefit of ICDs is more pronounced in patients with ICM, but ICDs may be less effective in patients with NICM.
Our findings are broadly consistent with the recent literature6, 12 suggesting that the effect of ICD may be attenuated in patients with NICM. Compared with ICM, NICM is known to be associated with a better left ventricular reverse remodelling,18 a better clinical outcome,19 and a lower background risk for ventricular arrhythmia.20, 21 Besides these differences between ICM and NICM, the evidence for the primary prevention of ICD among patients with NICM is less robust.4, 5, 6 The recent DANISH trial, which randomized 1116 patients with NICM to receive conventional therapy or an ICD, demonstrated no significant association between the presence of ICD and all‐cause mortality among NICM patients (HR: 0.87; 95% CI: 0.68 to 1.12; P = 0.28). Similarly to the WARCEF population, the rates of optimal medical therapy in DANISH trial participants were high. Studies have consistently shown that the optimal medical therapies, such as ACE‐Inhibitors,22 ARBs,23 beta‐blockers,24 mineralocorticoid‐receptor antagonists,25 or combinations of these medications,26 all reduce the rate of cardiovascular death or sudden death. It is, therefore, possible that sudden death in medically optimized HF patients may be less common, leading to diminished benefit from ICDs.
It may be surprising that in our analysis, ICD status predicted mortality in patients with ICM, but its association on sudden death was not found to be statistically significant. Conversely, ICD status did not predict mortality in patients with NICM in our analysis but did have a strong association with sudden death. However, these results regarding sudden death must be interpreted with caution, as the adjudication in WARCEF was not blinded to defibrillator status, and the number of sudden death events was small. In addition, the DANISH trial6 has shown that in the populations with NICM in which ICDs did not reduce overall mortality, ICDs may nonetheless have an apparent effect on sudden death (HR: 0.50; 95% CI: 0.31 to 0.82; P = 0.005).
Despite our findings, there remains uncertainty regarding the current recommendation for ICD implantation in patients with NICM. Subsequent analysis of the DANISH trial has shown that ICD implantation may be beneficial in NICM patients younger than 70 years of age.27 Compared with the DANISH trial in which the median age was 63 years old, NICM patients in the WARCEF trial were considerably younger, with a median age of 58 years old. It is therefore unclear if age or other clinical risk predictors might be helpful in guiding decision for ICD implantation in patients with NICM. Furthermore, statistical power remains a concern for both the DANISH trial and our analysis, as recent meta‐analyses continue to suggest a survival benefit for ICD implantation among patients with NICM7, 8, 9, 10, 11 even after the inclusion of the DANISH trial. Nonetheless, differences in patient clinical characteristics and contemporary background medical therapy may affect the validity of these findings, and thereby, future studies with carefully defined patient selection criteria will be needed to clarify whether ICD implantation can be beneficial in subgroups of patients with NICM.28
Limitations
First, our study was retrospective and therefore necessarily hypothesis generating. ICD status was not randomly assigned, which raised the potential for indication bias. To address this, we used a propensity‐matching approach, which can address confounding from known baseline covariates. However, residual confounding may still be present, because the decision to place an ICD is often nuanced and incorporates additional unmeasured patient clinical and psychosocial characteristics. Second, past medical history of cardiac arrest was not recorded at baseline, and it is possible that a small number of patients received ICDs for secondary prevention. However, these patients are more likely to benefit from ICDs,29 which therefore would not affect our null finding for patients with NICM. Third, we did not collect detailed information on CRT status at baseline, or whether patients received ICD and/or CRT during follow‐up. We also did not collect electrocardiogram data such as QRS duration or presence of left bundle branch block. We therefore were not able to control for these potential confounders. Fourth, WARCEF was limited to patients in sinus rhythm at baseline, which may limit the external validity of our analysis. Fifth, our propensity matching led to exclusion of more than half of the patients with NICM from the current analysis, and our findings therefore must be interpreted cautiously.
Conclusions
In this propensity‐matched, retrospective analysis of data from the WARCEF trial, we found that the presence of ICD at baseline conveyed a survival benefit in those with ICM but not in those with NICM. Our results are consistent with previous literature demonstrating that ICDs are beneficial in patients with ICM and corroborated the results of the DANISH trial, which suggested that beneficial effects of ICDs are attenuated in patients with NICM.
Conflict of interest
S.D.A. reports being a consultant for Bayer, Boehringer Ingelheim, Novartis, Stealth Peptides, Servier, Vifor, and Janssen (all for trial/registry steering committee work), and he has received research grants from Abbott Vascular and Vifor. S.H. reports being a consultant for St. Jude Medical, Daiichi Sankyo, Bristol‐Myers Squibb, and Pfizer. A.J.L. has received a research grant from Bristol‐Myers Squibb/Pfizer for the AREST trial. G.Y.H.L. has served as a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo and speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo. No fees are directly received personally. R.L.S. has received research grants from NINDS, NCATS, AHA, Evelyn McKnight Brain Foundation, and Boehringer Ingelheim. J.R.T. has received consulting fees/research grants from Actelion, Amgen, Bayer, Cytokinetics, Medtronic, Novartis, St. Jude Medical, and Trevena. The other authors have no conflicts of interest to report.
Funding
The WARCEF trial was supported by grants [U01‐NS‐043975 (to S.H.) and U01‐NS‐039143 (to J.L.P.T.)] from the National Institute of Neurological Disorders and Stroke. S.Y. is supported by a National Institutes of Health K23 career development award (K23‐HL‐121144). T.C.L. is supported by a grant from the Abe Fellowship Program administered by the Social Science Research Council and in cooperation with and with funds provided by the Japan Foundation Center for Global Partnership.
