Abstract
Aims
In the Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients study, a novel algorithm for heart failure (HF) monitoring was implemented. The HeartLogic (Boston Scientific) index combines data from multiple implantable cardioverter defibrillator (ICD)‐based sensors and has proved to be a sensitive and timely predictor of impending HF decompensation. The remote monitoring of HF patients by means of HeartLogic has never been described in clinical practice. We report post‐implantation data collected from sensors, the combined index, and their association with clinical events during follow‐up in a group of patients who received a HeartLogic‐enabled device in clinical practice.
Methods and results
Patients with ICD and cardiac resynchronization therapy ICD were remotely monitored. In December 2017, the HeartLogic feature was activated on the remote monitoring platform, and multiple ICD‐based sensor data collected since device implantation were made available: HeartLogic index, heart rate, heart sounds, thoracic impedance, respiration, and activity. Their association with clinical events was retrospectively analysed. Data from 58 patients were analysed. During a mean follow‐up of 5 ± 3 months, the HeartLogic index crossed the threshold value (set by default to 16) 24 times (over 24 person‐years, 0.99 alerts/patient‐year) in 16 patients. HeartLogic alerts preceded five HF hospitalizations and five unplanned in‐office visits for HF. Symptoms or signs of HF were also reported at the time of five scheduled visits. The median early warning time and the time spent in alert were longer in the case of hospitalizations than in the case of minor events of clinical deterioration of HF. HeartLogic contributing sensors detected changes in heart sound amplitude (increased third sound and decreased first sound) in all cases of alerts. Patients with HeartLogic alerts during the observation period had higher New York Heart Association class (P = 0.025) and lower ejection fraction (P = 0.016) at the time of activation.
Conclusions
Our retrospective analysis indicates that the HeartLogic algorithm might be useful to detect gradual worsening of HF and to stratify risk of HF decompensation.
Keywords: ICD, CRT, Decompensation, Heart failure, Telemedicine
Introduction
Implantable cardioverter defibrillators (ICDs) and defibrillators together with cardiac resynchronization therapy (CRT‐Ds) have been demonstrated to improve the outcome of selected heart failure (HF) patients and has been included in the current guidelines for the management of chronic HF.1 Modern cardiac devices enable patients' data to be accessed through remote monitoring systems. Moreover, devices can continuously monitor the integrity of the implanted device, as well as measure clinical variables, thus potentially providing early warning of safety issues or changes in clinical status. Many studies have investigated the ability of ICD diagnostics to identify patients at risk of HF events, with contradictory results.2, 3, 4, 5, 6 In the Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients (MultiSENSE) study,7 a novel algorithm for HF monitoring was implemented. The HeartLogic (Boston Scientific, St. Paul, Minnesota) index combines data from multiple ICD‐based and CRT‐D‐based sensors and has proved to be a sensitive and timely predictor of impending HF decompensation.
We report the first experience with HeartLogic‐enabled ICDs in clinical practice. Data were collected post‐implantation from sensors, as was the combined index; their association with clinical events during follow‐up was evaluated.
Methods
In December 2017, the HeartLogic feature was made available on the LATITUDE (Boston Scientific) remote monitoring platform. At the study centres, HeartLogic was activated in all patients who had received a HeartLogic‐enabled ICD or CRT‐D device (RESONATE family, Boston Scientific) and were enrolled in LATITUDE for remote monitoring. On the first remote data review after activation, the full report of automatic diagnostics was available. This included sensor data collected since device implantation and the composite HeartLogic index.
Heart sound trends, HeartLogic index and alert were not available before their activation with consequent previous unavailability of any clinical related reaction. Data on the clinical events that had occurred since implantation were collected at the study centres in the framework of a prospective registry and were retrieved by operators blinded to the ICD diagnostics. The Institutional Review Boards approved the study, and all patients provided written informed consent for data storage and analysis.
Sensor data and HeartLogic index
The HeartLogic algorithm combines data from multiple sensors: accelerometer‐based first and third heart sounds, intrathoracic impedance, respiration rate, the ratio of respiration rate to tidal volume, night heart rate, and patient activity. Each day the device calculates the degree of worsening in sensors from their moving baseline and computes a composite index. As initialization is required, the HeartLogic index does not become available until 30–37 days after data collection begins. An alert is issued when the index crosses a programmable threshold (nominal value 16). When the index enters into an alert state, the threshold is automatically dropped to a recovery value (nominal value 6).
Objectives
The objective of the study was to measure the rate of HeartLogic threshold crossings and the time spent in the alert state. We also evaluated the association between HeartLogic alerts and clinical events.
