Abstract
Aims
Vitamin D deficiency is prevalent in heart failure (HF), but its relevance in early stages of heart failure with preserved ejection fraction (HFpEF) is unknown. We tested the association of 25‐hydroxyvitamin D [25(OH)D] serum levels with mortality, hospitalizations, cardiovascular risk factors, and echocardiographic parameters in patients with asymptomatic diastolic dysfunction (DD) or newly diagnosed HFpEF.
Methods and results
We measured 25(OH)D serum levels in outpatients with risk factors for DD or history of HF derived from the DIAST‐CHF study. Participants were comprehensively phenotyped including physical examination, echocardiography, and 6 min walk test and were followed up to 5 years. Quality of life was evaluated by the Short Form 36 (SF‐36) questionnaire. We included 787 patients with available 25(OH)D levels. Median 25(OH)D levels were 13.1 ng/mL, mean E/e′ medial was 13.2, and mean left ventricular ejection fraction was 59.1%. Only 9% (n = 73) showed a left ventricular ejection fraction <50%. Fifteen per cent (n = 119) of the recruited participants had symptomatic HFpEF. At baseline, participants with 25(OH)D levels in the lowest tertile (≤10.9 ng/L; n = 263) were older, more often symptomatic (oedema and fatigue, all P ≤ 0.002) and had worse cardiac [higher N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and left atrial volume index, both P ≤ 0.023], renal (lower glomerular filtration rate, P = 0.012), metabolic (higher uric acid levels, P < 0.001), and functional (reduced exercise capacity, 6 min walk distance, and SF‐36 physical functioning score, all P < 0.001) parameters. Increased NT‐proBNP, uric acid, and left atrial volume index and decreased SF‐36 physical functioning scores were independently associated with lower 25(OH)D levels. There was a higher risk for lower 25(OH)D levels in association with HF, DD, and atrial fibrillation (all P ≤ 0.004), which remained significant after adjusting for age. Lower 25(OH)D levels (per 10 ng/mL decrease) tended to be associated with higher 5 year mortality, P = 0.05, hazard ratio (HR) 1.55 [1.00; 2.42]. Furthermore, lower 25(OH)D levels (per 10 ng/mL decrease) were related to an increased rate of cardiovascular hospitalizations, P = 0.023, HR = 1.74 [1.08; 2.80], and remained significant after adjusting for age, P = 0.046, HR = 1.63 [1.01; 2.64], baseline NT‐proBNP, P = 0.048, HR = 1.62 [1.01; 2.61], and other selected baseline characteristics and co‐morbidities, P = 0.043, HR = 3.60 [1.04; 12.43].
Conclusions
Lower 25(OH)D levels were associated with reduced functional capacity in patients with DD or HFpEF and were significantly predictive for an increased rate of cardiovascular hospitalizations, also after adjusting for age, NT‐proBNP, and selected baseline characteristics and co‐morbidities.
Keywords: Vitamin D, Diastolic dysfunction, Heart failure, HFpEF, NT‐proBNP
Introduction
Vitamin D deficiency is commonly observed, and the prevalence is as high as almost 50% among the elderly.1, 2 The main vitamin D source is sunlight‐induced vitamin D production in the skin. Vitamin D deficiency can be caused by limited sun exposure and consequential reduced ultraviolet B‐induced vitamin D production in the skin.1, 3 Naturally, after synthesis in the skin, vitamin D becomes metabolized by a hepatic hydroxylase into 25‐hydroxyvitamin D [25(OH)D], which is the main circulating vitamin D metabolite and is used for the classification of vitamin D status by most guidelines.3, 4 Furthermore, 25(OH)D becomes hydroxylated by a renal 1α‐hydroxylase into calcitriol (1,25‐dihydroxyvitamin D), which is the most active vitamin D metabolite, which has a high affinity to the vitamin D receptor, and which is rather an indicator of calcium homeostasis and kidney function than a good indicator of vitamin D status.1, 3, 4
Vitamin D suppresses activation of the cardiac renin–angiotensin system and of the natriuretic peptides, regulates the extracellular matrix turnover, calcium flux, and myocardial contractility, and affects the differentiation and proliferation of cardiomyocytes, which may mediate antihypertrophic and antihypertensive effects of vitamin D and protect against myocardial dysfunction.2, 3 Mice with a systemic knockout of the vitamin D receptor developed cardiac hypertrophy and dysfunction with increased activation of the cardiac renin–angiotensin system, and in vitamin D‐deficient rats, cardiac hypertrophy with increased myocardial collagen content and shortening of the QT interval was also demonstrated.3
A lack of vitamin D has been reported to be associated with increased cardiovascular risk and various diseases, including hypertension, diabetes, obesity, vascular inflammation, left ventricular (LV) hypertrophy, and congestive heart failure (HF).5, 6, 7, 8
Heart failure is a major health problem in our society.9 Clinical studies demonstrated that a low vitamin D status is prevalent in patients with HF.2, 4, 10, 11 A significant proportion of patients with HF is marked by preserved left ventricular ejection fraction (LVEF) and diastolic dysfunction (DD). The prevalence of this subgroup, termed HF with preserved ejection fraction (HFpEF), accounts for more than 50% of all HF cases, and the prevalence of DD and HFpEF is increasing.12, 13, 14 Several studies have already proved an association between vitamin D deficiency and a poor outcome in patients with HF and reduced ejection fraction (HFrEF).4, 6, 10 In contrast, a correlation between vitamin D status and the outcome in patients with asymptomatic DD or HFpEF has not been investigated so far. Therefore, the aim of our study was to determine whether an insufficient vitamin D status is associated with poor prognosis (i.e. mortality and hospitalizations) in a large cohort of patients with asymptomatic DD or HFpEF. Furthermore, we aimed to evaluate cross‐sectional associations between 25(OH)D and echocardiographic measures, cardiovascular risk factors, and co‐morbidities. Additionally, the diagnostic and prognostic value of vitamin D, in comparison with the generally accepted parameter N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) in patients with HF, was investigated.