Supporting information
Appendix S1. Supporting information.
Lee T. C., Qian M., Mu L., Di Tullio M. R., Graham S., Mann D. L., Nakanishi K., Teerlink J. R., Lip G. Y. H., Freudenberger R. S., Sacco R. L., Mohr J. P., Labovitz A. J., Ponikowski P., Lok D. J., Estol C., Anker S. D., Pullicino P. M., Buchsbaum R., Levin B., Thompson J. L. P., Homma S., Ye S., and for the WARCEF Investigators (2019) Association between mortality and implantable cardioverter‐defibrillators by aetiology of heart failure: a propensity‐matched analysis of the WARCEF trial, ESC Heart Failure, 6: 297–307. 10.1002/ehf2.12407.
The investigators in the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Study Group are listed in the Supporting Information, Appendix S1 .
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation , American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial III . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial I . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 5. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH, Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation I . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350: 2151–2158. [DOI] [PubMed] [Google Scholar]
- 6. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp‐Pedersen C, Pehrson S, Investigators D. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Khatib SM, Fonarow GC, Joglar JA, Inoue LYT, Mark DB, Lee KL, Kadish A, Bardy G, Sanders GD. Primary prevention implantable cardioverter defibrillators in patients with nonischemic cardiomyopathy: a meta‐analysis. JAMA Cardiol 2017; 2: 685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golwala H, Bajaj NS, Arora G, Arora P. Implantable cardioverter‐defibrillator for nonischemic cardiomyopathy: an updated meta‐analysis. Circulation 2017; 135: 201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolodziejczak M, Andreotti F, Kowalewski M, Buffon A, Ciccone MM, Parati G, Scicchitano P, Uminska JM, De Servi S, Bliden KP, Kubica J, Bortone A, Crea F, Gurbel P, Navarese EP. Implantable cardioverter‐defibrillators for primary prevention in patients with ischemic or nonischemic cardiomyopathy: a systematic review and meta‐analysis. Ann Intern Med 2017; 167: 103–111. [DOI] [PubMed] [Google Scholar]
- 10. Stavrakis S, Asad Z, Reynolds D. Implantable cardioverter defibrillators for primary prevention of mortality in patients with nonischemic cardiomyopathy: a meta‐analysis of randomized controlled trials. J Cardiovasc Electrophysiol 2017; 28: 659–665. [DOI] [PubMed] [Google Scholar]
- 11. Shun‐Shin MJ, Zheng SL, Cole GD, Howard JP, Whinnett ZI, Francis DP. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta‐analysis of 8567 patients in the 11 trials. Eur Heart J 2017; 38: 1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barra S, Boveda S, Providencia R, Sadoul N, Duehmke R, Reitan C, Borgquist R, Narayanan K, Hidden‐Lucet F, Klug D, Defaye P, Gras D, Anselme F, Leclercq C, Hermida JS, Deharo JC, Looi KL, Chow AW, Virdee M, Fynn S, Le Heuzey JY, Marijon E, Agarwal S, French UKSCRTN. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J Am Coll Cardiol 2017; 69: 1669–1678. [DOI] [PubMed] [Google Scholar]
- 13. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R, Investigators W. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012; 366: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol 2001; 2: 169–188. [Google Scholar]
- 15. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79: 516–524. [Google Scholar]
- 17. Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42: 1–28. [Google Scholar]
- 18. Follath F, Cleland JG, Klein W, Murphy R. Etiology and response to drug treatment in heart failure. J Am Coll Cardiol 1998; 32: 1167–1172. [DOI] [PubMed] [Google Scholar]
- 19. Bart BA, Shaw LK, McCants CB Jr, Fortin DF, Lee KL, Califf RM, O'Connor CM. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997; 30: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 20. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998; 98: 2334–2351. [DOI] [PubMed] [Google Scholar]
- 21. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001; 345: 1473–1482. [DOI] [PubMed] [Google Scholar]
- 22. Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta‐analysis of randomized clinical trials. J Am Coll Cardiol 1999; 33: 598–604. [DOI] [PubMed] [Google Scholar]
- 23. Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause‐specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2004; 110: 2180–2183. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Gobari M, El Khatib C, Pillon F, Gueyffier F. Beta‐blockers for the prevention of sudden cardiac death in heart failure patients: a meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord 2013; 13: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bapoje SR, Bahia A, Hokanson JE, Peterson PN, Heidenreich PA, Lindenfeld J, Allen LA, Masoudi FA. Effects of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with left ventricular systolic dysfunction: a meta‐analysis of randomized controlled trials. Circ Heart Fail 2013; 6: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining risk of sudden death in heart failure. N Engl J Med 2017; 377: 41–51. [DOI] [PubMed] [Google Scholar]
- 27. Elming M, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, Olesen L, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen J, Høfsten DE, Torp‐Pedersen C, Pehrson S, Køber L, Thune J. Age and outcomes of primary prevention implantable cardioverter‐defibrillators in patients with nonischemic systolic heart failure. Circulation 2017; 136: 1772–1780. [DOI] [PubMed] [Google Scholar]
- 28. Leyva F, Zegard A, Acquaye E, Gubran C, Taylor R, Foley PWX, Umar F, Patel K, Panting J, Marshall H, Qiu T. Outcomes of cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 2017; 70: 1216–1227. [DOI] [PubMed] [Google Scholar]
- 29. Santini M, Lavalle C, Ricci R. Primary and secondary prevention of sudden cardiac death: who should get an ICD? Heart 2007; 93: 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