Statistical analysis
To analyse the association between alerts and clinical events, we used the same criteria adopted in the MultiSENSE study7; that is, alerts were classified as true positive if the alert began before a clinical event and did not reset earlier than 30 days before the event. Moreover, we included in analysis HF events if they occurred at least 45 days after the initiation of sensor data collection (period required to establish a sensor baseline). For the analysis of the sensed parameters that contribute to the calculation of the HeartLogic index, the device data were downloaded, dates of nominal alert threshold crossings were identified, and worsening indices of six data trends on those dates were extracted: third heart sound amplitude (S3), third/first heart sound amplitude ratio (S3/S1), thoracic impedance, median respiratory rate, ratio of respiration rate to tidal volume (rapid shallow breathing index), and night heart rate. Descriptive statistics are reported as means ± standard deviation for normally distributed continuous variables or medians with range in the case of skewed distribution. Normality of distribution was tested by means of the non‐parametric Kolmogorov–Smirnov test. Differences between mean data were compared by means of a t‐test for Gaussian variables, using the F‐test to check the hypothesis of equality of variance. The Mann–Whitney non‐parametric test was used to compare non‐Gaussian variables. Differences in proportions were compared by applying χ2 analysis or Fisher's exact test, as appropriate. A P‐value < 0.05 was considered significant for all tests. All statistical analyses were performed by means of STATISTICA software, version 7.1 (StatSoft, Inc., Tulsa, OK).
Results
From December 2017 to April 2018, HeartLogic was activated in 67 patients who had received an ICD or CRT‐D since April 2017. At the time of activation, HeartLogic initialization had been completed in 58 patients, and daily index values were available for analysis over a mean observation period of 5 ± 3 months (a total of 24 person‐years). The HeartLogic index crossed the threshold value (set by default to 16) 24 times (0.99 alerts/patient‐year) in 16 patients. Overall, the time in the alert state (i.e. the number of weeks when the HeartLogic index was above the threshold) was 153 weeks (12% of the total observation period). Table 1 shows the baseline clinical variables of all patients in analysis and of the groups with and without HeartLogic alerts during the observation period.
Table 1.
Demographics and baseline clinical parameters of the study population and of the groups with and without HeartLogic alerts during the observation period
| Parameter |
Total N = 58 |
Alerts N = 16 |
No alerts N = 42 |
P |
|---|---|---|---|---|
| Male gender, n (%) | 47 (81) | 13 (81) | 34 (80) | 0.980 |
| Age, years | 71 ± 9 | 72 ± 11 | 70 ± 9 | 0.773 |
| Ischaemic aetiology, n (%) | 21 (37) | 7 (44) | 14 (33) | 0.461 |
| QRS duration, ms | 153 ± 25 | 147 ± 24 | 156 ± 25 | 0.340 |
| NYHA class | ||||
| Class I, n (%) | 2 (4) | 1 (6) | 1 (2) | 0.025 |
| Class II, n (%) | 29 (50) | 4 (25) | 26 (62) | |
| Class III, n (%) | 26 (44) | 11 (69) | 14 (33) | |
| Class IV, n (%) | 1 (2) | 0 (0) | 1 (2) | |
| LV ejection fraction, % | 30 ± 8 | 26 ± 6 | 31 ± 8 | 0.016 |
| AF history, n (%) | 23 (40) | 9 (56) | 14 (34) | 0.111 |
| Diabetes, n (%) | 18 (30) | 9 (53) | 9 (22) | 0.010 |
| COPD, n (%) | 9 (17) | 3 (21) | 6 (16) | 0.675 |
| Chronic kidney disease, n (%) | 14 (24) | 6 (36) | 8 (20) | 0.142 |
| Hypertension, n (%) | 48 (82) | 13 (80) | 35 (83) | 0.851 |
| β‐Blocker use, n (%) | 55 (94) | 13 (84) | 42 (100) | 0.004 |
| ACE‐inhibitor use, n (%) | 32 (55) | 10 (62) | 22 (53) | 0.489 |
| Diuretic use, n (%) | 53 (92) | 16 (100) | 37 (87) | 0.149 |
| Antiarrhythmic use, n (%) | 10 (17) | 3 (18) | 7 (16) | 0.851 |
| Ivabradine use, n (%) | 6 (10) | 0 (0) | 6 (14) | 0.110 |
| Primary prevention, n (%) | 55 (95) | 14 (87) | 41 (97) | 0.120 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; LV, left ventricular; NYHA, New York Heart Association.
Clinical events
During the observation period, five hospitalizations in three patients with a primary or secondary diagnosis of HF and requiring at least one overnight stay occurred in the study population (rate: 0.21 per patient‐year) (Table 2) (Figure 1). In addition, five unplanned in‐office visits in three patients occurred for HF, that is, symptoms (dyspnoea, fatigue, etc.) and signs of clinical deterioration of HF (e.g. pulmonary congestion and increased body weight). Symptoms or signs of clinical deterioration of HF were also reported at the time of five scheduled visits in five patients. In all these cases, the HeartLogic index crossed the threshold before the event and recovered after HF treatment or restoration of therapy (in one patient, the index crossed the threshold before two consecutive hospitalizations, separated by 49 days, remained above the recovery value between the two events, and finally recovered after the second discharge) (Figure 2, 3, 4, 5).
Table 2.