Methods
Patient population
We recruited outpatients aged 50 to 85 years in 2004 and 2005 for the prospective, multicentre, observational DIAST‐CHF study, which is part of the German Competence Network of Heart Failure. The participants had either more than or equal to one risk factor(s) for DD (hypertension, diabetes mellitus, sleep apnea syndrome, and coronary artery disease) or a previous diagnosis or history of HF. Candidates were identified by primary care physicians. Exclusion criteria were unwillingness to participate or inability for logistic reasons. Detailed description of the study concept has been published previously.15
For the present analysis, participants with missing data of diastolic function were excluded. Additionally, we excluded those patients in whom vitamin D parameter [serum levels of 25(OH)D] was not ascertainable. All study subjects gave written informed consent. The DIAST‐CHF study complies with the Declaration of Helsinki and was approved by the responsible Ethics Committees.
Clinical assessment
All participants underwent a complete physical examination, electrocardiogram, echocardiography, standardized 6 min walk test, and blood sampling. Medical history, including signs and symptoms of HF, co‐morbidities, and medication, was determined. Echocardiography, particularly comprehensive evaluation of diastolic function with tissue Doppler technics, was performed in accordance with the guidelines of the American Society of Echocardiography.16 A standard operating procedure was used, and randomly chosen examinations were verified by the echo core laboratory of the Competence Network Heart Failure at the University of Essen. DD was determined as described previously.15, 17 Quality of life was assessed by the 36‐Item Short Form Health Survey (SF‐36). Follow‐up was after 5 years.
Routine laboratory and neurohumoral activation measurements have been described in a previous publication.15 Classifications of the vitamin D status are usually performed according to serum levels of 25(OH)D. In detail, serum 25(OH)D measurements were performed by a competitive binding protein assay (DiaSorin, Stillwater, MN, USA) with an interassay coefficient of variation of 10–15%. This assay measures both 25‐hydroxyvitamin D2 and 25‐hydroxyvitamin D3. In addition, NT‐proBNP was measured with a commercially available electrochemiluminescence immunoassay on an Elecsys analyser (Roche Diagnostics GmbH, Mannheim, Germany).15
The primary outcome measurements of the present analysis of the DIAST‐CHF study were 5 year mortality, hospitalization, and cardiovascular hospitalization.
Statistical analysis
As there is no disease‐specific consensus about vitamin D cut‐off values, we categorized patients into tertiles of 25(OH)D serum levels, calculated according to the 25(OH)D concentrations of the whole study population. In our analyses, we performed comparisons between the first tertile and the upper tertiles because previous studies indicate that detrimental effects of vitamin D become only apparent at severely depressed 25(OH)D levels.18 Results were expressed as means with standard deviations or as median with interquartile range. Categorical variables were given as percentage or number of observations. Metric variables were tested with univariate ANOVA, ordinal‐scaled variables were tested with Kendall's rank correlation coefficient τ‐B, nominal variables were tested by a χ2 test, and index variables were calculated with Fisher's exact test.
To further elucidate associations of 25(OH)D concentrations with selected demographic, clinical, and biochemical variables, we performed logistic regression analyses, with 25(OH)D concentrations ≤10.9 and >10.9 ng/mL as the binary outcome variable. Beside univariate analysis, we performed a multivariate analysis as well, with results presented as odds ratios (ORs) with 95% confidence intervals.
Differences in the primary outcome measures between the first and the upper tertiles of 25(OH)D were calculated with Kaplan–Meier curves (log–rank tests) and Cox proportional hazard regression models using crude models as well as models adjusted for potential confounders. Results are presented as hazard ratios (HRs) with 95% confidence intervals. A P‐value ≤0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Study sample
25‐hydroxyvitamin D serum levels were available in 787 outpatients of the DIAST‐CHF study with risk factors for DD or with previous diagnosis of HF. Baseline characteristics, echocardiographic and exercise capacity parameters, of all patients and according to 25(OH)D tertiles (first tertile vs. the upper tertiles) are presented in Table 1. The median 25(OH)D serum level was 13.1 ng/mL (interquartile range = 7.5). Overall, 33.4% (n = 263, first tertile) of the patients had 25(OH)D concentrations ≤10.9 ng/mL. The mean age was 67.2 years, 47% were female, and mean LVEF was 59.1%. The mean value of E/e′ medial was 13.2, and 15% of the patients had HFpEF according to the Paulus criteria. Only 9% (n = 73) of all patients had a reduced LVEF <50%. Patients with lower 25(OH)D concentrations (≤10.9 ng/mL) were older (P = 0.002) and more symptomatic (oedema and fatigue, both P < 0.001; nocturnal cough, P = 0.039; New York Heart Association II–IV, P = 0.008) than those with 25(OH)D levels >10.9 ng/mL. Lower 25(OH)D levels were also associated with higher body mass index (P = 0.002) and heart rate (P < 0.001), lower glomerular filtration rate (P = 0.012) and HDL (P = 0.044), higher uric acid (P < 0.001), and higher NT‐proBNP (P = 0.023). Patients with lower 25(OH)D also showed reduced exercise capacity (6 min walk distance and SF‐36 physical functioning score, both P < 0.001), had higher values of left atrial volume index (LAVI) (P = 0.005), and showed HFpEF according to the Paulus criteria more often (P = 0.003). In addition, patients with lower 25(OH)D concentrations suffered from chronic obstructive pulmonary disease (P = 0.015) or atrial fibrillation (P = 0.024) more often and used angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers (P = 0.001), diuretics, and vitamin K antagonists or other anticoagulants (all P < 0.001) more frequently.
Table 1.
Baseline characteristics according to 25‐hydroxyvitamin D serum levels
| Vitamin D (ng/L) | Total | ≤10.9 | >10.9 | P‐value |
|---|---|---|---|---|
| No. of subjects | (N = 787) | (N = 263) | (N = 524) | |
| Clinical characteristics | ||||
| Age (years)—mean ± SD | 67.2 ± 8.2 | 68.5 ± 8.4 | 66.6 ± 8.0 | 0.002 |
| Female sex—N (%) | 367 (47) | 118 (45) | 249 (48) | 0.50 |
| Body mass index (kg/m2)—mean ± SD | 29.4 ± 5.0 | 30.1 ± 5.4 | 29.0 ± 4.7 | 0.002 |
| Systolic blood pressure (mmHg)—mean ± SD | 148 ± 21 | 148 ± 21 | 149 ± 21 | 0.53 |
| Diastolic blood pressure (mmHg)—mean ± SD | 83 ± 12 | 83 ± 12 | 84 ± 12 | 0.33 |
| Heart rate (1/min)—mean ± SD | 67 ± 12 | 69 ± 13 | 65 ± 12 | <0.001 |
| 6 min walk distance (m)—mean ± SD | 500 ± 118 | 477 ± 125 | 510 ± 114 | <0.001 |
| Signs and symptoms | ||||
| NYHA functional class—N (%) | <0.001 | |||
| No HF | 635 (81) | 185 (71) | 450 (86) | |
| NYHA I | 40 (5) | 26 (10) | 14 (3) | |
| NYHA II | 76 (10) | 33 (13) | 43 (8) | |
| NYHA III | 30 (4) | 15 (6) | 15 (3) | |
| NYHA IV | 1 (0) | — | 1 (0) | |
| NYHA II–IV—N (%) | 107 (14) | 48 (18) | 59 (11) | 0.008 |
| Oedema—N (%) | 175 (22) | 80 (30) | 95 (18) | <0.001 |
| Nycturia—N (%) | 475 (60) | 165 (63) | 310 (59) | 0.35 |
| Nocturnal cough—N (%) | 48 (6) | 23 (9) | 25 (5) | 0.039 |
| Fatigue—N (%) | 198 (25) | 88 (33) | 110 (21) | <0.001 |
| Co‐morbidities | ||||
| Coronary artery disease—N (%) | 187 (24) | 65 (25) | 122 (23) | 0.66 |
| Hypertension—N (%) | 710 (90) | 234 (89) | 476 (91) | 0.37 |
| Hyperlipidaemia—N (%) | 374 (48) | 122 (46) | 252 (48) | 0.71 |
| Diabetes mellitus—N (%) | 220 (28) | 81 (31) | 139 (27) | 0.24 |
| Smoking behaviour—N (%) | 0.09 | |||
| Non‐smoker | 377 (48) | 112 (43) | 265 (51) | |
| Former smoker | 328 (42) | 123 (47) | 205 (39) | |
| Smoker | 81 (10) | 28 (11) | 53 (10) | |
| COPD—N (%) | 68 (9) | 32 (12) | 36 (7) | 0.015 |
| Atrial fibrillation—N (%) | 34 (4) | 18 (7) | 16 (3) | 0.024 |
| Depression—N (%) | 79 (10) | 25 (10) | 54 (10) | 0.80 |
| Medication | ||||
| ACE‐I or ARB—N (%) | 522 (67) | 195 (75) | 327 (63) | 0.001 |
| Betablocker—N (%) | 400 (51) | 128 (49) | 272 (52) | 0.45 |
| Diuretics—N (%) | 431 (55) | 168 (64) | 263 (50) | <0.001 |
| Vitamin K antagonists/other anticoagulants—N (%) | 76 (10) | 40 (15) | 36 (7) | <0.001 |
| Antidepressants—N (%) | 60 (8) | 12 (5) | 48 (9) | 0.023 |
| Laboratory parameters | ||||
| NT‐proBNP (pg/mL)—median (IQR) | 116 (57 to 252) | 145 (63 to 292) | 106 (56 to 226) | 0.023 |
| Potassium (mmol/L)—mean ± SD | 4.3 ± 0.6 | 4.4 ± 0.6 | 4.2 ± 0.5 | 0.004 |
| HDL cholesterol (mg/dL)—mean ± SD | 53 ± 17 | 52 ± 18 | 54 ± 16 | 0.044 |
| Haemoglobin (g/dL)—mean ± SD | 14.0 ± 1.3 | 14.0 ± 1.3 | 14.0 ± 1.2 | 0.50 |
| GFR Clearance MDRD (mL/min)—mean ± SD | 73 ± 19 | 70 ± 19 | 74 ± 19 | 0.012 |
| Uric acid (mg/dL)—mean ± SD | 6.2 ± 1.6 | 6.6 ± 1.6 | 6.0 ± 1.5 | <0.001 |
| Quality of life | ||||
| PHQ‐9 score—mean ± SD | 4.9 ± 4.2 | 5.6 ± 4.4 | 4.6 ± 4.0 | 0.003 |
| SF‐36 physical functioning score—mean ± SD | 71 ± 25 | 62 ± 27 | 74 ± 24 | <0.001 |
| Echocardiographic parameters | ||||
| LVEF (%)—mean ± SD | 59.1 ± 8.3 | 58.3 ± 9.0 | 59.5 ± 7.9 | 0.05 |
| LVD(ED) (mm)—mean ± SD | 49.8 ± 6.3 | 50.2 ± 6.5 | 49.6 ± 6.2 | 0.22 |
| LV mass index—male—mean ± SD | 130 ± 30 | 131 ± 31 | 130 ± 29 | 0.67 |
| LV mass index—female—mean ± SD | 109 ± 25 | 110 ± 24 | 109 ± 25 | 0.56 |
| LA (end‐systolic) (mm)—mean ± SD | 42.0 ± 6.5 | 43.4 ± 6.2 | 41.3 ± 6.5 | <0.001 |
| LAVI (mL/m2)—mean ± SD | 25.6 ± 10.0 | 27.2 ± 10.8 | 24.6 ± 9.4 | 0.005 |
| E/e′ medial—mean ± SD | 13.2 ± 4.6 | 13.4 ± 5.4 | 13.1 ± 4.1 | 0.58 |
| HFpEF according to the Paulus scheme—N (%) | 119 (15) | 54 (21) | 65 (12) | 0.003 |
P‐value: Fisher's exact test for nominal data and t‐test/Welch test for metric data. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; E/e′ medial, mitral wave peak early filling velocity to (medial) mitral annular velocity ratio; GFR, glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; LA, left atrium; LAVI, left atrium volume index; LV, left ventricle; LVD(ED), left ventricular end diastolic diameter; MDRD, modification of diet in renal disease equation for estimating glomular filtration rate LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PHQ‐9, Patient Health Questionnaire‐Depression module; SD, standard deviation; SF‐36, 36‐Item Short‐Form Health Survey.