Clinical events that occurred during the observation period
| Event reported | Early warninga (days) | Time from event to crossingc (days) | Time in alert state (days) | Maximum HeartLogic index value | Sensors with worsening on the day of the alert threshold crossing | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S3 | S3/S1 | TI | RR | RSBI | NHR | |||||
| 1. HF hospitalization | b | — | 54 | 28 | NA | NA | x | x | x | x |
| 2. HF hospitalization | 8 | — | 71 | 43 | x | x | ||||
| 3. HF hospitalizationd | 58 | — | 71 | 22 | x | x | ||||
| 4. HF hospitalization | 69 | — | 70 | 40 | x | x | ||||
| 5. Hospitalization for pleuritis (secondary diagnosis HF) | 17 | — | 61 | 40 | x | x | x | |||
| 6. Patient‐reported weight gain | 28 | — | 40 | 18 | x | x | x | |||
| 7. Patient‐reported weight gain | 1 | — | 18 | 22 | x | x | x | |||
| 8. Patient‐reported weight gain and worsened dyspnoea | 15 | — | 52 | 38 | x | x | ||||
| 9. Patient‐reported fatigue and worsened dyspnoea | 1 | — | 16 | 19 | x | x | x | x | ||
| 10. Patient‐reported worsened nocturnal dyspnoea | 0 | — | 8 | 16 | x | x | x | x | ||
| 11. Large increase in BNP measured at scheduled visit | 14 | — | 61 | 25 | x | x | x | x | ||
| 12. Large increase in BNP measured at scheduled visit | 42 | — | 139 | 31 | x | x | x | x | ||
| 13. Worsened HF signs at scheduled visit | 0 | — | 46 | 22 | x | x | x | x | ||
| 14. Worsened HF signs at scheduled visit | 20 | — | 31 | 39 | x | x | x | NA | NA | x |
| 15. Patient‐reported weight gain after reduction of diuretic dose | 10 | 11 | 31 | 25 | x | x | x | x | ||
| 16. Reduction of diuretic dose (no symptoms) | — | 60 | 38 | 20 | x | x | x | |||
| 17. Reduction of diuretic dose (no symptoms) | — | 54 | 15 | 30 | x | x | x | x | x | |
| 18. Reduction of diuretic dose (no symptoms) | — | 5 | 24 | 20 | x | x | x | |||
| 19. Reduction of diuretic dose (no symptoms) | — | 20 | 22 | 30 | x | x | x | x | x | |
| 20. Discontinuation of ACE‐inhibitor therapy (no symptoms) | — | 33 | 31 | 36 | x | x | x | x | x | x |
| 21. New‐onset atrial fibrillation | — | 37 | 133 | 39 | x | x | x | x | ||
| 22. Change in activity and sleep habits | — | 20 | 30 | 17 | x | x | x | |||
| 23. Discontinuation of multi‐site LV pacing for PNS | — | 6 | 15 | 16 | x | x | ||||
| 24. Twiddler's syndrome | — | 27 | 17 | 24 | x | x | x | x | ||
| 25. Right ventricular lead dislodgement | — | 0 | 7 | 18 | x | x | x | |||
ACE, angiotensin‐converting enzyme; BNP, brain natriuretic peptide; HF, heart failure; LV, left ventricular; NHR, night heart rate; PNS, phrenic nerve stimulation; RR, median respiratory rate; RSBI, rapid shallow breathing index; S3, third heart sound amplitude; S3/S1, third/first heart sound amplitude ratio; TI, thoracic impedance.
After the first hospitalization, the index remained above the recovery threshold and rose further at the time of the worsening of conditions that led to the second admission. The early warning is calculated starting from the same crossing, while the maximum index is the value reached at the time of the second hospitalization. The index finally recovered after the second discharge.
Time between HeartLogic crossing and event.
Admission within 45 days of implantation (before sensor baseline establishment).
Time between trigger event and HeartLogic crossing.
HF hospitalizations no. 2 and no. 3 occurred consecutively in the same patient.
Figure 1.