Selected baseline parameters, co‐morbidities, drugs, and 25‐hydroxyvitamin D serum level
In multiple linear regression analysis, increased values of NT‐proBNP (P = 0.001), uric acid (P < 0.001), and LAVI (P = 0.001) as well as decreased SF‐36 physical functioning scores (P < 0.001) and New York Heart Association class >I (P = 0.026) were independent determinants of lower 25(OH)D levels (per 10 ng/mL decrease). These findings remained significant after adjusting for age (Table 2A ).
Table 2A.
Selected baseline parameters and 25‐hydroxyvitamin D levels (per 10 ng/mL decrease)
| Variable | Unadjusted | Adjusted by age | ||
|---|---|---|---|---|
| B [95% CI] | P‐value | B [95% CI] | P‐value | |
| NT‐proBNP (geometric) | 1.44 [1.16; 1.79] | 0.001 | 1.29 [1.06; 1.56] | 0.011 |
| Uric acid (mg/mL) | 0.66 [0.39; 0.94] | <0.001 | 0.63 [0.35; 0.9] | <0.001 |
| 6 min walk distance (m) | −23.31 [−48.52; 1.9] | 0.07 | −11.72 [−35.13; 11.68] | 0.33 |
| SF‐36 physical functioning scale (points) | −11.3 [−16.1; −6.5] | <0.001 | −10.08 [−14.8; −5.35] | <0.001 |
| LA end‐systolic (mm) | 3.72 [2.5; 4.94] | <0.001 | 3.6 [2.37; 4.82] | <0.001 |
| LAVI (mL/m2) | 3.15 [1.32; 4.98] | 0.001 | 2.76 [0.94; 4.57] | 0.003 |
| NYHA >I | 0.09 [0.01; 0.16] | 0.026 | 0.08 [0.001; 0.152] | 0.047 |
B, regression coefficient; CI, confidence interval; LA, left atrium; LAVI, left atrium volume index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SF‐36, 36‐Item Short‐Form Health Survey.
Logistic regression analysis showed a statistically higher risk for lower 25(OH)D levels (per 10 ng/mL decrease) in association with DD, OR 1.84 [1.24; 2.73]; P = 0.002, or HF (history of HF, verified by cardiologists or primary care physicians, OR 2.54 [1.73; 3.72]; P < 0.001). Furthermore, selected drugs and co‐morbidities, notably the presence of atrial fibrillation, OR 3.2 [1.44; 7.10]; P = 0.004, were associated with decreased 25(OH)D levels. These associations remained significant after adjusting for age. The tested co‐morbidities and drugs are completely listed in Table 2B .
Table 2B.
Heart failure, co‐morbidities, drugs, and 25‐hydroxyvitamin D levels (per 10 ng/mL decrease)
| Variable | Unadjusted | Adjusted by age | ||
|---|---|---|---|---|
| Odds ratio [95% CI] | P‐value | Odds ratio [95% CI] | P‐value | |
| Heart failure | 2.54 [1.73; 3.72] | <0.001 | 2.38 [1.62; 3.50] | <0.001 |
| DD by Paulus criteria | 1.84 [1.24; 2.73] | 0.002 | 1.58 [1.06; 2.36] | 0.025 |
| Atrial fibrillation | 3.20 [1.44; 7.10] | 0.004 | 2.76 [1.23; 6.20] | 0.014 |
| Oedema | 2.31 [1.62; 3.28] | <0.001 | 2.16 [1.51; 3.07] | <0.001 |
| Diuretics | 1.57 [1.22; 2.02] | 0.001 | 1.46 [1.13; 1.89] | 0.004 |
| Vitamin K antagonists or other anticoagulants | 2.31 [1.39; 3.84] | 0.001 | 2.06 [1.23; 3.44] | 0.006 |
CI, confidence interval; DD, diastolic dysfunction.
Prognostic value of 25‐hydroxyvitamin D
During 5 years of follow‐up, 77 (9.8%) patients died, 227 (28.8%) patients were hospitalized, and 62 (7.9%) patients were lost to follow‐up. There was no significant difference between the group with 25(OH)D level ≤10.9 ng/mL and the group that had 25(OH)D level >10.9 ng/mL relating to the endpoint mortality (log–rank test P = 0.142) (Figure 1 ). Additionally, the number of first hospitalizations increased over the time, but we detected no significant difference between both 25(OH)D groups regarding the endpoint first hospitalization (log–rank test P = 0.172). However, cardiovascular hospitalization increased significantly in the lowest compared with the upper two 25(OH)D tertiles (log–rank test P = 0.012) (Figure 2 ).