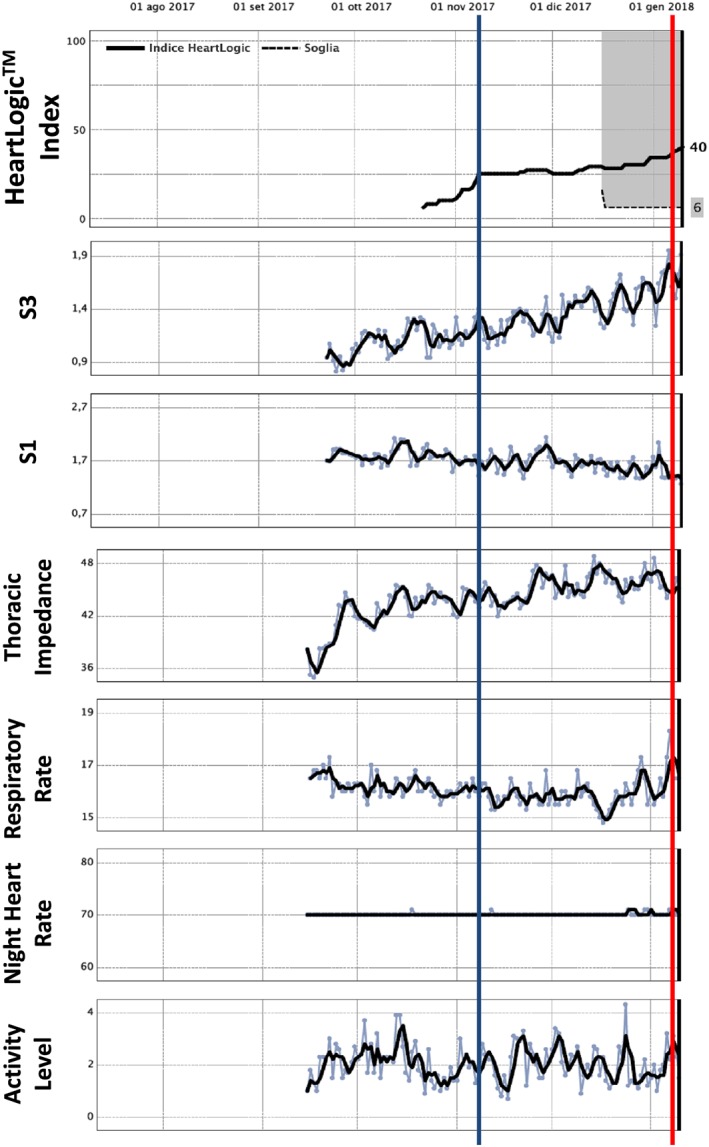
A 65‐year‐old male with non‐ischaemic dilated cardiomyopathy, left bundle brunch block, and paroxysmal atrial fibrillation was implanted with a cardiac resynchronization therapy defibrillator in September 2017. After dismission, the patient was hospitalized again on 11 January 2018 (red line) for severe heart failure. At endovascular catheterization, an elevated left ventricular filling pressure was found. The retrospective HeartLogic index evaluation did show a previous number 16 crossing already on 2 November 2017 (blue line). Thus, an early warning for heart failure development did appear already 70 days before symptoms appearance that was mainly due to heart sounds (third, first) intensity modification. Patient died on 17 January 2018 despite resuscitation attempts.
Figure 2.

A 74‐year‐old man with non‐ischaemic dilated cardiomyopathy, 22% ejection fraction, left bundle brunch block, and persistent atrial fibrillation was implanted with a cardiac resynchronization therapy defibrillator for primary prevention on May 2017 (Table 2, event 15). During follow‐up on 1 November 2017 (red line), he discontinued diuretic therapy. At a subsequent in‐office medical control on 4 December 2018, he reported weight gain of 4 kg within 7 days; therefore, diuretic therapy was restored (green line). HeartLogic index analysis showed crossing of the alarm threshold value already on 25 November 2017 (blue line) with thus an early warning 10 days in advance compared with clinical evaluation. That index normalized after therapy restoration. Main sensor contributing were heart sounds and thoracic impedance.
Figure 3.
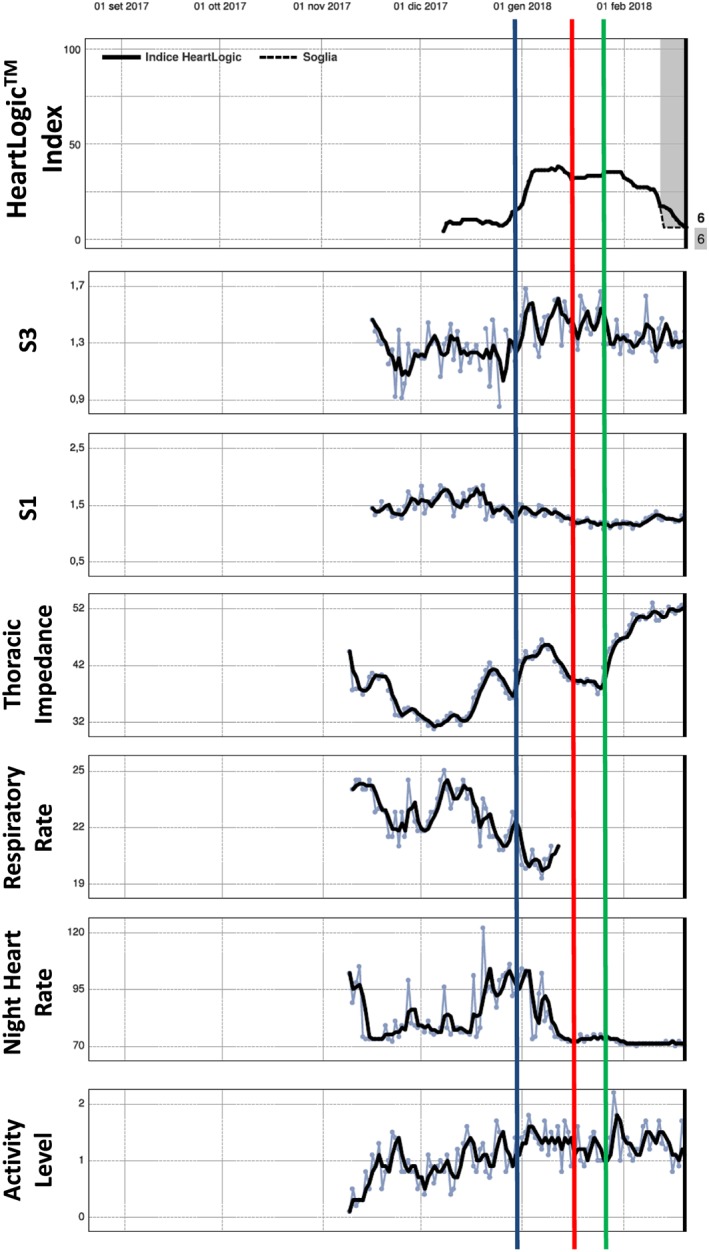