Figure 1.
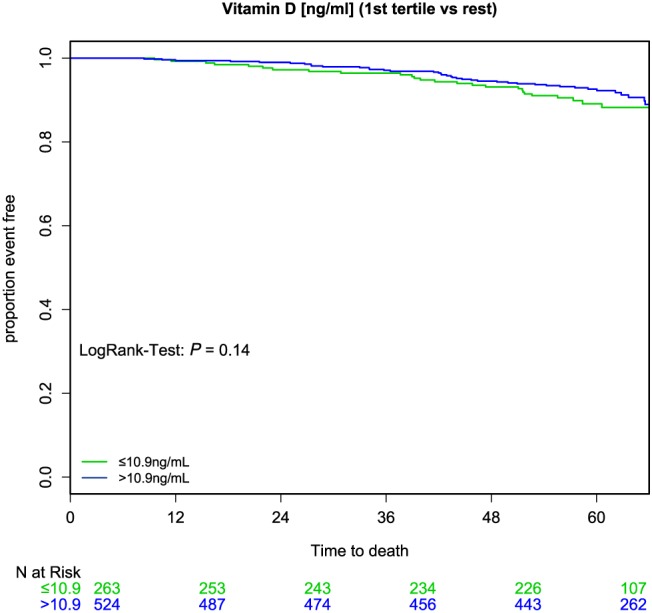
25‐hydroxyvitamin D and mortality. There was no significant difference between the lowest (≤10 ng/mL, green) and the upper (>10 ng/mL, blue) 25‐hydroxyvitamin D tertiles relating to the endpoint mortality (log–rank test P = 0.142).
Figure 2.
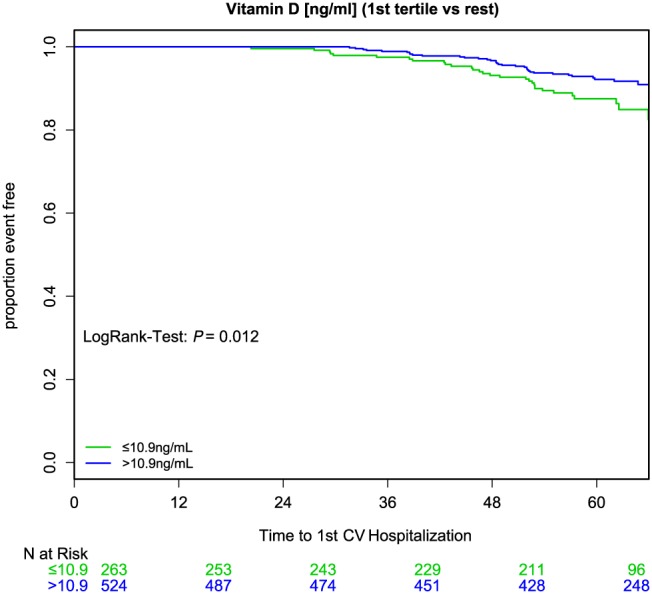
25‐hydroxyvitamin D and cardiovascular (CV) hospitalization. Cardiovascular hospitalization increased significantly in the lowest (≤10 ng/mL, green) compared with the upper (>10 ng/mL, blue) two 25‐hydroxyvitamin D tertiles (log–rank test P = 0.012).
Multivariable Cox regression analysis showed that lower 25(OH)D concentrations (per 10 ng/mL decrease) tended to be associated with higher 5 year mortality, P = 0.05, HR = 1.55 [1.00; 2.42], but there was no significant association between lower 25(OH)D levels and first hospitalization, P = 0.28, HR = 1.14 [0.90; 1.46]. After adjusting for age, NT‐proBNP, and several baseline characteristics, co‐morbidities, and drugs, there was also no significant correlation between lower 25(OH)D levels and the endpoints 5 year mortality or first hospitalization. However, lower 25(OH)D concentrations (per 10 ng/mL decrease) were significantly and independently associated with first cardiovascular hospitalization, P = 0.023, HR = 1.74 [1.08; 2.80], and remained statistically significant after adjusting for age, P = 0.046, HR = 1.63 [1.01; 2.64], NT‐proBNP at baseline, P = 0.048, HR = 1.62 [1.01; 2.61], and selected baseline characteristics, co‐morbidities, and drugs, P = 0.043, HR = 3.60 [1.04; 12.43] (all variables and results are shown in detail in Table 3 , Model 1).
Table 3.
25‐hydroxyvitamin D, NT‐proBNP, and outcome
| Model | Variable and model | 5 year mortality | First hospitalization | First CV hospitalization | |||
|---|---|---|---|---|---|---|---|
| HR [95% CI] | P‐value | HR [95% CI] | P‐value | HR [95% CI] | P‐value | ||
| 1a | Vitamin D (per 10 ng/mL decrease), unadjusted | 1.55 [1.00; 2.42] | 0.05 | 1.14 [0.90; 1.46] | 0.28 | 1.74 [1.08; 2.80] | 0.023 |
| 1b | Vitamin D (per 10 ng/mL decrease), adjusted for age | 1.16 [0.74; 1.82] | 0.51 | 1.15 [0.90; 1.46] | 0.27 | 1.63 [1.01; 2.64] | 0.046 |
| 1c | Vitamin D (per 10 ng/mL decrease), adjusted for NT‐proBNP | 1.10 [0.70; 1.74] | 0.67 | 1.14 [0.90; 1.46] | 0.28 | 1.62 [1.01; 2.61] | 0.048 |
| 1d | Vitamin D (per 10 ng/mL decrease), adjusted for NT‐proBNP and age | 1.04 [0.66; 1.63] | 0.87 | 1.15 [0.90; 1.46] | 0.27 | 1.59 [0.98; 2.56] | 0.06 |
| 1e | Vitamin D (per 10 ng/mL decrease), adjusted for ¥ | 0.70 [0.33; 1.49] | 0.35 | 1.23 [0.77; 1.97] | 0.38 | 3.60 [1.04; 12.43] | 0.043 |
| 1f | Vitamin D (per 10 ng/mL decrease), adjusted for ¥ and age | 0.68 [0.31; 1.46] | 0.32 | 1.20 [0.75; 1.92] | 0.44 | 3.37 [0.97; 11.74] | 0.06 |
| 2a | NT‐proBNP (per two‐fold increase), unadjusted | 1.72 [1.54; 1.93] | <0.001 | 1.01 [0.93; 1.09] | 0.86 | 1.19 [1.04; 1.36] | 0.013 |
| 2b | NT‐proBNP (per two‐fold increase), adjusted for age | 1.56 [1.37; 1.77] | <0.001 | 1.00 [0.92; 1.10] | 0.93 | 1.14 [0.98; 1.33] | 0.08 |
| 2c | NT‐proBNP (per two‐fold increase), adjusted for Vitamin D | 1.71 [1.53; 1.93] | <0.001 | 1.00 [0.92; 1.08] | 0.98 | 1.16 [1.01; 1.33] | 0.033 |
| 2d | NT‐proBNP (per two‐fold increase), adjusted for Vitamin D and age | 1.56 [1.37; 1.77] | <0.001 | 1.00 [0.92; 1.09] | 1.00 | 1.13 [0.97; 1.31] | 0.12 |
| 2e | NT‐proBNP (per two‐fold increase), adjusted for * | 1.58 [1.20; 2.09] | 0.001 | 1.02 [0.86; 1.21] | 0.79 | 1.12 [0.80; 1.55] | 0.52 |
| 2f | NT‐proBNP (per two‐fold increase), adjusted for * and age | 1.46 [1.11; 1.93] | 0.007 | 0.99 [0.83; 1.18] | 0.90 | 1.06 [0.76; 1.49] | 0.73 |
¥ represents NT‐proBNP, heart failure, diastolic dysfunction by Paulus, atrial fibrillation, oedema, diuretics, vitamin K antagonists or anticoagulants, uric acid, 6 min walk distance, Short Form 36 physical functioning scale, left atrium (end‐systolic), and left atrium volume index. * represents vitamin D, heart failure, diastolic dysfunction by Paulus, atrial fibrillation, oedema, diuretics, vitamin K antagonists or anticoagulants, uric acid, 6 min walk distance, Short Form 36 physical functioning scale, left atrium (end‐systolic), and left atrium volume index. CI, confidence interval; CV, cardiovascular; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
In contrast, increased NT‐proBNP levels (per two‐fold increase) had a predictive impact on 5 year mortality, P < 0.001, HR 1.72 [1.54; 1.93], and first cardiovascular hospitalization, P = 0.013, HR 1.19 [1.04; 1.36]. But after adjusting for age, vitamin D, and different baseline characteristics, co‐morbidities, and drugs, increased NT‐proBNP concentrations (per two‐fold increase) remained significantly and independently associated with 5 year mortality only, age: P < 0.001, HR 1.56 [1.37; 1.77]; vitamin D: P < 0.001, HR 1.71 [1.53; 1.93]; baseline characteristics: P = 0.001, HR 1.58 [1.20; 2.09] (all variables and results are shown in detail in Table 3, Model 2).
Discussion
To our knowledge, this is the first study to elucidate the association of 25(OH)D serum levels with mortality and hospitalizations as well as with cardiovascular risk factors and echocardiographic measures of LV function especially in patients with asymptomatic DD or HFpEF. The present study demonstrated that vitamin D deficiency, as defined as the lowest tertile, is highly prevalent in our cohort: 33.4% (n = 263, first tertile) of all patients had 25(OH)D concentrations ≤10.9 ng/mL. Several previous studies reported vitamin D deficiency to be highly prevalent in HF patients2, 4, 7, 10, 11, 19 and indicated poor outcome of these individuals.4, 6, 7, 10, 19 There is no consensus about a definite cut‐off level for vitamin D deficiency; nevertheless, 25(OH)D levels <10 ng/mL (multiply by 2.496 to convert ng/mL to nmol/L) are considered by several previous studies as definite vitamin D deficiency, which is comparable with our cut‐off.2, 6, 7
We found no significant association between the vitamin D status of patients with DD or HFpEF and the endpoints mortality and first hospitalization. However, cardiovascular hospitalization rates increased significantly with decreasing vitamin D serum level. Multivariable Cox regression analysis showed that lower 25(OH)D concentrations (per 10 ng/mL decrease) tended to be associated with higher 5 year mortality but not significantly with first hospitalization. However, lower 25(OH)D concentrations were significantly and independently associated with a first cardiovascular hospitalization of patients with DD or HFpEF and remained statistically significant after adjusting for age, baseline values of NT‐proBNP, and selected baseline characteristics and co‐morbidities. Vitamin D deficiency seems to be a predictor for increased cardiovascular hospitalizations.
In contrast, increased NT‐proBNP levels were predictive of 5 year mortality risk as well as of first cardiovascular hospitalizations. However, after adjusting for age, vitamin D, and selected baseline characteristic and co‐morbidities, increased NT‐proBNP concentration remained significant and independently associated only with 5 year mortality. NT‐proBNP, which is actually an important marker in diagnosis and therapy of HF, seems to reflect the overall mortality risk in our analysis.