A 70‐year‐old man with ischaemic dilated cardiomyopathy, 30% ejection fraction, and permanent atrial fibrillation was implanted with a cardiac resynchronization therapy defibrillator for primary prevention in November 2017 (Table 2, event 8). In 20 December, the night heart rate was very high (122 b.p.m.); the rate persisted high (90/min) even in the following weeks. In 10 January 2018 at an outpatient routine control, the therapy was revisited in order to improve the rate control, thus allowing a better percentage of biventricular pacing. Night heart rate consequently lowered in the following days. On 15 January, patient reported rest dyspnoea (red line); therefore, on 25 January, diuretic dosages were increased (green line). Looking retrospectively to the HeartLogic index, we saw a value above 16 already on 31 December (blue line), thus giving a 15 day warning prior to symptoms. Contemporary to the night heart rate increment, there were also drops in S1 and S3 sound elevation. Index improved after potentiation of diuretics.
Figure 4.
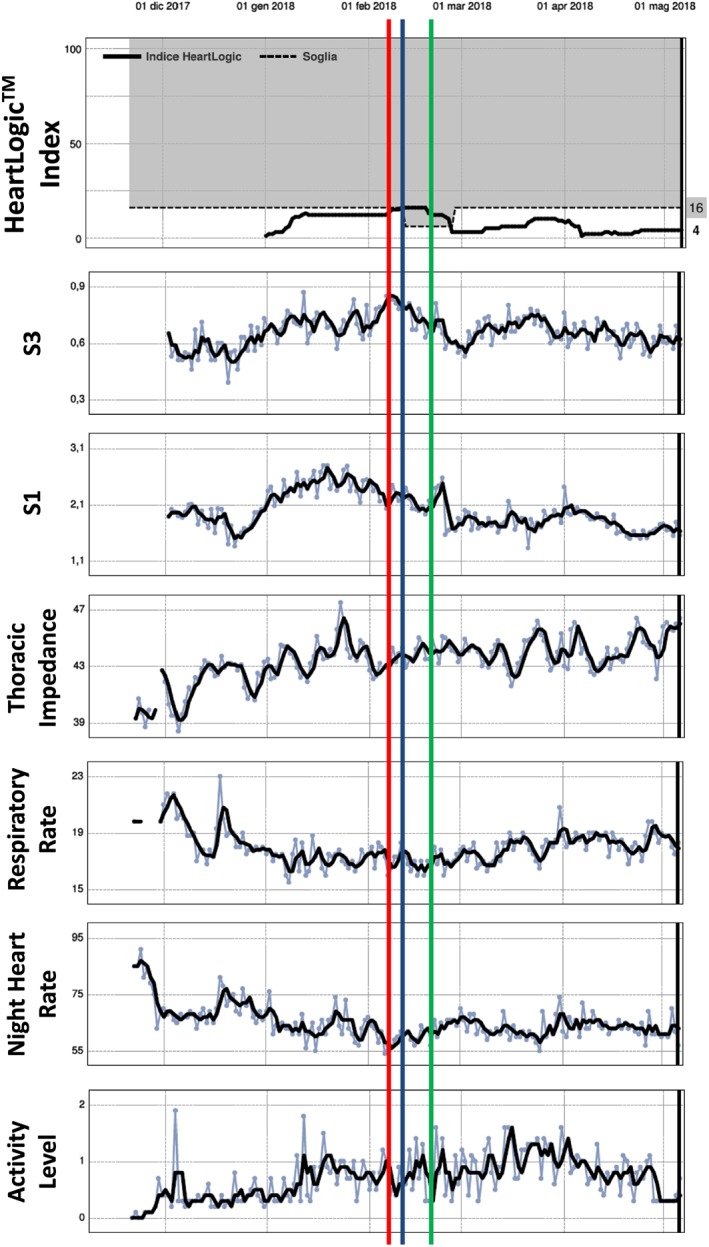
A 78‐year‐old man with ischaemic cardiomyopathy and 26% ejection fraction underwent a cardiac resynchronization therapy defibrillator implantation for primary prevention in December 2017 (Table 2, event 23). He complained of phrenic nerve stimulation‐related symptoms on 2 February 2018; therefore, the multi‐site ventricular stimulation was turned off. On 11 February, HeartLogic index crossed the 16 value (blue line). On 23 February at a subsequent in‐office control, a suitable new pacing stimulation mode was settled and the multi‐site pacing was restored (green line). As a consequence of a better ventricular stimulation setting, the index decreased to below 6. Heart sounds were the main contributing sensors.
Figure 5.
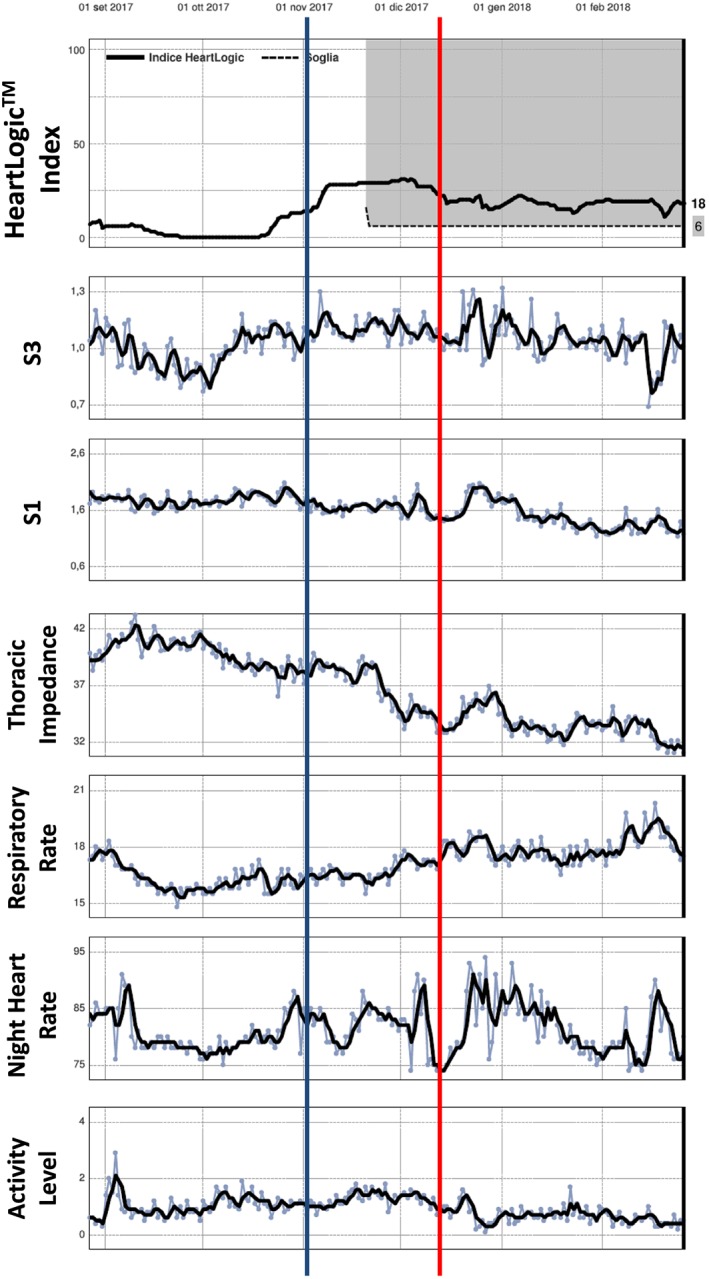
A 70‐year‐old male with ischaemic heart disease, 26% ejection fraction, and persistent atrial fibrillation was implanted with a cardiac resynchronization therapy defibrillator for primary prevention in April 2017 (Table 2, event 12). At a scheduled medical control on 18 December, he reported worsening of heart failure symptoms (red line). The NT‐proBNP was very high (8619 pg/mL), and the diuretic dosages were increased. The patient was poorly compliant, and he modified the dosages only by the end of February 2018. HeartLogic index crossed the 16 value already on 1 November (blue line) that means 45 days before symptoms appearance. The long persistence of high values was possibly related to the delay in therapy adjustment. All heart sounds, thoracic impedance, respiratory rate, and night heart rate contributed to the HeartLogic index behaviour.
The median early warning time (i.e. the time between threshold crossing and the event) was 38 days (25th–75th percentile, 15 to 61 days) in the case of hospitalizations and 12 days (25th–75th percentile, 1 to 19 days) in that of minor events reflecting clinical deterioration of HF. The median time spent in the alert state was 70 days (25th–75th percentile, 61 to 71 days) in the case of hospitalizations and 36 days (25th–75th percentile, 21 to 51 days) in that of minor HF events. The maximum HeartLogic index value was 40 (25th–75th percentile, 28 to 40) in the case of hospitalizations and 24 (25th–75th percentile, 20 to 30) in that of minor HF events.