Several studies have already examined the association between vitamin D status and outcome in patients with HFrEF or HF in general and were predominantly in line with our results, according to a poor outcome in consequence of a lack of vitamin D. Liu et al. measured 25(OH)D levels in 548 HF patients with reduced LVEF. After a mean follow‐up of 18 months, all‐cause mortality increased significantly with decreasing 25(OH)D tertiles. HF rehospitalizations increased numerically, across decreasing 25(OH)D tertiles (first tertile: vitamin D <12 ng/mL, second tertile: vitamin D 12–17.6 ng/mL, third tertile: vitamin D >17.6 ng/mL), although this increase did not reach statistical significance.10 In line with this, Schierbeck et al. examined levels of 25(OH)D in a prospective study of 148 HF outpatients with reduced LVEF with a follow‐up after 3.5 years: decreased level of 25(OH)D (vitamin D insufficient: 25(OH)D <20 ng/mL, vitamin D deficient: 25(OH)D <10 ng/mL) was independently associated with all‐cause and cardiovascular mortality in a population of outpatients with HF, independent of other HF markers such as glomerular filtration rate, LVEF, NT‐proBNP, and age.6 A similar study of Zitterman et al. investigated the association between the vitamin D metabolite calcitriol and the prognosis in 383 end‐stage congestive HF patients with an LVEF below 35%. There was a high prevalence of low calcitriol [1,25(OH)2D] levels, and deficient calcitriol levels were associated with poor clinical outcome in these patients.4 Gotsman et al. demonstrated that vitamin D deficiency [25(OH)D <10 ng/mL] had a higher prevalence in patients with HF compared with the control group (28% vs. 22%) and was a significant predictor of reduced survival. Seventy‐five per cent of those HF patients had an ischaemic heart disease, but there was no differentiation relating to LVEF.7 Pilz et al. reported in the framework of the LURIC study that in patients who were routinely referred to coronary angiography, low levels of 25(OH)D (severe deficiency <10 ng/mL, moderate deficiency 10–20 ng/mL, and insufficiency 20–30 ng/mL) and 1,25(OH)2D were associated with HF, deaths due to HF, and sudden cardiac death, but they did not differentiate between HFrEF and HFpEF.2
Trials that elucidate the correlation between vitamin D status and outcome in patients with asymptomatic DD or HFpEF are sparse to date. In the framework of the Hoorn study, Pilz et al. measured 25(OH)D levels and performed LV echocardiograms of 614 older men and women.20 25(OH)D serum levels were not significantly associated with LV structure and function. However, they found a moderate but non‐significant trend towards increased prevalence of DD in participants with vitamin D deficiency.20 Nevertheless, their echocardiographic evaluation did not include tissue Doppler examination for diagnosing DD. Also, van Ballegooijen et al. (the Hoorn study 2012) showed no strong association of 25(OH)D with myocardial structure and function.21 Fall et al. measured 25(OH)D levels and performed echocardiography in subjects without prior HF at age 70 in the PIVUS study. Higher circulating vitamin D concentrations were associated with better systolic LV function and smaller left ventricular end systolic diameter (LVESD) at baseline. Over 5 years of follow‐up, measures of cardiac geometry tended to increase, and measures of LV systolic and diastolic function tended to decrease. No significant association was observed between 25(OH)D at baseline and changes of LV measures over the time. They only measured vitamin D concentrations at the age of 70, and therefore, they could not analyse, whether there exists a correlation between changes of vitamin D levels over the time of 5 years and changes of echocardiography LV measures.22
In the present analyses, we could find a higher probability for the development of decreased 25(OH)D levels in case of DD or HF. Low vitamin D levels in these patients could be explained as a consequence of reduced sun exposure due to disease‐related sedentary lifestyle, limited mobility, and reduced outdoor activities or in case of malnutrition.2, 20 Baseline parameters of NT‐proBNP, uric acid, and LAVI, as well as selected co‐morbidities and drugs, were independent determinants of lower 25(OH)D levels.
Our results provide the basis for future studies to further evaluate the significance of vitamin D as a marker for diagnosis and therapy of cardiovascular diseases, particularly asymptomatic DD and HFpEF. So far, the effects of the treatment with vitamin D supplements on asymptomatic DD and HFpEF are not known. Previous studies reported that vitamin D therapy is relatively easy, cheap, and safe.3, 19 We need further trials to prove the effect of regular vitamin D supplementation in prevention or treatment of DD and HFpEF.
Limitations
A limitation of our study is that our data are based on a typical cardiovascular risk cohort, despite representing data from almost 800 individuals. Our findings are also limited by significantly different results in an individual level and compared with other studies that is due to the approach that patients were categorized accordingly to 25(OH)D levels. Furthermore, in our cohort, we did not consider possible seasonal variations of 25(OH)D. Although our findings were adjusted for a selection of potential confounders, other impacting factors, such as an unhealthy mode of life (sedentary lifestyle, reduced outdoor activities, and obesity), might have been overlooked. Therefore, the generalizability of our findings to other populations may be limited. Moreover, the observational nature of our work precludes final conclusions regarding causality. In contrast, the main strengths of our analysis are the use of standardized 25(OH)D, the inclusion of comprehensively characterized patients with DD and HFpEF, and the 5 year follow‐up period.
Conclusions
Patients with lower levels of 25(OH)D and preserved LVEF were older and more symptomatic. They demonstrated reduced exercise capacity as well as manifested HFpEF significantly more often. Furthermore, decreased 25(OH)D concentrations were significantly and independently associated with first cardiovascular hospitalization and remained statistically significant after adjusting for age, NT‐proBNP, and several co‐morbidities and baseline characteristics. To elucidate whether vitamin D supplementation is useful for treatment and prevention of DD and HFpEF, further trials are needed.