During follow‐up, 10 additional HeartLogic threshold crossings were reported in seven patients. No clinical events were detected after these warnings. Thus, according to the definition adopted in the MultiSENSE study, these could be regarded as unexplained alerts. Their rate would be 0.41 per patient‐year, and the proportion of alerts that were positively associated with HF events, that is, positive predictive value, would be 58% (14/24). However, analysis of these patients' charts revealed that these 10 alerts had occurred after discontinuation or reduction of prescribed HF therapy (n = 5) or after events with a direct impact on clinical status or on sensor data collection (Table 2). The median time from the trigger event to the HeartLogic alert threshold crossing was 20 days (25th–75th percentile, 9 to 35 days). The median alert time was 24 days (25th–75th percentile, 22 to 31 days), and the maximum HeartLogic index value was 24 (25th–75th percentile, 19 to 30).
The HeartLogic sensors detected changes in the amplitude of heart sounds (increase in the third sound and decrease in the first sound) in all cases of warnings; worsening of respiratory rate or rapid shallow breathing was measured for 12 alerts, decreased thoracic impedance for 11 alerts, and increased night heart rate for 15 alerts.
Discussion
In the present report, we describe the first experience of the use of HeartLogic in clinical practice. The strong association between HeartLogic alerts and HF‐related clinical events in our study is consistent with the high sensitivity in early detection of worsening HF demonstrated in the validation phase of the MultiSENSE study.7
In our blinded analysis, we found a rate of about one alert per patient‐year with a nominal threshold value of 16. Overall, in our series, the time spent in the alert state was 12% of the total observation period. For comparison, in the MultiSENSE study, HeartLogic had sensitivity of 70%, with a median early warning of 34 days before the HF events, an unexplained alert rate of 1.47 per patient‐year at the nominal threshold, and the time spent in alert state of 17% of the total observation period. In that study, HF events included hospitalizations with a primary cause of worsening HF. Nonetheless, additional occurrences of worsening HF were picked up by clinicians, and corrective therapies were delivered (e.g. oral medication changes) that mitigated more severe events. The alerts associated with these corrective HF therapies were considered to be HF‐related alerts, because the patients' HF decline was appropriately detected and the alerts were consistent with the intended behaviour and use of the algorithm. Similarly, in the present study, HeartLogic alerts were associated not only with major HF hospitalizations but also with initial signs (e.g. body weight increase) or symptoms of HF.
HF hospitalization rate in this study was 0.21 per patient‐year and is consistent with event rates observed in previous device studies.2, 3 According to our analysis, the early warning window was longer in cases of major HF hospitalizations. This suggests that there is disease progression that may finally result in a fully decompensated status. In contrast, when patients or physicians could identify early worsening of HF by detecting initial signs or symptoms, prompt management prevented further worsening; this resulted in shorter duration of the alert state and was associated with a lower maximum HeartLogic index value. Presumably, the availability of the HeartLogic alert, if it had been active at the time of the events, would have allowed clinical decompensation to be detected even earlier. However, the potential benefits of a preventive treatment approach based on the HeartLogic alerts alone are still under investigation.8 In our cases, we recorded the recovery of the HeartLogic index after HF treatment or restoration of an interrupted therapy, together with positive changes in the contributing signals. This further confirms relevance of HeartLogic to monitoring HF status.
During follow‐up, we also recorded HeartLogic alerts that occurred after discontinuation or decrease of a prescribed HF therapy. In these cases, except for one patient who complained of weight gain, no signs or symptoms of HF were reported, and the therapy was restored as soon as the lack of compliance with the physician's prescription had been noticed. Indeed, in these cases in which therapy was restored before signs or symptoms of HF occurred, the alert time was short, and HeartLogic index values were low. We can therefore hypothesize that enabling the HeartLogic alert would result in an even prompter medical reaction and more stable patient conditions. Nonetheless, even if these events were classified as unexplained alerts, according to the strict definition adopted in the MultiSENSE study, their rate would be 0.41 per patient‐year, thus favourably comparing with the rate reported in that study (i.e. 1.47 per patient‐year). Moreover, applying this classification, the positive predictive value would be 58 vs. 11.3% in the MultiSENSE study. This is likely attributable to the real‐time evaluation of HeartLogic alerts and thus an opportunity to identify more instances of early worsening HF. These results also compare favourably with the performance of previous HF diagnostics based on intrathoracic impedance monitoring. Indeed, in the Sensitivity of the InSync Sentry OptiVol Feature for the Prediction of Heart Failure study,2 the reported positive predictive value was only 4.7% in the blinded validation phase and 38.1% in the subsequent unblinded phase.