Conflict of interest
During the conduct of the study, Dr. Edelmann reports grants from German Research Foundation, Dr. Herrmann‐Lingen and Dr. Holzendorf report grants from German Ministry of Education and Research (BMBF no. 81X2800119), and Dr. Wachter reports grants from Bundesministerium für Bildung und Forschung. The other authors had nothing to disclose.
Funding
This work was supported by grants from the German Federal Ministry of Education and Research (German Heart Failure Network, TP 7) (BMBF no. 81X2800119).
Nolte K., Herrmann‐Lingen C., Platschek L., Holzendorf V., Pilz S., Tomaschitz A., Düngen H.‐D., Angermann C. E., Hasenfuß G., Pieske B., Wachter R., and Edelmann F. (2019) Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction, ESC Heart Failure, 6: : 262–270. 10.1002/ehf2.12413.
References
- 1. Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6: 621–630. [DOI] [PubMed] [Google Scholar]
- 2. Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner‐Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross‐sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93: 3927–3935. [DOI] [PubMed] [Google Scholar]
- 3. Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 2010; 54: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 4. Zittermann A, Schleithoff SS, Götting C, Dronow O, Fuchs U, Kuhn J, Kleesiek K, Tenderich G, Koerfer R. Poor outcome in end‐stage heart failure patients with low circulating calcitriol levels. Eur J Heart Fail 2008; 10: 321–327. [DOI] [PubMed] [Google Scholar]
- 5. Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008; 52: 1949–1956. [DOI] [PubMed] [Google Scholar]
- 6. Schierbeck LL, Jensen TS, Bang U, Jensen G, Køber L, Jensen JE. Parathyroid hormone and vitamin D—markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail 2011; 13: 626–632. [DOI] [PubMed] [Google Scholar]
- 7. Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 2012; 14: 357–366. [DOI] [PubMed] [Google Scholar]
- 8. Holick MF. The vitamin D epidemic and its health consequences. J Nutr 2005; 135: 2739S–2748S. [DOI] [PubMed] [Google Scholar]
- 9. Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: trends in incidence and survival in a 10‐year period. Arch Intern Med 1999; 159: 29–34. [DOI] [PubMed] [Google Scholar]
- 10. Liu LC, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK, Silljé HH, van Gilst WH, Jaarsma T, de Boer RA. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 2011; 13: 619–625. [DOI] [PubMed] [Google Scholar]
- 11. Zittermann A, Schleithoff SS, Frisch S, Götting C, Kuhn J, Koertke H, Kleesiek K, Tenderich G, Koerfer R. Circulating calcitriol concentrations and total mortality. Clin Chem 2009; 55: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 12. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 13. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 14. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 15. Edelmann F, Stahrenberg R, Polzin F, Kockskämper A, Düngen HD, Duvinage A, Binder L, Kunde J, Scherer M, Gelbrich G, Hasenfuss G, Pieske B, Wachter R, Herrmann‐Lingen C. Impaired physical quality of life in patients with diastolic dysfunction associates more strongly with neurohumoral activation than with echocardiographic parameters: quality of life in diastolic dysfunction. Am Heart J 2011; 161: 797–804. [DOI] [PubMed] [Google Scholar]
- 16. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography‐summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol 2003; 42: 954–970. [DOI] [PubMed] [Google Scholar]
- 17. Edelmann F, Schmidt AG, Gelbrich G, Binder L, Herrmann‐Lingen C, Halle M, Hasenfuss G, Wachter R, Pieske B. Rationale and design of the ‘aldosterone receptor blockade in diastolic heart failure’ trial: a double‐blind, randomized, placebo‐controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure (Aldo‐DHF). Eur J Heart Fail 2010; 12: 874–882. [DOI] [PubMed] [Google Scholar]
- 18. Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njølstad I, Løchen ML, März W, Kleber ME, Tomaschitz A, Grübler M, Eiriksdottir G, Gudmundsson EF, Harris TB, Cotch MF, Aspelund T, Gudnason V, Rutters F, Beulens JW, van 't Riet E, Nijpels G, Dekker JM, Grove‐Laugesen D, Rejnmark L, Busch MA, Mensink GB, Scheidt‐Nave C, Thamm M, Swart KM, Brouwer IA, Lips P, van Schoor NM, Sempos CT, Durazo‐Arvizu RA, Škrabáková Z, Dowling KG, Cashman KD, Kiely M, Pilz S. Vitamin D and mortality: individual participant data meta‐analysis of standardized 25‐hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017; 12: e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilz S, Tomaschitz T. Vitamin D status: to be considered in heart failure patients! Eur J Heart Fail 2011; 13: 595–596. [DOI] [PubMed] [Google Scholar]
- 20. Pilz S, Henry RM, Snijder MB, van Dam RM, Nijpels G, Stehouwer CD, Kamp O, Tomaschitz A, Pieber TR, Dekker JM. Vitamin D deficiency and myocardial structure and function in older men and women: the Hoorn study. J Endocrinol Invest 2010; 33: 612–617. [DOI] [PubMed] [Google Scholar]
- 21. van Ballegooijen AJ, Snijder MB, Visser M, van den Hurk K, Kamp O, Dekker JM, Nijpels G, Stehouwer CD, Henry RM, Paulus WJ, Brouwer IA. Vitamin D in relation to myocardial structure and function after eight years of follow‐up: the Hoorn study. Ann Nutr Metab 2012; 60: 69–77. [DOI] [PubMed] [Google Scholar]
- 22. Fall T, Shiue I, Bergeå af Geijerstam P, Sundström J, Ärnlöv J, Larsson A, Melhus H, Lind L, Ingelsson E. Relations of circulating vitamin D concentrations with left ventricular geometry and function. Eur J Heart Fail 2012; 14: 985–991. [DOI] [PubMed] [Google Scholar]