Interestingly, our comparison of patients' characteristics revealed more frequent diabetes and more severe functional impairment and systolic dysfunction at the time of activation in patients who received HeartLogic alerts during the observation period. This finding provides further evidence of the ability of the index to stratify risk, because patients at higher risk of HF events are plausibly those in worse clinical condition at the baseline. A sub‐analysis of the MultiSENSE study9 showed that HeartLogic maintained its predictive value for HF events even after correction for baseline variables in a multivariate model that included ejection fraction, New York Heart Association class, and diabetes, together with other variables.
In patients with alerts, we also observed less frequent use of β‐blockers. This, together with a trend towards lower ivabradine use, may be an indicator of low adherence to the prescribed therapy, which was also detected in some patients during follow‐up. Moreover, less use of heart rate‐lowering drugs may also explain the occurrence of HeartLogic alerts, because high heart rate contributes to increasing the index.
Among the sensed parameters that contribute to the calculation of the HeartLogic index, accelerometer‐based heart sounds seemed to correlate well with HF status. Specifically, the third heart sound is detected in order to provide an objective measure of elevated filling pressure, while the first heart sound is taken as a surrogate for left ventricular contractility, because it has been shown to correlate with the maximum pressure derivative.10 In agreement with previous studies,11 we also observed changes in the respiratory pattern at the time of HF events. Moreover, decreased thoracic impedance was observed only in a minority of events, in agreement with previous findings on the low sensitivity of the parameter.2 Previously proposed diagnostics for the clinical management of HF have mainly been based on monitoring intrathoracic impedance to detect fluid accumulation.12 However, it has been shown that, instead of improving the management of HF, these diagnostics are associated with a relatively high rate of false‐positive detections and a consequent increase in hospital admissions.2, 3
HeartLogic was developed in accordance with the hypothesis that an algorithm that combines multiple physiological sensors that evaluate different aspects of HF physiology would be superior to monitoring a single sensor. In this first description of the use of HeartLogic in clinical practice, the algorithm demonstrated its ability to detect gradual worsening of HF. Therefore, activation of the associated alert may allow clinical worsening to be detected early and enable action to be taken in patients who are deteriorating but not yet critical, thereby potentially preventing severe events.
Limitations
The main limitations of this study are the small sample size and the short duration of observation. Although HeartLogic has been shown to be accurate in detecting HF events, further studies are needed in order to establish whether this HF alert, associated with an appropriate intervention strategy, may improve patient outcomes.
Conflict of interest
M. Campari and S. Valsecchi are employees of Boston Scientific. The other authors report no conflicts.
Acknowledgement
Our sincere thanks go to Viktoria Averina (Boston Scientific, St. Paul, Minnesota) for her support in the analysis of data, which made this study possible.
Capucci A., Santini L., Favale S., Pecora D., Petracci B., Calò L., Molon G., Cipolletta L., Bianchi V., Schirripa V., Santobuono V. E., La Greca C., Campari M., Valsecchi S., Ammirati F., and D'Onofrio A. (2019) Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: a retrospective case series report, ESC Heart Failure, 6: 308–318. 10.1002/ehf2.12394.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE‐HF trial. Eur Heart J 2011; 32: 2266–2273. [DOI] [PubMed] [Google Scholar]
- 3. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M, DOT‐HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon‐Moreau L, Proff J, Gerds TA, Anker SD, Torp‐Pedersen C. Daily remote monitoring of implantable cardioverter‐defibrillators: insights from the pooled patient‐level data from three randomized controlled trials (IN‐TIME, ECOST, TRUST). Eur Heart J 2017; 38: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, Mangoni Di S, Stefano L, Burri H, MORE‐CARE Study Investigators . Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE‐CARE multicentre randomized controlled trial. Eur J Heart Fail 2017; 19: 416–425. [DOI] [PubMed] [Google Scholar]
- 6. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017; 38: 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail 2017; 5: 216–225. [DOI] [PubMed] [Google Scholar]
- 8. Multiple Cardiac Sensors for the Management of Heart Failure (MANAGE‐HF) . ClinicalTrials.gov identifier: NCT03237858
- 9. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C, Thakur PH, An Q, Wehrenberg S, Hammill EF, Zhang Y, Boehmer JP. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circ Heart Fail 2018; 11: e004669. [DOI] [PubMed] [Google Scholar]
- 10. Thakur PH, An Q, Swanson L, Zhang Y, Gardner RS. Haemodynamic monitoring of cardiac status using heart sounds from an implanted cardiac device. ESC Heart Fail 2017; 4: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forleo GB, Santini L, Campoli M, Malavasi M, Scaccia A, Menichelli M, Riva U, Lamberti F, Carreras G, Orazi S, Ribatti V, Di Biase L, Lovecchio M, Natale A, Valsecchi S, Romeo F. Long‐term monitoring of respiratory rate in patients with heart failure: the Multiparametric Heart Failure Evaluation in Implantable Cardioverter‐Defibrillator Patients (MULTITUDE‐HF) study. J Interv Card Electrophysiol 2015; 43: 135–144. [DOI] [PubMed] [Google Scholar]
- 12. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005; 112: 841–848. [DOI] [PubMed] [Google Scholar]


